Web Applications for Teaching the Respiratory System: Content Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Application and Modules’ Description
2.1.1. Anatomy and Physiology Module
2.1.2. Work of Breathing Indexes Module
2.2. Validation Process
2.2.1. Anatomy and Physiology Module
Description of the Database
Analysis
2.2.2. Work of Breathing Indexes Module
Description of the Database
Analysis
2.2.3. Delphi Validation
2.3. Statistical Techniques
3. Results
3.1. Anatomy and Physiology Module
3.1.1. Statistical Analysis
Population Analysis
Individual Analysis
3.1.2. Delphi Validation
3.2. Work of Breathing Indexes Module
3.2.1. Statistical Analysis
Individual Analysis
3.2.2. Delphi Validation
4. Discussion
4.1. Anatomy and Physiology Module
4.1.1. Statistical Analysis
Population Analysis
Individual Analysis
4.1.2. Delphi Validation
4.2. Work of Breathing Indexes Model
4.2.1. Statistical Analysis
Population Analysis
Individual Analysis
4.2.2. Delphi Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| C | Respiratory system compliance |
| Ccw | Chest wall compliance |
| CL | Dynamic lung compliance |
| DB | Database |
| MV | Mechanical ventilation |
| Pao | Pressure at the airway opening |
| PEEP | Positive end-expiratory pressure |
| PImax | Maximal inspiratory pressure |
| Pmusc | Pressure developed by respiratory muscles |
| PS | Pressure support ventilation |
| PSV | Pressure support ventilation |
| R | Respiratory system resistance |
| Raw | Airway resistance |
| RR | Respiratory rate |
| RSBI | Rapid shallow breathing index or Tobin Index |
| V | Volume |
| VT | Tidal volume |
| VTmax | Maximal tidal volume |
| Airflow | |
| WOB | Work of breathing |
References
- Barret, K.E.; Barman, S.M.; Boitano, S.; Brooks, H. GANONG Fisiología médica, 24th ed.; McGraw-Hill: Mexico City, Mexico, 2012. [Google Scholar]
- Kasper, D.; Fauci, A.; Hauser, S.; Longo, D.; Jameson, J.L.; Loscalzo, J. Harrison. Principios de Medicina Interna, 19th ed.; McGraw-Hill: New York, NY, USA, 2016. [Google Scholar]
