Characteristics of Conventional and Microwave Sintered Iron Ore Preform
Abstract
:1. Introduction
2. State of Art
3. Methodology
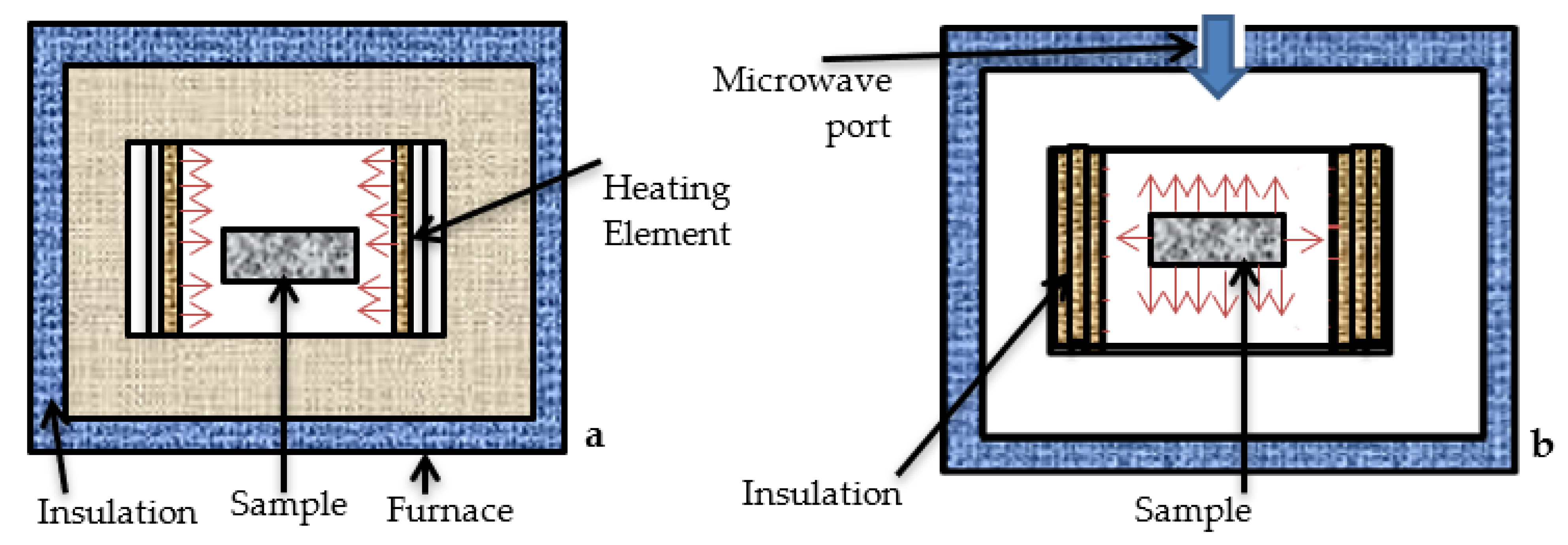
3.1. Conventional Sintering of the Preforms
3.2. Microwave Sintering of the Preforms
4. Modules of Data Collection
5. Results and Discussions
5.1. Density and Porosity
5.2. Cold Crushing Strength (CCS)
5.3. Microhardness Test
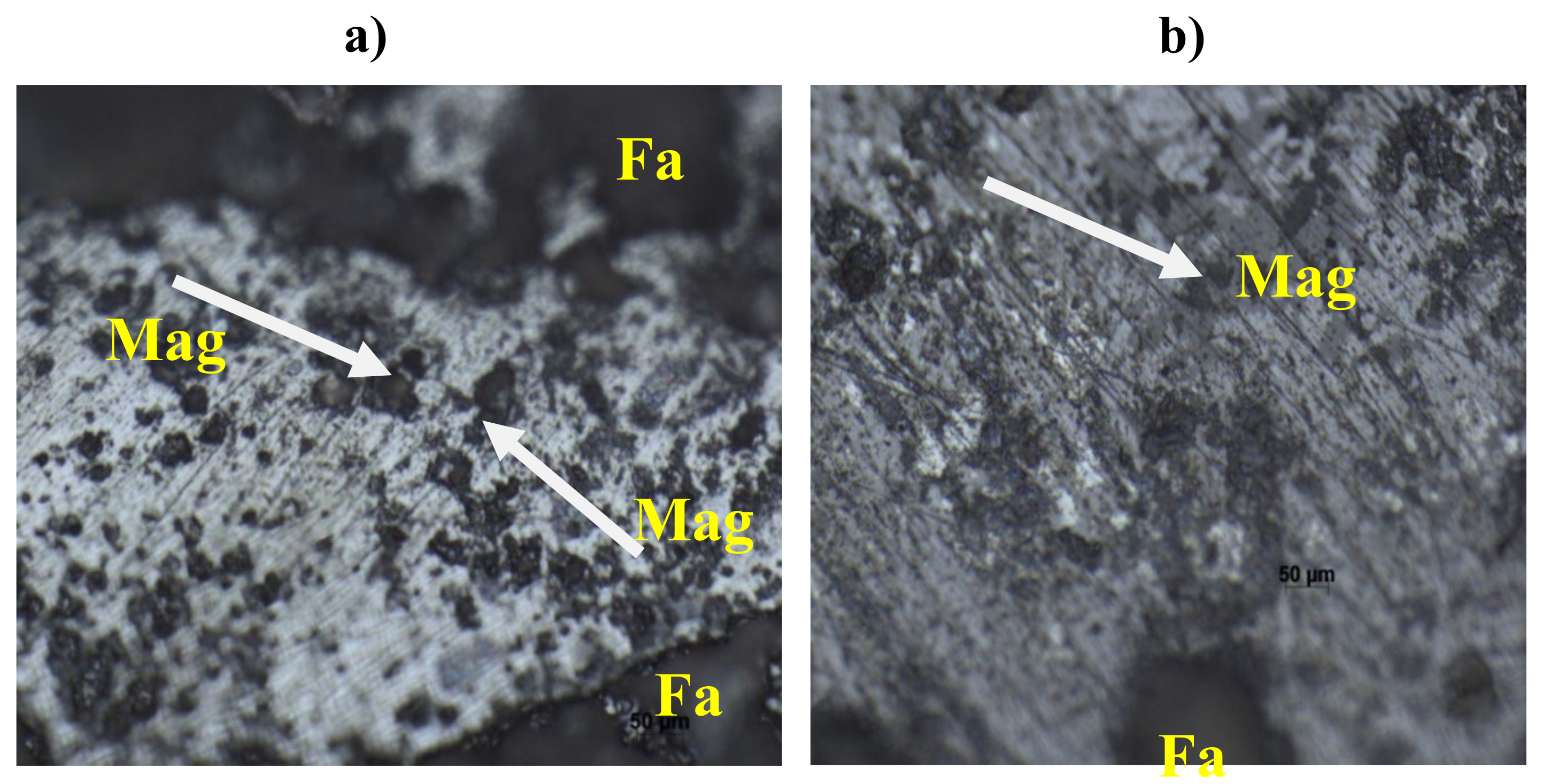
5.4. Optical Microscopy
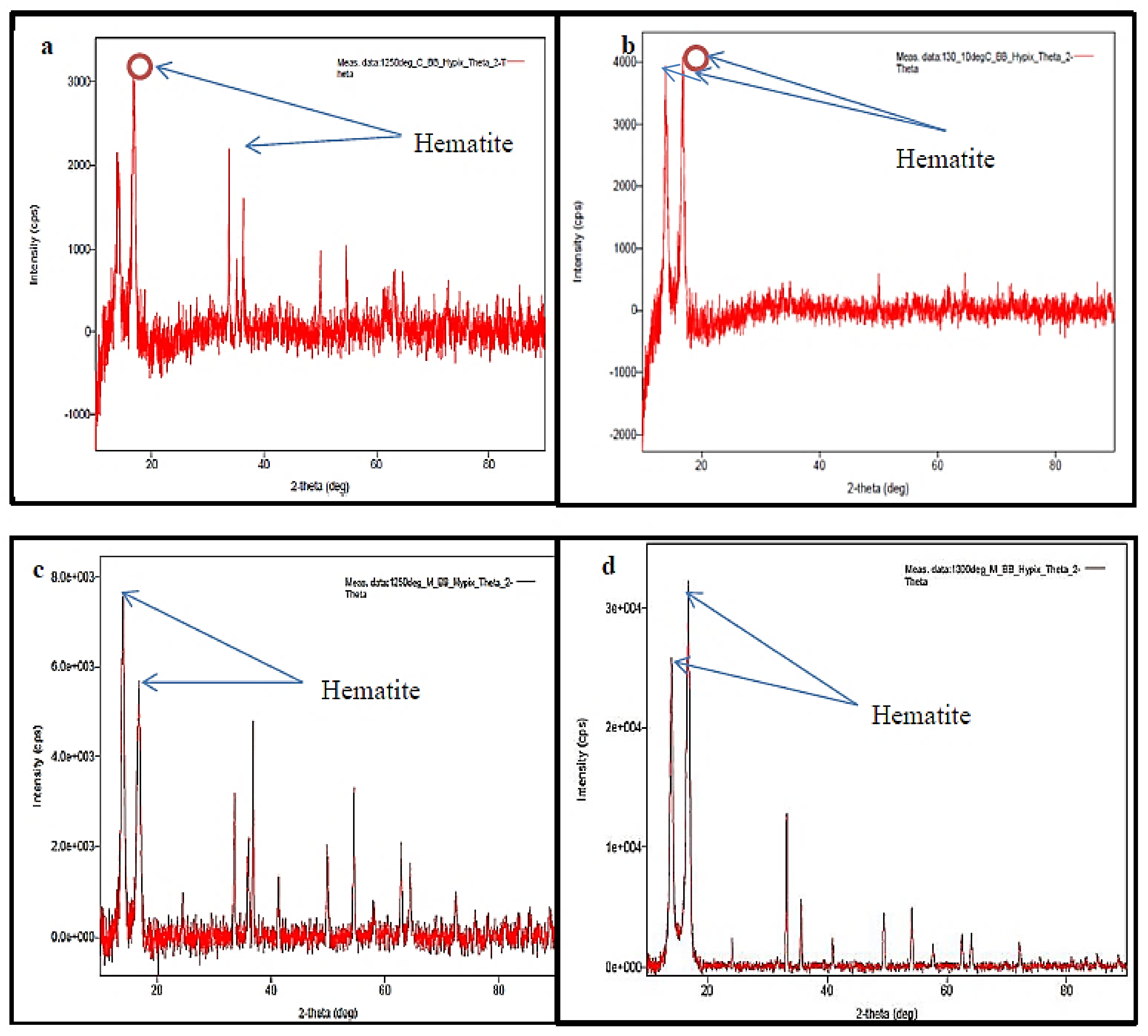
5.5. XRD Analysis
6. Conclusions
- Microwave sintering is found to be a superior method since it takes less sintering time and produces sintered preforms with better microstructure.
- Microwave sintered preforms have less porosity and have a higher density than conventionally sintered preforms.
- Microwave sintered preforms have improved iron ore diffusion which provides more strength and micro hardness to them.
- Due to reduced porosity and higher density, microwave sintered preforms have relatively higher cold crushing strength.
- The microwave sintering method provides uniform heating of the powder material and also improves hematite distribution, as evident from the XRD analysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.; Tang, W.; Xue, X. Effect of TiO2 on the sintering behavior of low-grade vanadiferous titanomagnetite ore. Materials 2021, 14, 4376. [Google Scholar] [CrossRef] [PubMed]
- Buckner, B.; Mali, H. Extended analyses of iron ore sinter by image processing. Steel Res. Int. 2020, 91, 2000236. [Google Scholar] [CrossRef]
- John, A.; Slotwinski, P.E.; Stutzman, S.S.; Watson, E.J.; Garboczi, M.A.P.; Chiara, F.F. Physical and chemical characterization techniques for metallic powders. AIP Conf. Proc. 2014, 119, 460–493. [Google Scholar]
- Onyishi, H.O.; Oluah, C.K. An overview of the state of the art and applications of sintered metals. IOSR J. Mech. Civ. Eng. 2020, 17, 1–10. [Google Scholar]
