Vegetables and Their Bioactive Compounds as Anti-Aging Drugs
Abstract
:1. Introduction
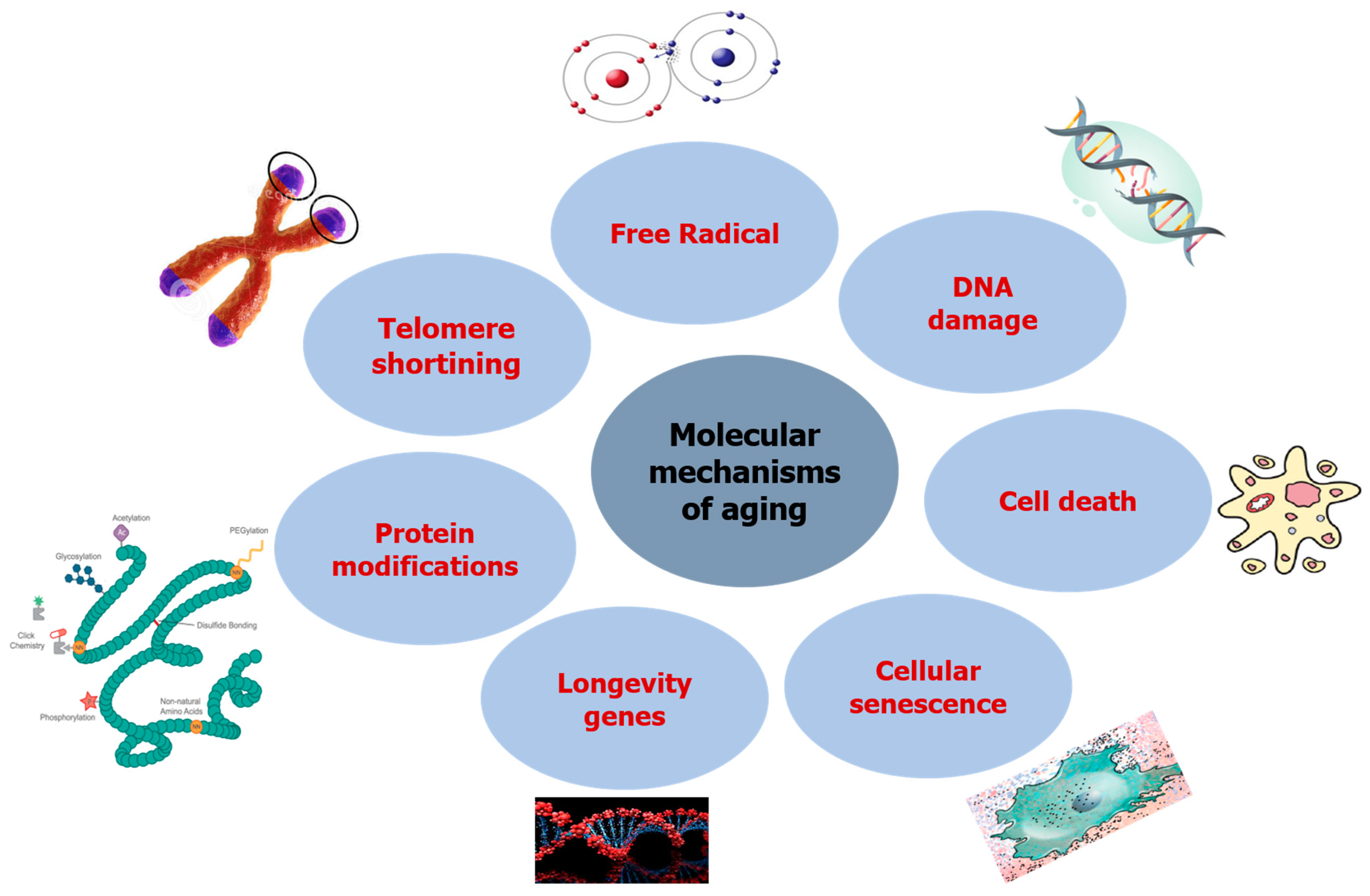
2. Aging and Its Molecular Mechanism
2.1. DNA Damage
2.2. Free Radicals
2.3. Telomere Shortening
2.4. Protein Modifications
2.5. Longevity Genes
2.6. Cellular Senescence
2.7. Cell Death
3. Anti-aging Effects of Vegetables
3.1. Allium cepa (Onion)
3.2. Allium sativum (Garlic)
3.3. Allium tuberosum (Chinese Chives, Buchu)
3.4. Dioscorea aimadoim (Yam)
3.5. Dioscorea opposite (Yam)
3.6. Asparagus cochinchinensis (Chinese Asparagus)
3.7. Asparagus officinalis (Asparagus)
3.8. Amaranthus tricolor (Red Spinach)
3.9. Cynara scolymus (Artichoke)
3.10. Taraxacum officinalis (Dandelion)
3.11. Brassica oleracea L. var. capitata F. rubra (Red Cabbage)
3.12. Brassica oleracea L. var. italica Plenck (Broccoli)
3.13. Raphanus sativus (Radish)
3.14. Cucumis sativus (Cucumber)
3.15. Cucurbita moschata (pumpkin)
3.16. Glycine max (Soybean)
3.17. Vigna angularis (Red Bean)
3.18. Phaseolus vulgaris (Black Bean)
3.19. Vigna angularis
3.20. Abelmoschus esculentus (Okra)
3.21. Rheum rhabarbarum (Rhubarb)
3.22. Rumex crispus (Curly Dock)
3.23. Daucus carota (Carrot)
4. Anti-Aging Natural Compounds from Vegetables
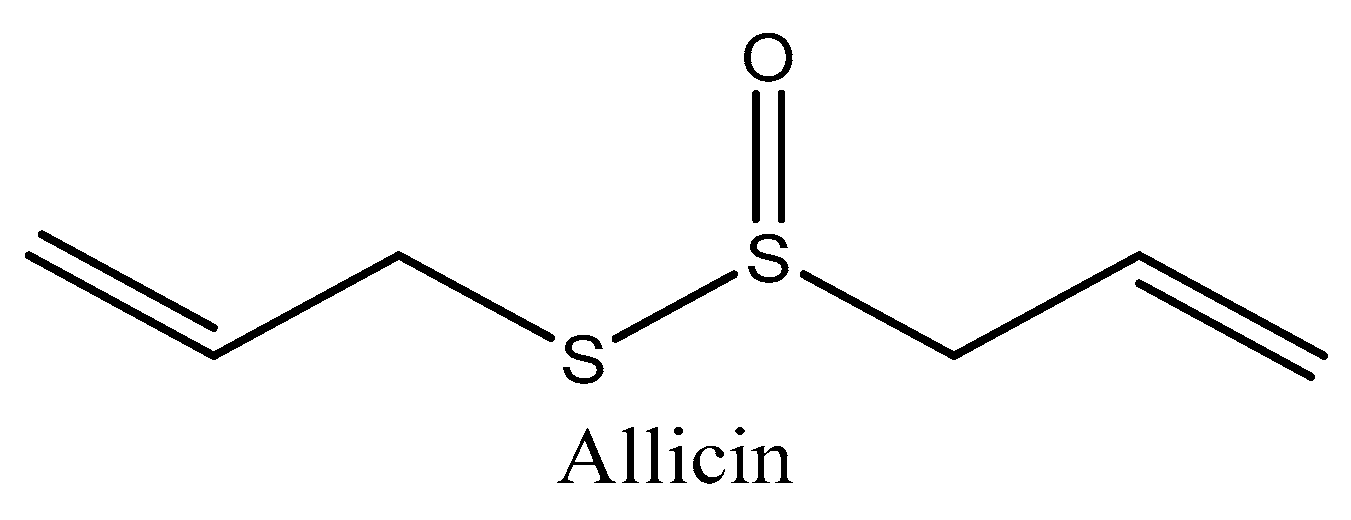
4.1. Allicin (Allium sativum)
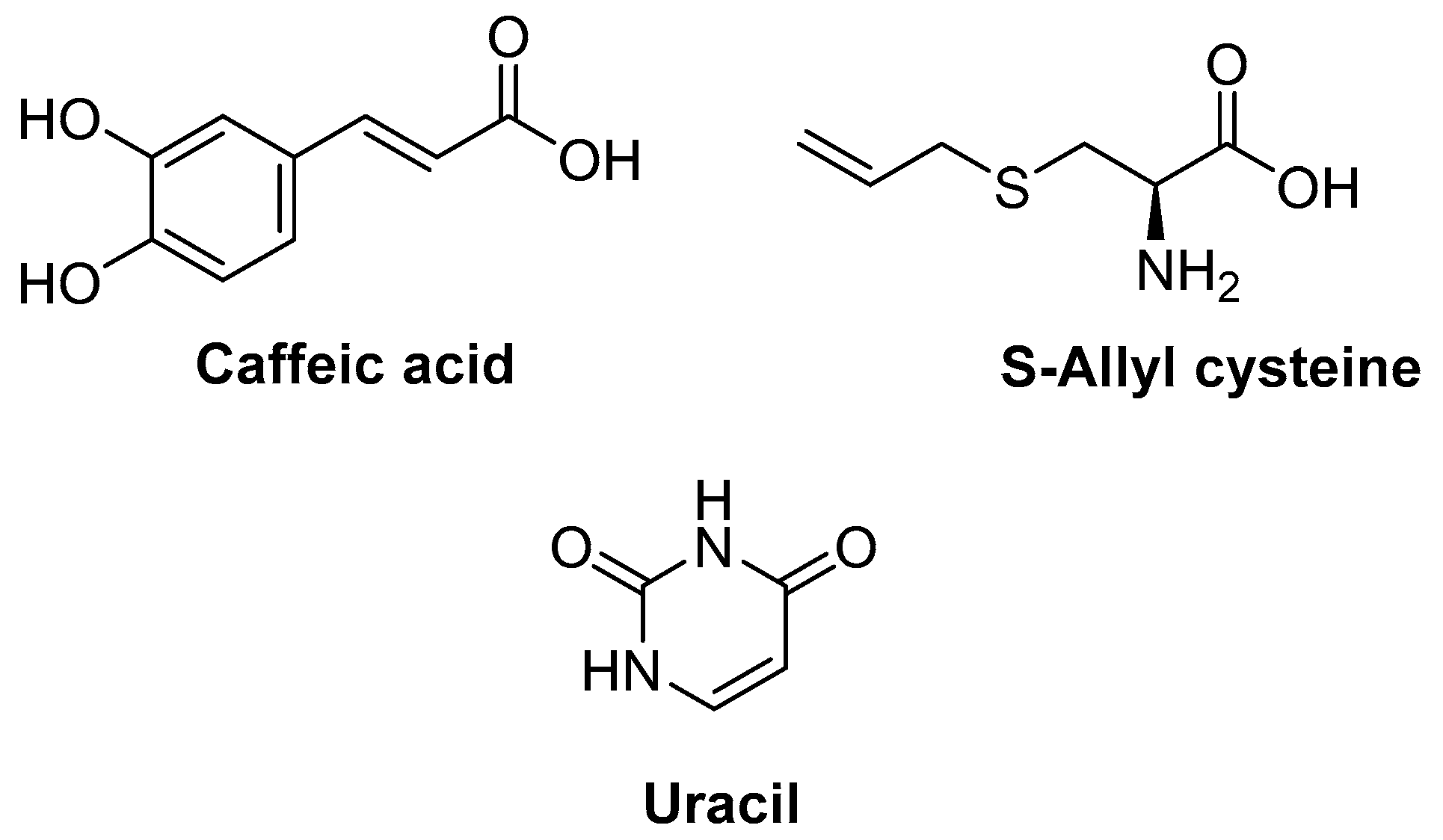
Caffeic Acid, S-Allyl Cysteine, and Uracil (Allium sativum)
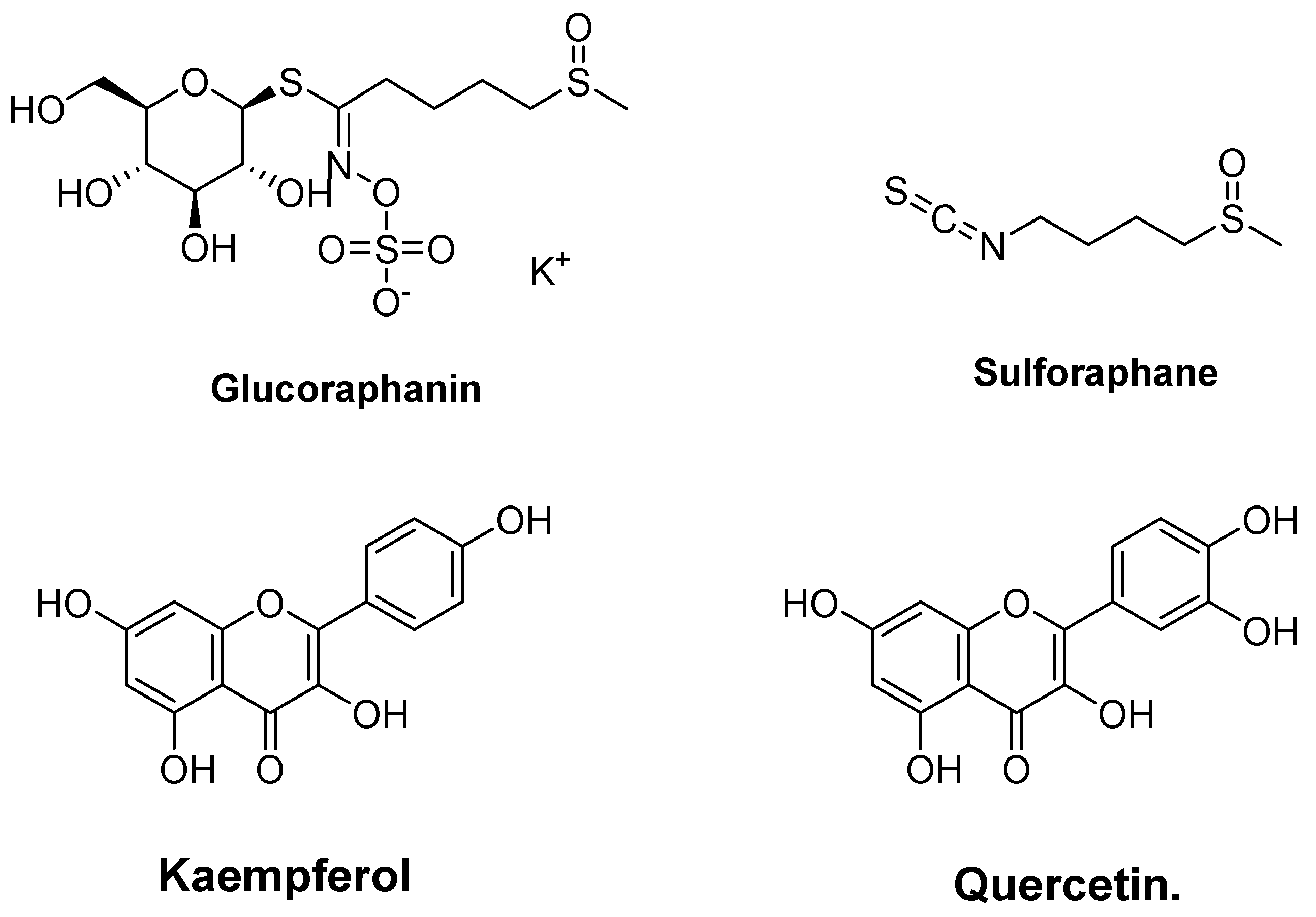
4.2. Glucosinolate (Glucoraphanin and Sulforaphane) and Phenolic (Kaempferol and Quercetin) from Broccoli (Brassica oleracea)
4.3. Water-Soluble Protein (Vicia faba)
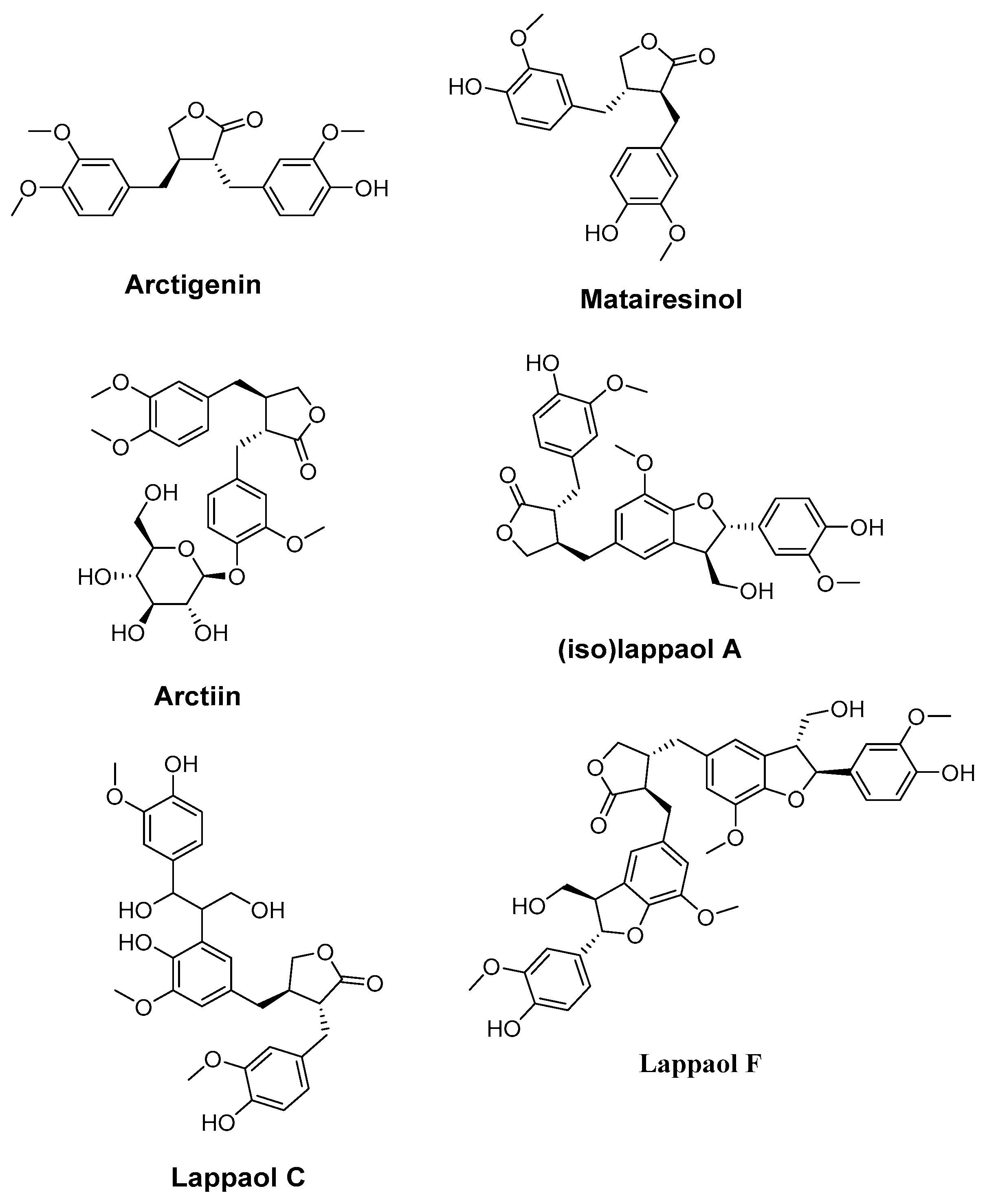
4.4. Arctigenin, Matairesinol, Arctiin, (iso)lappaol A, lappaol C, and lappaol F. (Arctium lappa)
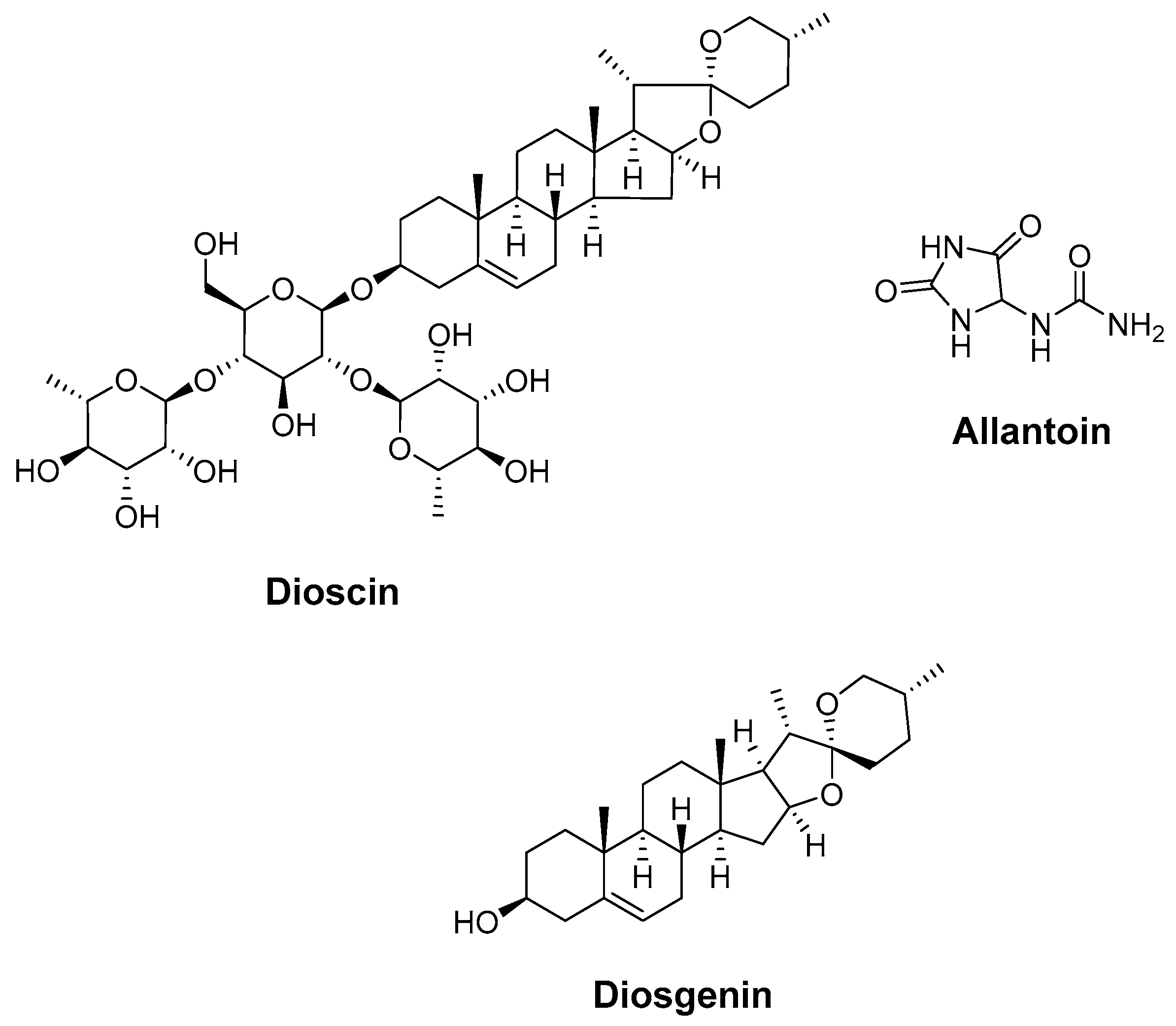
4.5. Dioscin, Allantoin, and Diosgenin (Dioscoreae Rhizoma)
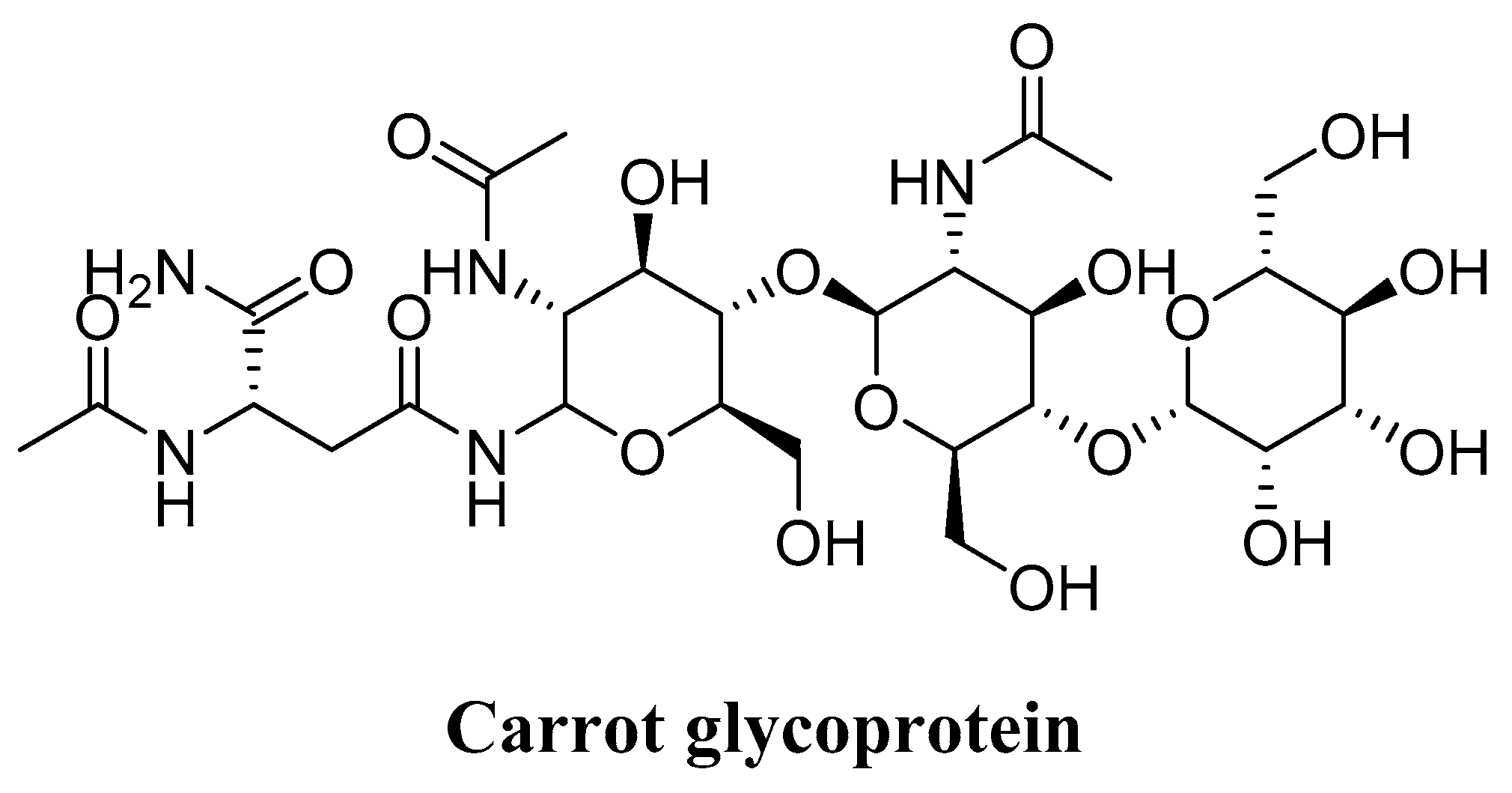
4.6. Glycoprotein (Daucus carota)
4.7. Trp-Pro-Lys (WPK) and Ala-Tyr-Leu-His (AYLH) Glycine max (Soybean)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid |
| ACN | Anthocyanin |
| AMPK | AMP-Activated Protein Kinase |
| AOC | Antioxidant Capacity |
| AT | Ataxia-Telangiectasia |
| CAT | Catalase |
| COX-2 | Cyclooxygenase-2 |
| Cu/Zn-SOD | Cupro-Zinc Superoxide Dismutase |
| CYP | Chinese Yam Polysaccharides |
| DNA | Deoxyribonucleic Acid |
| DDR | DNA Damage Response |
| DPPH | 1,1-Diphenyl-2-Picrylhydrazyl |
| ECM | Extracellular Matrix |
| EXO1 | Exonuclease 1 |
| FRAP | Ferric Reducing Antioxidant Power |
| FT-IR | Fourier Transform Infrared |
| GO | Gene Ontology |
| GPXs | Glutathione Peroxidase |
| GSH | Glutathione |
| HDL | High-Density Lipoprotein |
| HRIPT | Human Repeat Insult Patch Testing |
| HPLC-MS | High-Performance Liquid Chromatography–Mass Spectrometry |
| HRSA | Hydroxyl Radical Scavenging Activity |
| IC50 | 50% Inhibitory Concentration |
| IL-1 | Interleukin-1 |
| iNOS | Inducible Nitric Oxide Synthase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LDL | Low-Density Lipoprotein |
| LF | Lipofuscin |
| MIC | Minimum Inhibitory Concentration |
| mtDNA | Mitochondrial DNA |
| MMPs | Matrix Metallo-Proteinases |
| Mn-SOD | Manganese Superoxide Dismutase |
| mRNA | Messenger RNA |
| NAD | Nicotinamide Adenine Dinucleotide |
| NF-κB | Nuclear Factor-κB |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| ORAC | Oxygen Radical Absorbance Capacity |
| OS | Oxidative Stress |
| PMS2 | Post-Meiotic Segregation increased 2 |
| PPI | Protein–Protein Interaction |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| RP-HPLC-DAD | Reversed-Phase High-Performance Liquid Chromatography with a Diode Array Detector |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SA-gal | Senescence-Associated Galactosidase |
| SCF | Supercritical Fluid |
| SOD | Superoxide Dismutase |
| SPF | Sun Protection Factor |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TC-NER | Transcription-Coupled Nucleotide Excision Repair |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| TNF-α | Tumor Necrosis Factor-A |
| TPC | Total Phenolic Content |
| UVB | Ultraviolet B |
| WRN | Werner’s Syndrome Protein |
| WSP | Water Soluble Protein |
References
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and Functions of Cellular Senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.M. Keynote: Mechanisms of Senescence—Complificationists versus Simplificationists. Mech. Ageing Dev. 2002, 123, 65–73. [Google Scholar] [CrossRef]
- Pedro de Magalhães, J. From Cells to Ageing: A Review of Models and Mechanisms of Cellular Senescence and Their Impact on Human Ageing. Exp. Cell Res. 2004, 300, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Romandini, M.; Citterio, F.; Romano, F.; Aimetti, M. Periodontitis and Accelerated Biological Aging: A Geroscience Approach. J. Dent. Res. 2022, 101, 125–132. [Google Scholar] [CrossRef]
- Brahadeeswaran, S.; Sivagurunathan, N.; Calivarathan, L. Inflammasome Signaling in the Aging Brain and Age-Related Neurodegenerative Diseases. Mol. Neurobiol. 2022, 1–17. [Google Scholar] [CrossRef]
- Ding, Y.-N.; Wang, H.-Y.; Chen, H.-Z.; Liu, D.-P. Targeting Senescent Cells for Vascular Aging and Related Diseases. J. Mol. Cell. Cardiol. 2022, 162, 43–52. [Google Scholar] [CrossRef]
- Lee, S.; Schmitt, C.A. The Dynamic Nature of Senescence in Cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Assemian, I.C.C.; Abrini, J.; Dakka, N. In Vitro Antiproliferative Activity of Selected Medicinal Plants from the North-West of Morocco on Several Cancer Cell Lines. Eur. J. Integr. Med. 2018, 18, 23–29. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Bakri, Y.; Dakka, N. Les huiles essentielles comme agents anticancéreux: Actualité sur le mode d’action. Phytothérapie 2018, 16, 254–267. [Google Scholar] [CrossRef]
- Bouyahya, A.; Belmehdi, O.; Benjouad, A.; Ameziane El Hassani, R.; Amzazi, S.; Dakka, N.; Bakri, Y. Pharmacological Properties and Mechanism Insights of Moroccan Anticancer Medicinal Plants: What Are the next Steps? Ind. Crops Prod. 2020, 147, 112198. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N.; et al. Essential Oils of Mentha Viridis Rich Phenolic Compounds Show Important Antioxidant, Antidiabetic, Dermatoprotective, Antidermatophyte and Antibacterial Properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chadon Assemian, I.C.; Mouzount, H.; Bourais, I.; Et-Touys, A.; Fellah, H.; Benjouad, A.; Dakka, N.; Bakri, Y. Could Volatile Compounds from Leaves and Fruits of Pistacia Lentiscus Constitute a Novel Source of Anticancer, Antioxidant, Antiparasitic and Antibacterial Drugs? Ind. Crops Prod. 2019, 128, 62–69. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula Stoechas Essential Oil from Morocco as Novel Source of Antileishmanial, Antibacterial and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Chuang, S.-Y.; Lin, C.-H.; Fang, J.-Y. Natural Compounds and Aging: Between Autophagy and Inflammasome. BioMed Res. Int. 2014, 2014, e297293. [Google Scholar] [CrossRef] [Green Version]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential Role of Natural Compounds Against Skin Aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef]
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; Cabo, R. Measuring Biological Aging in Humans: A Quest. Aging Cell 2020, 19, e13080. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.R.; Yousefzadeh, M.J.; Rozgaja, T.A.; Wang, J.; Li, X.; Tilstra, J.S.; Feldman, C.H.; Gregg, S.Q.; Johnson, C.H.; Skoda, E.M.; et al. Spontaneous DNA Damage to the Nuclear Genome Promotes Senescence, Redox Imbalance and Aging. Redox Biol. 2018, 17, 259–273. [Google Scholar] [CrossRef]
