Pholiota nameko Polysaccharides Protect against Ultraviolet A-Induced Photoaging by Regulating Matrix Metalloproteinases in Human Dermal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Measurement of Elastase Activity
2.4. Cell Culture and UVA Irradiation
2.5. Cell Viability
2.6. Determining the Protective Ability of PNPs against UVA-Induced Cell Aging
2.7. Morphological Analysis
2.8. ROS Measurement
2.9. β-Galactosidase Staining
2.10. Western Blot
2.11. Nuclear Magnetic Resonance
2.12. Statistical Analysis
3. Results
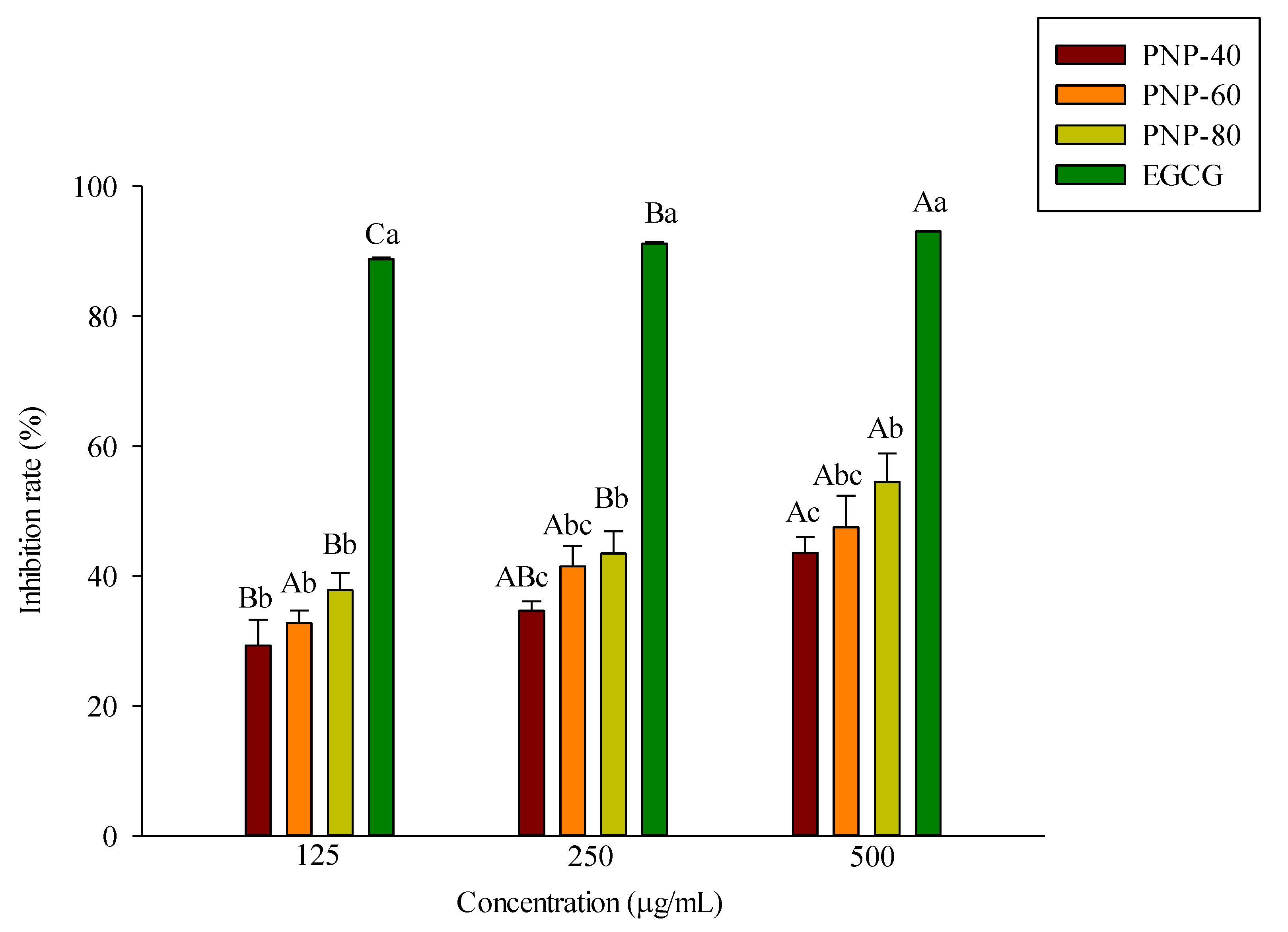
3.1. Inhibition of Elastase Activity by PNPs
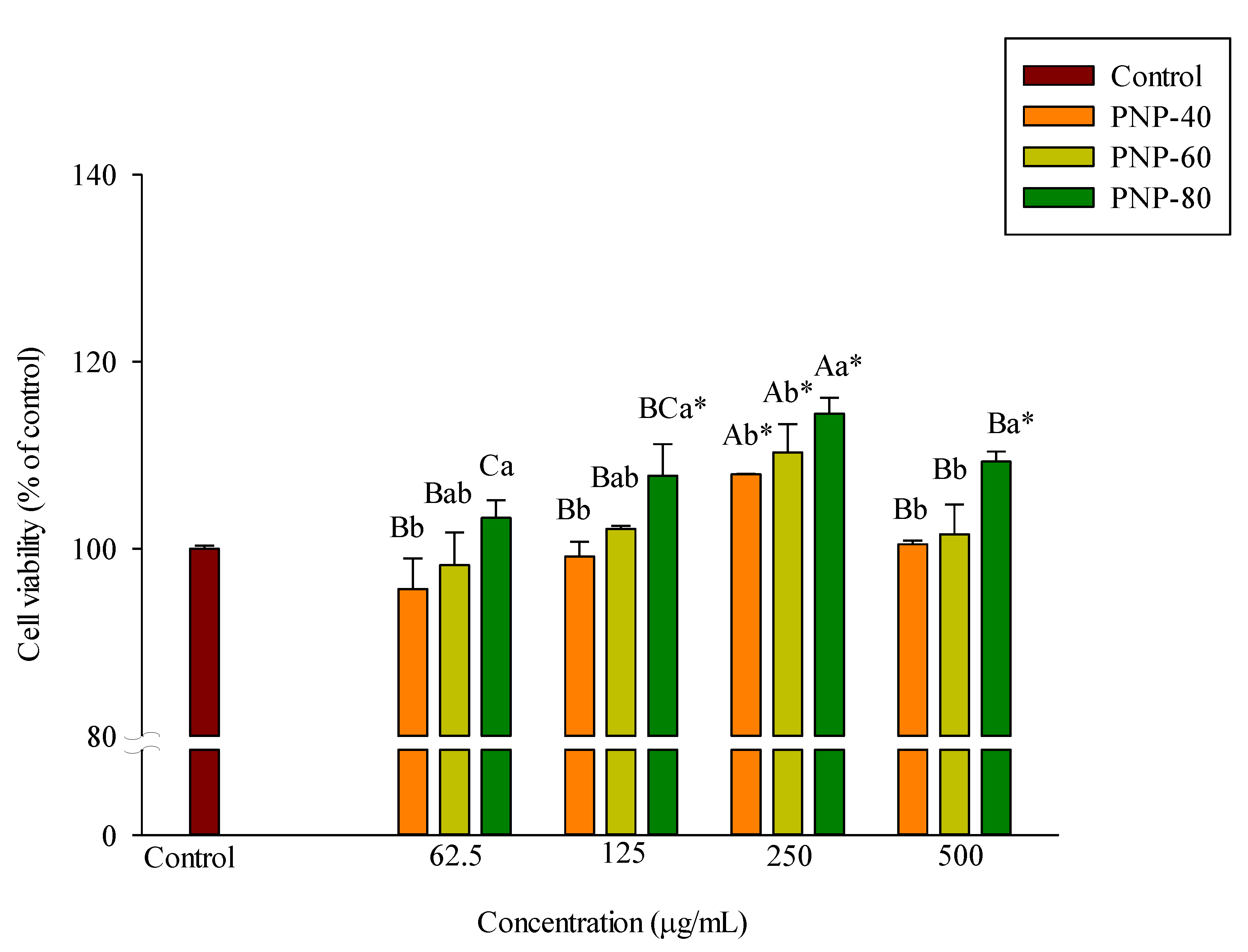
3.2. Effect of PNPs on Hs68 Cell Viability
3.3. UVA Exposure Dose-Dependently Reduces Fibroblast Viability
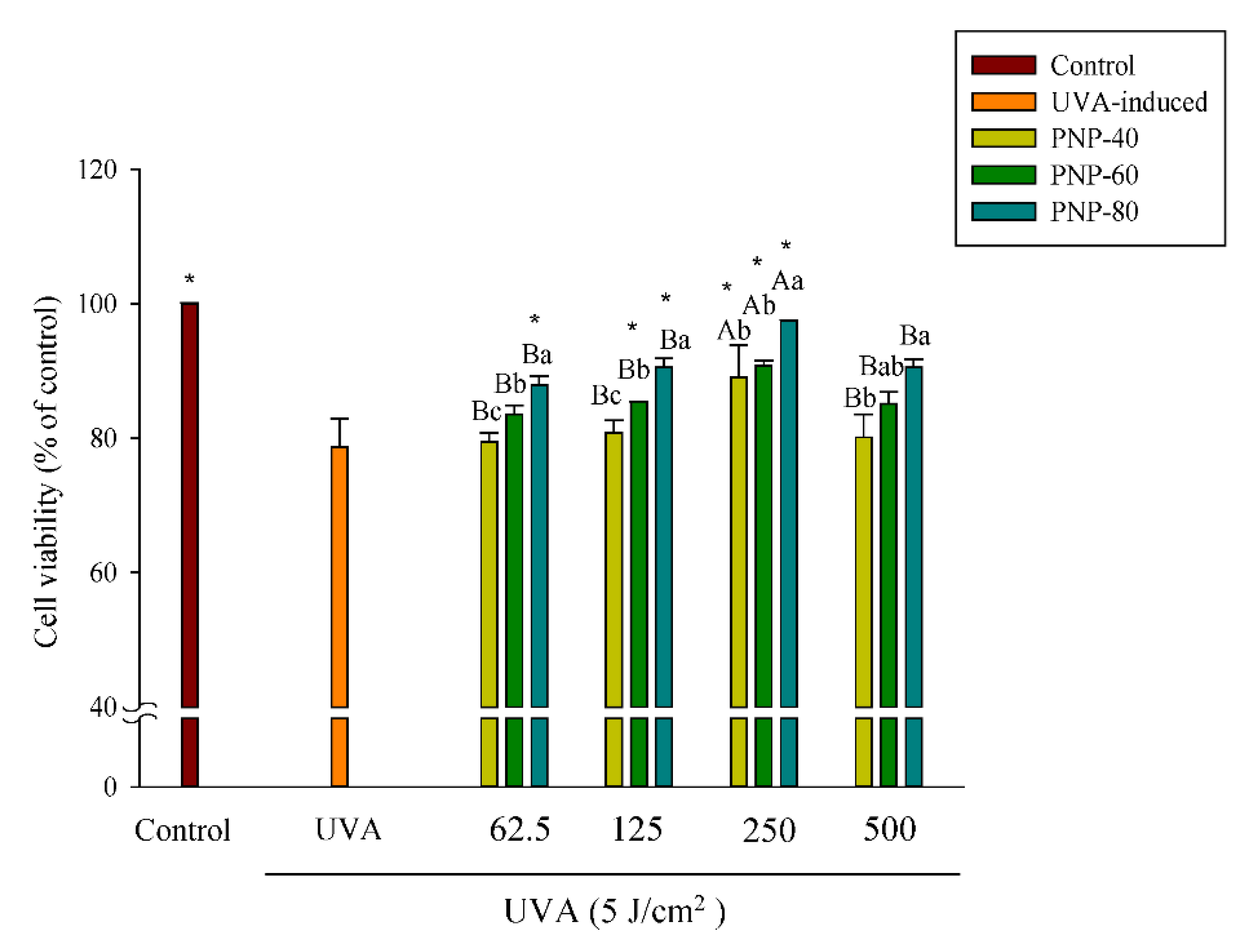
3.4. PNPs Protect Fibroblasts against UVA-Induced Cell Death
3.5. Effect of PNPs on UVA-Induced ROS Production in Hs68 Cells
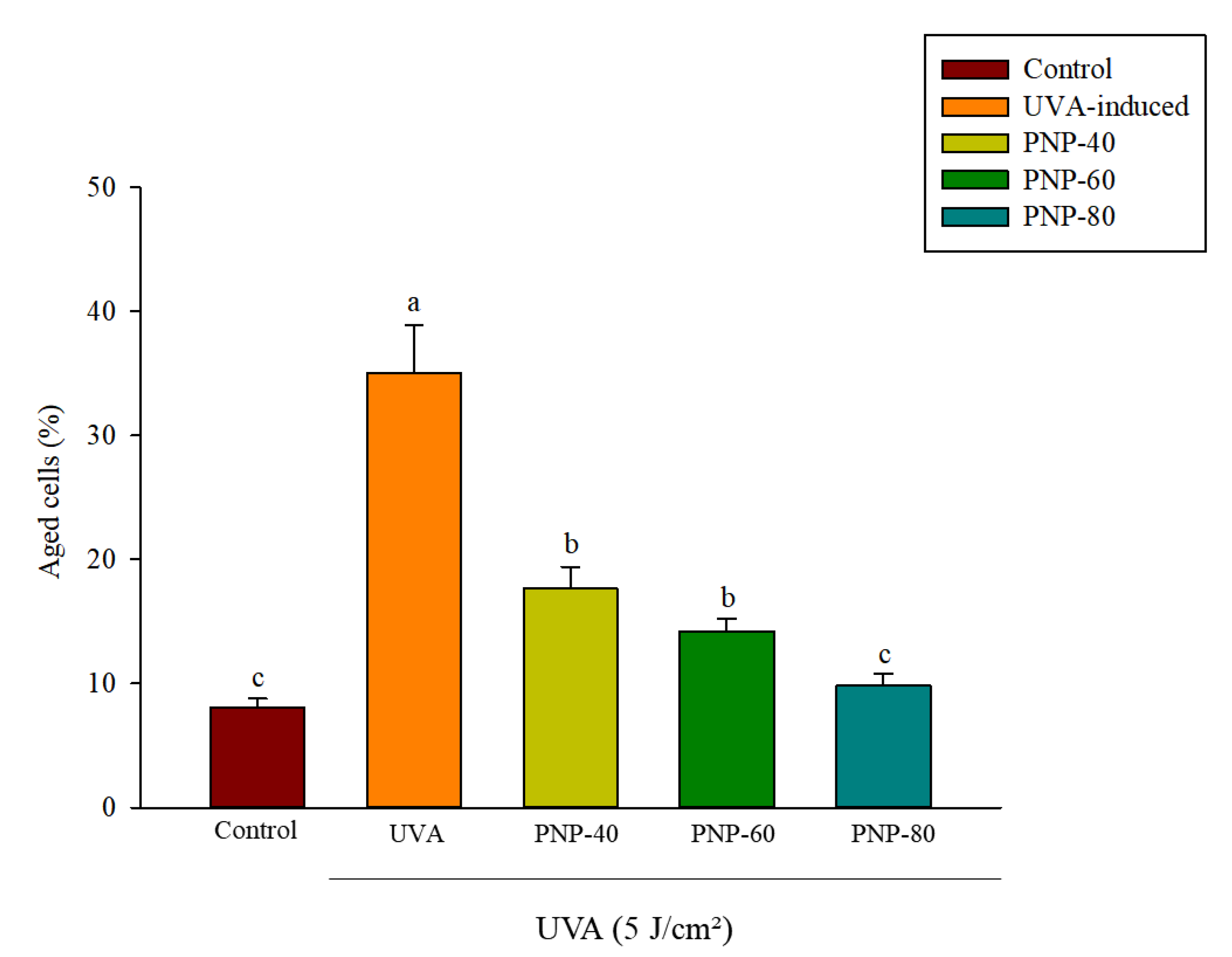
3.6. Effect of PNPs on UVA-Induced Senescence in Hs68 Cells
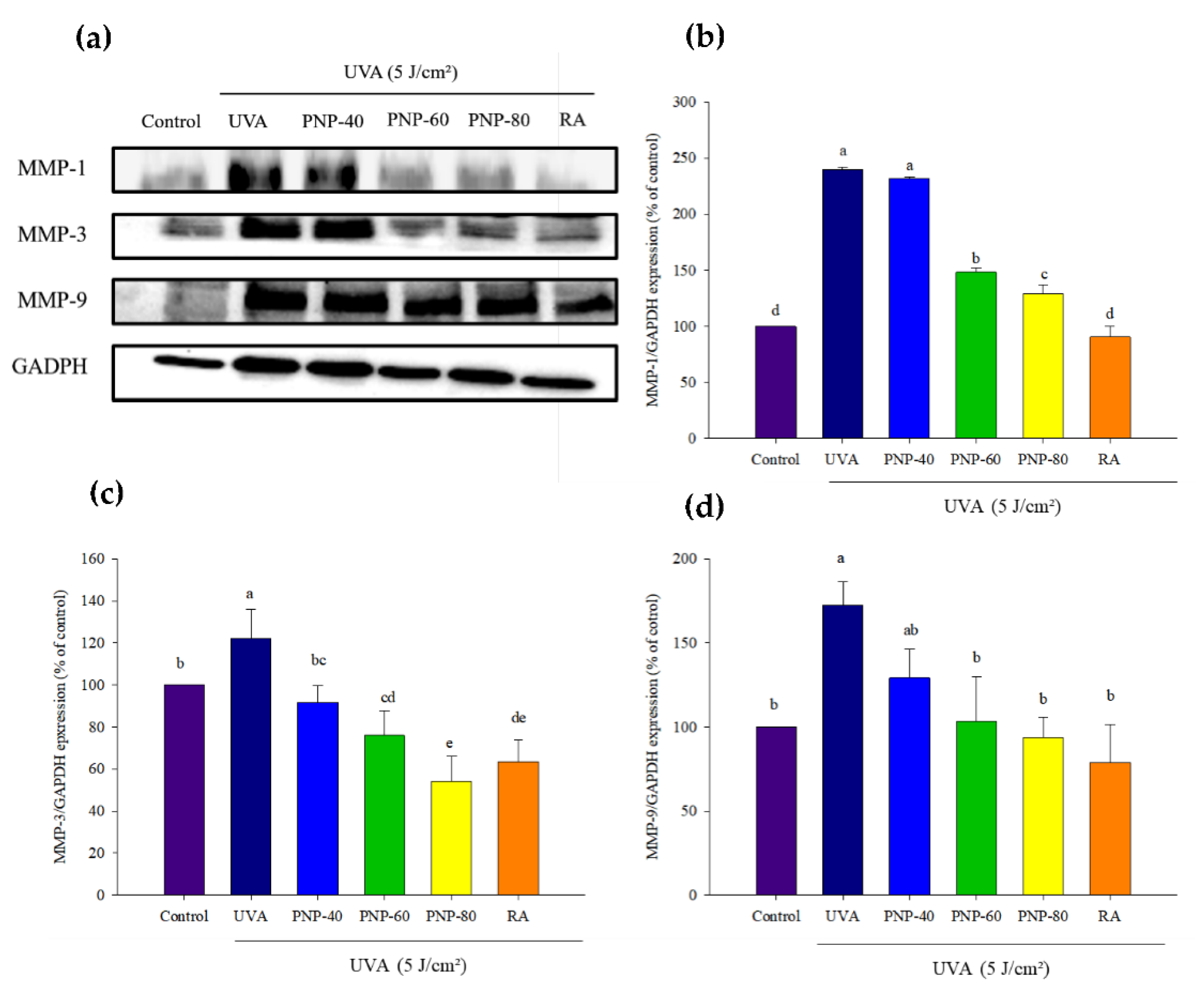
3.7. PNPs Downgrade UVA-Induced MMP-1, -3, -9 Expression in Hs68 Cells
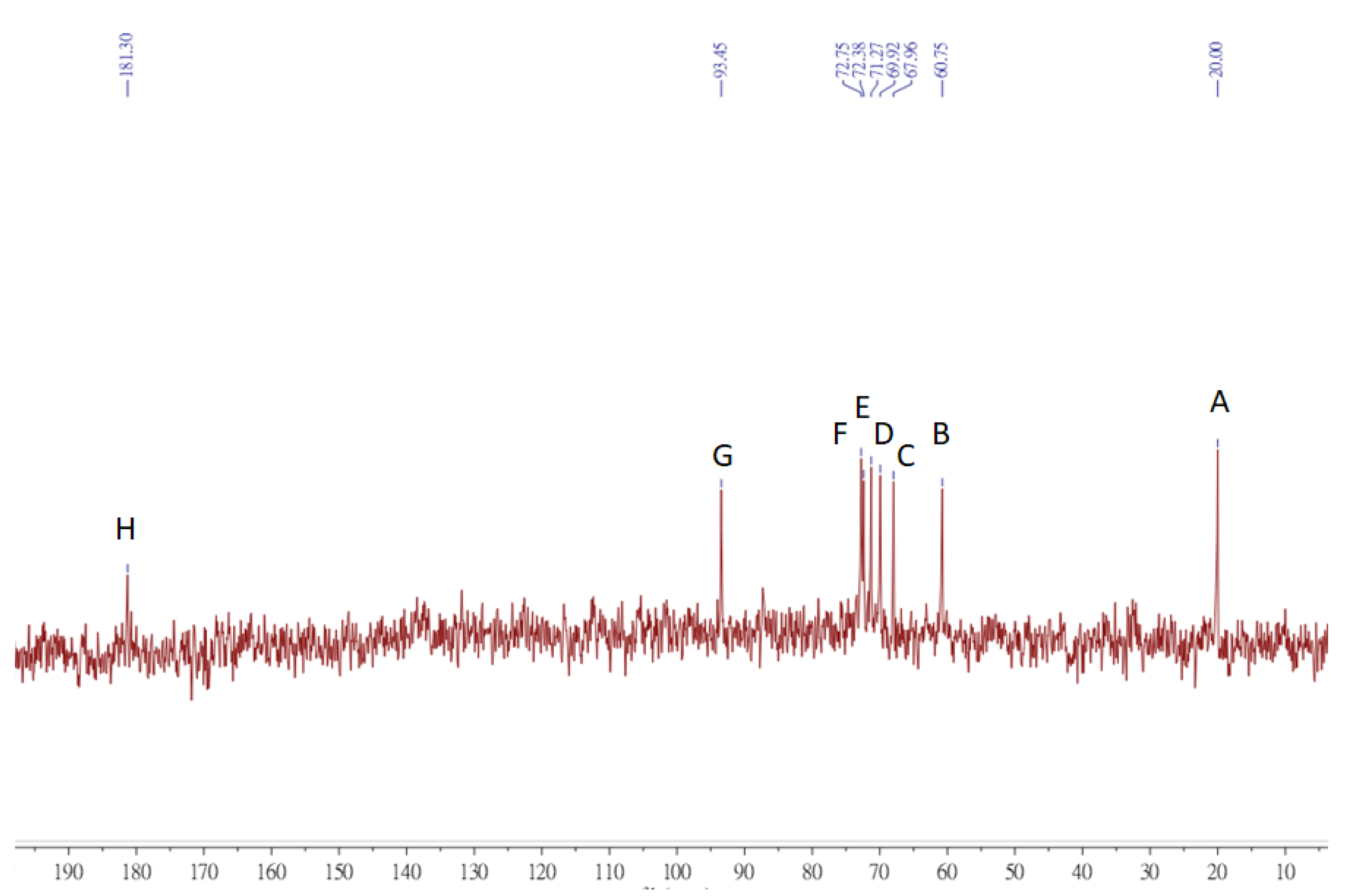
3.8. NMR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, K.-D.; Cheng, K.-C. From nutraceutical to clinical trial: Frontiers in Ganoderma development. Appl. Microbiol. Biotechnol. 2018, 102, 9037–9051. [Google Scholar] [CrossRef] [PubMed]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. Pharmanutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Hsu, K.-D.; Wu, S.-P.; Lin, S.-P.; Lum, C.-C.; Cheng, K.