- Akbar, H.; Reddy, M.P.; Ubaid, F.; Shakoor, R.A.; Mohamed, A.M.A. Scanning electron microscopic studies of microwave sintered al-sic nano-composites and their properties. Scanning 2018, 3, 7546573. [Google Scholar]
- Morteza, O.; Omid, M. Microwave versus Conventional sintering: A review of fundamentals, advantages and applications. J. Alloy. Compd. 2010, 494, 175–189. [Google Scholar]
- Matli, P.R.; Mohamed, A.M.A.; Rajuru, R.R. Microwave fast sintering of double perovskite ceramic materials. In Advanced Ceramic Processing; Intech Open Book Series; InTech Open: London, UK, 2015. [Google Scholar]
- Anklekar, R.M.; Bauer, K.; Agrawal, D.K.; Roy, R. Improved mechanical properties and microstructural development of microwave sintered copper and nickel steel PM parts. J. Powder Metall. 2005, 48, 39–46. [Google Scholar] [CrossRef]
- Ayyappadas, C.; Annamalai, A.R.; Agrawal, D.; Muthuchamy, A. Conventional and microwave assisted sintering of copper-silicon carbide metal matrix composites: A comparison. J. Metall. Res. Technol. 2017, 114, 506. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, X.; Yu, J.; Wang, H.; Huang, Z. Microwave and conventional sintering of SiC/SiC composites: Flexural properties and microstructures. J. Ceram. Int. 2015, 41, 11651–11654. [Google Scholar] [CrossRef]
- Ramesh, S.; Zulkifli, N.; Tan, C.Y.; Wong, Y.H.; Tarlochan, F.; Ramesh, S.; Teng, W.D.; Sopyan, I.; Bang, L.T.; Sarhan, A.D. Comparison between microwave and conventional sintering on the properties and microstructural evolution of tetragonal zirconia. J. Ceram. Int. 2018, 44, 8922–8927. [Google Scholar] [CrossRef]
- Yang, M.; Wang, H.; Chen, R.; Gong, Y.; Huang, Z.; Guangming, R.; Chen, X.; Lu, T.; Xiao, C. Comparison of the microwave and conventional sintering of Li2TiO3 ceramic pebbles. J. Ceram. Int. 2018, 44, 19672–19677. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Zeng, J.Q. Study on the microwave reduction of carbon-containing iron ore fines. J. Iron Steel 2004, 39, 1–5. [Google Scholar]
