Introduction
The pseudopterosins are a class of diterpene glycosides isolated from the sea whip
Pseudopterogorgia elisabethae [
1–
3]. As with many marine natural products, diverse congeners of the pseudopterosins are found in different locations and there are presently fifteen known pseudopterosin derivatives (A–O). All of the known pseudopterosins contain the amphilectane skeleton with a glycosidic linkage at either C-9 or C-10.
The identity of the sugar and the degree of acetylation account for the additional structural variation of this family of diterpenes. Pseudopterosins A–D (
1–
4), from Sweetings Cay in the Bahamas, possess the amphilectane skeleton with an attached xylose sugar which is acetylated at different locations (
Figure 1).
The seco-pseudopterosins A–D are a related group of compounds (
5–
8) belonging to the serrulatane class of diterpenes initially isolated from
Pseudopterogorgia kallos in the Florida Keys [
4]. More recently, novel seco-pseudopterosins were reported to co-occur with pseudopterosins in
P. elisabethae [
3]. The pseudopterosin and seco-pseudopterosin classes of diterpenes exhibit potent anti-inflammatory and analgesic activity [
3,
5]. The pseudopterosins are pharmacologically distinct from typical NSAIDs and they appear to act by a novel mechanism of action [
6,
7]. The commercial market for the pseudopterosins, presently as ingredients in a skin cream, indicates a need for the development of a sustainable supply of these compounds. Consequently, a continuing goal in our laboratory is to elucidate all steps in the biosynthetic pathway leading to the pseudopterosins.
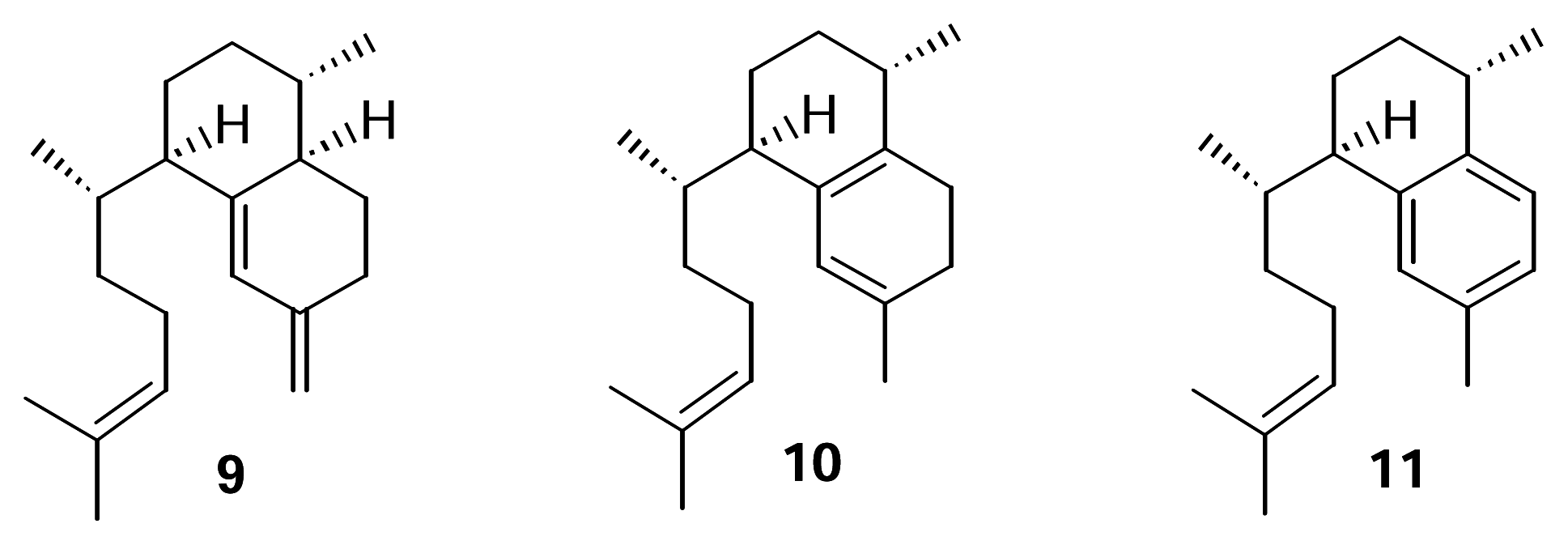
We recently confirmed the identity of the diterpene cyclase product leading to the pseudopterosins as elisabethatriene (
9) (
Figure 2).