-C. Enhanced active extracellular polysaccharide production from Ganoderma formosanum using computational modeling. J. Food Drug Anal. 2017, 25, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-K.; Ho, W.-J.; Chang, S.-L.; Yeh, C.-H.; Liang, Z.-C.; Hsu, T.-H.; Hsieh, C. Fractionation, characterization and antioxidant activity of exopolysaccharide from fermentation broth of a Xylaria nigripes. Bioact. Carbohydr. Diet. Fibre 2018, 16, 37–42. [Google Scholar] [CrossRef]
- Jhan, M.-H.; Yeh, C.-H.; Tsai, C.-C.; Kao, C.-T.; Chang, C.-K.; Hsieh, C. Enhancing the Antioxidant Ability of Trametes versicolor Polysaccharopeptides by an Enzymatic Hydrolysis Process. Molecules 2016, 21, 1215. [Google Scholar] [CrossRef]
- Wen, L.; Gao, Q.; Ma, C.; Ge, Y.; You, L.; Liu, R.H.; Fu, X.; Liu, D. Effect of polysaccharides from Tremella fuciformis on UV-induced photoaging. J. Funct. Foods 2016, 20, 400–410. [Google Scholar] [CrossRef]
- Watson, R.; Gibbs, N.K.; Griffiths, C.; Sherratt, M. Damage to Skin Extracellular Matrix Induced by UV Exposure. Antioxid. Redox Signal. 2014, 21, 1063–1077. [Google Scholar] [CrossRef]
- Dwivedi, A.; Tripathi, A.K.; Singh, J.; Pal, M.K. Ultraviolet Radiation (UVR): An Introduction in Photocarcinogenesis & Photoprotection; Springer: Singapore, 2018; pp. 1–8. [Google Scholar]
- Lopes, D.M.; McMahon, S. Ultraviolet Radiation on the Skin: A Painful Experience? CNS Neurosci. Ther. 2016, 22, 118–126. [Google Scholar] [CrossRef]