- Park, J.H.; Yoo, Y.; Park, Y.J. Epigenetics: Linking Nutrition to Molecular Mechanisms in Aging. Prev. Nutr. Food Sci. 2017, 22, 81. [Google Scholar]
- Gompertz, B. On the Nature of the Function Expressive of the Law of Human Mortality, and on a New Mode of Determining the Value of Life Contingencies. Philos. Trans. R. Soc. Lond. 1825, 115, 513–583. [Google Scholar]
- Ventura, S.; Peters, K.; Martin, J.; Maurer, J. Births and Deaths: United States, 1996. Mon. Vital Stat. Rep. 1997, 46, 1–40. [Google Scholar]
- Kaeberlein, M.; McVey, M.; Guarente, L. Using Yeast to Discover the Fountain of Youth. Sci. Aging Knowl. Environ. Sage Ke 2001, 2001, pe1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shock, N.W. Normal Human Aging: The Baltimore Longitudinal Study of Aging; U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Aging, Gerontology Research Center: Baltimore, MD, USA, 1984. [Google Scholar]
- Schaie, K.W.; Hofer, S.M. Longitudinal Studies in Aging Research. In Handbook of the Psychology of Aging, 5th ed.; Academic Press: San Diego, CA, USA, 2001; pp. 53–77. ISBN 978-0-12-101262-5. [Google Scholar]
- Lakatta, E.G. Changes in Cardiovascular Function with Aging. Eur. Heart J. 1990, 11, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.R.; Anderson, S. The Aging Kidney: Physiological Changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkwood, T.B.L. Human Senescence. BioEssays 1996, 18, 1009–1016. [Google Scholar] [CrossRef]
- Dodig, S.; Čepelak, I.; Pavić, I. Hallmarks of Senescence and Aging. Biochem. Med. 2019, 29, 483–497. [Google Scholar] [CrossRef]
- Rozhok, A.; DeGregori, J. A Generalized Theory of Age-Dependent Carcinogenesis. eLife 2019, 8, e39950. [Google Scholar] [CrossRef]
- Piotrowski, I.; Kulcenty, K.; Suchorska, W.M.; Skrobała, A.; Skórska, M.; Kruszyna-Mochalska, M.; Kowalik, A.; Jackowiak, W.; Malicki, J. Carcinogenesis Induced by Low-Dose Radiation. Radiol. Oncol. 2017, 51, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Karasik, D.; Demissie, S.; Cupples, L.A.; Kiel, D.P. Disentangling the Genetic Determinants of Human Aging: Biological Age as an Alternative to the Use of Survival Measures. J. Gerontol. Ser. A 2005, 60, 574–587. [Google Scholar] [CrossRef] [Green Version]
- Fraga, M.F. Genetic and Epigenetic Regulation of Aging. Curr. Opin. Immunol. 2009, 21, 446–453. [Google Scholar] [CrossRef]
- Gredilla, R.; Sánchez-Román, I.; Gómez, A.; López-Torres, M.; Barja, G. Mitochondrial Base Excision Repair Positively Correlates with Longevity in the Liver and Heart of Mammals. GeroScience 2020, 42, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Niedernhofer, L.J.; Gurkar, A.U.; Wang, Y.; Vijg, J.; Hoeijmakers, J.H.J.; Robbins, P.D. Nuclear Genomic Instability and Aging. Annu. Rev. Biochem. 2018, 87, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Gee, P.; Ho, L.; Hsu, R.K.; Kane, C.J. Genomic Heterogeneity of DNA Repair. Role in Aging. Ann. N. Y. Acad. Sci. 1992, 663, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nebel, A.; Flachsbart, F.; Till, A.; Caliebe, A.; Blanché, H.; Arlt, A.; Häsler, R.; Jacobs, G.; Kleindorp, R.; Franke, A.; et al. A Functional EXO1 Promoter Variant Is Associated with Prolonged Life Expectancy in Centenarians. Mech. Ageing Dev. 2009, 130, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ryu, S.; Moskowitz, D.M.; Rothenberg, D.; Leahy, D.J.; Atzmon, G.; Barzilai, N.; Suh, Y. Discovery of Novel Non-Synonymous SNP Variants in 988 Candidate Genes from 6 Centenarians by Target Capture and next-Generation Sequencing. Mech. Ageing Dev. 2013, 134, 478–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimitou, E.P.; Symington, L.S. Sae2, Exo1 and Sgs1 Collaborate in DNA Double-Strand Break Processing. Nature 2008, 455, 770–774. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Huang, S.; Lee, L.; Davalos, A.; Schiestl, R.H.; Campisi, J.; Oshima, J. WRN, the Protein Deficient in Werner Syndrome, Plays a Critical Structural Role in Optimizing DNA Repair. Aging Cell 2003, 2, 191–199. Available online: https://onlinelibrary.wiley.com/doi/full/10.1046/j.1474-9728.2003.00052.x (accessed on 28 September 2021). [CrossRef]
- de Boer, J. Premature Aging in Mice Deficient in DNA Repair and Transcription. Science 2002, 296, 1276–1279. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.T.; Stark, Z.; Sutton, R.E.; Danda, S.; Ekbote, A.V.; Elsayed, S.M.; Gibson, L.; Goodship, J.A.; Jackson, A.P.; Keng, W.T.; et al. The Cockayne Syndrome Natural History (CoSyNH) Study: Clinical Findings in 102 Individuals and Recommendations for Care. Genet. Med. Off. J. Am. Coll. Med. Genet. 2016, 18, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Kudlow, B.A.; Kennedy, B.K.; Monnat, R.J. Werner and Hutchinson-Gilford Progeria Syndromes: Mechanistic Basis of Human Progeroid Diseases. Nat. Rev. Mol. Cell Biol. 2007, 8, 394–404. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Rossi, M.L.; Singh, D.K.; Dunn, C.; Ramamoorthy, M.; Croteau, D.L.; Liu, Y.; Bohr, V.A. RECQL4, the Protein Mutated in Rothmund-Thomson Syndrome, Functions in Telomere Maintenance. J. Biol. Chem. 2012, 287, 196–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croteau, D.L.; Singh, D.K.; Hoh Ferrarelli, L.; Lu, H.; Bohr, V.A. RECQL4 in Genomic Instability and Aging. Trends Genet. TIG 2012, 28, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, D. The Aging Process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Sohal, R.S.; Weindruch, R. Oxidative Stress, Caloric Restriction, and Aging. Science 1996, 273, 59–63. Available online: https://www.science.org/doi/abs/10.1126/science.273.5271.59 (accessed on 28 September 2021). [CrossRef] [Green Version]
- Sen, C.K.; Packer, L. Antioxidant and Redox Regulation of Gene Transcription. FASEB J. 1996, 10, 709–720. [Google Scholar] [CrossRef]
- Suzuki, Y.J. Oxidant-Mediated Protein Amino Acid Conversion. Antioxidants 2019, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.J.; Forman, H.J.; Sevanian, A. Oxidants as Stimulators of Signal Transduction. Free Radic. Biol. Med. 1997, 22, 269–285. [Google Scholar] [CrossRef]
- Barja, G. Free Radicals and Aging. Trends Neurosci. 2004, 27, 595–600. [Google Scholar] [CrossRef]
- Sohal, R.S.; Svensson, I.; Sohal, B.H.; Brunk, U.T. Superoxide Anion Radical Production in Different Animal Species. Mech. Ageing Dev. 1989, 49, 129–135. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zainal, T.A.; Oberley, T.D.; Allison, D.B.; Szweda, L.I.; Weindruch, R. Caloric Restriction of Rhesus Monkeys Lowers Oxidative Damage in Skeletal Muscle. FASEB J. 2000, 14, 1825–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnane, A.W.; Zhang, C.; Baumer, A.; Nagley, P. Mitochondrial DNA Mutation and the Ageing Process: Bioenergy and Pharmacological Intervention. Mutat. Res. 1992, 275, 195–208. [Google Scholar] [CrossRef]
- Su, T.; Turnbull, D.M.; Greaves, L.C. Roles of Mitochondrial DNA Mutations in Stem Cell Ageing. Genes 2018, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, A.; Zuryn, S. The Cellular Mitochondrial Genome Landscape in Disease. Trends Cell Biol. 2019, 29, 227–240. [Google Scholar] [CrossRef]
- Ozawa, T. Genetic and Functional Changes in Mitochondria Associated with Aging. Physiol. Rev. 1997, 77, 425–464. [Google Scholar] [CrossRef]
- Miquel, J. Role of Mitochondria in Cell Aging. In Molecular Basis of Aging; CRC Press: Boca Raton, FL, USA, 1995; Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9780203711309-7/role-mitochondria-cell-aging-jaime-miquel (accessed on 28 September 2021).
- Katayama, M.; Tanaka, M.; Yamamoto, H.; Ohbayashi, T.; Nimura, Y.; Ozawa, T. Deleted Mitochondrial DNA in the Skeletal Muscle of Aged Individuals. Biochem. Int. 1991, 25, 47–56. [Google Scholar]