- Tulaimat, A.; Patel, A.; Wisniewski, M.; Gueret, R. The validity and reliability of the clinical assessment of increased work of breathing in acutely ill patients. J. Crit. Care 2016, 34, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Y.M.; Muñoz, I.C.; Hernández, A.M. Work of Breathing Dynamics under Changes of PEEP and Pressure Support in Non-invasive Mechanical Ventilation. In Applied Computer Sciences in Engineering; Springer: Cham, Switzerland, 2018; pp. 408–417. [Google Scholar]
- Grinnan, D.C.; Truwit, J.D. Clinical review: Respiratory mechanics in spontaneous and assisted ventilation. Crit. Care 2005, 9, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.L.; Tobin, M.J. A Prospective Study of Indexes Predicting the Outcome of Trials of Weaning from Mechanical Ventilation. N. Eng. J. Med. 1991, 324, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.C.; Enright, P.L.; Stukovsky, K.D.; Jiang, R.; Barr, R.G. Performance of maximum inspiratory pressure tests and maximum inspiratory pressure reference equations for 4 race/ethnic groups. Respir. Care 2009, 54, 1321–1328. [Google Scholar] [PubMed]
- Naureckas, E.T.; Solway, J. Disturbances of Respiratory Function. In Harrison’s Principles of Internal Medicine, 20th ed.; McGraw-Hill Education: New York, NY, USA, 2018; Chapter 279. [Google Scholar]
- Levitzky, M.G. Mechanics of Breathing. In Pulmonary Physiology, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2017; Chapter 2. [Google Scholar]
- Lipscombe, T.C.; Mungan, C.E. Breathtaking Physics: Human Respiration as a Heat Engine. Phys. Teach. 2020, 58, 150–151. [Google Scholar] [CrossRef]
- García-Prieto, E.; Amado-Rodríguez, L.; Albaiceta, G. Monitorization of respiratory mechanics in the ventilated patient. Med. Intensiv. (Engl. Ed.) 2014, 38, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.; Matínez, A.I.; Otero, M.C. Ventajas del uso de las TICs en el proceso de enseñanza-aprendizaje desde la óptica de los docentes universitarios españoles. Edutec. Rev. Electrón. Tecnol. Educ. 2009, 29, 127–145. [Google Scholar] [CrossRef]
