Abiotic Stresses in Plants and Their Markers: A Practice View of Plant Stress Responses and Programmed Cell Death Mechanisms
Abstract
:1. Introduction
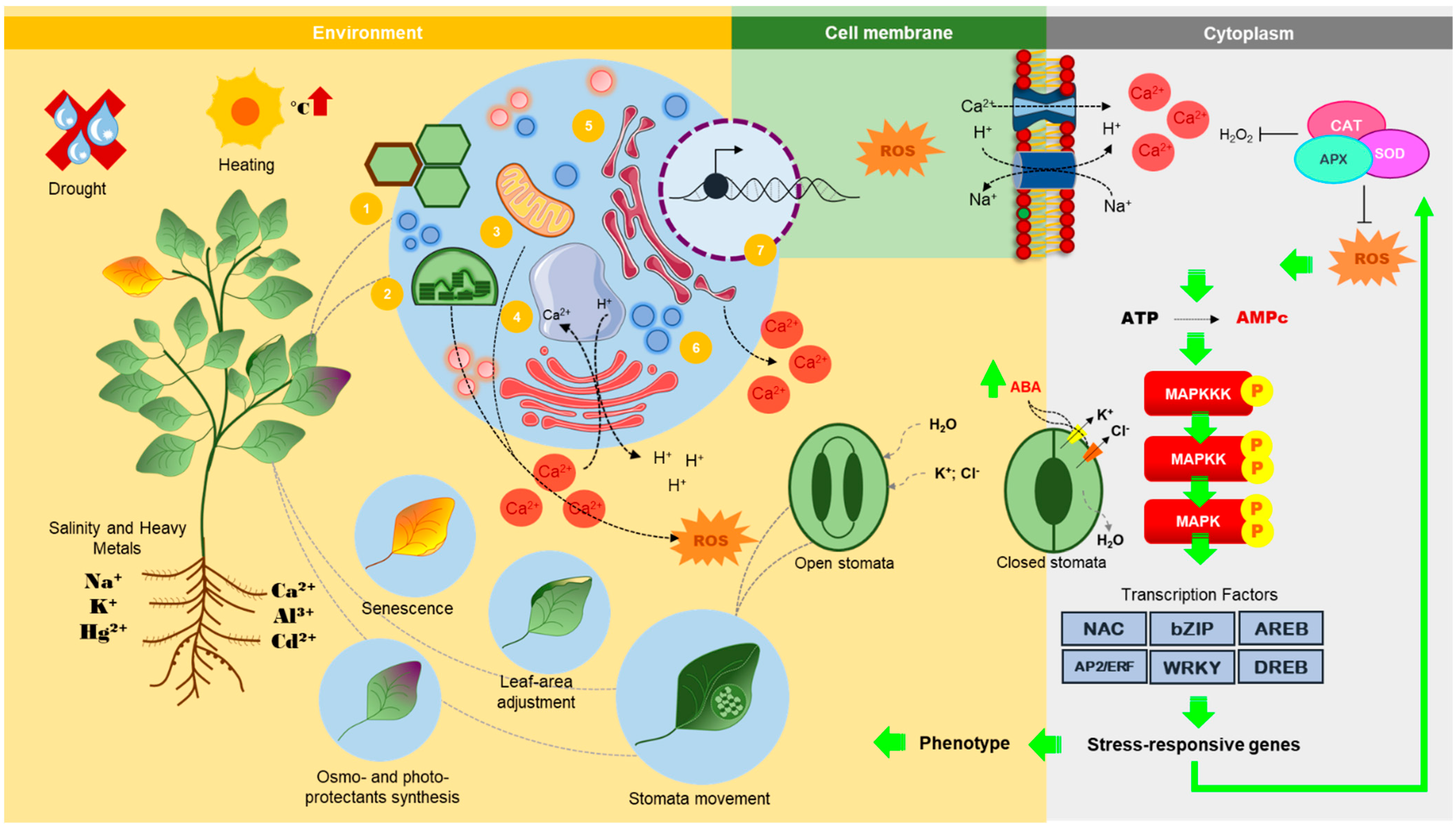
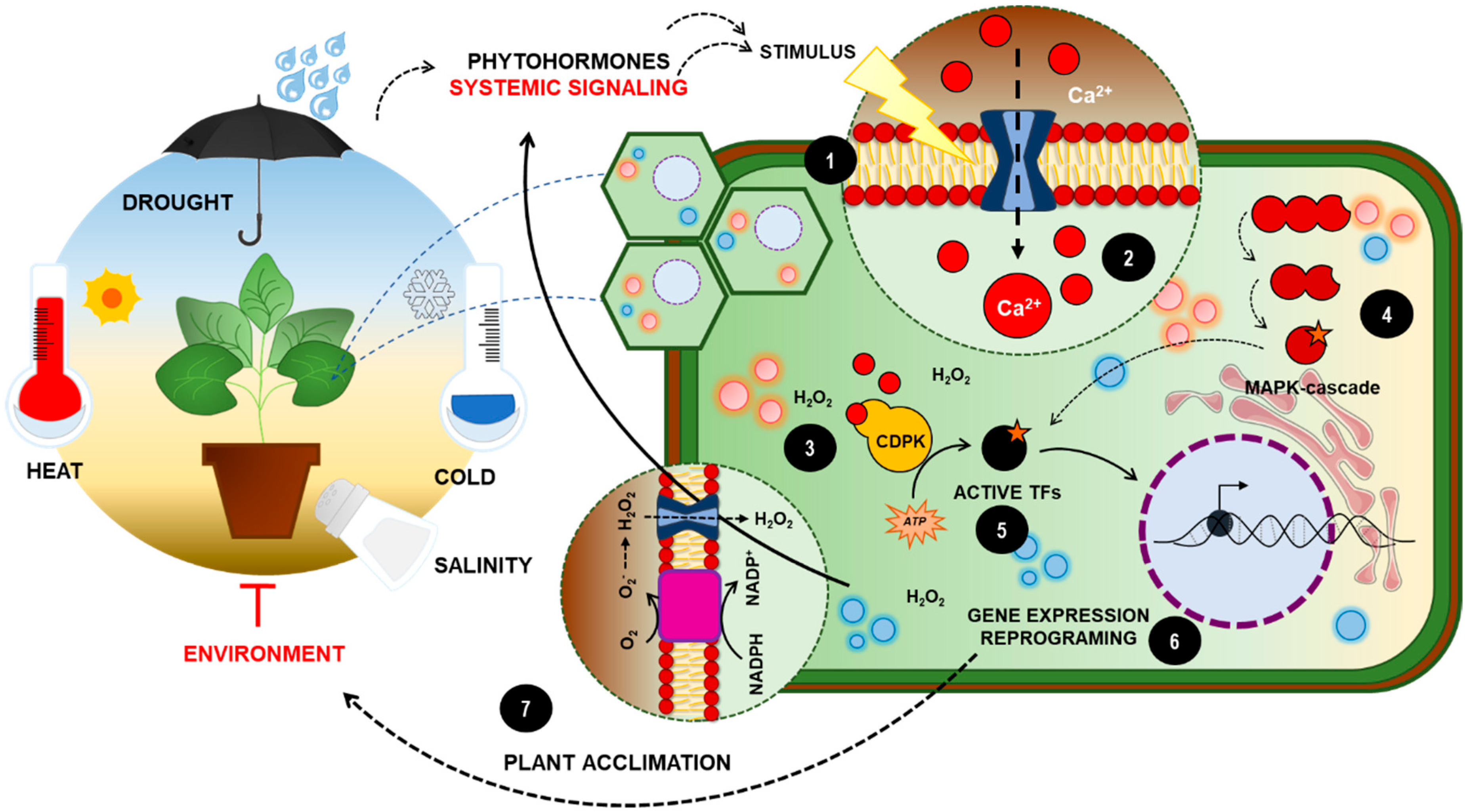
2. Plants in Adverse Environments—General Sensing and Signaling
2.1. Signaling Pathways in Abiotic Stresses in Plants
2.2. Stress-Sensor Mechanisms in Plants
2.3. Reactive Oxygen Species (ROS) and Their Role in Stress Signaling
2.4. Ca2+ and Calcium-Dependent Protein Kinases (CDPKs) Cascade
2.5. SnRKs and MAPK Cascade
3. Integrative Hormone, ROS and Kinases Signaling axis and the Signal Transduction in Abiotic Stresses
4. Secondary Metabolites as Signaling and Effector Molecules in Plant Abiotic Stresses
5. Mechanisms of Stress-Induced Senescence
6. Biochemical and Physiological Markers of Abiotic Stresses—A Practice View of Plant Phenotyping and Molecular Analyses
7. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Jannat, A.; Munir, F.; Fatima, N.; Amir, R. Biochemical and Molecular Mechanisms of Abiotic Stress Tolerance. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; ISBN 978-981-15-2171-3. [Google Scholar]
- Lichtenthaler, H.K. The Stress Concept in Plants: An Introduction. Ann. N. Y. Acad. Sci. 1998, 851, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Fraga, O.T.; de Melo, B.P.; Quadros, I.P.S.; Reis, P.A.B.; Fontes, E.P.B. Senescence-associated glycine max (Gm)nac genes: Integration of natural and stress-induced leaf senescence. Int. J. Mol. Sci. 2021, 22, 8287. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.P.; de Moura, S.M.; Morgante, C.V.; Pinheiro, D.H.; Alves, N.S.F.; Rodrigues-Silva, P.L.; Lourenço-Tessutti, I.T.; Andrade, R.V.; Fragoso, R.R.; Grossi-de-Sa, M.F. Regulated promoters applied to plant engineering: An insight over promising soybean promoters under biotic stress and their cis-elements. Biotechnol. Res. Innov. 2021, 5, e2021005. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Yadav, I.S.; Arora, N.K.; Boora, R.S.; Mittal, M.; Kaur, P.; Erskine, W.; Chhuneja, P.; Gill, M.I.S.; Singh, K. RNA-sequencing based gene expression landscape of guava cv. Allahabad Safeda and comparative analysis to colored cultivars. BMC Genom. 2020, 21, 1–19. [Google Scholar] [CrossRef]
- Peng, B.; Guan, K.; Tang, J.; Ainsworth, E.A.; Asseng, S.; Bernacchi, C.J.; Cooper, M.; Delucia, E.H.; Elliott, J.W.; Ewert, F.; et al. Towards a multiscale crop modelling framework for climate change adaptation assessment. Nat. Plants 2020, 6, 338–348. [Google Scholar] [CrossRef]
- Gilliham, M.; Able, J.A.; Roy, S.J. Translating knowledge about abiotic stress tolerance to breeding programmes. Plant J. 2017, 90, 898–917. [Google Scholar] [CrossRef] [Green Version]
- Nicolia, A.; Manzo, A.; Veronesi, F.; Rosellini, D. An overview of the last 10 years of genetically engineered crop safety research. Crit. Rev. Biotechnol. 2014, 34, 77–88. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, P.; Suneja, P. Biotechnological interventions for inducing abiotic stress tolerance in crops. Plant Gene 2021, 27, 100315. [Google Scholar] [CrossRef]
- Que, Q.; Chilton, M.D.M.; de Fontes, C.M.; He, C.; Nuccio, M.; Zhu, T.; Wu, Y.; Chen, J.S.; Shi, L. Trait stacking in transgenic crops: Challenges and opportunities. GM Crops 2010, 1, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Rajeevkumar, S.; Anunanthini, P.; Sathishkumar, R. Epigenetic silencing in transgenic plants. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. Biological Parts for Engineering Abiotic Stress Tolerance in Plants. BioDesign Res. 2022, 2022, 1–41. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi-Moqadam, M.-R.; MohseniFard, E.; Shekari, F.; Jafary, H.; Moradi, P.; Pucci, M.; Abate, G.; Mastinu, A. Physiological and Molecular Aspects of Two Thymus Species Differently Sensitive to Drought Stress. BioTech 2022, 11, 8. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Xun, H.; Wang, W.; Ding, X.; Tian, H.; Hussain, S.; Dong, Q.; Li, Y.; Cheng, Y.; Wang, C.; et al. Mutation of GmAITR Genes by CRISPR/Cas9 Genome Editing Results in Enhanced Salinity Stress Tolerance in Soybean. Front. Plant Sci. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Tran, M.T.; Doan, D.T.H.; Kim, J.; Song, Y.J.; Sung, Y.W.; Das, S.; Kim, E.J.; Son, G.H.; Kim, S.H.; Van Vu, T.; et al. CRISPR/Cas9-based precise excision of SlHyPRP1 domain(s) to obtain salt stress-tolerant tomato. Plant Cell Rep. 2021, 40, 999–1011. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2018, 18, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-León, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Z.; Wang, X.; Han, X.; Yu, D.; Wang, C.; Song, W.; Zheng, X.; Chen, C.; Zhang, Y. Knockout of the OsNAC006 transcription factor causes drought and heat sensitivity in rice. Int. J. Mol. Sci. 2020, 21, 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santosh Kumar, V.V.; Verma, R.K.; Yadav, S.K.; Yadav, P.; Watts, A.; Rao, M.V.; Chinnusamy, V. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol. Mol. Biol. Plants 2020, 26, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Qin, X.; Luo, L.; Han, Y.; Wang, X.; Usman, B.; Nawaz, G.; Zhao, N.; Liu, Y.; Li, R. CRISPR/Cas9-Induced Mutagenesis of Semi-Rolled Leaf1,2 Confers Curled Leaf Phenotype and Drought Tolerance by Influencing Protein Expression Patterns and ROS Scavenging in Rice (Oryza sativa L.). Agronomy 2019, 9, 728. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Watanabe, T.; Sugano, S.S.; Ueta, R.; Ishihara, R.; Shinozaki, K.; Osakabe, K. Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- STUBER, C.W. Biochemical and Molecular Markers in Plant Breeding. In Plant Breeding Reviews; Wiley: Oxford, UK, 2010; pp. 37–61. [Google Scholar]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [Green Version]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [Green Version]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Wang, P.; Xiong, Y. Target of rapamycin signaling in plant stress responses. Plant Physiol. 2020, 182, 1613–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luoni, S.B.; Astigueta, F.H.; Nicosia, S.; Moschen, S.; Fernandez, P.; Heinz, R. Transcription factors associated with leaf senescence in crops. Plants 2019, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Tuteja, N. Abiotic Stress Signaling in Plants-An Overview. Abiotic Stress Response Plants 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Akpinar, B.A.; Avsar, B.; Lucas, S.J.; Budak, H. Plant abiotic stress signaling. Plant Signal. Behav. 2012, 7, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, X.; Marshall, R.S.; Paez-Valencia, J.; Lacey, P.; Vierstra, R.D.; Otegui, M.S. Reticulon proteins modulate autophagy of the endoplasmic reticulum in maize endosperm. Elife 2020, 9, 1–27. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Jojoa-Cruz, S.; Saotome, K.; Murthy, S.E.; Tsui, C.C.A.; Sansom, M.S.P.; Patapoutian, A.; Ward, A.B. Cryo-EM structure of the mechanically activated ion channel OSCA1.2. Elife 2018, 7, 1–17. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Sun, L. Structure of the hyperosmolality-gated calcium-permeable channel OSCA1.2. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Maity, K.; Heumann, J.M.; McGrath, A.P.; Kopcho, N.J.; Hsu, P.K.; Lee, C.W.; Mapes, J.H.; Garza, D.; Krishnan, S.; Morgan, G.P.; et al. Cryo-EM structure of OSCA1.2 from Oryza sativa elucidates the mechanical basis of potential membrane hyperosmolality gating. Proc. Natl. Acad. Sci. USA 2019, 116, 14309–14318. [Google Scholar] [CrossRef] [Green Version]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Rennie, E.A.; Ebert, B.; Miles, G.P.; Cahoon, R.E.; Christiansen, K.M.; Stonebloom, S.; Khatab, H.; Twell, D.; Petzold, C.J.; Adams, P.D.; et al. Identification of a sphingolipid α-glucuronosyltransferase that is essential for pollen function in Arabidopsis. Plant Cell 2014, 26, 3314–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y.; et al. The SOS2-SCaBP8 Complex Generates and Fine-Tunes an AtANN4-Dependent Calcium Signature under Salt Stress. Dev. Cell 2019, 48, 697–709.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 2018, 28, 666–675.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Zayed, O.; Yu, Z.; Jiang, W.; Zhu, P.; Hsu, C.C.; Zhang, L.; Andy Tao, W.; Lozano-Durán, R.; Zhu, J.K. Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 13123–13128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature Review. New Phytol. 2005, 181, 275–294. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, D.; Jiang, L.; Ye, L. Genome-wide identification and characterization of SnRK family genes in Brassica napus. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Wu, Y.; Luo, Q.; Zhang, Y.; Qiu, D.; Han, J.; Su, P.; Xiong, Z.; Chang, J.; et al. TaSnRK2.9, a sucrose non-fermenting 1-related protein kinase gene, positively regulates plant response to drought and salt stress in transgenic tobacco. Front. Plant Sci. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Jia, Y.; Shi, Y.; Zhang, X.; Song, C.; Gong, Z.; Yang, S. OST 1-mediated BTF 3L phosphorylation positively regulates CBF s during plant cold responses. EMBO J. 2018, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Lv, J.; Shi, Y.; Gao, J.; Hua, J.; Song, C.; Gong, Z.; Yang, S. EGR 2 phosphatase regulates OST 1 kinase activity and freezing tolerance in Arabidopsis. EMBO J. 2019, 38, 1–17. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. Ros regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N. ROS as Key Players of Abiotic Stress Responses in Plants. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-20420-8. [Google Scholar]
- Martí, M.C.; Stancombe, M.A.; Webb, A.A.R. Cell-and stimulus type-specific intracellular free Ca2+ signals in Arabidopsis. Plant Physiol. 2013, 163, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry & Molecular Biology of Plants; 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-0-470-71421-8. [Google Scholar]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hua, D.; Wang, C.; He, J.; Liao, H.; Duan, Y.; Zhu, Z.; Guo, Y.; Chen, Z.; Gong, Z. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 2012, 24, 2546–2561. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]
- Geiger, D.; Scherzer, S.; Mumm, P.; Marten, I.; Ache, P.; Matschi, S.; Liese, A.; Wellmann, C.; Al-Rasheid, K.A.S.; Grill, E.; et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 2010, 107, 8023–8028. [Google Scholar] [CrossRef] [Green Version]