- Mohamed, H.R.H. Alleviation of Cadmium Chloride–Induced Acute Genotoxicity, Mitochondrial DNA Disruption, and ROS Generation by Chocolate Coadministration in Mice Liver and Kidney Tissues. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Mann, V.M.; Cooper, J.M.; Dexter, D.; Daniel, S.E.; Jenner, P.; Clark, J.B.; Marsden, C.D. Anatomic and Disease Specificity of NADH CoQ1 Reductase (Complex I) Deficiency in Parkinson’s Disease. J. Neurochem. 1990, 55, 2142–2145. [Google Scholar] [CrossRef]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adav, S.S.; Park, J.E.; Sze, S.K. Quantitative Profiling Brain Proteomes Revealed Mitochondrial Dysfunction in Alzheimer’s Disease. Mol. Brain 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S. Senile Dementia and Alzheimer’s Disease. Brain Blood Flow and Metabolism. Prog. Neuropsychopharmacol. Biol. Psychiatry 1986, 10, 447–478. [Google Scholar] [CrossRef]
- Carmo, C.; Naia, L.; Lopes, C.; Rego, A.C. Mitochondrial Dysfunction in Huntington’s Disease. In Polyglutamine Disorders; Nóbrega, C., Pereira de Almeida, L., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; pp. 59–83. ISBN 978-3-319-71779-1. [Google Scholar]
- Beal, M.F. Neurochemistry and Toxin Models in Huntington’s Disease. Curr. Opin. Neurol. 1994, 7, 542–547. [Google Scholar] [CrossRef]
- Li, H.; Slone, J.; Fei, L.; Huang, T. Mitochondrial DNA Variants and Common Diseases: A Mathematical Model for the Diversity of Age-Related MtDNA Mutations. Cells 2019, 8, 608. [Google Scholar] [CrossRef] [Green Version]
- Lawless, C.; Greaves, L.; Reeve, A.K.; Turnbull, D.M.; Vincent, A.E. The Rise and Rise of Mitochondrial DNA Mutations. Open Biol. 2020, 10, 200061. [Google Scholar] [CrossRef]
- Herrmann, M.; Pusceddu, I.; März, W.; Herrmann, W. Telomere Biology and Age-Related Diseases. Clin. Chem. Lab. Med. CCLM 2018, 56, 1210–1222. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Peng, S.-H.; Ning, X.-H.; Li, T.; Liu, S.-J.; Liu, J.-Y.; Hong, B.-A.; Qi, N.-N.; Peng, X.; Zhou, B.-W.; et al. Shorter Telomere Length Increases Age-Related Tumor Risks in von Hippel-Lindau Disease Patients. Cancer Med. 2017, 6, 2131–2141. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [Green Version]
- Harley, C.B. Telomere Loss: Mitotic Clock or Genetic Time Bomb? Mutat. Res. 1991, 256, 271–282. [Google Scholar] [CrossRef]
- Smith, E.M.; Pendlebury, D.F.; Nandakumar, J. Structural Biology of Telomeres and Telomerase. Cell. Mol. Life Sci. 2020, 77, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.P.; Levine, B.L.; June, C.H.; Hodes, R.J. Human Naive and Memory T Lymphocytes Differ in Telomeric Length and Replicative Potential. Proc. Natl. Acad. Sci. USA 1995, 92, 11091–11094. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, H.; Schächter, F.; Uchida, I.; Wei, L.; Zhu, X.; Effros, R.; Cohen, D.; Harley, C.B. Loss of Telomeric DNA during Aging of Normal and Trisomy 21 Human Lymphocytes. Am. J. Hum. Genet. 1993, 52, 661–667. [Google Scholar] [PubMed]
- Shu, Y.; Wu, M.; Yang, S.; Wang, Y.; Li, H. Association of Dietary Selenium Intake with Telomere Length in Middle-Aged and Older Adults. Clin. Nutr. 2020, 39, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhan, Y.; Pedersen, N.L.; Fang, F.; Hägg, S. Telomere Length and All-Cause Mortality: A Meta-Analysis. Ageing Res. Rev. 2018, 48, 11–20. Available online: https://www.sciencedirect.com/science/article/pii/S1568163718301235 (accessed on 29 September 2021). [CrossRef] [PubMed]
- Hemann, M.T.; Strong, M.A.; Hao, L.-Y.; Greider, C.W. The Shortest Telomere, Not Average Telomere Length, Is Critical for Cell Viability and Chromosome Stability. Cell 2001, 107, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Experimental Elongation of Telomeres Extends the Lifespan of Immortal x Normal Cell Hybrids. EMBO J. 1996, 15, 1734–1741. [CrossRef]
- Marks, D.S.; Hopf, T.A.; Sander, C. Protein Structure Prediction from Sequence Variation. Nat. Biotechnol. 2012, 30, 1072–1080. Available online: https://www.nature.com/articles/nbt.2419 (accessed on 29 September 2021). [CrossRef] [PubMed] [Green Version]
- Petropoulos, I.; Friguet, B. Maintenance of Proteins and Aging: The Role of Oxidized Protein Repair. Free Radic. Res. 2006, 40, 1269–1276. [Google Scholar] [CrossRef]
- Schöneich, C. Protein Modification in Aging: An Update. Exp. Gerontol. 2006, 41, 807–812. [Google Scholar] [CrossRef]
- Rattan, S.I.S.; Derventzi, A.; Clark, B.F.C. Protein Synthesis, Posttranslational Modifications, and Aginga. Ann. N. Y. Acad. Sci. 1992, 663, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-Y.; Lin, Y.-Y.; Zhu, H.; Chuang, L.-M.; Boeke, J.D. Protein Acetylation and Aging. Aging 2011, 3, 911–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch, C.E.; Tanzi, R.E. Genetics of Aging. Science 1997, 278, 407–411. Available online: https://www.science.org/doi/abs/10.1126/science.278.5337.407 (accessed on 29 September 2021). [CrossRef] [PubMed]
- Singh, P.P.; Demmitt, B.A.; Nath, R.D.; Brunet, A. The Genetics of Aging: A Vertebrate Perspective. Cell 2019, 177, 200–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadra, M.; Howell, P.; Dutta, S.; Heintz, C.; Mair, W.B. Alternative Splicing in Aging and Longevity. Hum. Genet. 2020, 139, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Johnson, T.E. A Genetic Pathway Conferring Life Extension and Resistance to UV Stress in Caenorhabditis Elegans. Genetics 1996, 143, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. daf-2, an Insulin Receptor-Like Gene That Regulates Longevity and Diapause in Caenorhabditis Elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis Elegans and Its Role in Metabolic Remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. HSF-1 and SIR-2.1 Linked Insulin-like Signaling Is Involved in Goji Berry (Lycium Spp.) Extracts Promoting Lifespan Extension of Caenorhabditis Elegans. Food Funct. 2021, 12, 7851–7866. [Google Scholar] [CrossRef]
- Jing, H.; Lin, H. Sirtuins in Epigenetic Regulation. Chem. Rev. 2015, 115, 2350–2375. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-E.; Mostoslavsky, R. Sirtuins, Metabolism, and DNA Repair. Curr. Opin. Genet. Dev. 2014, 26, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 Complex and SIR2 Alone Promote Longevity in Saccharomyces Cerevisiae by Two Different Mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Cao, J.; Hu, K.; He, X.; Yun, D.; Tong, T.; Han, L. Sirtuins and Their Biological Relevance in Aging and Age-Related Diseases. Aging Dis. 2020, 11, 927. [Google Scholar] [CrossRef]
- Tong, L.; Denu, J.M. Function and Metabolism of Sirtuin Metabolite O-Acetyl-ADP-Ribose. Biochim. Biophys. Acta BBA—Proteins Proteom. 2010, 1804, 1617–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tissenbaum, H.A. Chapter One—DAF-16: FOXO in the Context of C. Elegans. In Current Topics in Developmental Biology; Ghaffari, S., Ed.; Forkhead FOXO Transcription Factors in Development and Disease; Academic Press: Cambridge, MA, USA, 2018; Volume 127, pp. 1–21. [Google Scholar]
- Lin, K.; Dorman, J.B.; Rodan, A.; Kenyon, C. Daf-16: An HNF-3/Forkhead Family Member That Can Function to Double the Life-Span of Caenorhabditis Elegans. Science 1997, 278, 1319–1322. Available online: https://www.science.org/doi/abs/10.1126/science.278.5341.1319 (accessed on 29 September 2021). [CrossRef] [PubMed] [Green Version]
- Lithgow, G.J.; Andersen, J.K. The Real Dorian Gray Mouse. Bioessays 2000, 22, 410–413. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1521-1878(200005)22:5%3C410::AID-BIES2%3E3.0.CO;2-C (accessed on 29 September 2021). [CrossRef]
- Flurkey, K.; Papaconstantinou, J.; Harrison, D.E. The Snell Dwarf Mutation Pit1dw Can Increase Life Span in Mice. Mech. Ageing Dev. 2002, 123, 121–130. [Google Scholar] [CrossRef]