- Arenas Márquez, F.J.; Domingo Carillo, M.A.; Molleda Jimena, G.; Ríos Martín, M.A.; Ruiz del Castillo, J.C. Aprendizaje interactivo en la educación superior a través de sitios web. Un estudio empírico. Pixel-Bit. Rev. Medios Educ. 2009, 35, 127–145. [Google Scholar]
- Sargent, R.G. Verification and validation of simulation models. In Proceedings of the Winter Simulation Conference, Syracuse, NY, USA, 5–8 December 2010. [Google Scholar]
- Hillston, J. Model Validation and Verification. In Teaching Notes; Technical Report; The University of Edinburgh Scotland: Edinburgh, UK, 2003. [Google Scholar]
- Rozanek, M.; Roubik, K. Design of the Mathematical Model of the Respiratory System Using Electroacoustic Analogy. Int. J. Math. Comput. Physical Electri. Comput. Eng. 2008, 2, 783–786. [Google Scholar]
- Heili Frades, S.; Peces Barba, G.; Rodríguez Nieto, M.J. Design of a Lung Simulator for Teaching Lung Mechanics in Mechanical Ventilation. Arch. Bronconeumol. 2007, 43, 674–679. [Google Scholar] [CrossRef]
- Al-Naggar, N.Q. Modelling and Simulation of Pressure Controlled Mechanical Ventilation System. Biomed. Sci. Eng. 2015, 8, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.; Da Silva, L.M.; Berton, D.C.; Cipriano, G.; Pitta, F.; O’Donnell, D.E.; Neder, J.A. Maximal Inspiratory Pressure: Does the Choice of Reference Values Actually Matter? Chest 2017, 152, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.C.; Hernández, A.M. Respiratory muscular response to obstructive maneuvers in non-invasively ventilated healthy subjects. Respir. Physiol. Neurobiol. 2018, 258, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Daza Lesmes, J. Evaluación Clínico-Funcional del Movimiento Corporal Humano; Editorial médica Panamericana: Colombia, Bogotá, 2007. [Google Scholar]
- Paramothayan, S. Essential Respiratory Medicine; Wiley: Hoboken, NJ, USA; Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Grippi, M.A.; Elias, J.; Fishman, J.A.; Kotloff, R.M.; Pack, A.I.; Senior, R.M.; Siegel, M.D. Fishman’s Pulmonary Diseases and Disorders, 5th ed.; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.J.; Rodrigues, V.R.; Davenport, P.W. Characterizing and Modeling Breathing Dynamics: Flow Rate, Rhythm, Period, and Frequency. Front. Physiol. 