- Shukla, M.; Ghosh, S.; Dandapat, N.; Mandal, A.K.; Balla, V.K. Comparative study on conventional sintering with microwave sintering and vacuum sintering of Y2O3-Al2O3-ZrO2 Ceramics. J. Mater. Sci. Chem. Eng. 2016, 4, 71–78. [Google Scholar]
- Padmavathi, C.; Upadhyaya, A.; Agrawal, D. Effect of microwave and conventional heating on sintering behaviour and properties of Al-Mg-Si-Cu alloy. J. Mater. Chem. Phys. 2011, 130, 449–457. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, Y.; Liu, X.; Yan, Y.; Liu, G. Effects of microwave sintering processing parameters on properties of BaTiO3 ceramics. J. Ferroelectr. 2016, 504, 237–241. [Google Scholar] [CrossRef]
- Brosnan, K.H.; Messing, G.L.; Agrawal, G.K. Microwave sintering of alumina at 2.45 GHz. J. Am. Ceram. Soc. 2003, 86, 1307–1312. [Google Scholar] [CrossRef]
- Li, J.; Pu, Y.P.; Wang, Z.; Dai, J. A comparative study of Sr0.7Ba0.3Nb2O6 relaxor ferroelectric ceramics prepared by conventional and microwave sintering techniques. Int. J. Ceram. 2013, 39, 5069–5075. [Google Scholar] [CrossRef]
- Mahajan, S.; Thakur, O.P.; Bhattacharya, D.K.; Sreenivas, K. A comparative study of Ba0.95Ca0.05Zr0.25Ti0.75O3 relaxor ceramics prepared by conventional and microwave sintering techniques. J. Mater. Chem. Phys. 2008, 112, 858–862. [Google Scholar] [CrossRef]
- Panda, S.S.; Singh, V.; Upadhyaya, A.; Agrawal, D. Sintering response of austenitic (316L) and ferritic (434L) stainless steel consolidated in conventional and microwave furnaces. J. Scr. Mater. 2006, 54, 2179–2183. [Google Scholar] [CrossRef]
- Chun, T.; Long, H.; Di, Z.; Wang, P.; Meng, Q. Influence of microwave heating on the microstructures of iron ore pellets with coal during reduction. J. Iron Mak. Steel Mak. Processes Prod. Appl. 2017, 7, 486–491. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Tiwari, S.K.; Mishra, P. Microwave sintering of W-Ni-Fe alloy. J. Scr. Mater. 2007, 56, 5–8. [Google Scholar] [CrossRef]