- Perls, T.; Levenson, R.; Regan, M.; Puca, A. What Does It Take to Live to 100? Mech. Ageing Dev. 2002, 123, 231–242. [Google Scholar] [CrossRef]
- Messaris, G.A.; Hadjinicolaou, M.; Karahalios, G.T. Why Do We Live for Much Less than 100 Years? A Fluid Mechanics View and Approach. Phys. Fluids 2017, 29, 081903. Available online: https://aip.scitation.org/doi/abs/10.1063/1.4998717 (accessed on 29 September 2021). [CrossRef]
- Bartlett, Z. The Limited In Vitro Lifetime of Human Diploid Cell Strains; Hayflick, L., Ed.; Academic Press: Cambridge, MA, USA, 1964. [Google Scholar]
- Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N.; Majid, N.M.N. Anti–Elastase, Anti–Tyrosinase and Matrix Metalloproteinase–1 Inhibitory Activity of Earthworm Extracts as Potential New Anti–Aging Agent. Asian Pac. J. Trop. Biomed. 2014, 4, S348–S352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsatsou, F.; Trakatelli, M.; Patsatsi, A.; Kalokasidis, K.; Sotiriadis, D. Extrinsic Aging: UV-Mediated Skin Carcinogenesis. Dermatoendocrinology 2012, 4, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierkötter, A.; Krutmann, J. Environmental Influences on Skin Aging and Ethnic-Specific Manifestations. Dermatoendocrinology 2012, 4, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.R. Free Radical Chemistry. Sports Med. 1988, 5, 156–170. [Google Scholar] [CrossRef]
- Sárdy, M. Role of Matrix Metalloproteinases in Skin Ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Riese, R.J.; Shi, G.-P. Emerging Roles for Cysteine Proteases in Human Biology. Annu. Rev. Physiol. 1997, 59, 63–88. [Google Scholar] [CrossRef] [Green Version]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-Collagenase, Anti-Elastase and Anti-Oxidant Activities of Extracts from 21 Plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.S. Review an Update Review of Tyrosinase Inhibitors Intern. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of Tyrosinase Inhibitory, Antioxidant, Antimicrobial, and Anti-aging Activities of Magnolia Officinalis Extracts after Aspergillus Niger Fermentation. BioMed Res. Int. 2018, 2018, 5201786. [Google Scholar] [CrossRef] [Green Version]
- Sturm, G.; Cardenas, A.; Bind, M.-A.; Horvath, S.; Wang, S.; Wang, Y.; Hägg, S.; Hirano, M.; Picard, M. Human Aging DNA Methylation Signatures Are Conserved but Accelerated in Cultured Fibroblasts. Epigenetics 2019, 14, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C. Cell Proliferation, Cell Death and Aging. Aging Clin. Exp. Res. 1989, 1, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. Available online: https://www.annualreviews.org/doi/abs/10.1146/annurev-physiol-030212-183653 (accessed on 29 September 2021). [CrossRef] [PubMed] [Green Version]
- Tomita, K.; Aida, J.; Izumiyama-Shimomura, N.; Nakamura, K.; Ishikawa, N.; Matsuda, Y.; Arai, T.; Ishiwata, T.; Kumasaka, T.; Takahashi-Fujigasaki, J.; et al. Changes in Telomere Length with Aging in Human Neurons and Glial Cells Revealed by Quantitative Fluorescence in Situ Hybridization Analysis. Geriatr. Gerontol. Int. 2018, 18, 1507–1512. [Google Scholar] [CrossRef]
- Nanba, D. Human Keratinocyte Stem Cells: From Cell Biology to Cell Therapy. J. Dermatol. Sci. 2019, 96, 66–72. [Google Scholar] [CrossRef]
- Bierman, E.L. The Effect of Donor Age on the in Vitro Life Span of Cultured Human Arterial Smooth-Muscle Cells. In Vitro 1978, 14, 951–955. [Google Scholar] [CrossRef]
- Tassin, J.; Malaise, E.; Courtois, Y. Human Lens Cells Have an in Vitro Proliferative Capacity Inversely Proportional to the Donor Age. Exp. Cell Res. 1979, 123, 388–392. [Google Scholar] [CrossRef]
- Mueller, S.N.; Rosen, E.M.; Levine, E.M. Cellular Senescence in a Cloned Strain of Bovine Fetal Aortic Endothelial Cells. Science 1980, 207, 889–891. Available online: https://www.science.org/doi/abs/10.1126/science.7355268 (accessed on 29 September 2021). [CrossRef]
- Tice, R.R.; Schneider, E.L.; Kram, D.; Thorne, P. Cytokinetic Analysis of the Impaired Proliferative Response of Peripheral Lymphocytes from Aged Humans to Phytohemagglutinin. J. Exp. Med. 1979, 149, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.S.; Van Deursen, J.M.; et al. Naturally Occurring P16Ink4a-Positive Cells Shorten Healthy Lifespan. Nature 2016, 530, 184–189. Available online: https://www.nature.com/articles/nature16932 (accessed on 29 September 2021). [CrossRef] [Green Version]
- Nguyen, T.T.M.; Gillet, G.; Popgeorgiev, N. Caspases in the Developing Central Nervous System: Apoptosis and Beyond. Front. Cell Dev. Biol. 2021, 9, 702404. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8322698/ (accessed on 29 September 2021). [CrossRef] [PubMed]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed Cell Death as a Defence against Infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Lockshin, R.A.; Zakeri, Z. Programmed Cell Death and Apoptosis: Origins of the Theory. Nat. Rev. Mol. Cell Biol. 2001, 2, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed Cell Death in Animal Development and Disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiebert, P.R.; Granville, D.J. Granzyme B in Injury, Inflammation, and Repair. Trends Mol. Med. 2012, 18, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Tower, J. Programmed Cell Death in Aging. Ageing Res. Rev. 2015, 23, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Chernukha, I.; Fedulova, L.; Vasilevskaya, E.; Kulikovskii, A.; Kupaeva, N.; Kotenkova, E. Antioxidant Effect of Ethanolic Onion (Allium cepa) Husk Extract in Ageing Rats. Saudi J. Biol. Sci. 2021, 28, 2877–2885. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, A.R.; Kim, M.J.; Park, S.N. Antibacterial, Antioxidative and Anti-aging Effects of Allium Cepa Peel Extracts. Appl. Chem. Eng. 2011, 22, 178–184. [Google Scholar]
- Abdel-reheim, E.S.; Abdel-Hafeez, H.A.-H. Onion and its active constituents against aging. Egypt. J. Biochem. Mol. Biol. 2014, 32, 206–219. [Google Scholar]
- Jeong, E.J.; Jegal, J.; Jung, Y.-S.; Chung, K.W.; Chung, H.Y.; Yang, M.H. Fermented Onions Extract Inhibits Tyrosinase and Collagenase-1 Activities as a Potential New Anti–Photoaging Agent. Nat. Prod. Commun. 2017, 12, 1934578X1701200711. [Google Scholar] [CrossRef] [Green Version]
- Moriguchi, T.; Saito, H.; Nishiyama, N. Anti-ageing Effect of Aged Garlic Extract in the Inbred Brain Atrophy Mouse Model. Clin. Exp. Pharmacol. Physiol. 1997, 24, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K. Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes. Nutrients 2016, 8, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svendsen, L.; Rattan, S.I.; Clark, B.F. Testing Garlic for Possible Anti-Ageing Effects on Long-Term Growth Characteristics, Morphology and Macromolecular Synthesis of Human Fibroblasts in Culture. J. Ethnopharmacol. 1994, 43, 125–133. [Google Scholar] [CrossRef]
- Lee, M.-J.; Ryu, B.-M.; Kim, M.-H.; Lee, Y.-S.; Moon, G.-S. Protective Effect of Dietary Buchu (Chinese chives) against Oxidative Damage from Aging and Ultraviolet Irradiation in ICR Mice Skin. Prev. Nutr. Food Sci. 2002, 7, 238–244. [Google Scholar] [CrossRef]
- Kim, D.-S.; Jeon, B.-K.; Mun, Y.-J.; Kim, Y.-M.; Lee, Y.-E.; Woo, W.-H. Effect of Dioscorea Aimadoimo on Anti-Aging and Skin Moisture Capacity. J. Physiol. Pathol. Korean Med. 2011, 25, 425–430. [Google Scholar]
- Wang, X.; Huo, X.; Liu, Z.; Yang, R.; Zeng, H. Investigations on the Anti-Aging Activity of Polysaccharides from Chinese Yam and Their Regulation on Klotho Gene Expression in Mice. J. Mol. Struct. 2020, 1208, 127895. [Google Scholar] [CrossRef]