- Masaki, H.J. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davó, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Yuan, W.; Mori, Y.; Varga, J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene 2000, 19, 3546–3555. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Cho, M.K.; Perry, D.; Quan, T. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: Implication for human skin connective tissue aging. J. Biomed. Sci. 2015, 22, 62. [Google Scholar] [CrossRef]
- Li, H.; Zhao, P.; Wang, F.; Huai, L.; Zhu, R.; Li, G.; Xu, Y. A polysaccharide from the culinary-medicinal mushroom Pholiota nameko (Agaricomycetes) inhibits the NF-κB pathway in dendritic cells through the TLR2 receptor. Int. J. Med. Mushrooms 2016, 18, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Abreu, H.; Simas, F.F.; Smiderle, F.R.; Sovrani, V.; Dallazen, J.L.; Maria-Ferreira, D.; Werner, M.F.; Cordeiro, L.M.; Iacomini, M. Gelling functional property, anti-inflammatory and antinociceptive bioactivities of β-D-glucan from the edible mushroom Pholiota nameko. Int. J. Biol. Macromol. 2019, 122, 1128–1135. [Google Scholar] [CrossRef]
- Chou, C.-H.; Sung, T.-J.; Hu, Y.-N.; Lu, H.-Y.; Yang, L.-C.; Cheng, K.-C.; Lai, P.-S.; Hsieh, C. Chemical analysis, moisture-preserving, and antioxidant activities of polysaccharides from Pholiota nameko by fractional precipitation. Int. J. Biol. Macromol. 2019, 131, 1021–1031. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Zhao, P.; Zhi, D.; Gao, X.; Zhao, X.; Li, M. Effect of ultrasound-assisted extraction on physicochemical properties and TLR2-affinity binding of the polysaccharides from Pholiota nameko. Int. J. Biol. Macromol. 2019, 135, 1020–1027. [Google Scholar] [CrossRef]