This hydrocarbon, with the serrulatane skeleton, was isolated from extracts of
P. elisabethae collected in the Florida Keys and in Sweetings Cay, Bahamas. The utilization of
9 in pseudopterosin biosynthesis was confirmed through biosynthetic experiments [
8]. Erogorgiaene (
11) was recently reported from a collection of
P. elisabethae off Colombia [
9]. Given the structure of the pseudopterosin class of diterpenes and our report of the transformation of
9 to
1–4, it seems reasonable to suggest that
11 is an intermediate in this biosynthetic pathway. Further, it seems plausible that an endocyclic isomer of
9 such as
10 could be an intermediate in the conversion of
9 to
11.
This report describes the results of experiments directed at testing the hypothesis that isoelisabethatriene (10) and erogorgiaene (11) are early intermediates in pseudopterosin biosynthesis. Our approach was to identify these in our P. elisabethae extracts through the synthesis of standard samples of 10 and 11 from 9 and if present, test the compounds as metabolic intermediates.
Experimental Section
General
[1-3H]-Geranylgeranyl diphosphate (60 Ci/mol) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). All other chemicals and reagents were purchased from Fisher Scientific, Sigma Chemical Co., or Aldrich Chemical Co. The 1H-NMR spectra (one- and two-dimensional) were recorded in C6D6 on a Varian 500 NMR spectrometer at 500 MHz. Mass spectral measurements were conducted at the Midwest Center for Mass Spectrometry at University of Nebraska-Lincoln. TLC was performed using silica gel, GF254 pre-coated plates and HPLC was performed using either on an HP 1090 or using a Perkin Elmer Series 410 pump with a Perkin Elmer LC-30 RI detector.
Collection and Identification of Pseudopterogorgia elisabethae
P. elisabethae from Sweetings Cay, Bahamas was collected by SCUBA in the month of May in 1998–2002 at depths of 10–15 m. Specimens of the gorgonian were identified by standard morphological analysis and pseudopterosins A–D identified by analyzing the crude extract by analytical TLC. Samples were flash frozen with liquid nitrogen and stored at −80°C. P. elisabethae from the Florida Keys was collected by SCUBA in 1999–2001 from Tennessee reef at a depth of 25 m. Organisms were flash frozen with liquid nitrogen and stored at −80°C. Specimens collected from Florida were identified as P. elisabethae by Fredrick M. Bayer, Department of Invertebrate Zoology, Natural Museum of Natural History, Smithsonian Institution, Washington, D.C. A voucher specimen (USNM100430) has been deposited in this institute.
