Regional Drought Conditions Control Quercus brantii Lindl. Growth within Contrasting Forest Stands in the Central Zagros Mountains, Iran
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.2. Sampling and Chronology Development
2.3. Climate Data
2.4. Climate–Growth Response
3. Results
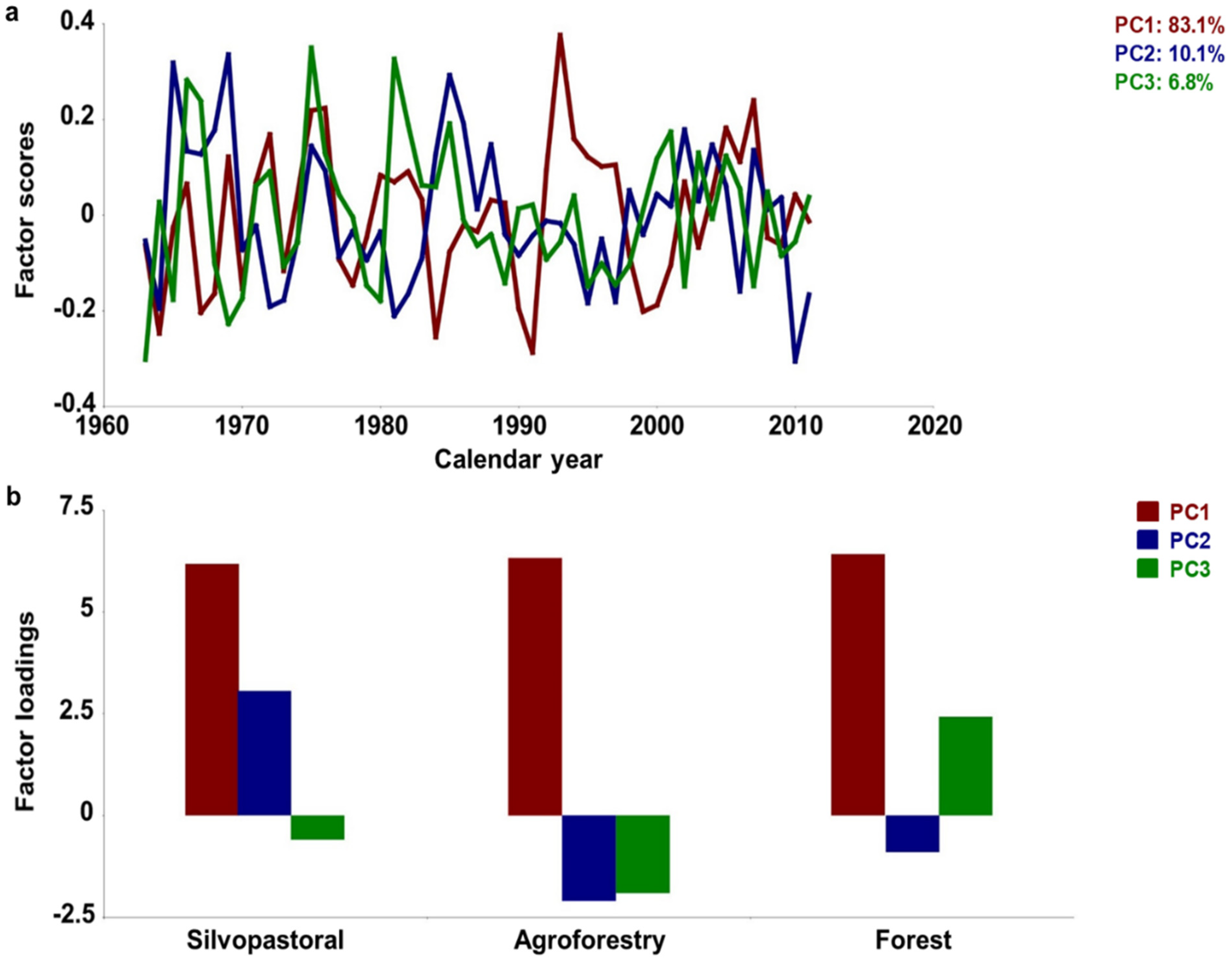
3.1. Chronology Characteristics
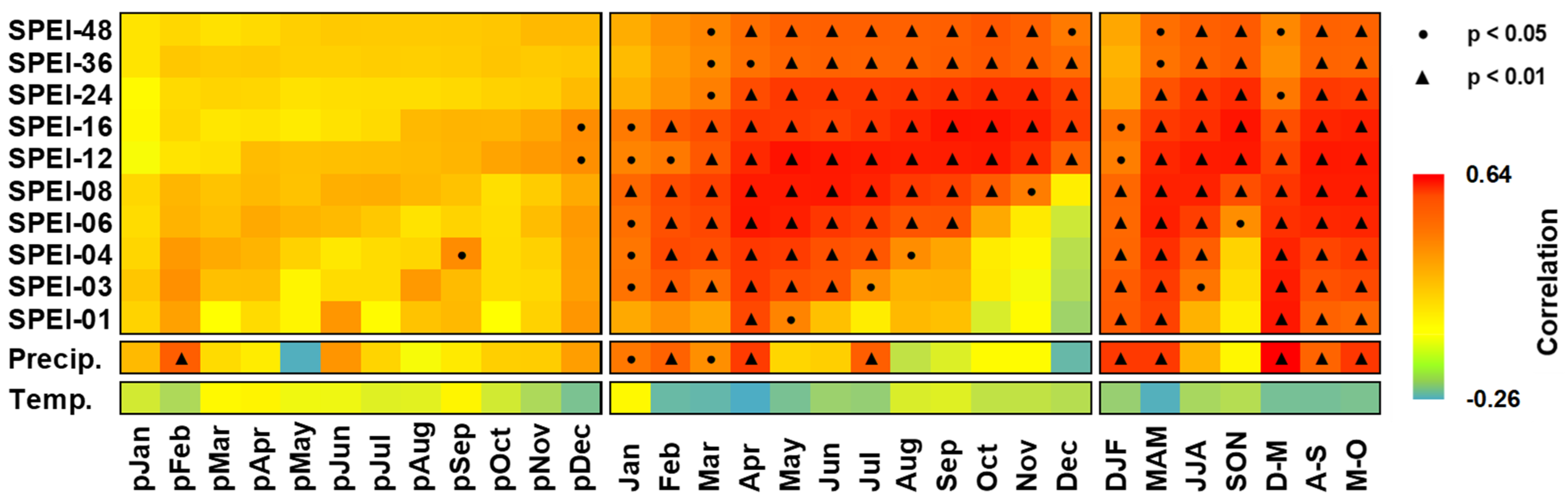
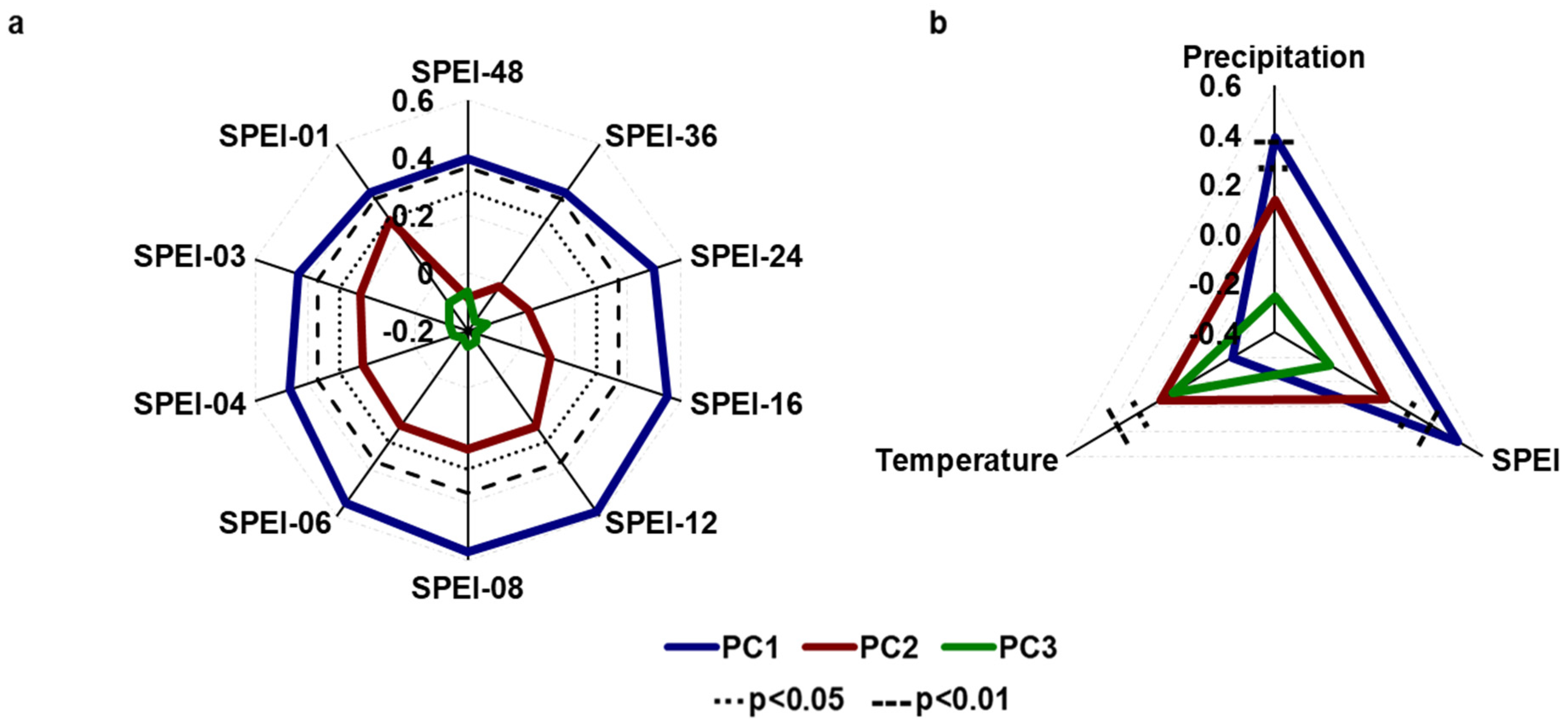
3.2. Growth–Climate Relationships
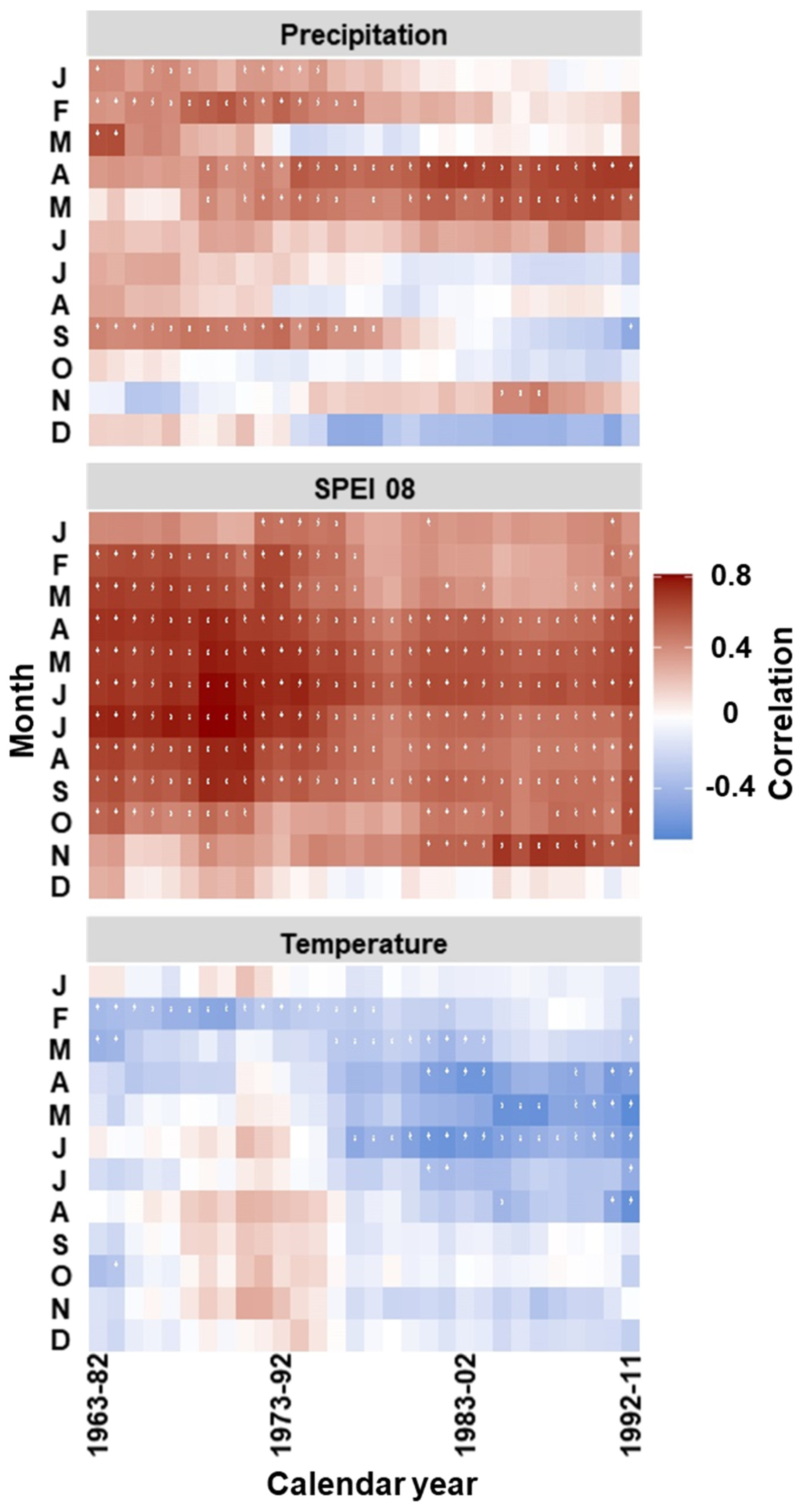