- Xiong, D.; Yu, L.-X.; Yan, X.; Guo, C.; Xiong, Y. Effects of Root and Stem Extracts of Asparagus Cochinchinensis on Biochemical Indicators Related to Aging in the Brain and Liver of Mice. Am. J. Chin. Med. 2011, 39, 719–726. [Google Scholar] [CrossRef]
- Lei, L.; Ou, L.; Yu, X. The Antioxidant Effect of Asparagus cochinchinensis (Lour.) Merr. Shoot in d-Galactose Induced Mice Aging Model and in Vitro. J. Chin. Med. Assoc. 2016, 79, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Chen, Y.; Ou, L.; Xu, Y.; Yu, X. Aqueous Root Extract of Asparagus cochinchinensis (Lour.) Merr. Has Antioxidant Activity in D-Galactose-Induced Aging Mice. BMC Complement. Altern. Med. 2017, 17, 469. [Google Scholar] [CrossRef] [Green Version]
- Sriyab, S.; Laosirisathian, N.; Punyoyai, C.; Anuchapreeda, S.; Tima, S.; Chiampanichayakul, S.; Chaiyana, W. Nutricosmetic Effects of Asparagus Officinalis: A Potent Matrix Metalloproteinase-1 Inhibitor. Sci. Rep. 2021, 11, 8772. [Google Scholar] [CrossRef]
- Shirato, K.; Takanari, J.; Koda, T.; Sakurai, T.; Ogasawara, J.; Ohno, H.; Kizaki, T. A Standardized Extract of Asparagus Officinalis Stem Prevents Reduction in Heat Shock Protein 70 Expression in Ultraviolet-B-Irradiated Normal Human Dermal Fibroblasts: An in Vitro Study. Environ. Health Prev. Med. 2018, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Amelia; Girsang, E.; Nasution, A.N.; Ginting, C.N. Anti-Aging Effectiveness of Red Spinach Extract Ointment (Amaranthus tricolor L.) Against Collagen, Elasticity, Hydration, Sebum, and Pigment Levels in Wistar Rats. In Proceedings of the 2021 IEEE International Conference on Health, Instrumentation & Measurement, and Natural Sciences (InHeNce), Medan, Indonesia, 14–16 July 2021; IEEE: Medan, Indonesia, 2021; pp. 1–6. [Google Scholar]
- Song, S.; Zhao, L.; Feng, L.; Wang, W.; Cong, T.; He, H. Using aging rats model to investigate anti-oxidative ability of artichoke (Cynara scolymus L.) leaf extract. Acta Hortic. 2012, 944, 113–122. [Google Scholar] [CrossRef]
- Marques, P.; Marto, J.; Gonçalves, L.M.; Pacheco, R.; Fitas, M.; Pinto, P.; Serralheiro, M.L.M.; Ribeiro, H. Cynara scolymus L.: A Promising Mediterranean Extract for Topical Anti-Aging Prevention. Ind. Crops Prod. 2017, 109, 699–706. [Google Scholar] [CrossRef]
- Sukoyan, G.V.; Gongadze, N.V.; Demina, N.B.; Golovach, V.V.; Tsivtsivadze, E.T.; Bakuridze, A.D. Ageing Induced Hyperproduction of Reactive Oxygen Species and Dysbalance in Enzymatic Link of Antioxidant Defense System of Skin and Therapeutic Efficacy of Artichoke Extract. Eur. J. Med. Plants 2019, 27, 1–10. [Google Scholar] [CrossRef]
- Sukoyan, G.; Tsivtsivadze, E.; Golovach, V.; Kezeli, T.; Demina, N. Anti-Aging Effect of Cynara cardunculus L. var. Cynara scolymus L. Extract in D-Galactose-Induced Skin Aging Model in Rats. Pharmacol. Amp Pharm. 2018, 9, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Noh, Y.-H.; Kim, D.-H.; Kim, J.Y.; Park, J.; Kim, O.H.; Han, D.; Kim, W.-Y.; Kim, S.-S.; Lee, M.-Y.; Heo, S.-H.; et al. Improvement of Andropause Symptoms by Dandelion and Rooibos Extract Complex CRS-10 in Aging Male. Nutr. Res. Pract. 2012, 6, 505. [Google Scholar] [CrossRef]
- Krishnan, G.M.; Arijana, I.K.; Sugiritama, I.W. The Ethanolic Extract of Red Cabbage (Brassica oleracea L. Var, Capitata f. Rubra) in Cream Preparation to the Dermal-Thickness of Male Wistar Mice (Rattus norvegicus) after Ultraviolet-B Exposure. Intisari Sains Medis 2018, 9. [Google Scholar] [CrossRef]
- Jusuf, N.K.; Bachtiar, A.; Hadisahputra, S.; Soebono, H. Effect of Broccoli Flower Extract (Brassica oleracea L. Var. italica Plenck) on Inhibition of Photoaging Viewed from Matrix Metalloproteinase-1 Expression in Human Skin Fibroblast. J. Biol. Agric. Healthc. 2014, 4, 54–60. [Google Scholar]
- Kim, H.K. Development of Anti-Aging Products (Anti-Wrinkle) like Epidermal Growth Factor(EGF) Materials Using Supercritical Heat-Treated Extract Radish. J. Converg. Cult. Technol. 2018, 4, 197–207. [Google Scholar] [CrossRef]
- Nema, N.K.; Maity, N.; Sarkar, B.; Mukherjee, P.K. Cucumis Sativus Fruit-Potential Antioxidant, Anti-Hyaluronidase, and Anti-Elastase Agent. Arch. Dermatol. Res. 2011, 303, 247–252. [Google Scholar] [CrossRef]
- Muntafiah, L.; Shabrina, B.A.; Sulistyowati, D.; Asshagab, M.R.N.; Jenie, R.I. Anti-Aging Activity Of Cucurbita Moschata Ethanolic Extract Towards NIH3T3 Fibroblast Cells Induced By Doxorubicin. Indones. J. Cancer Chemoprev. 2016, 7, 49–53. [Google Scholar] [CrossRef]
- Amakye, W.K.; Hou, C.; Xie, L.; Lin, X.; Gou, N.; Yuan, E.; Ren, J. Bioactive Anti-Aging Agents and the Identification of New Anti-Oxidant Soybean Peptides. Food Biosci. 2021, 42, 101194. [Google Scholar] [CrossRef]
- Waqas, M.; Akhtar, N.; Rasul, A.; Rashid, S.; Mustafa, R.; Khan, B.; Murtaza, G. In Vivo Evaluation of a Cosmetic Emulsion Containing Soybean Extract for Anti-Aging. Trop. J. Pharm. Res. 2014, 13, 1401. [Google Scholar] [CrossRef] [Green Version]
- Corpuz, H.M.; Arimura, M.; Chawalitpong, S.; Miyazaki, K.; Sawaguchi, M.; Nakamura, S.; Katayama, S. Oral Administration of Okara Soybean By-Product Attenuates Cognitive Impairment in a Mouse Model of Accelerated Aging. Nutrients 2019, 11, 2939. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.-J.; Pyo, Y.-H. Effect of Monascus-Fermented Soybean Extracts on Antioxidant and Skin Aging-Related Enzymes Inhibitory Activities. Prev. Nutr. Food Sci. 2017, 22, 376–380. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Fan, Y.; Li, S.; Zhou, X.; Park, K.-Y.; Zhao, X.; Liu, H. Antioxidant and Anti-Aging Effects of Fermented Soybean Milk. In Functional Effects of Fermented Soybean Food in China; Book Publisher International: London, UK, 2021; pp. 89–114. [Google Scholar]
- Wu, Y.H.; Liu, E.Q.; Zhang, J.P.; Chen, S.L.; Li, Y.; Geng, Z.H. In Vivo Antioxidant Activity of Black Soybean Peptide in Aging Mice Caused by D-Galactose. Appl. Mech. Mater. 2014, 618, 421–425. [Google Scholar] [CrossRef]
- Prasetyo, B.E.; Rafika, D.; Laila, L.; Aminah, F. Physical Evaluation and Anti-Aging Effect of Red Bean Ethanolic Extract (Vigna angularis (Wild.) Ohwi & Ohashi) Peel-Off Gel Mask. Open Access Maced. J. Med. Sci. 2019, 7, 3907–3910. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, D.F.; Orozco-Avila, I.; Lugo-Cervantes, E.; Mojica, L. Black Bean (Phaseolus vulgaris L.) Phenolic Extract Exhibits Antioxidant and Anti-Aging Potential. Curr. Dev. Nutr. 2020, 4, 24. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Lee, H.J.; Sun, Z.; Lee, T.Y.; Song, H.G.; Shin, H.-S.; Yi, T.H. Vigna Angularis Water Extracts Protect Against Ultraviolet B-Exposed Skin Aging In Vitro and In Vivo. J. Med. Food 2014, 17, 1339–1349. [Google Scholar] [CrossRef]
- Jumnongprakhon, P.; Pasakawee, K.; Banjongsinsiri, P.; Donrung, N.; Daodee, S.; Chonpathompikunlert, P. The Effects of Ethanolic Extract of Okra Fruit, Abelmoschus esculentus (L.) Moench on Cellular Senescence in Aging. Neuron. Songklanakarin J. Sci. Technol. 2020, in press. [Google Scholar]
- Ying, R.; Chongshun, S.; Jing, G. Experimental Research on the Mechanisms of the Effect of Compound Rhubarb Anti-aging Preparation for Enhancing Memory. J.-Beijing Univ. Tradit. Chin. Med. 2003, 26, 35–37. [Google Scholar]
- Uzun, M.; Demirezer, L.O. Anti-aging Power of Rumex crispus L.: Matrixmetalloproteinases Inhibitor, Sun Protective and Antioxidant. S. Afr. J. Bot. 2019, 124, 364–371. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Fouda, K.; Hamed, I.M.; Abdelgayed, S.S. Protective Effect of Kumquat Fruits and Carrot Seeds Extracts against Brain Aging in Rats. J. Herbmed Pharmacol. 2019, 8, 287–294. [Google Scholar] [CrossRef]