- Brandt, B.; Munemasa, S.; Wang, C.; Nguyen, D.; Yong, T.; Yang, P.G.; Poretsky, E.; Belknap, T.F.; Waadt, R.; Alemań, F.; et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in arabidopsis guard cells. Elife 2015, 4, 1–25. [Google Scholar] [CrossRef]
- Barajas-Lopez, J. de D.; Moreno, J.R.; Gamez-Arjona, F.M.; Pardo, J.M.; Punkkinen, M.; Zhu, J.K.; Quintero, F.J.; Fujii, H. Upstream kinases of plant SnRKs are involved in salt stress tolerance. Plant J. 2018, 93, 107–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shou, H.; Bordallo, P.; Wang, K. Expression of the Nicotiana protein kinase (NPK1) enhanced drought tolerance in transgenic maize. J. Exp. Bot. 2004, 55, 1013–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danquah, A.; De Zélicourt, A.; Boudsocq, M.; Neubauer, J.; Frei Dit Frey, N.; Leonhardt, N.; Pateyron, S.; Gwinner, F.; Tamby, J.P.; Ortiz-Masia, D.; et al. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J. 2015, 82, 232–244. [Google Scholar] [CrossRef]
- de Zelicourt, A.; Colcombet, J.; Hirt, H. The Role of MAPK Modules and ABA during Abiotic Stress Signaling. Trends Plant Sci. 2016, 21, 677–685. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Obata, T.; Feil, R.; Lunn, J.E.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Fernie, A.R. The role of abscisic acid signaling in maintaining the metabolic balance required for arabidopsis growth under nonstress conditions. Plant Cell 2019, 31, 84–105. [Google Scholar] [CrossRef] [Green Version]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Matsuo, M.; Johnson, J.M.; Hieno, A.; Tokizawa, M.; Nomoto, M.; Tada, Y.; Godfrey, R.; Obokata, J.; Sherameti, I.; Yamamoto, Y.Y.; et al. High REDOX RESPONSIVE TRANSCRIPTION FACTOR1 Levels Result in Accumulation of Reactive Oxygen Species in Arabidopsis thaliana Shoots and Roots. Mol. Plant 2015, 8, 1253–1273. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Li, H.; Zhang, X.; Xie, Q.; Gong, Z.; Yang, S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in arabidopsis. Dev. Cell 2015, 32, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Barrero-Gil, J.; Salinas, J. CBFs at the Crossroads of Plant Hormone Signaling in Cold Stress Response. Mol. Plant 2017, 10, 542–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Ding, Y.; Yang, S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 2011, 129, 1619–1622. [Google Scholar] [CrossRef]
- Wu, A.; Allu, A.D.; Garapati, P.; Siddiqui, H.; Dortay, H.; Zanor, M.I.; Asensi-Fabado, M.A.; Munná-Bosch, S.; Antonio, C.; Tohge, T.; et al. JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 2012, 24, 482–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baier, M.; Bittner, A.; Prescher, A.; van Buer, J. Preparing plants for improved cold tolerance by priming. Plant Cell Environ. 2019, 42, 782–800. [Google Scholar] [CrossRef]
- Yao, Y.; He, R.J.; Xie, Q.L.; Zhao, X.H.; Deng, X.M.; He, J.B.; Song, L.; He, J.; Marchant, A.; Chen, X.Y.; et al. ETHYLENE RESPONSE FACTOR 74 (ERF74) plays an essential role in controlling a respiratory burst oxidase homolog D (RbohD)-dependent mechanism in response to different stresses in Arabidopsis. New Phytol. 2017, 213, 1667–1681. [Google Scholar] [CrossRef]
- Suzuki, N.; Bajad, S.; Shuman, J.; Shulaev, V.; Mittler, R. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J. Biol. Chem. 2008, 283, 9269–9275. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.; Tang, G.; De Dios Barajas-Lopez, J.; et al. GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Yu, D. BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in arabidopsis. Plant Cell 2014, 26, 4394–4408. [Google Scholar] [CrossRef] [Green Version]
- Nolan, T.M.; Vukasinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 298–318. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Tang, J.; Liu, J.; Hu, J.; Liu, J.; Chen, Y.; Cai, Z.; Wang, X. Abscisic Acid Signaling Inhibits Brassinosteroid Signaling through Dampening the Dephosphorylation of BIN2 by ABI1 and ABI2. Mol. Plant 2018, 11, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.; Yu, J.Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Gao, C.J.; Song, L.X.; Zhou, Y.H.; Shi, K.; Yu, J.Q. Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ. 2014, 37, 2036–2050. [Google Scholar] [CrossRef]
- Shang, Y.; Dai, C.; Lee, M.M.; Kwak, J.M.; Nam, K.H. BRI1-Associated Receptor Kinase 1 Regulates Guard Cell ABA Signaling Mediated by Open Stomata 1 in Arabidopsis. Mol. Plant 2016, 9, 447–460. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Signal transduction networks during stress combination. J. Exp. Bot. 2020, 71, 1734–1741. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef]
- Olvera-Carrillo, Y.; Van Bel, M.; Van Hautegem, T.; Fendrych, M.; Huysmans, M.; Simaskova, M.; van Durme, M.; Buscaill, P.; Rivas, S.; Coll, N.S.; et al. A conserved core of programmed cell death indicator genes discriminates developmentally and environmentally induced programmed cell death in plants. Plant Physiol. 2015, 169, 2684–2699. [Google Scholar] [CrossRef]