- Sung, T.-J.; Wang, Y.-Y.; Liu, K.-L.; Chou, C.-H.; Lai, P.-S.; Hsieh, C. Pholiota nameko Polysaccharides Promotes Cell Proliferation and Migration and Reduces ROS Content in H2O2-Induced L929 Cells. Antioxidants 2020, 9, 65. [Google Scholar] [CrossRef]
- Jackson, J.K.; Zhao, J.; Wong, W.; Burt, H.M. The inhibition of collagenase induced degradation of collagen by the galloyl-containing polyphenols tannic acid, epigallocatechin gallate and epicatechin gallate. J. Mater. Sci. Mater. Med. 2010, 21, 1435–1443. [Google Scholar] [CrossRef]
- Shirzad, M.; Hamedi, J.; Motevaseli, E.; Modarressi, M.H. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1051–1061. [Google Scholar] [CrossRef]
- Hahn, H.J.; Kim, K.B.; Bae, S.; Choi, B.G.; An, S.; Ahn, K.J.; Kim, S.Y. Pretreatment of Ferulic Acid Protects Human Dermal Fibroblasts against Ultraviolet A Irradiation. Ann. Dermatol. 2016, 28, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective effect of curcumin against ultraviolet A irradiation-induced photoaging in human dermal fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhu, S.; Han, X.; Feng, Y.; Huang, H.; Ren, G.; Pan, L.; Zhang, Y.; Lu, J.; Huang, B. BMP4-Smad Signaling Pathway Mediates Adriamycin-induced Premature Senescence in Lung Cancer Cells. J. Biol. Chem. 2009, 284, 12153–12164. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhou, F.; Lei, L.; Chen, J.; Lu, J.; Zhou, J.; Cao, K.; Gao, L.; Xia, F.; Ding, S.; et al. Ganoderma lucidum polysaccharides protect fibroblasts against UVB-induced photoaging. Mol. Med. Rep. 2016, 15, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Xuan, S.H.; Park, S.N. Licoricidin, an isoflavonoid isolated from Glycyrrhiza uralensis Fisher, prevents UVA-induced photoaging of human dermal fibroblasts. Int. J. Cosmet. Sci. 2016, 39, 133–140. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.; Li, Z.; Bai, W.; Zhao, J.; Huang, C.; Li, Q.; Fan, C.; Deng, L.; Lu, D. The effect of Cyanidin-3-o-glucoside on UVA-induced damage in human dermal fibroblasts. Photodermatol. Photoimmunol. Photomed. 2018, 34, 224–231. [Google Scholar] [CrossRef]
- Nie, S.-P.; Cui, S.W.; Phillips, A.O.; Xie, M.-Y.; Phillips, G.O.; Al-Assaf, S.; Zhang, X.-L. Elucidation of the structure of a bioactive hydrophilic polysaccharide from Cordyceps sinensis by methylation analysis and NMR spectroscopy. Carbohydr. Polym. 2011, 84, 894–899. [Google Scholar] [CrossRef]
- Madan, K.; Nanda, S. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Hung, Y.-T.; Fang, A.-H.; Ching-Shuang, W. Effects of irradiance on UVA-induced skin aging. J. Dermatol. Sci. 2019, 94, 220–228. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Pan, L.-C.; Han, D.; Sun, H.-Q.; Chen, L.-J. Structural properties and antioxidant activities of polysaccharide from fruit bodies of Pholiota nameko. Nat. Prod. Res. 2019, 33, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Unravelling Glycobiology by NMR Spectroscopy, in Glycosylation; IntechOpen: London, UK, 2012; pp. 63–98. [Google Scholar]