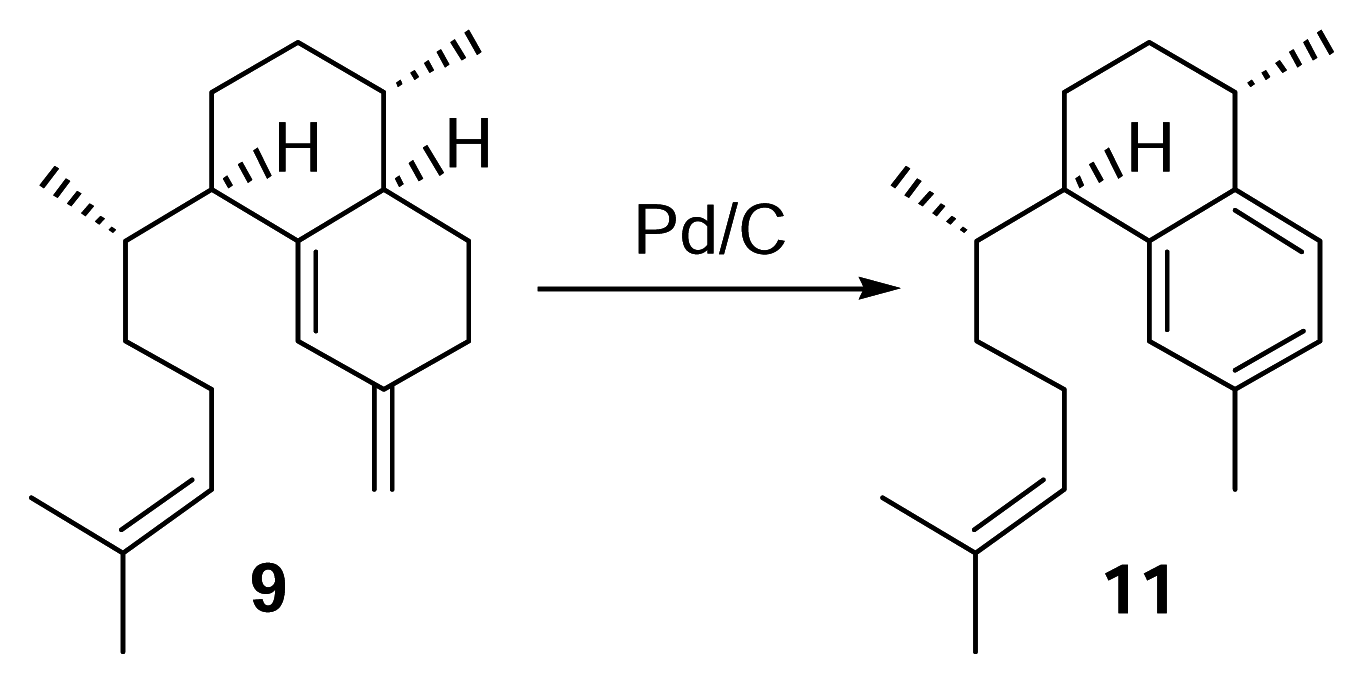
Identification of erogorgiaene (11) in P. elisabethae collected in the Florida Keys
Elisabethatriene (9) (0.5 mg) was reacted with a spatula tip of Pd/C in 500 μL of triethylene glycol dimethyl ether (triglyme), and the mixture refluxed under nitrogen for 3 hours. Following the reaction, the sample was filtered and the solvent evaporated under nitrogen. Analysis was then performed by reversed phase HPLC using a diode array detector (λ = 215 nm) with 100% methanol (2 mL/min.). To identify the compound in the Florida Keys’ P. elisabethae extract, the hexane layer (~6 g) obtained from partitioning a crude methylene chloride/ethyl acetate extract with hexanes and methanol/water (9:1) was passed through a small silica column with 100% hexanes. The solvent was evaporated using a rotary evaporator and fractionated by HPLC using a diode array detector (λ = 215 nm) with 100% methanol (2 mL/min.). The peak corresponding to a retention time of 22 min. was subjected to NMR analysis.
Identification of compound 10 in P. elisabethae collected in the Florida Keys
A crude methylene chloride/methanol extract of P. elisabethae (Florida Keys) was partially purified through a silica column eluted with 100% hexanes. The sample was further purified using preparative TLC with hexanes as the eluent and the band corresponding to an Rf of approximately 0.6 was isolated. Further fractionation was achieved by reversed phase HPLC using a refractive index detector and 100% methanol (2 mL/min.). The peak corresponding to a retention time of 27 min. was subjected to NMR analysis. Compound 10: UV (MeOH) λmax 245 nm; 1H-NMR (500 MHz, C6D6) δ 6.44 (1 H, s), 5.23 (1 H, t), 3.02 (1 H, m), 1.72 (3 H, s), 1.68 (3 H, s), 1.57 (3 H, s), 1.02 (3 H, d, J = 6 Hz), 0.90 (3 H, d, J = 6 Hz).
Purification of 3H-labeled 9 from incubation of [1-3H]-GGPP
Two separate reactions were conducted by incubating 20 μCi of [1-3H]-GGPP with each of two 500 μL aliquots of a Sweetings Cay P. elisabethae cell-free extract for 1 hour at 29°C and 200 rpm. A Sweetings Cay P. elisabethae cell-free extract that had been partially purified by DEAE-cellulose anion-exchange chromatography was also incubated with 10 μCi of [1-3H]-GGPP for 1 hour at 29°C and 200 rpm. The samples were extracted with hexanes and passed through a small silica pasteur pipet column (5 cm). Elisabethatriene (300,000 DPM) was rigorously purified by reversed phase HPLC using a refractive index detector and 100% methanol (2 mL/min.). The radioactivity was measured using a liquid scintillation counter.