- Desikan, R.; Last, K.; Harrett-Williams, R.; Tagliavia, C.; Harter, K.; Hooley, R.; Hancock, J.T.; Neill, S.J. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006, 47, 907–916. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, Y.; Zhang, X.; Xin, X.; Qi, L.; Guo, H.; Li, J.; Yang, S. PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E6695–E6702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovtun, Y.; Chiu, W.-L.; Tena, G.; Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; An, F.; Feng, Y.; Li, P.; Xue, L.; A, M.; Jiang, Z.; Kim, J.-M.; To, T.K.; Li, W.; et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 12539–12544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, P.J.; Calvert, C.; Atzorn, R.; Wasternack, C.; Leyser, H.M.O.; Bowles, D.J. Ethylene as a Signal Mediating the Wound Response of Tomato Plants. Science 1996, 274, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Piqueras, R.; Sánchez-Serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 Integrates Signals from Ethylene and Jasmonate Pathways in Plant Defense[W]. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front. Plant Sci. 2017, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 1–25. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Stanius, Ž. Effect of External and Internal Factors on Secondary Metabolites Accumulation in St. John’s Worth. Bot. Lith. 2013, 18, 101–108. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Fatani, S.H.; Mohamed Nour Eldin, E.E.; Wink, M. Natural Products Modulate the Multifactorial Multidrug Resistance of Cancer. Pharmacol. Amp; Pharm. 2015, 06, 146–176. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plant Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Lqbal, M.; Hussain, I.; Rasheed, R. Physiological and biochemical approaches for salinity tolerance. In Managing Salt Tolerance in Plants: Molecular and Genomic Perspectives; Wani, S.H., Hossain, M.A., Eds.; CRC Press, 2015; pp. 79–113. [Google Scholar]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234–235, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Depaepe, T.; Hendrix, S.; Janse van Rensburg, H.C.; Van den Ende, W.; Cuypers, A.; Van Der Straeten, D. At the Crossroads of Survival and Death: The Reactive Oxygen Species–Ethylene–Sugar Triad and the Unfolded Protein Response. Trends Plant Sci. 2021, 26, 338–351. [Google Scholar] [CrossRef]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van Den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Ljung, K.; Nemhauser, J.L.; Perata, P. New mechanistic links between sugar and hormone signalling networks. Curr. Opin. Plant Biol. 2015, 25, 130–137. [Google Scholar] [CrossRef]
- Emanuelle, S.; Doblin, M.S.; Stapleton, D.I.; Bacic, A.; Gooley, P.R. Molecular Insights into the Enigmatic Metabolic Regulator, SnRK1. Trends Plant Sci. 2016, 21, 341–353. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A Key Organic Osmolyte Effectively Involved in Plant Abiotic Stress Tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Albert, N.W.; Zhou, Y.; Schwinn, K.E. Functions of Flavonoid and Betalain Pigments in Abiotic Stress Tolerance in Plants. In Annual Plant Reviews Online; Wiley: Hoboken, NJ, USA, 2018; Volume 1, pp. 21–62. ISBN 9781119312994. [Google Scholar]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NTMYB4 and NTCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Zhan, X.; Shen, Q.; Chen, J.; Yang, P.; Wang, X.; Hong, Y. Rice sulfoquinovosyltransferase SQD2.1 mediates flavonoid glycosylation and enhances tolerance to osmotic stress. Plant Cell Environ. 2019, 42, 2215–2230. [Google Scholar] [CrossRef]
- Kim, J.; Lee, W.J.; Vu, T.T.; Jeong, C.Y.; Hong, S.W.; Lee, H. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep. 2017, 36, 1215–1224. [Google Scholar] [CrossRef]
- Cui, L.G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014, 80, 1108–1117. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, D.; Kim, Y.S.; Hörtensteiner, S.; Paek, N.C. Arabidopsis STAYGREEN-LIKE (SGRL) promotes abiotic stress-induced leaf yellowing during vegetative growth. FEBS Lett. 2014, 588, 3830–3837. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; An, K.; Liao, Y.; Zhou, X.; Cao, Y.; Zhao, H.; Ge, X.; Kuai, B. Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in Arabidopsis. Plant Physiol. 2007, 144, 1429–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Blumwald, E. Stress-induced chloroplast degradation in arabidopsis is regulated via a process independent of autophagy and senescence-associated vacuoles. Plant Cell 2014, 26, 4875–4888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prins, A.; Van Heerden, P.D.R.; Olmos, E.; Kunert, K.J.; Foyer, C.H. Cysteine proteinases regulate chloroplast protein content and composition in tobacco leaves: A model for dynamic interactions with ribulose-1,5- bisphosphate carboxylase/oxygenase (Rubisco) vesicular bodies. J. Exp. Bot. 2008, 59, 1935–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simova-Stoilova, L.; Vaseva, I.; Grigorova, B.; Demirevska, K.; Feller, U. Proteolytic activity and cysteine protease expression in wheat leaves under severe soil drought and recovery. Plant Physiol. Biochem. 2010, 48, 200–206. [Google Scholar] [CrossRef]
- Martínez, D.E.; Costa, L.; Guiamét, J.J. Activities of Vacuolar Cysteine Proteases in Plant Senescence. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; pp. 283–297. ISBN 978-1-4939-7672-0. [Google Scholar]
- Nadarajah, K.K. Ros homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef]