2022, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Lee, K. A Comparison of the Validity of Three Exercise Tests for Estimating Maximal Oxygen Uptake in Korean Adults Aged 19–64 Years. Appl. Sci. 2022, 12, 1371. [Google Scholar] [CrossRef]
- Tamburrano, P.; Sciatti, F.; Distaso, E.; Lorenzo, L.D.; Amirante, R. Validation of a Simulink Model for Simulating the Two Typical Controlled Ventilation Modes of Intensive Care Units Mechanical Ventilators. Appl. Sci. 2022, 12, 2057. [Google Scholar] [CrossRef]
- Chan, P. An Empirical Study on Data Validation Methods of Delphi and General Consensus. Data 2022, 7, 18. [Google Scholar] [CrossRef]
- Hess, D.R. Respiratory Mechanics in Mechanically Ventilated Patients. Respir. Care 2014, 59, 1773–1794. [Google Scholar] [CrossRef] [Green Version]
- García Río, F.; Lores, V.; Rojo, B. Evaluación funcional respiratoria (obstrucción y atrapamiento). Arch. Bronconeumol. 2007, 43, 8–14. [Google Scholar] [CrossRef]
- Sauleda Roig, J. Consecuencias clínicas de la disfunción muscular en la enfermedad pulmonar obstructiva crónica. Nutr. Hosp. 2006, 21, 69–75. [Google Scholar] [PubMed]
- García Prieto, E.; Amado Rodríguez, L.; Albaiceta, G.M. Monitorización de la mecánica respiratoria en el paciente ventilado. Puesta Med. Intensiv. Vent. Mec. Difer. Entid. 2014, 38, 49–55. [Google Scholar] [CrossRef]
- Briscoe, W.A.; Dubois, A.B. The relationship between airway resistance, airway conductance and lung volume in subjects of different age and body size. J. Clin. Investig. 1958, 37, 1279–1285. [Google Scholar] [CrossRef] [Green Version]
- Hagberg, C.A. Benumof and Hagberg’s Airway Management; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Suter, P.M.; Fairley, H.B.; Isenberg, M.D. Effect of Tidal Volume and Positive End-Expiratory Pressure on Compliance during Mechanical Ventilation. Chest 1978, 73, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Chiappero, G.R.; Ríos, F.; Setten, M. Ventilación Mecánica, 3rd ed.; Editorial Médica Panamericana: Bogotá, Colombia, 2018. [Google Scholar]