Incubation of Florida Keys’ P. elisabethae Cell-free Extract with 3H-9 and Purification of 3H-11 and 3H-10
3H-Elisabethatriene (300,000 DPM) was collected from HPLC purification and the solvent was evaporated under a stream of N2. Following the addition of 1 mL of assay buffer and 0.05% Tween 20, the sample was sonicated for 10 min. and cell-free extract was added to a total volume of 40 mL. The sample was then incubated at 29°C and 200 rpm for 1 hour. The sample was lyophilized and partitioned between hexanes and methanol/H20 (9:1). After partial purification through a silica column (7 cm) with 100% hexanes, the sample was purified using reversed phase HPLC with 100% methanol (2 mL/min.). Compounds 9–11 were collected in separate vials and the solvent evaporated under N2. Ten percent of each sample was then reinjected and fractions were collected before and after each peak. The solvent was evaporated from the fractions and they were subjected to liquid scintillation counting. For compound 10, another 40% was added to the sample and the liquid scintillation counting repeated. The remaining amount (90%) of 9 (48,740 DPM) and 11 (1,860 DPM) was then reinjected to purify further.
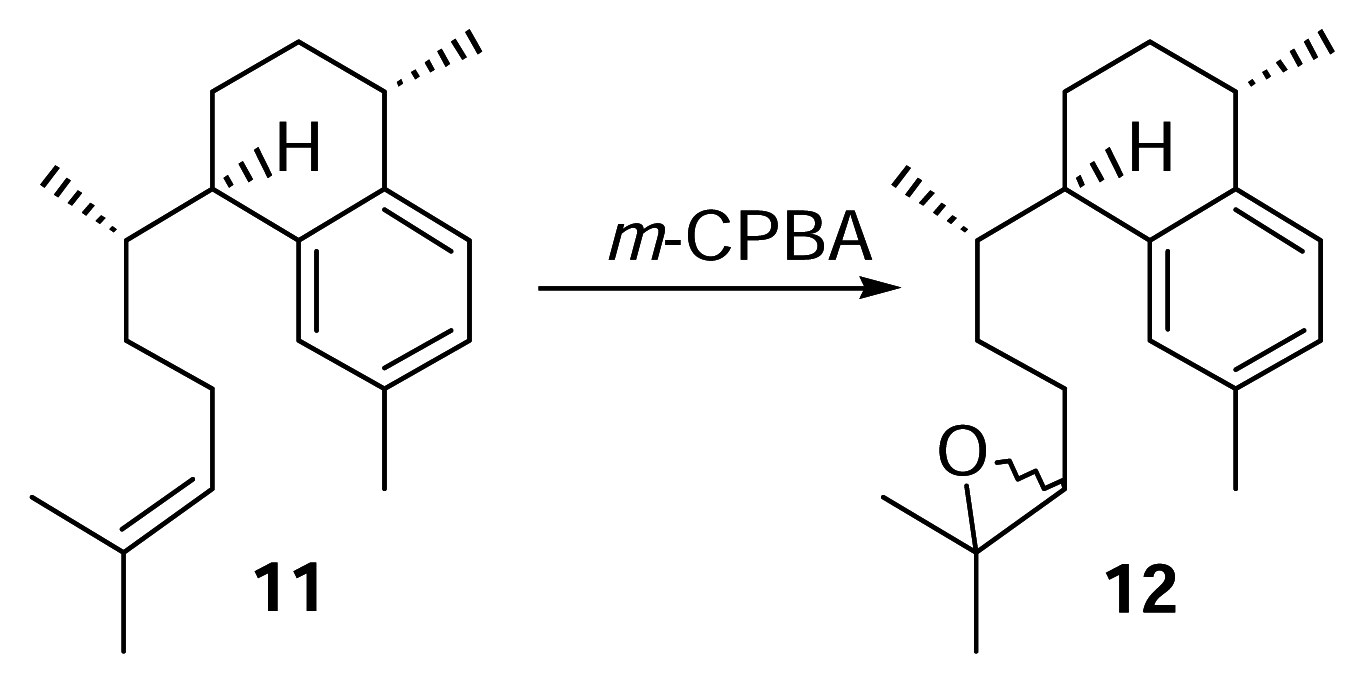
Derivitization of 11 to 12
meta-Chloroperoxybenzoic acid (m-CPBA, 76.2%, 3.5 mg) was dissolved in dry chloroform (5 mL). A portion of this mixture (1 mL, 4.6 μmol, 1.04 mg m-CPBA) was pipetted into a small separatory funnel and added to a stirred vial of 11 (4.6 μmol, 1.25 mg) over 10 minutes. The reaction was conducted at 0°C for 2.5 hours. The reaction mixture was concentrated under N2 and purified by reversed phase HPLC with 100% methanol (2 mL/min.) to afford 12 (1.25 mg) as a colorless oil. Compound 12: UV (MeOH) λmax 220 nm; HRMS calcd for C20H30O (M+) 286.2297, found 286.2297; 1H-NMR (500 MHz, C6D6) δ 7.12 (1 H, d), 7.06 (1 H, s), 6.95 (1 H, d, J = 7.5), 2.88 (1 H, m), 2.83 (1 H, m), 2.65 (1 H, m), 2.57 (1 H, dd), 2.21 (3 H, br s), 1.22 (3 H, d, J = 6.5), 1.17 (3 H, br s), 1.14 (3 H, br. d, J = 3.5), 0.67 (3 H, d, J = 3 Hz), 0.66 (3 H, d, J = 3 Hz). EIMS m/z [M]+ 183 (28), 175 (31), 174 (31), 173 (67), 159 (83), 157 (100), 145 (28), 69 (33).
Synthesis and Purification of Radioactive 12