- Ou, M.C.; Liu, Y.H.; Sun, Y.W.; Chan, C.F. The composition, antioxidant and antibacterial activities of cold-pressed and distilled essential oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid. Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Im, K.H.; Baek, S.A.; Choi, J.; Lee, T.S. Antioxidant, Anti-Melanogenic and Anti-Wrinkle Effects of Phellinus vaninii. Mycobiology 2019, 47, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Yong, B.; Lee, H.H. Antioxidant and physiological activities of Coriolus versicolor fruit body crude extracts. J. Korea Acad. Ind. Coop. Soc. 2016, 17, 415–422. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 17, 1–28. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Tang, Y.; Ren, G.; Zhang, Y.; Ren, X. Concentrated growth factor inhibits UVA-induced photoaging in human dermal fibroblasts via the MAPK/AP-1 pathway. Biosci. Rep. 2020, 40, 40. [Google Scholar] [CrossRef]
- Kuboyama, N.; Ohta, M.; Sato, Y.; Abiko, Y. Anti-inflammatory activities of light emitting diode irradiation on collagen-induced arthritis in mice. Nippon Laser Igakkaishi 2012, 33, 19–25. [Google Scholar] [CrossRef][Green Version]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef]
- Xiao, L.; Mochizuki, M.; Nakahara, T.; Miwa, N. Hydrogen-Generating Silica Material Prevents UVA-ray-Induced Cellular Oxidative Stress, Cell Death, Collagen Loss and Melanogenesis in Human Cells and 3D Skin Equivalents. Antioxidants 2021, 10, 76. [Google Scholar] [CrossRef]
- He, R.; Zhao, Y.; Zhao, R.; Sun, P. Antioxidant and antitumor activities in vitro of polysaccharides from E. sipunculoides. Int. J. Biol. Macromol. 2015, 78, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, X.; Wang, F.; Xu, J.; Tang, X.; Li, N. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018, 111, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.-Y.; Sun, P.-P.; Li, H.-R.; Zhu, Z.-Y. Effects of Na2SeO3 on growth, metabolism, antioxidase and enzymes involved in polysaccharide synthesis of Cordyceps militaris. Process Biochem. 2020, 97, 64–71. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Ma, G. Hypolipidemic effect of the polysaccharide from Pholiota nameko. Nutrition 2010, 26, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhai, G.; Zhang, J.; Wang, L.; Ma, Z.; Jia, M.; Jia, L. Antihyperlipidemic and hepatoprotective activities of mycelia zinc polysaccharide from Pholiota nameko SW-. Int. J. Biol. Macromol. 2014, 70, 523–529. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljević, D.; Todorović, N.; Wan-Mohtar, W.A.a.I.; Nikšić, M. Ganoderma lucidum as a cosmeceutical: Antiradical potential and inhibitory effect on hyperpigmentation and skin extracellular matrix degradation enzymes. Arch. Biol. Sci. 2019, 71, 253–264. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Bai, D.; Cai, C.; Li, J.; Yan, C.; Zhang, S.; Wu, Z.; Hao, J.; Yu, G. Photoprotective effect of Astragalus membranaceus polysaccharide on UVA-induced damage in HaCaT cells. PLoS ONE 2020, 15, e0235515. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.; Redmond, C.; Poisson, R.; Bowman, D.; Couture, J.; Dimitrov, N.V.; Wolmark, N.; Wickerham, D.L.; Fisher, E.R.; et al. A Randomized Clinical Trial Evaluating Tamoxifen in the Treatment of Patients with Node-Negative Breast Cancer Who Have Estrogen-Receptor–Positive Tumors. N. Engl. J. Med. 1989, 320, 479–484. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Zaid, M.A.; Afaq, F.; Syed, D.N.; Dreher, M.; Mukhtar, H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem. Photobiol. 2007, 83, 882–888. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Cheng, K.-C.; Lin, J.-A.; Hsieh, L.-P.; Chou, C.-H.; Wang, Y.-Y.; Lai, P.-S.; Chu, P.-C.; Hsieh, C.-W. Pholiota nameko Polysaccharides Protect against Ultraviolet A-Induced Photoaging by Regulating Matrix Metalloproteinases in Human Dermal Fibroblasts. Antioxidants 2022, 11, 739. https://doi.org/10.3390/antiox11040739
Lin H, Cheng K-C, Lin J-A, Hsieh L-P, Chou C-H, Wang Y-Y, Lai P-S, Chu P-C, Hsieh C-W. Pholiota nameko Polysaccharides Protect against Ultraviolet A-Induced Photoaging by Regulating Matrix Metalloproteinases in Human Dermal Fibroblasts. Antioxidants. 2022; 11(4):739. https://doi.org/10.3390/antiox11040739
Chicago/Turabian StyleLin, His, Kuan-Chen Cheng, Jer-An Lin, Liang-Po Hsieh, Chun-Hsu Chou, Yu-Ying Wang, Ping-Shan Lai, Po-Cheng Chu, and Chang-Wei Hsieh. 2022. "Pholiota nameko Polysaccharides Protect against Ultraviolet A-Induced Photoaging by Regulating Matrix Metalloproteinases in Human Dermal Fibroblasts" Antioxidants 11, no. 4: 739. https://doi.org/10.3390/antiox11040739
APA StyleLin, H., Cheng, K.-C., Lin, J.-A., Hsieh, L.-P., Chou, C.-H., Wang, Y.-Y., Lai, P.-S., Chu, P.-C., & Hsieh, C.-W. (2022). Pholiota nameko Polysaccharides Protect against Ultraviolet A-Induced Photoaging by Regulating Matrix Metalloproteinases in Human Dermal Fibroblasts. Antioxidants, 11(4), 739. https://doi.org/10.3390/antiox11040739