- Pangastuti, A.; Indriwati, S.E.; Amin, M. Investigation of the Anti-Aging Properties of Allicin from Allium sativum L Bulb Extracts by a Reverse Docking Approach. Trop. J. Pharm. Res. 2018, 17, 635–639. [Google Scholar] [CrossRef]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-Wrinkle and Anti-Inflammatory Effects of Active Garlic Components and the Inhibition of MMPs via NF-ΚB Signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikmawati, V.F.; Alam, F.M.; Ainnayah, J.S.; Fatchiyah, F. Virtual Prediction of The Effect Phenolic And Glucosinolate Compounds In Broccoli (Brassica oleracea) On Anti-Aging As Stimulant Nrf-2. J. Exp. Life Sci. 2021, 10, 104–112. [Google Scholar] [CrossRef]
- Lee, M.-J.; Jeong, N.-H.; Jang, B.-S. Antioxidative Activity and Anti-aging Effect of Carrot Glycoprotein. J. Ind. Eng. Chem. 2015, 25, 216–221. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, M. Effect of a Radical Scavenger “Water Soluble Protein” from Broad Beans (Vicia Faba) on Antioxidative Enzyme Activity in Cellular Aging. J. Nutr. Sci. Vitaminol. 2000, 46, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Wink, M. Natural Lignans from Arctium Lappa as Anti-aging Agents in Caenorhabditis Elegans. Phytochemistry 2015, 117, 340–350. [Google Scholar] [CrossRef]
- Chen, G.; Huang, C.; Shi, P.; Xu, H.; Gao, S.; Luo, D.; Chen, T.; Xie, Y.; Huang, R.; Song, H. Mechanism of Chinese Yam for the Treatment of Aging-Related Diseases Based on Network Pharmacology. Eur. J. Integr. Med. 2021, 41, 101254. [Google Scholar] [CrossRef]

| Vegetables (Common Names) | Extract Types | Models Used | Methods | Key Results | References |
|---|---|---|---|---|---|
| Amaryllideae | |||||
| Allium cepa L. (Onion) | Ethanolic onion husk extract | Aged male Wistar albino rats (17 months) | Ferric reducing antioxidant power (FRAP) assay Quantitative determination of reduced glutathione (GSH) Estimation of catalase (CAT) and superoxide dismutase (SOD) activity | Affected the antioxidant system of the liver and brain (for 188 days of treatment) without affecting blood and plasma | [133] |
| Allium cepa L. (Onion) | Ethyl acetate extract from onion peel | Activities investigated in vitro | Antibacterial effect against skin resident flora Staphylococcus aureus, Propionibacterium acnes, Pityrosporum ovale, and Escherichia coli1,1-Diphenyl-2-picrylhydrazyl radical, (DPPH) assay Tyrosinase and elastase inhibitory activity | Induced MIC values of 0.06% on skin resident flora Induced excellent DPPH radical scavenging activity (FSC50 = 5.05 µg/mL) Induced significant ROS scavenging activity (OSC50 = 0.05 µg/mL) Inhibited tyrosinase activity (IC50 = 9.16 µg/mL) | [134] |
| Allium cepa L. (Onion) | Onion oil | Male aged rats (1.5–2 years old) | A treatment period of 4 weeks Biochemical measurements | Reduced the elevated levels of all liver function markersReduced total protein and albumin levels Decreased the concentration of urea and creatinine Reduced cholesterol, triglyceride, and LDL levels Increased monoamine levels in aged rats Increased testosterone levels in aged rats | [135] |
| Allium cepa L. (Onion) | Fermented onions extract | B16F10 melanoma cells and HaCaT keratinocyte cells | Cytotoxicity test Melanin content assay Western blot analysis Hyaluronic acid production assay Phytochemical analysis by HPLC-MS | Inhibited melanin formation, at a dose of 100 μg/mL Downregulated collagenase-1 expression and upregulated type I collagen level in UVB-irradiated HaCaT keratinocyte cells Enhanced hyaluronic acid synthesis | [136] |
| Allium sativum L. (Garlic) | Aged garlic extract (AGE) | The SAMPIONS and SAMRl/HS substrains of SAM mice model (a strain of senescence-accelerated mouse (SAM) characterized by age-related brain atrophy) | Evaluation of senescence degree Motor activity Spatial memory test Macroscopic brain morphometry | Prevented the increase in the grading score of SAMPlO and SAMR1 Improved learning and memory deficits of SAMPlO Prevented decreased brain weight and atrophic frontal brain changes at 12 months of age | [137] |
| Allium sativum L. (Garlic) | Hydroethanolic extract | Immortalized human keratinocyte cell line | Antioxidant activity Cell culture and UV irradiation Quantitative real-time RT-PCR MMP-1 production Cytokine determinations | Induced strong DPPH radical scavenging activity (IC50 = 2.50 mg/mL) Induced strong NO scavenging activity (IC50 = 4.38 mg/mL) Attenuated UVB-induced intracellular ROS production Reduced MMP-1 level and MMP-1 mRNA and protein expressions Inhibited the production of UV-induced pro-inflammatory cytokines (IL-6 and IL-1β) Enhanced SA-β-gal and SIRT1 activities in UV-irradiated HaCaT human keratinocytes Inhibited photoaging due to increased cellular senescence in HaCaT cells | [138] |
| Allium sativum L. (Garlic) | Garlic aqueous extract | Human skin fibroblasts | Cell culture and lifespan estimation Macromolecular synthesis | Sustained serial subcultures for more than 55 population doublings in 475 days Prevented the development of malignant cells | [139] |
| Allium tuberosum Rottler ex Spreng. (Chinese chives, Buchu) | Buchu powder | Male ICR mice | 12-month diets containing 2% or 5% buchu Measurement of skin lipid peroxides, protein oxidation, antioxidant enzyme activities, and GSH levels Measurement of insoluble collagen in the skin Ultraviolet irradiation of skin homogenates | Reduced protein carbonyl levels in the skin Maintained enzyme activities and GSH concentrations at youthful levels Increased, over time, the activity of SOD, GPx, and CAT, as well as the total GSH contents Reduced lipid peroxidation and protein oxidation in ICR mouse skin homogenates Reduced the synthesis of insoluble collagen in the skin of mice | [140] |
| Dioscoreaceae | |||||
| Dioscorea aimadoimo (Yam) | Ethanolic extract | Human dermal fibroblast neonatal (HDFn) | DPPH assay SOD activity Collagenase inhibition Measurement of skin moisturizing effect Cell proliferation rate measurement Effect on cell migration and fibroblast proliferation | Reduced the activity of collagenase Increased skin water content (38–45%) Raised cell proliferation to 114% Induced cell migration in HDFn | [141] |
| Dioscorea opposite Thunb. (Yam) | Yam polysaccharides | Mice | Polysaccharide characterization and determination of their content FT-IR spectroscopy Visceral index and biochemical assay RNA isolation RT-PCR Western blot analysis | Improved the learning abilities of mice and helped them recover from spatial memory deficits Inhibited malondialdehyde generation and increased SOD, CAT, and GPx activities in multiple organs Reduced damage caused by d-galactose in different tissues Upregulated anti-aging klotho gene expression in brain and kidney Restored organ function and improved klotho gene expression | [142] |
| Asparagaceae | |||||
| Asparagus cochinchinensis (Lour.) Merr. (Chinese asparagus) | Polysaccharides roots and stems aqueous extracts | Mice | SOD activity Malonaldehyde (MDA) and total protein content in the brain, liver, and plasma | Improved spleen index and SOD activity by lowering MDA levels and slowing the aging process Reduced SOD activity and increased MDA accumulation in mouse brain and liver | [143] |
| Asparagus cochinchinensis (Lour.) Merr. (Chinese asparagus) | Shoot aqueous extract | Kun Ming mice | Mouse treated with d-galactose, vitamin C, and extract Measurement of blood cells, nitric oxide synthase (NOS), CAT, SOD, and nitric oxide (NO) activities, and MDA concentration Histopathology | Exhibited good DPPH and ABTS radical scavenging capabilities Increased NOS, CAT, and SOD activities and NO content Reduced MDA content Improved microstructure of mouse viscera Increased expressions of NOS, SOD, and GPX | [144] |