- Raff, H.; Levitzky, M. Fisiología Médica. Un Enfoque por Aparatos y Sistemas; McGraw-Hill Interamericana de España: New York, NY, USA, 2013. [Google Scholar]
- Nicolò, A.; Marcora, S.; Bazzucchi, I.; Sacchetti, M. Differential control of respiratory frequency and tidal volume during high-intensity interval training. Exp. Physiol. 2017, 102, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Girardi, M.; Sacchetti, M. Control of the depth and rate of breathing: Metabolic vs. non-metabolic inputs. J.Physiol. 2017, 595, 6363–6364. [Google Scholar] [CrossRef] [Green Version]
- García, F. Control de la respiración. Arch. Bronconeumol. 2017, 40, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Monpín, J. Introducción a la Bioingeniería; Marcombo Boixareu Editores: Barcelona, Spain, 1988. [Google Scholar]
- Buchanan, G.F. Timing, Sleep, and Respiration in Health and Disease. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 8. [Google Scholar]
- Alexander, P. Respiratory Physiology for Intensivists. In Critical Heart Disease in Infants and Children; Elsevier: Amsterdam, The Netherlands, 2019; pp. 134–149.e2. [Google Scholar]
- Muñoz, I.C.; Hernández, A.M. Cambios en la mecánica ventilatoria debidos a variaciones de la PEEP y la presión soporte: Estudio en sujetos sanos bajo ventilación mecánica no invasiva. Rev. Fac. Med. 2017, 65, 321–328. [Google Scholar] [CrossRef]
- Sahetya, S.K.; Goligher, E.C.; Brower, R.G. FiftyYearsofResearchinARDS.Setting Positive End-Expiratory Pressure in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Ravagnan, I.; Giurati, G.; Panigada, M.; Bottino, N.; Tredici, S.; Eccher, G.; Gattinoni, L. Positive End-expiratory Pressure Improves Respiratory Function in Obese but not in Normal Subjects during Anesthesia and Paralysis. Anesthesiology 1999, 91, 1221. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M. Principles and Practice of Mechanical Ventilation; McGraw-Hill Medical: New York, NY, USA, 2013. [Google Scholar]