- Niu, F.; Cui, X.; Zhao, P.; Sun, M.; Yang, B.; Deyholos, M.K.; Li, Y.; Zhao, X.; Jiang, Y.Q. WRKY42 transcription factor positively regulates leaf senescence through modulating SA and ROS synthesis in Arabidopsis thaliana. Plant J. 2020, 104, 171–184. [Google Scholar] [CrossRef]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.C.; Chen, J.; Miao, C.; Song, C.P. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef] [Green Version]
- Gepstein, S.; Glick, B.R. Strategies to ameliorate abiotic stress-induced plant senescence. Plant Mol. Biol. 2013, 82, 623–633. [Google Scholar] [CrossRef]
- Tran, L.S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Role of cytokinin responsive two-component system in ABA and osmotic stress signalings. Plant Signal. Behav. 2010, 5, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Qi, Y.; Xu, J.; Dai, X.; Chen, J.; Dong, C.H.; Xiang, F. Arabidopsis WRKY71 regulates ethylene-mediated leaf senescence by directly activating EIN2, ORE1 and ACS2 genes. Plant J. 2021, 107, 1819–1836. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.R.; Silva, P.A.; Mendes, G.C.; Alves, J.R.; Caetano, H.D.N.; Machado, J.P.B.; Brustolini, O.J.B.; Carpinetti, P.A.; Melo, B.P.; Silva, J.C.F.; et al. The Stress-Induced Soybean NAC Transcription Factor GmNAC81 Plays a Positive Role in Developmentally Programmed Leaf Senescence. Plant Cell Physiol. 2016, 57, 1098–1114. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.S.; Reis, P.A.B.; Dadalto, S.P.; Faria, J.A.Q.A.; Fontes, E.P.B.; Fietto, L.G. A novel transcription factor, ERD15 (Early Responsive to Dehydration 15), connects endoplasmic reticulum stress with an osmotic stress-induced cell death signal. J. Biol. Chem. 2011, 286, 20020–20030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, P.A.B.; Carpinetti, P.A.; Freitas, P.P.J.; Santos, E.G.D.; Camargos, L.F.; Oliveira, I.H.T.; Silva, J.C.F.; Carvalho, H.H.; Dal-Bianco, M.; Soares-Ramos, J.R.L.; et al. Functional and regulatory conservation of the soybean ER stress-induced DCD/NRP-mediated cell death signaling in plants. BMC Plant Biol. 2016, 16, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.D.L.; Reis, P.A.B.; Valente, M.A.S.; Irsigler, A.S.T.; Carvalho, C.M.; Loureiro, M.E.; Aragão, F.J.L.; Boston, R.S.; Fietto, L.G.; Fontes, E.P.B. A new branch of endoplasmic reticulum stress signaling and the osmotic signal converge on plant-specific asparagine-rich proteins to promote cell death. J. Biol. Chem. 2008, 283, 20209–20219. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.O.; Fraga, O.T.; Pimenta, M.R.; Caetano, H.D.N.; Machado, J.P.B.; Carpinetti, P.A.; Brustolini, O.J.B.; Quadros, I.P.S.; Reis, P.A.B.; Fontes, E.P.B. GmNAC81 Inversely Modulates Leaf Senescence and Drought Tolerance. Front. Genet. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Reis, P.A.A.; Rosado, G.L.; Silva, L.A.C.; Oliveira, L.C.; Oliveira, L.B.; Costa, M.D.L.; Alvim, F.C.; Fontes, E.P.B. The binding protein BiP attenuates stress-induced cell death in soybean via modulation of the N-RICH protein-mediated signaling pathway. Plant Physiol. 2011, 157, 1853–1865. [Google Scholar] [CrossRef] [Green Version]
- Quadros, I.P.S.; Madeira, N.N.; Loriato, V.A.P.; Saia, T.F.F.; Silva, J.C.; Soares, F.A.F.; Carvalho, J.R.; Reis, P.A.B.; Fontes, E.P.B.; Clarindo, W.R.; et al. Cadmium-mediated toxicity in plant cells is associated with the DCD/NRP-mediated cell death response. Plant. Cell Environ. 2022, 45, 556–571. [Google Scholar] [CrossRef]
- Pieruschka, R.; Schurr, U. Plant phenotyping: Past, present, and future. Plant Phenomics 2019, 2019. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Furbank, R.T.; Tester, M. Phenomics—Technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, F.; Schurr, U. Future Scenarios for Plant Phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos Silva, M.L.; De Sousa, H.G.; Dos Santos Silva, M.L.; De Lacerda, C.F.; Gomes-Filho, E. Growth and photosynthetic parameters of saccharine sorghum plants subjected to salinity. Acta Sci. Agron. 2019, 41, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Palmeros-Suárez, P.A.; Massange-Sánchez, J.A.; Sánchez-Segura, L.; Martínez-Gallardo, N.A.; Espitia Rangel, E.; Gómez-Leyva, J.F.; Délano-Frier, J.P. AhDGR2, an amaranth abiotic stress-induced DUF642 protein gene, modifies cell wall structure and composition and causes salt and ABA hyper-sensibility in transgenic Arabidopsis. Planta 2017, 245, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Wang, L.; Hu, P.; Wang, Y.; Jia, Y.; Zhang, C.; Zhang, Y.; Zhang, Y.; Wang, C.; et al. Expression of the MYB transcription factor gene BplMYB46 affects abiotic stress tolerance and secondary cell wall deposition in Betula platyphylla. Plant Biotechnol. J. 2017, 15, 107–121. [Google Scholar] [CrossRef]
- Verma, R.K.; Kumar, V.V.S.; Yadav, S.K.; Kumar, T.S.; Rao, M.V.; Chinnusamy, V. Overexpression of Arabidopsis ICE1 enhances yield and multiple abiotic stress tolerance in indica rice. Plant Signal. Behav. 2020, 15. [Google Scholar] [CrossRef]
| Cell Markers | |||
|---|---|---|---|
| Marker | Biological Function | Variations | Analysis |