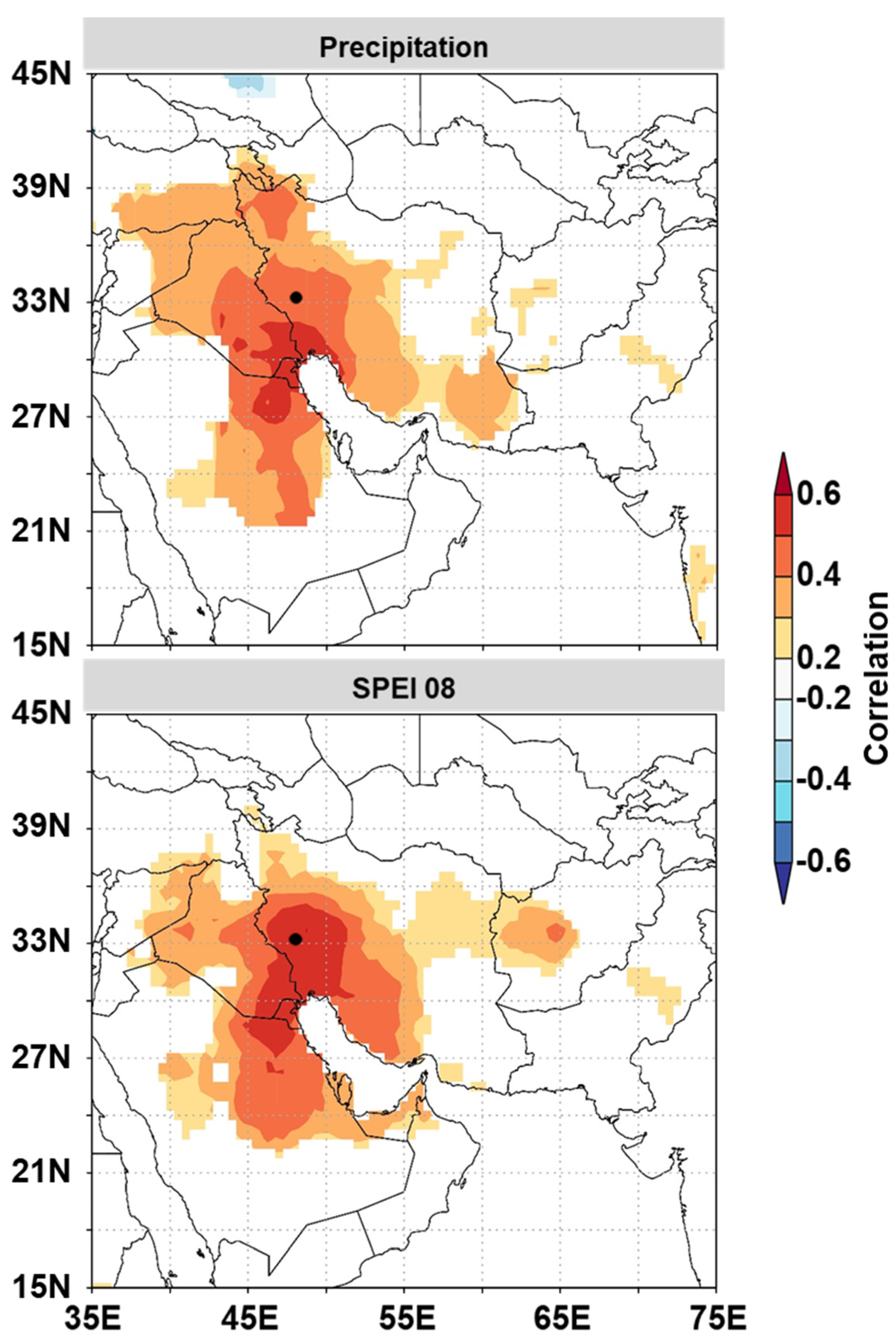
3.3. Temporal Stability and Spatial Extent of the Hydroclimate Signal
4. Discussion
4.1. Data Quality
4.2. Drought Signal
4.3. Growth–Stand Density Relationships
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Temp. | Precip. | SPEI 01 | SPEI 03 | SPEI 04 | SPEI 06 | SPEI 08 | SPEI 12 | SPEI 16 | SPEI 24 | SPEI 36 | SPEI 48 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JAN | −2.25 * | −0.59 | −0.44 | −0.70 | −0.44 | −0.54 | −0.49 | −1.23 | −0.42 | −1.39 | −2.4 * | −2.22 * |