| Asparagus cochinchinensis (Lour.) Merr. (Chinese asparagus) | Root aqueous extract | Kun Ming mice | DPPH assay d-galactose induced mouse aging model Measurement of SOD, CAT, NOS, MDA, and NO contents Histopathology | Induced strong antioxidant activity Increased white blood cell count Improved SOD, CAT, and NOS activities in aging mice Increased NO content Reduced MDA content | [145] |
| Asparagus officinalis L. (asparagus) | Spear powder ethanol extract | Peripheral blood mononuclear cells (PBMCs) | 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and ferric reducing ability of plasma (FRAP) assays Matrix metalloproteinase-1 (MMP-1) inhibitory activity Elastase inhibitory activity Hyaluronidase inhibitory activity Cytotoxicity effect | Inhibited the elevated levels of MMP-1, elastase, and hyaluronidase by 83.4 ± 1.5%, 70.4 ± 4.1%, and 75.2 ± 1.0%, respectively Presented an attractive source of natural anti-wrinkle ingredients | [146] |
| Asparagus officinalis L. (asparagus) | Aqueous stem extract | Normal human dermal fibroblasts | UV-B-irradiated NHDFs cells RT-PCR Western blot analysis Measurement of telomere length | Increased HSP70 mRNA levels in NHDFs Reduced HSP70 expression at mRNA and protein levels Preserved HSP70 quantity in UV-B-irradiated NHDFs Induced anti-photoaging effects by suppressing HSP70 expression in UV-irradiated dermal fibroblasts | [147] |
| Amaranthaceae | |||||
| Amaranthus tricolor L. (Chinese Spinach) | Red Spinach Extract Ointment | Wistar Rats | Measurement of collagen, elasticity, hydration, sebum, and pigment levels in animals | At 10%, increased skin hydration levels (64.84%) At 10%, increased skin collagen levels (56.25%) At 10%, increased skin elasticity levels (46.30%) At 10%, increased skin pigmentation levels (35.97%) At 10%, decreased sebum levels (40%) | [148] |
| Asteraceae | |||||
| Cynara scolymus L. (Artichoke) | Leaf extract | d-galactose-induced aging rats | Rat model of d-galactose-induced aging Measurement of hematological parameters Evaluation of SOD, GPx, and CAT activities, and MDA and LF (lipofuscin) levels in serum, liver, and brain | Increased SOD activity in brain, liver, and GSH-Px Decreased MDA content in serum, LF in brain and liverExhibited anti-aging proprieties | [149] |
| Cynara scolymus L. (Artichoke) | Bract aqueous extract | Immortalized human keratinocyte cell line (HaCaT) | High-performance liquid chromatography (RP-HPLC-DAD) DPPH assay Reactive oxygen species (ROS) scavenging activityIn vitro sun protection factor (SPF) measurement Human repeat insult patch testing (HRIPT) Chromameter evaluation of UV radiation-induced oxidative stress | Exhibited antioxidant and photoprotective activity | [150] |
| Cynara scolymus L. (Artichoke) | Leaf extract | Wistar rat | Determination of activities of enzymatic part of skin endogenous antioxidant defense system d-gal-induced aging changes in skin Total SOD activity and superoxide anion generation | Restored skin relative weight Decreased the generation rate of O2•−, H2O2, and LPx Activated the enzyme link in the innate antioxidant defense system in the d-galactosidase-induced skin aging model | [151] |
| Cynara scolymus L. (Artichoke) | Leaf extract | Wistar rats | Antioxidant capacity (AOC) determination Skin edema evaluation Total collagen (hydroxyproline) and hexosamine contents determination NF-κB determination | Restored skin relative weight Induced elevated solubility in neutral salt and acid Decreased pepsin solubility collagen fraction Restored the hexosamine/collagen ratio Decreased NF-κB activity Improved collagen metabolism Attenuated the progression of inflammation in the skin aging model | [152] |
| Taraxacum officinale F.H. Wigg. (Dandelion) | Aqueous extract | TM3 cells, an immature mouse Leydig cell line, and 18-week-old male Sprague Dawley rats | Western blot analysis Measurement of serum testosterone level Swimming retention test Measurement of sperm count and activity | Protected TM3 cells from serum restriction and oxidative stress via activation of ERK and Akt pathways Improved testosterone level and activation of spermatogenesis in rats Improved physical locomotion Improved quality of life for aging males | [153] |
| Brassicaceae | |||||
| Brassica oleracea L. var. capitata F. rubra (red cabbage) | Ethanol extract | Male Wistar mice | UV-B exposure Dermis-elastic fiber thickness assessment | No alteration in the thickness of the dermal layer following UV-B exposure | [154] |
| Brassica oleracea L. var. italica Plenck (Broccoli) | Ethanol extract of flowers | Normal human fibroblast cells | Ultraviolet irradiation RNA extraction RT-PCR ELISA | Decreased MMP-1 expression at both MMP-1 mRNA and MMP-1 protein expression Prevented UVB-induced MMP-1 expression at both mRNA and protein levels | [155] |
| Raphanus sativus L. (Radish) | Supercritical heat-treated radish skin and green extract | UV-induced Hos: HRM-2 wrinkled mouse | Evaluation of skin thickness, elasticity, and wrinkles induced by UVB lamp | Increased depth of wrinkles Increased expression of MMP-2 and MMP-2 genes Inhibited MMP-2 expression Improved skin wrinkles | [156] |
| Cucurbitaceae | |||||
| Cucumis sativus L. (Cucumber) | Juice | In vitro enzymatic assays | HPLC analysis DPPH assay Hyaluronidase inhibition assay Elastase inhibition assay | Exhibited DPPH free radical and superoxide radical scavenging activity Induced strong anti-hyaluronidase and anti-elastase activity | [157] |
| Cucurbita moschata Duchesne (pumpkin) | Seed petroleum ether extract | Fibroblast cell lines | Cytotoxicity assay SA-βgal (Senescence-Associated Beta Galactosidase) activity Molecular docking | Reduced the % of cell senescence in a dose-dependent manner | [158] |
| Fabaceae | |||||
| Glycine max (L.) Merr. (Soybean) | Protein hydrolysate extract | d-galactose-induced specific pathogen-free (SPF) Kunming mice | DPPH assay Hydroxyl radical scavenging activity (HRSA) Reducing power assay Characterization of peptides Evaluation of antioxidant activity (in vitro) of synthesized peptides | Reversed learning and memory impairments associated with aging Induced significant DPPH scavenging activity | [159] |
| Glycine max (L.) Merr. (Soybean) | Extract of (ethanol: hexane) | Male human volunteers | DPPH assay Anti-aging study (skin microrelief, skin elasticity, and skin capacitance) | Affected the skin elasticity and moisture contents Diminished skin scaliness, skin wrinkles, skin smoothness, and skin roughness Exerted potential skin anti-aging effects | [160] |
| Glycine max (L.) Merr. (Soybean) | Okara (soy pulp) | Male SAMP8 and senescence-accelerated resistant mouse 1 (SAMR1) mice | Barnes maze test Microbiota sequencing and analysis qRT-PCR Western blot analysis Immunohistochemical analysis Measurement of acetylcholine concentration | Decreased the inflammatory cytokine TNF-α Increased brain-derived neurotrophic factor (BDNF) Increased the expression of acetylcholine synthesis enzyme Increased the level of acetylcholine in the brain Prevented cognitive decline without dramatically altering the gut microbiome | [161] |
| Glycine max (L.) Merr. (Soybean) | Monascus-Fermented Soybean Extracts | In vitro assay of inhibitory enzyme activities | Tyrosinase inhibition assay Hyaluronidase inhibition assay Elastase inhibition assay Trolox equivalent antioxidant capacity (TEAC) assay | Increased antioxidant capacities depending on the dose Inhibited the activity of tyrosinase, hyaluronidase, and elastase Inhibited the activity of skin aging-related enzymes | [162] |