| Stomatal closure | The stomatal movement, characterized by opening and closing, is a process regulated by the hormone ABA as a function of temperature fluctuation and/or water availability. The turgidity of guard cells is controlled by potassium (K+) and chloride (Cl−) channels and, according to the osmotic flow of water, the stomata may be open (high turbidity—greater osmotic potential inside the cells) or closed (low turbidity—greater osmotic potential outside the cells). The stomatal closure decreases the photosynthetic rate and transpiration. In consequence of the lower electronic flow through the photosynthetic apparatus, the generation of ROS decreases. (*) | Open/Closed | Evaluation of random fields on the abaxial surface of leaves by optical microscopy or confocal microscopy with staining of sections with propidium iodide and excitation at 543 nm [88,94,135]. (*) In cases of high light stress, ROS might to be generated, even with closed stomata, as a side effect of high electronic flow in photosystems and the imbalance in energy absorption and dissipation. |
| Leaf area adjustment | Leaf area adjustment is a process that, like stomatal closure, decreases the photosynthetic rate. The adjustment can be made by curling the ends or by leaf abscission. | - | Phenotypic evaluation. The profile of the leaves of a control plant (wild specimen or untreated specimen) must be carefully observed in relation to the treated specimens. To provide a quantitative parameter, it is possible to count the leaves with symptoms in relation to the asymptomatic leaves [151]. |
| Cell wall thickening | Stress-mediated ROS accumulation leads to cell wall thickening as a way to ensure greater mechanical rigidity. This thickening is achieved through the deposition and polymerization of phenolic compounds into lignin and by modifying the structure of polymers that make up the cell wall, such as hemicellulose. | Esterification between glycoproteins and phenolic compounds | Immunolocalization mediated by specific antibodies against glycoproteins and cell wall polymers and their esterified and non-esterified variations and analysis by confocal and/or electron microscopy [152,153]. |
| Lignification | Quantification of phenolic compounds by Follin reaction. Mapping of genes involved in the cell wall polymer biosynthesis process [152,153]. | ||
| Modifications of the lipid profile of the plasma membrane | Changes in the lipid profile of the plasma membrane in plant cells are mainly caused by temperature fluctuations and ROS-mediated lipid peroxidation. | Cold: increased concentration of unsaturated fatty acids | In practice, it is difficult to monitor changes in the lipid composition of membranes. The evaluation of these modifications can be done through indirect inference, evaluating the expression of genes related to fatty acid saturation or desaturation. HPLC (high-performance liquid chromatography) might to be an alternative with correct standardization. |
| Heat: increase in the concentration of saturated fatty acids | |||
| Increased concentration of malonic aldehyde (MDA) | Spectrophotometric quantification at 532 nm [49,53,80]. | ||
| Programmed cell death | When stress-response mechanisms fail to overcome it, plants trigger stress-induced senescence as a way of remobilizing their nutrients to reproductive organs and seeds in an attempt to perpetuate the species. The extent of senescence symptoms (chlorosis and leaf necrosis) is indicative of greater tolerance or susceptibility to stress. | Increased leaf chlorosis | Leaf chlorosis can be directly evaluated as a phenotypic parameter, observing the leaf area in which there is loss of chlorophyll or by direct spectrophotometric quantification of the pigment [134]. |
| Increased leaf and root necrosis | Leaf and root necrosis can be evaluated by testing the color of leaves and roots by vital dyes. In leaves, Trypan Blue or Evans Blue is normally used and the extent of cell death is directly associated with the intensity of blue staining, since the dye is only able to penetrate dead cells. In the root, this evaluation can be done by staining the roots with propidium iodide and its evaluation under confocal microscopy. In dead cells, cell wall and nucleus are stained and in living cells, only the wall is stained. Therefore, the higher incidence of stained nuclei in random fields indicates a greater extent of cell death in the root [49,153]. | ||
| Programmed cell death (continued) | (continuation) | Increased degradation of proteins and nucleic acids | Degradation of proteins and nucleic acids can be assessed by quantitative and qualitative methods. The electrophoresis of nucleic acids and the total protein extract of plants subjected to stress allow us to infer on the quality of the sample, which directly reflects on the rate of degradation. Large drag areas indicate a greater degree of degradation. In the case of proteins, the rate of protein decay can be estimated by quantitative methods, such as Bradford, comparing the control sample with the treated sample, assuming as absence of degradation the amount of protein in the control sample and calculating (relatively) the rate of degradation based on the quantification of proteins in the treated sample [130]. |
| Molecular Markers—Hormonal Metabolism | |||
| Marker | Biological Function | Variations | Analysis |