- Fiastro, J.F.; Habib, M.P.; Quan, S.F. Pressure Support Compensation for Inspiratory Work due to Endotracheal Tubes and Demand Continuous Positive Airway Pressure. Chest 1988, 93, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Meza, S.; Mendez, M.; Ostrowski, M.; Younes, M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J. Appl. Physiol. 1998, 85, 1929–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumb, A.B. Respiratory Support and Artificial Ventilation. In Nunn’s Applied Respiratory Physiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 451–478. [Google Scholar]
- Naik, S.; Greenough, A.; Giffin, F.J.; Baker, A. Manoeuvres to elevate mean airway pressure, effects on blood gases and lung function in children with and without pulmonary pathology. Eur. J. Pediatr. 1998, 157, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Baptistella, A.R.; Sarmento, F.J.; da Silva, K.R.; Baptistella, S.F.; Taglietti, M.; Zuquello, R.Á.; Filho, J.R.N. Predictive factors of weaning from mechanical ventilation and extubation outcome: A systematic review. J. Crit. Care 2018, 48, 56–62. [Google Scholar] [CrossRef] [PubMed]

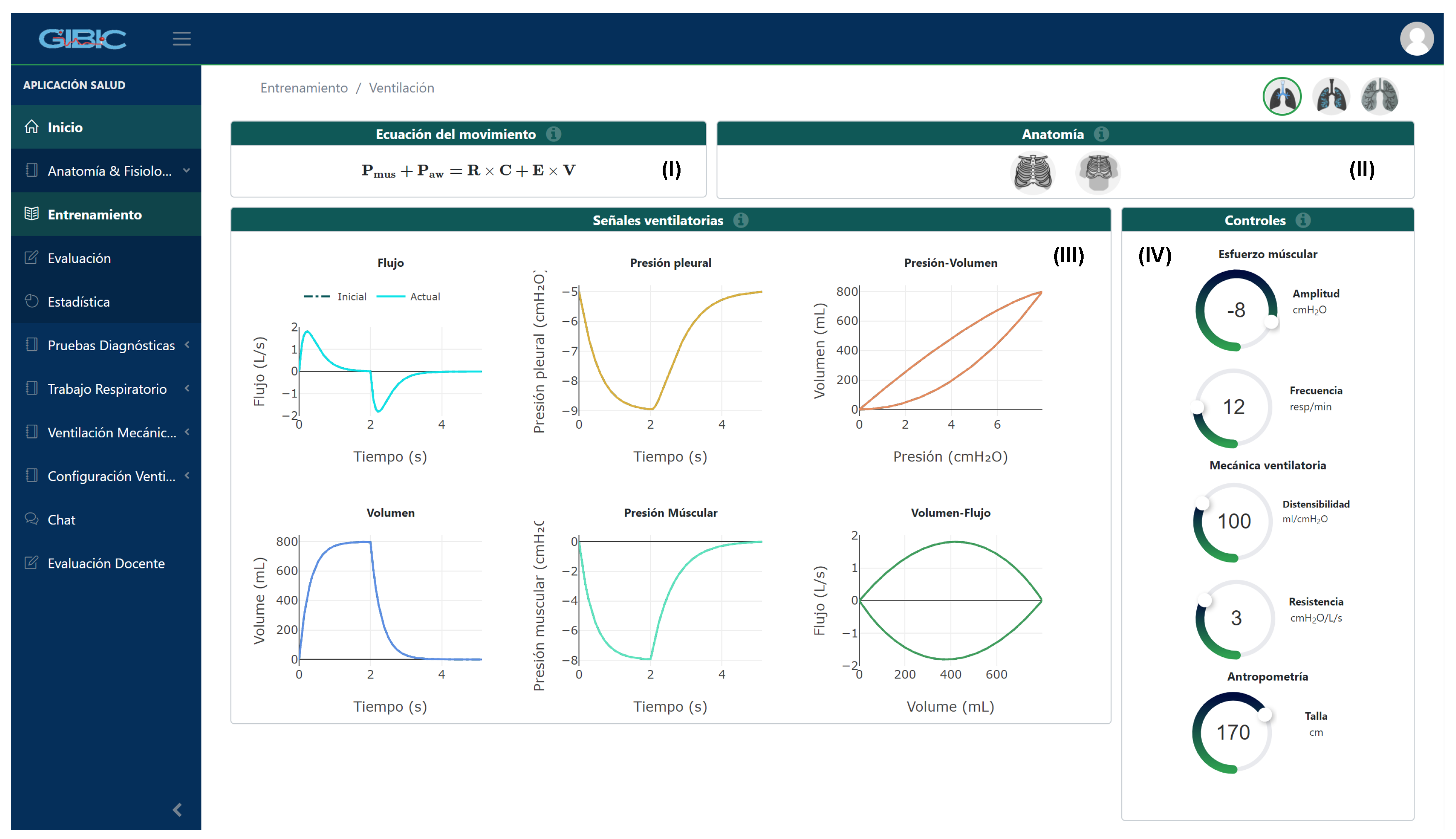

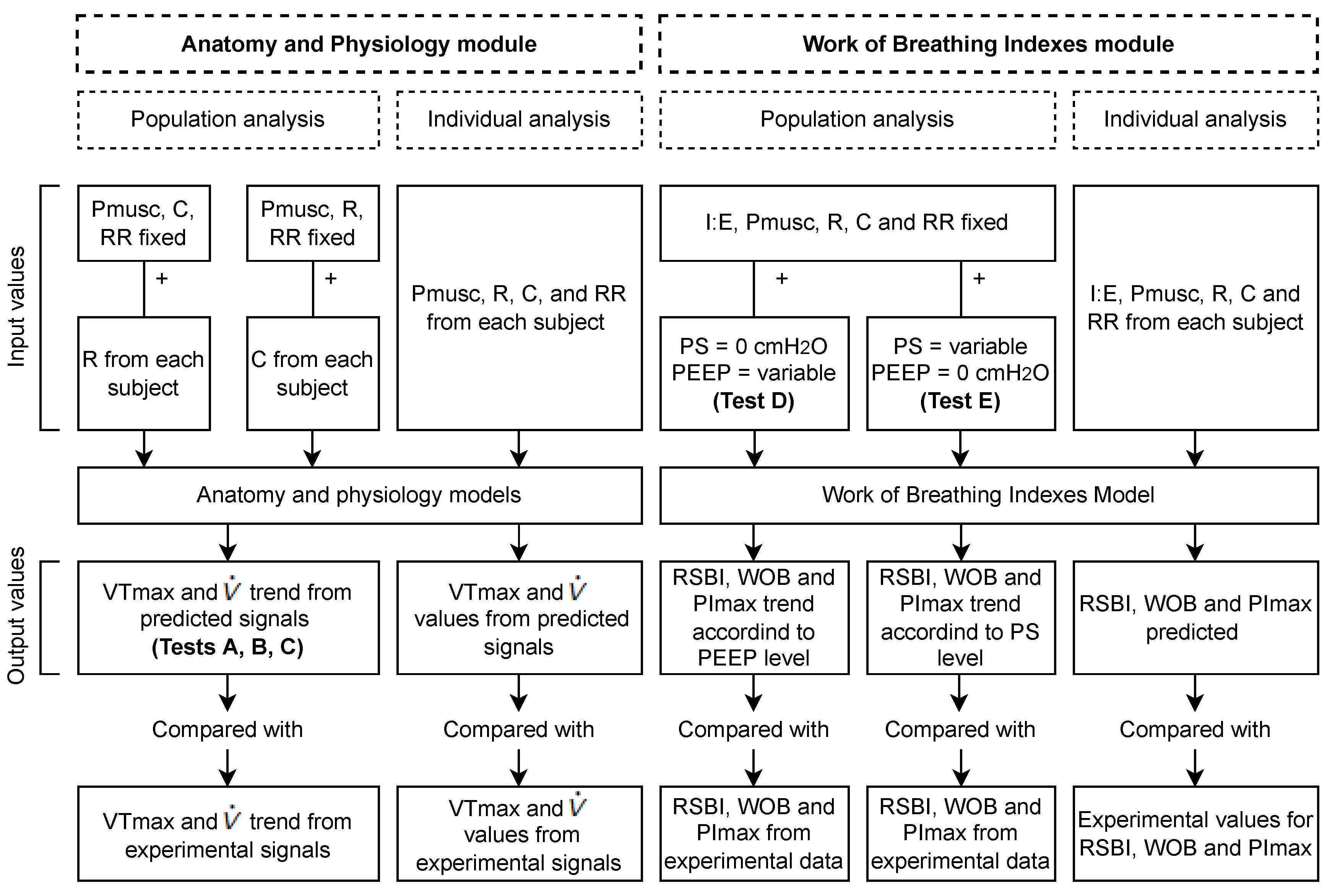
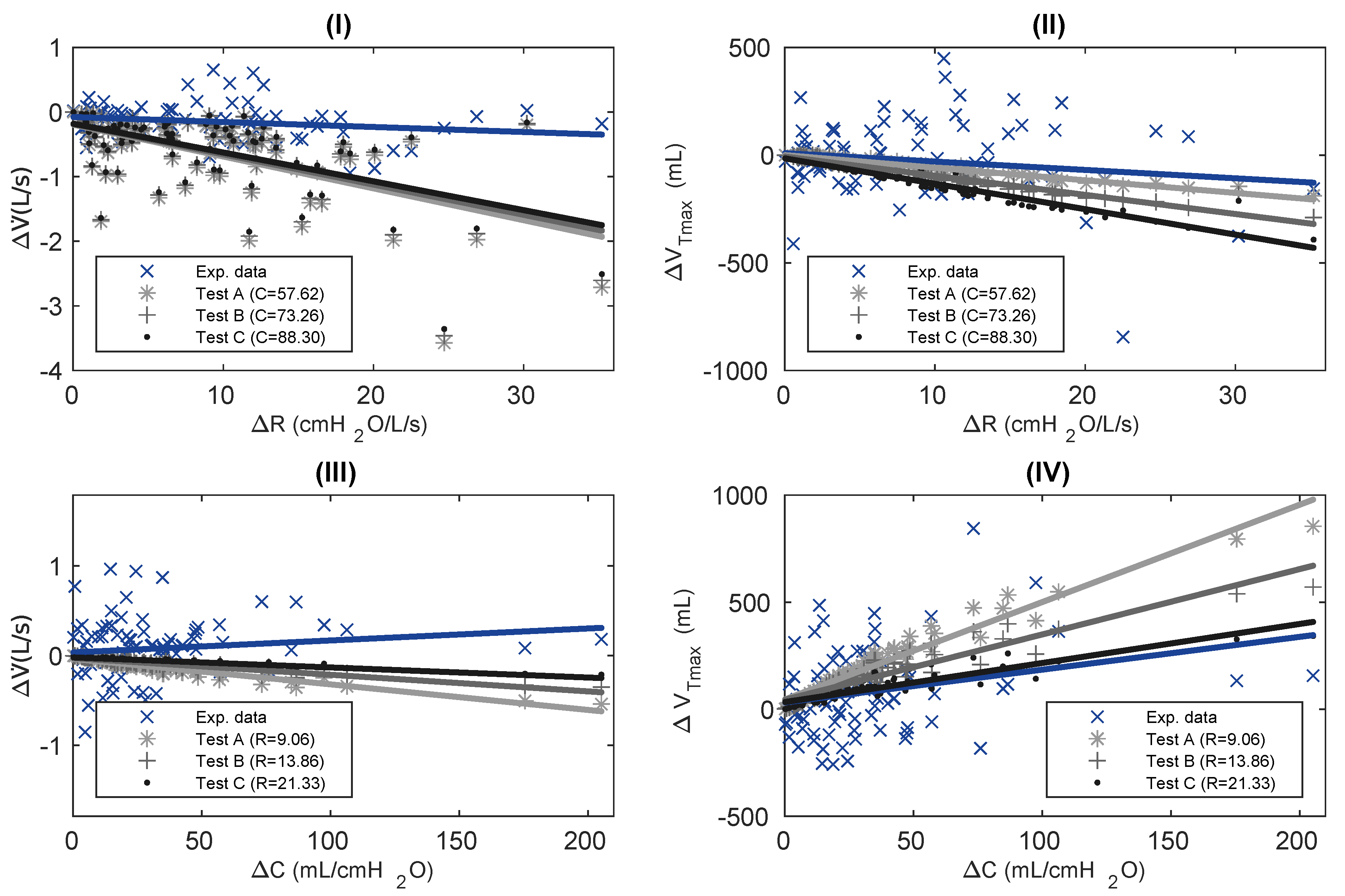
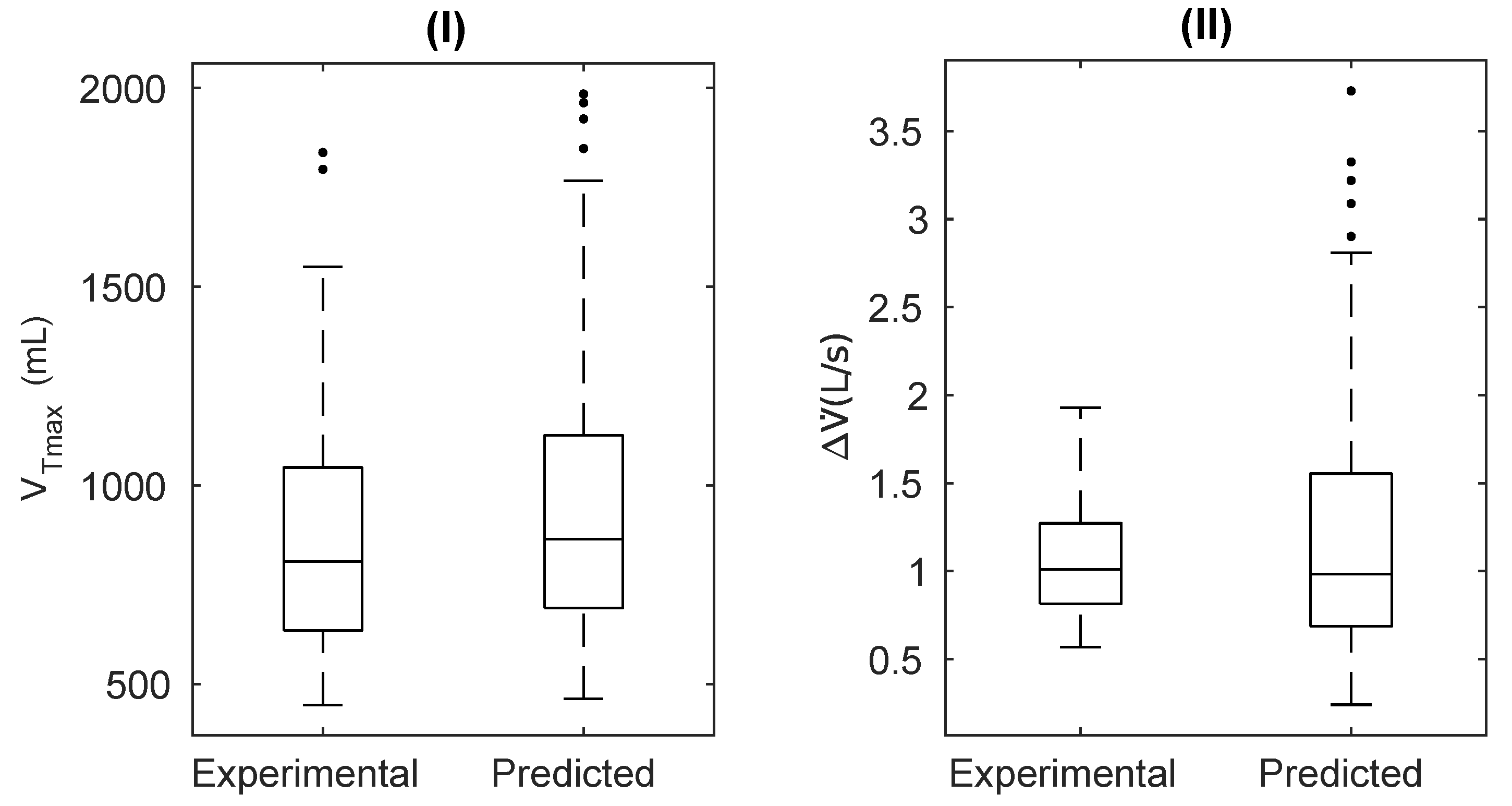

| Ventilatory Mechanics | Values |
|---|---|
| R (cmHO/L/s) | 13.86 (9.06–21.33) |
| C (mL/cmHO) | 73.26 (57.62–88.33) |
| Pmusc (cmHO) | 15.78 (12.45–21.81) |
| RR (bpm) | 13.58 (10.50–17.38) |
| Parameter | Test D | Test E |
|---|---|---|
| PEEP (cmHO) | 0, 2, 4, 6, 8, 10 | 0 |
| PS (cmHO) | 0 | 0, 2, 4, 6, 8, 10 |
| I:E | 1 : 0.83 (0.73–0.92) | 1 : 0.83 (0.75–0.93) |
| R (cmHO/L/s) | 9.67 (7.62–12.57) | 12.27 (9.11–15.01) |
| C (mL/cmHO) | 99.68 (80.00–139.42) | 74.99 (61.20–89.97) |
| Pmusc (cmHO) | 12.32 (9.07–16.12) | 11.58 (8.65–15.48) |
| RR (bpm) | 15.28 (12.17–17.40) | 15.98 (13.26–19.24) |
| Curve | Test | Slope | Median | Interquartile Range | Data Units |
|---|---|---|---|---|---|