| Glycine max (L.) Merr. (Soybean) | Fermented soybean milk by Lactobacillus plantarum | Mice with premature aging induced by d-galactose | DPPH assay Histopathology Biochemical analysis of GSH, CAT, SOD, GPx, and MDA levels RT-PCR | Presented better ability to scavenge free radicals Protected the skin, spleen, and liver Reduced oxidative damage and inflammation Upregulated GSH, CAT, SOD, and GPx levels Decreased MDA content in liver, brain, and serum Improved antioxidant capacity in mice with d-galactose-induced premature aging | [163] |
| Glycine max (L.) Merr. (Soybean) | Black soybean peptides | Aging mice induced by d-galactose | Antioxidant activity assessment (in vivo) | Increased SOD and GPx activity in liver and serumReduced MDA contents in serumExhibited significant antioxidant activity in mice | [164] |
| Vigna angularis (Wild.) Ohwi and Ohashi (Red Bean) | Ethanolic extract | Human volunteers | Gel Formulation evaluation Anti-aging test (moisture level, skin pore size, evenness) Dark spot test | Improved moisture level, pore size, evenness, and number of black spots Transformed into a peel-off gel mask with anti-aging properties | [165] |
| Phaseolus vulgaris L. (Black Bean) | Phenolic extract | In vitro assay of inhibitory enzyme activities | Total phenolic content (TPC) Total anthocyanin content (ACN) Supercritical fluid (SCF) Leaching extractions DPPH and ABTS assays Tyrosinase inhibition assay Elastase inhibition assay | For the DPPH scavenging assay: IC50 = 0.32 ± 0.01 mg GAE/g coat For the ABTS assay: IC50 = 0.40 ± 0.03 mg GAE/g coatFor the tyrosinase enzymatic inhibition assay: IC50 = 10.44 ± 1.32 mg GAE/g coat For the collagenase enzymatic inhibition assay: IC50 = 8.33 ± 0.65 mg GAE/g coat For the elastase enzymatic inhibition assay: IC50 = 0.11 ± 0.02 mg GAE/g coat | [166] |
| Vigna angularis (Wild.) Ohwi and Ohashi (Azuki beans) | Aqueous extract | Normal human dermal fibroblasts cells and hairless mice | DPPH assay UV irradiation ROS assay MTT assay Topical application Wrinkle measurement Measurement of physiological skin functions Histopathology Western blot analysis | Induced antioxidant activity in UVB-exposed human dermal fibroblasts Suppressed MMP-1 production (90%) Suppressed wrinkle formation and skin thickness Prevented skin photoaging accelerated by UVB radiation | [167] |
| Malvaceae | |||||
| Abelmoschus Esculentus (L.) Moench (Okra) | Ethanol extract | Human neuroblastoma (SK-N-SH) cell lines | Cell viability assay ROS assay SA-β-galactosidase enzyme assay | Promoted cell viability over reduced ROS content and SA-β-gal positive cells Developed synaptic plasticity by inhibiting AChE activity Attenuated the negative responses of aging neurons | [168] |
| Polygonaceae | |||||
| Rheum rhabarbarum L. (Rhubarb) | Rhubarb Preparation | Mice with cerebral malfunction induced by d-galactose | Determination of Ach and AchE levels Assessment of peroxidase level | Increased cortical CAT and GPx activities Decreased AchE activity and increased Ach level Increased cerebellar SOD, CuSOD, and Mn-SOD activities Lowered LPO level Increased cortical CAT activity Decreased the level of cerebellar LPO Improved memory with its ability Regulated the activities of CAT, GSH-px, and SOD Inhibited the activity of AchE Increased Ach level | [169] |
| Rumex crispus L. (Curly Dock) | Roots and leaves hydroethanolic extract | In vitro assay of inhibitory enzyme activities | Measurement of UV absorption DPPH and ABTS assays NO assay Phosphomolibdate assay MMP-1, MMP-8, and MMP-13 inhibitor screening tests | Exhibited the highest inhibitory effect on all MMP enzymes Presented high UV protection Exhibited strong antioxidant capabilities | [170] |
| Apiaceae | |||||
| Daucus carota L. | Seeds ethanol and petroleum ether extracts | Sprague Dawley rats’ brain aging induced by d-galactose | Carrot seed oil fatty acid assessment Anti-aging assessment | Removed both CAT reduction and MDA elevation Exhibited remarkable antioxidant and anti-inflammatory properties | [171] |
| Molecules (Origin) | Models Used | Methods | Key Results | References |
|---|---|---|---|---|
| Allicin (Allium sativum) | In silico molecular docking | Molecular docking | Presented the highest potential against premature aging Inhibited leukocyte elastase activity | [172] |
| Caffeic acid, S-allyl cysteine, and uracil (Allium sativum) | HR1 hairless mouse | Masson’s trichrome staining ROS assay Western blot analysis | Inhibited the degradation of y-type procollagen Inhibited the expression of matrix metalloproteinases in vivo Improved the histological disorder of collagen fibers and oxidative stress in vivo Decreased oxidative stress and inflammation via modulation of NF-κB and AP-1 activities | [173] |
| Glucosinolate (glucoraphanin and sulforaphane) and phenolics (kaempferol and quercetin) from broccoli (Brassica oleracea) | In silico molecular docking | Drug-likeness and bioactivity prediction Biological activity prediction using PASS online | Presented a strong interaction with Keap1 Inhibited Keap1-Nrf2 interaction Enhanced HMOXI expression Inhibited peroxidase | [174] |
| Glycoprotein (Daucus carota) | Fibroblasts of the dermis of the human body | DPPH assy Inhibition of lipid peroxidation Collagen type-1 creation promotion experiments Inhibition of MMP-1 expression | Exhibited good antioxidant activity Expressed high lipid peroxidation Promoted the generation of collagen type-1 Reduced the MMP-1 Eliminated ROS Acted as an antioxidant and anti-aging agent in skin exposed to solar ultraviolet light | [175] |
| Water-soluble protein (Vicia faba) | Human fibroblasts (TIG-1) | Effect of water-soluble protein against antioxidant enzyme activities and GSH concentration Biochemical analysis. | Decreased cytosolic SOD activity Presented elevated CAT activity in young and old cells | [176] |
| Arctigenin, matairesinol, arctiin, (iso) lappaol A, lappaol C, and lappaol F (Arctium lappa L.) | Caenorhabditis elegans | DPPH assay ROS assay Lifespan assay juglone-induced oxidative stress assay RT-PCR | Exhibited good antioxidant activity (strongest observed with matairesinol) Extended the average lifespan of C. elegans | [177] |
| Dioscin, allantoin, and diosgenin (Dioscoreae Rhizoma) | molecular docking | Constructing the protein–protein interaction (PPI) network Molecular docking Gene ontology (GO) functional enrichment analysis Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis | Regulated the expression of target proteins via enriched signaling pathways Inhibited tumor proliferation and metastasis Regulated metabolism Promoted nerve repair by regulating the expression of targets (MAPK3, HADC3, HADC1, RXRA, STAT3, etc.) | [178] |
| Trp-Pro-Lys (WPK) and Ala-Tyr-Leu-His (AYLH) Glycine max (Soybean) | d-galactose-induced specific pathogen-free (SPF) Kunming mice | Shuttle box test Biochemical analysis DPPH assay HRSA Reducing power assay Separation and purification of peptides. | Attenuated H2O2-induced oxidative damage in PC12 cells Prevented age-related learning and memory impairments and oxidative stress | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechchate, H.; El Allam, A.; El Omari, N.; El Hachlafi, N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules 2022, 27, 2316. https://doi.org/10.3390/molecules27072316
Mechchate H, El Allam A, El Omari N, El Hachlafi N, Shariati MA, Wilairatana P, Mubarak MS, Bouyahya A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules. 2022; 27(7):2316. https://doi.org/10.3390/molecules27072316
Chicago/Turabian StyleMechchate, Hamza, Aicha El Allam, Nasreddine El Omari, Naoufal El Hachlafi, Mohammad Ali Shariati, Polrat Wilairatana, Mohammad S. Mubarak, and Abdelhakim Bouyahya. 2022. "Vegetables and Their Bioactive Compounds as Anti-Aging Drugs" Molecules 27, no. 7: 2316. https://doi.org/10.3390/molecules27072316