| Abscisic acid (ABA) | ABA is the main hormone in the integration of environmental signals and adaptive physiology, controlling the expression of important transcription factors in ABA-dependent signaling pathways and stomatal closure. | Increase in ABA concentration | Spectrophotometric quantification based on immunodiagnostic kits (ELISA) or ultra-performance liquid chromatography (UPLC) [86,134,138]. |
| Molecular Markers—Oxidative Metabolism | |||
| Marker | Biological Function | Variations | Analysis |
| Reactive Oxygen Species (ROS) | ROS are products of metabolic processes (balanced or unbalanced), such as photosynthesis and cellular respiration, or products of the activity of peroxidases and oxidases in response to stresses. They can occur in chloroplasts, mitochondria, endoplasmic reticulum (ER) and peroxisomes. | Increase as a consequence of photosystem overload and decrease in stomatal conductance | Hydrogen peroxide (H2O2) is the most commonly analyzed form of ROS and reflects the global picture of the redox state of cells. In the qualitative evaluation, H2O2 reacts with diaminobenzamidine (DAB) or nitro-blue tetrazolium chloride (NBT) forming a brown and blue precipitate, respectively, in the leaves. Quantitatively, peroxide in acidic medium reacts with potassium iodide and is degraded to O2 and H2O. This degradation leads to a decrease in the absorbance of the reaction at 390 nm and its quantification can be performed based on a standard curve [86,134,135]. |
| Enzyme activity (CAT, SOD, APX and GPX) | In response to ROS accumulation, plants increase the transcription and activity of antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX) and glutathione-peroxidase (GPX). SOD converts superoxide radicals to H2O2 in chloroplasts. In turn, CAT and APX enzymes convert hydroxyl radicals to H2O and O2 in both chloroplasts and cytosol, as well as GPX. | Increase in enzyme activity | Enzyme activity assays based on enzyme-promoted ROS degradation and its effect on the enzymatic reaction [53,86]. |
| Nitrogen osmolytes | Nitrogen osmolytes, mostly represented by amino acids or derivatives, play an important role in the response to abiotic stresses, providing tolerance to water loss and ROS accumulation, in addition to acting as antifreeze in cases of extreme low temperatures. | Increased concentration of proline, glycine, glycine-betaine and γ-aminobutyric acid (GABA), in addition to polyamines | Proline quantification is the most widely used technique for the quantification of nitrogenous osmolytes. It is based on the reaction of proline in sulfosalicylic acid with ninhydrin, forming a bluish colored chromophore, whose reading is taken at 520 nm and the concentration calculated based on the absorbance of the samples and the molar extinction coefficient of proline. Fractional analyzes of amino acids and other nitrogen derivatives are conducted by HPLC [53,81]. |
| Soluble sugars | Soluble sugars, thanks to their reducing properties and their high solvation layer, are osmolytes synthesized by plants in response to drought and osmotic stress. When in high concentrations, they are able to optimize cellular osmotic potential, improve hydration status and protect lipid membranes against excessive heat and freezing. | Increased concentration of maltose, raffinose and trehalose | Detection based on oxide-reduction reactions. For the general detection of soluble sugars, the dinitrosalicylic acid (DNS) method is used, in which the reducing sugars react with the DNS when hot, oxidizing and forming a brownish-colored compound. Quantification is done by spectrophotometric reading at 540 nm and based on a monosaccharide standard curve. For quantification of specific sugars, high performance liquid chromatography is normally applied [126,130,134]. |
| Pigments—chlorophyll | Chlorophyll comprises a group of photosynthetic porphyrin pigments present in chloroplasts that impart a greenish color to leaves. Plants have chlorophyll a and b, which differ from each other by a methyl group instead of aldehyde at position 3 of the tetrapyrrole ring. | Decrease in chlorophyll ester in stress | Direct spectrophotometric analysis using extinction coefficients properly [130]. |
| Pigments—Carotenoids | Carotenoids are pigments of a lipidic nature, derived from terpenes. Due to their conjugated nature, they are excellent antioxidants and their abundance is associated with greater tolerance to the deleterious effects of ROS accumulation. | Decrease in carotenoid content due to oxidation | As well as chlorophyll, they are analyzed by direct spectrophotometry [130]. HPLC may also be indicated with correct standardization. |
| Pigments—anthocyanins | Anthocyanins are purplish pigments derived from flavonoids with a thermoprotective and antioxidant function. They are produced in response to excessive light and water deprivation. | Increased anthocyanin content in response to heat and drought | Extraction in hydrochloric acid alcohol and quantification by spectrophotometry with readings at 529 nm and 650 nm [48,80]. |
| Physiological Markers | |||
| Marker | Biological Function | Variations | Analysis |