- Annamalai, R.; Upadhyaya, A.; Agrawal, D. An investigation on microwave sintering of Fe, Fe-Cu and Fe-Cu-C alloys. J. Bull. Mater. Sci. 2013, 36, 447–456. [Google Scholar] [CrossRef]
- Gao, C.; Xu, P.; Ruan, F.; Yang, C. Realization of phase and microstructure control in Fe/Fe2SiO4-FeAl2O4 metal-ceramic by alternative microwave susceptors. Materials 2022, 15, 1905. [Google Scholar] [CrossRef] [PubMed]
- Verdeja, L.F.; Ferreira, S.G.; Ruiz-Bustinza, I. Iron ore sintering. Part 1. Theory and practice of the sintering process. Dyna 2013, 180, 152–171. [Google Scholar]
- Saragi, T.; Santika, A.S.; Permana, B.; Syakir, N.; Kartawidjaja, M. Risdiana, synthesis and properties of iron oxide particles prepared by hidrothermal method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 196, 012025. [Google Scholar] [CrossRef] [Green Version]
- Montealegre-Meléndez, I.; Arévalo, C.; Pérez-Soriano, E.M.; Kitzmantel, M.; Neubauer, E. Microstructural and XRD analysis and study of the properties of the system Ti-TiAl-B4C processed under different operational conditions. Metals 2018, 8, 367. [Google Scholar] [CrossRef] [Green Version]
- Machado, M.M.; Lagoeiro, L.E.; Graca, L. Phase and microstructural characterization of iron ore pellet and their relation with cold crushing strength test. Miner. Processing Extr. Metall. Rev. 2016, 37, 295–304. [Google Scholar]











| S. No. | Material | Composition |
|---|---|---|
| 1 | Iron ore (Fe2O3) | 85% |
| 2 | Limestone | 11% |
| 3 | Coke | 4% |
| Sl. No. | Samples and Sintering Temperature | Dry Weight (g) | Soaked Weight (g) | Suspended Weight (g) | Density (g/cm3) | Porosity (%) |
|---|---|---|---|---|---|---|
| 1 | A at 1250 °C | 8.2733 | 8.6820 | 6.2405 | 3.3886 | 16.739 |
| 2 | B at 1250 °C | 8.2853 | 8.7732 | 6.3280 | 3.4249 | 16.305 |
| 3 | A at 1300 °C | 8.6331 | 9.0615 | 6.4857 | 3.3516 | 16.632 |
| 4 | B at 1300 °C | 8.5764 | 8.9915 | 6.4396 | 3.3610 | 16.266 |
| Sl. No. | Samples and Sintering Temperature | Dry Weight (g) | Soaked Weight (g) | Suspended Weight (g) | Density (g/cm3) | Porosity (%) |