| vs. | Exp. data | −0.01 | −0.12 | (−0.28–0.00) | L/s |
| A * | −0.05 | −0.44 | (−0.85–−0.23) | ||
| B * | −0.05 | −0.42 | (−0.81–−0.22) | ||
| C * | −0.04 | −0.40 | (−0.77–−0.21) | ||
| vs. | Exp. data | −3.89 | −28.87 | (−101.08–111.33) | mL |
| A | −5.85 | −43.17 | (−80.78–−12.06) | ||
| B * | −8.89 | −72.53 | (−131.00–−25.20) | ||
| C * | −11.80 | −105.58 | (−181.39–−41.55) | ||
| vs. | Exp. data | 0.00 | 0.11 | (−0.12–0.28) | L/s |
| A * | 0.00 | −0.11 | (−0.17–−0.06) | ||
| B * | 0.00 | −0.08 | (−0.12–−0.04) | ||
| C * | 0.00 | −0.05 | (−0.07–−0.03) | ||
| vs. | Exp. data | 1.53 | 8.86 | (−75.35–136.12) | mL |
| A * | 4.55 | 170.45 | (85.88–290.27) | ||
| B * | 3.05 | 144.09 | (73.11–248.05) | ||
| C | 1.82 | 114.28 | (55.40–185.60) |
| Parameter | RR Condition | Median (%) | Interquartile Range (%) | n (Subjects) |
|---|---|---|---|---|
| VTmax | RR ≥ 12 bpm | 9.26 | (4.72–17.68) | 77 |
| RR < 12 bpm | 10.38 | (5.07–19.57) | 45 | |
| Total | 9.54 | (4.80–18.18) | 122 | |
| RR ≥ 12 bpm | 31.01 | (14.78–65.57) | 77 | |
| RR < 12 bpm | 22.62 | (12.54–37.52) | 45 | |
| Total | 25.38 | (13.15–51.35) | 122 |
| Section | Sub-Section | Score | n (Evaluated Items) |
|---|---|---|---|
| Ventilation | Equation of motion | 4.00 (3.95–4.30) | 6 |
| Anatomy | 4.60 (4.20–4.80) | 7 | |
| Ventilatory signals | 4.80 (4.60–5.00) | 26 | |
| Controls | 4.50 (4.25–4.80) | 8 |
| Content Type | Aspect | Score | n (Evaluated Items) |
|---|---|---|---|
| Theoretical | Relevance | 4.30 (4.05–4.85) | 4 |
| Sufficience | 4.80 (4.15–4.80) | 14 | |
| Veracity | 4.60 (4.50–4.85) | 6 |
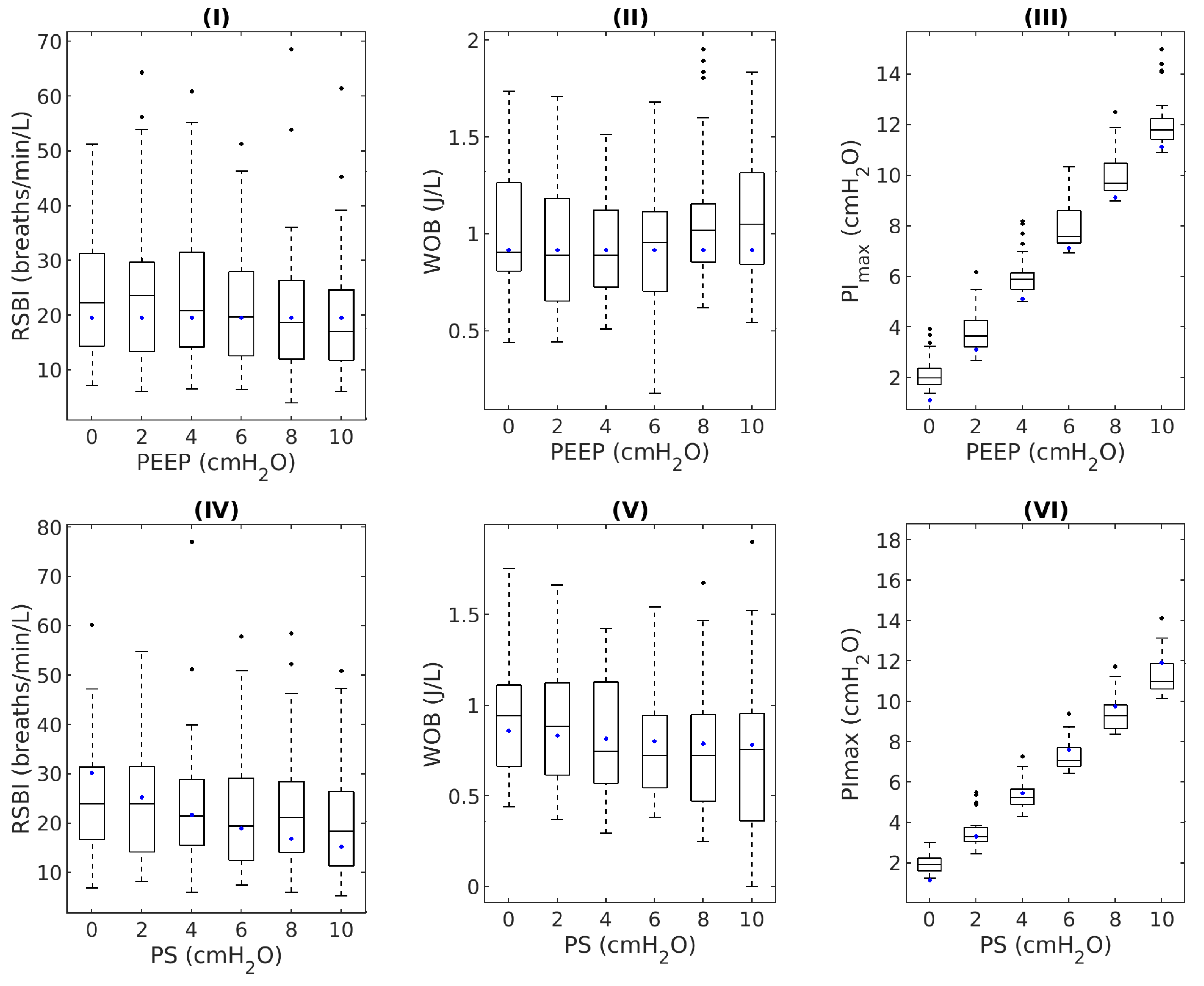
| Index | p-Value | Median (%) | Interquartile Range (%) |
|---|---|---|---|
| RSBI | 0.43 | 6.55 | (3.04–11.49) |
| WOB | 0.43 | 9.33 | (3.84–18.40) |
| PImax | 0.32 | 11.77 | (5.31–23.00) |
| Section | Sub-Section | Score | n (Evaluated Items) |
|---|---|---|---|
| Work Indexes | Assessment of dyspnea | 5.00 (4.60–5.00) | 40 |
| Campbell diagram | 5.00 (4.50–5.00) | 17 | |
| Ventilatory indexes | 5.00 (4.80–5.00) | 43 | |