| Stomatal conductance | The stomata are the main leaf structure involved in the control of gas exchange between the plant and the environment. Resistance and stomatal conductance are direct measures of the efficiency of these exchanges and reinforce the values analyzed for photosynthetic rate and transpiration, which should be lower in plants subjected to water and osmotic stress, for example. | Decrease in stomatal conductance to minimize water loss through transpiration | Infrared gas analyzer (IRGA) [154]. |
| CO2 fixation Photosynthesis + Transpiration | The consumption of CO2 has a direct relationship with the photosynthetic rate of plants, which is reduced in abiotic stress due to the reduction in leaf area and stomatal closing and reclusion mechanisms, as a side effect of the reduction in water loss through transpiration and optimization of the use of water inside the cells. | Decrease in photosynthetic rate and transpiration rate 1 | IRGA [154]. |
| (1) A decrease in the photosynthetic rate at a lower rate, compared to a decrease in the transpiration rate, and associated with a decrease in stomatal conductance, indicates the existence of adaptive mechanisms that optimize the use of water and reduce its loss, characteristics often associated with better performance of plants under water stress situation. | |||
| Relative water content | Relative water content refers to the water content of cells compared at two different points. It is generally indicative of the efficiency of water use in studies of osmotic stress, such as drought and salt stress, since higher relative water content indicates less transpiration, with a consequent improvement in the oxidative performance of cells and less leaf wilt. | Decrease in relative water content | The determination of the relative water content is done in a comparative situation, usually in irrigated plants and later submitted to water stress. The plant has its fresh mass determined and is subjected to drying to determine the dry mass and, consequently, the relative water content. [53,135]. The analysis can be also performed considering the weight of turgid leaves after water immersion in a standardized time. |
| Ionic flux due to electrolyte leakage | When a plant is subjected to a type of abiotic stress, normally, a flux of ions sets in across the plasma membrane in response to the stress. However, with the accumulation of ROS and the deleterious effects of stresses on the plasma membrane, there may be electrolyte leakage caused by membrane rupture. Therefore, electrolyte leakage is a parameter that infers membrane integrity. | Increased electrolyte leakage under stress | Comparative measurement in control plants and stressed plants or over time performed with a conductivity meter [95,126,134]. |
| Gene Markers | |||
| Gene name | Protein/Marker Type | Variation | Analysis |
| CAT | Catalase/Antioxidant enzyme | Increases | Gene expression analysis by qRT-PCR and expression calculation by 2-ΔCt or 2-ddCt comparative method [80,82,85,95,126,154]. |
| SOD | Superoxide dismutase/Antioxidant enzyme | Increases | |
| APX | Ascorbate Peroxidase/Antioxidant enzyme | Increases | |
| GPX | Glutathione Peroxidase/Antioxidant enzyme | Increases | |
| AREB-1 | ABA responsive element-binding/Transcription factor | Increases | |
| DREB1/2A | Dehydration responsive element-binding/Transcription factor | Increases | |
| RD29A | Responsive to desiccation 29A/Transcription factor | Increases | |
| RD29B | Responsive to desiccation 29B/Transcription factor | Increases | |
| RD20 | Responsive to desiccation 20/Transcription factor | Increases | |
| RAB18 | Ras-related protein 18/LEA protein | Increases | Gene expression analysis by qRT-PCR and expression calculation by 2-ΔCt or 2-ddCt comparative method. |
| PAL | Phenylalanine ammonia lyase/Phenylpropanoid pathway enzyme and phenolic compound synthesis | Increases | |
| NRP-1/2 | N-rich protein 1 and 2/Transcription factor | Increases | |
| VPE | Vacuolar processing enzyme/Caspase-like enzyme in cell death processes | Increases | |
| CNX | Calnexin/Calcium-dependent protein | Increases | |
| GLK1 | Golden like-1 protein/Chloroplast maintenance | Decreases | |
| NYC1 | Non-yellow Coloring 1/Chlorophyll degradation | Increases | |
| PaO | Pheophorbide Oxidase/Chlorophyll degradation | Increases | |
| BFN1 | Bifunctional nuclease 1/Ac. nucleic and proteins | Increases | |
| SINA1 | Seven in Absentia/Protease | Increases | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paes de Melo, B.; Carpinetti, P.d.A.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; de Camargos, L.F.; Ferreira, M.F.d.S. Abiotic Stresses in Plants and Their Markers: A Practice View of Plant Stress Responses and Programmed Cell Death Mechanisms. Plants 2022, 11, 1100. https://doi.org/10.3390/plants11091100
Paes de Melo B, Carpinetti PdA, Fraga OT, Rodrigues-Silva PL, Fioresi VS, de Camargos LF, Ferreira MFdS. Abiotic Stresses in Plants and Their Markers: A Practice View of Plant Stress Responses and Programmed Cell Death Mechanisms. Plants. 2022; 11(9):1100. https://doi.org/10.3390/plants11091100
Chicago/Turabian StylePaes de Melo, Bruno, Paola de Avelar Carpinetti, Otto Teixeira Fraga, Paolo Lucas Rodrigues-Silva, Vinícius Sartori Fioresi, Luiz Fernando de Camargos, and Marcia Flores da Silva Ferreira. 2022. "Abiotic Stresses in Plants and Their Markers: A Practice View of Plant Stress Responses and Programmed Cell Death Mechanisms" Plants 11, no. 9: 1100. https://doi.org/10.3390/plants11091100