|---|---|---|---|---|---|---|
| 1 | A at 1250 °C | 8.2108 | 8.9354 | 6.0504 | 2.8460 | 25.11 |
| 2 | B at 1250 °C | 8.3453 | 8.8799 | 6.0003 | 2.8772 | 20.65 |
| 3 | A at 1300 °C | 8.0988 | 8.7598 | 5.9844 | 2.9181 | 23.82 |
| 4 | B at 1300 °C | 8.1567 | 8.7975 | 6.0132 | 2.9953 | 23.00 |
| Sl. No. | Samples | Load at the Point of Failure (kg) | Cross Sectional Area (cm2) | Crushing Strength (kg/cm2) |
|---|---|---|---|---|
| 1 | Conventional sintering, B (1250 °C) | 202.920 | 3.1415 | 64.593 |
| 2 | Conventional sintering, B (1300 °C) | 227.396 | 3.1415 | 72.384 |
| 3 | Microwave sintering, B (1250 °C) | 498.641 | 3.1415 | 158.727 |
| 4 | Microwave sintering, B (1300 °C) | 530.250 | 3.1415 | 168.789 |
| Loads ► | 50 gf | 100 gf | 200 gf | 300 gf |
|---|---|---|---|---|
| Samples ▼ | ||||
| Conventional sintering, B (1250 °C) | 24.4 HV | 30.4 HV | 48.7 HV | 70.5 HV |
| Conventional sintering, B (1300 °C) | 29.8 HV | 41.0 HV | 53.1 HV | 71.8 HV |
| Microwave sintering, B (1250 °C) | 33.5 HV | 43.8 HV | 67.8 HV | 91.0 HV |
| Microwave sintering, B (1300 °C) | 33.8 HV | 69.3 HV | 75.5 HV | 98.9 HV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Equbal, A.; Ali, M.; Equbal, M.A.; Srivastava, S.C.; Khan, Z.A.; Equbal, M.I.; Badruddin, I.A.; El-Hady, K.M.; Kamangar, S. Characteristics of Conventional and Microwave Sintered Iron Ore Preform. Materials 2022, 15, 2655. https://doi.org/10.3390/ma15072655
Equbal A, Ali M, Equbal MA, Srivastava SC, Khan ZA, Equbal MI, Badruddin IA, El-Hady KM, Kamangar S. Characteristics of Conventional and Microwave Sintered Iron Ore Preform. Materials. 2022; 15(7):2655. https://doi.org/10.3390/ma15072655
Chicago/Turabian StyleEqubal, Azhar, Mohammad Ali, Md. Asif Equbal, S. C. Srivastava, Zahid A. Khan, Md. Israr Equbal, Irfan Anjum Badruddin, Khalid Mohamed El-Hady, and Sarfaraz Kamangar. 2022. "Characteristics of Conventional and Microwave Sintered Iron Ore Preform" Materials 15, no. 7: 2655. https://doi.org/10.3390/ma15072655