3H-Labeled 9 (45.6 μg, 169 nmol, 1,420 DPM) was treated with m-CPBA (39.7 μg, 175 nmol) in dry CHCl3 at 0°C for 2.5 hours as described previously. The reaction mixture was purified by reversed phase HPLC as described (methanol, 2 mL/min.) to afford 8.8 nmol (5.2%) of 12 and the radioactivity measured. Quantities of the compounds were obtained by integration of HPLC peaks.
Synthetic Conversion of 9 to 11
H
5PMo
10V
2O
40− (60 mg) was stirred with 1,2-dichloroethane (1 mL) and tetraglyme (65 μL) in a 2 mL conical vial at 70°C for 5 min [
12]. Elisabethatriene (
9) (0.46 mg) was reacted with the mixture under oxygen atmosphere for 1.5 hours. The 1,2-dichloroethane was evaporated and the mixture partially purified through a small silica pasteur pipet column with hexanes. Reversed phase HPLC of the reaction (methanol, 2 mL/min.) showed the presence of erogorgiaene in approximately 80% yield.
Synthesis and Purification of Radioactive 11
3H-9 (48,740 DPM) purified from the incubation with [1-3H] GGPP was treated with H5PMo10V2O40− (26.5 mg) in tetraglyme (55 μL) and 1,2-dichloroethane at 70°C for 2 hours as described above. The sample was purified as described in the previous section and 5% was reinjected along with a small amount of “cold” erogorgiaene (11). Fractions were collected before and after the erogorgiaene peak and subjected to liquid scintillation counting. The other 95% was reinjected separately for purification.
Incubation of Cell-free Extract with 3H-11 and Purification of 3H-[1–4]
3H-11 (5,130 DPM) was added to a plastic conical vial using hexanes and the solvent evaporated. Following the addition of glycerol (4 mL, 10% v/v) and Tween 20 (20 μL, 0.05%), the sample was sonicated for 10 min. and a Sweetings Cay P. elisabethae cell-free extract was added to a total volume of 40 mL. The sample was incubated at 200 rpm and 29°C for 24 hours. Solvent partitioning was performed between hexanes and methanol/water (9:1) and then methanol/water (1:1) and methylene chloride. The pseudopterosins A–D were subsequently purified from the methylene chloride layer by normal phase HPLC with a hexane/ethyl acetate gradient (60:40 to 100% ethyl acetate over 35 min., λ=283 nm). Radioactivity was monitored using a liquid scintillation counter.