| Obstructive Pattern | Clinical evaluation | 3.80 (3.30–4.30) | 15 |
| Pulmonary function charts and ventilatory monitoring | 4.50 (4.00–5.00) | 17 | |
| Ventilatory loops | 5.00 (3.80–5.00) | 7 | |
| Diagnostic tests | 4.80 (4.50–5.00) | 35 | |
| Restrictive Pattern | Clinical evaluation | 4.40 (3.58–4.88) | 12 |
| Pulmonary function charts and ventilatory monitoring | 4.80 (4.00–5.00) | 15 | |
| Ventilatory loops | 5.00 (4.80–5.00) | 7 | |
| Diagnostic tests | 4.15 (3.50–4.68) | 12 | |
| Mixed Pattern | Clinical evaluation | 4.30 (4.00–4.80) | 16 |
| Pulmonary function charts and ventilatory monitoring | 5.00 (4.30–5.00) | 15 | |
| Ventilatory loops | 5.00 (4.80–5.00) | 7 | |
| Diagnostic tests | 5.00 (2.80–5.00) | 18 |
| Content-Type | Aspect | Score | n (Evaluated Items) |
|---|---|---|---|
| Relevance | 4.75 (4.31–5.00) | 28 | |
| Theoretical | Sufficience | 4.25 (3.75–4.75) | 31 |
| Veracity | 4.75 (4.00–5.00) | 147 | |
| Definition | 4.80 | 2 | |
| Graphic | Ordering | 5.00 (4.50–5.00) | 3 |
| Veracity | 5.00 (4.75–5.00) | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía, S.; Muñoz, I.C.; Serna, L.Y.; Sarmiento, C.A.; Bravo, C.L.; Hernández, A.M. Web Applications for Teaching the Respiratory System: Content Validation. Appl. Sci. 2022, 12, 4289. https://doi.org/10.3390/app12094289
Mejía S, Muñoz IC, Serna LY, Sarmiento CA, Bravo CL, Hernández AM. Web Applications for Teaching the Respiratory System: Content Validation. Applied Sciences. 2022; 12(9):4289. https://doi.org/10.3390/app12094289
Chicago/Turabian StyleMejía, Susana, Isabel Cristina Muñoz, Leidy Yanet Serna, Carlos Andrés Sarmiento, Carlos Leonardo Bravo, and Alher Mauricio Hernández. 2022. "Web Applications for Teaching the Respiratory System: Content Validation" Applied Sciences 12, no. 9: 4289. https://doi.org/10.3390/app12094289
APA StyleMejía, S., Muñoz, I. C., Serna, L. Y., Sarmiento, C. A., Bravo, C. L., & Hernández, A. M. (2022). Web Applications for Teaching the Respiratory System: Content Validation. Applied Sciences, 12(9), 4289. https://doi.org/10.3390/app12094289