| FEB | −1.1 | −1.11 | −1.08 | −0.46 | −0.78 | −0.75 | −0.77 | −1.41 | −1.01 | −1.61 | −2.44 * | −2.51 * |
| MAR | −2.39 * | 0.13 | 0.00 | −0.85 | −0.37 | −0.58 | −0.68 | −1.65 | −1.63 | −1.58 | −2.4 * | −2.46 * |
| APR | −2.21 * | −0.11 | −0.42 | −0.85 | −1.03 | −0.53 | −0.42 | −0.92 | −1.51 | −1.58 | −2.46 * | −2.27 * |
| MAY | −1.46 | −2.09 * | −1.47 | −0.96 | −1.39 | −1.41 | −0.96 | −0.89 | −1.78 | −1.68 | −2.34 * | −2.42 * |
| JUN | −0.86 | 0.80 | −1.18 | −1.18 | −1.16 | −1.75 | −0.89 | −0.94 | −1.59 | −1.80 | −2.35 * | −2.39 * |
| JUL | −1.72 | 1.14 | −0.44 | −1.59 | −1.13 | −1.41 | −1.41 | −1.03 | −1.41 | −1.73 | −2.37 * | −2.39 * |
| AUG | −1.21 | 1.13 | −0.23 | −0.72 | −1.54 | −1.06 | −1.63 | −1.04 | −1.15 | −1.72 | −2.22 * | −2.32 * |
| SEP | −2.1 * | −0.07 | 0.04 | −0.13 | −0.77 | −1.11 | −1.54 | −0.96 | −0.99 | −1.80 | −2.23 * | −2.27 * |
| OCT | −2.4 * | 0.13 | −0.03 | −0.42 | −0.53 | −1.73 | −1.58 | −1.41 | −1.32 | −1.68 | −2.27 * | −2.59 ** |
| NOV | −4.45 *** | 0.54 | 0.61 | −0.04 | 0.08 | −0.18 | −0.59 | −1.75 | −1.32 | −2.08 * | −2.37 * | −2.58 ** |
| DEC | −2.1 * | −0.49 | −0.53 | 0.06 | 0.06 | −0.20 | −1.13 | −1.72 | −1.37 | −2.15 * | −2.8 ** | −2.73 ** |
| A-S | −1.6 | −0.85 | −0.97 | −1.22 | −1.28 | −1.25 | −1.15 | −0.92 | −1.53 | −1.78 | −2.28 * | −2.39 * |
| M-O | −1.82 | −1.04 | −1.54 | −1.47 | −1.54 | −1.66 | −1.20 | −0.85 | −1.56 | −1.85 | −2.27 * | −2.46 * |
| Annual | −2.32 * | −2.01 * | −1.49 | −1.66 | −1.73 | −1.54 | −1.16 | −1.25 | −1.56 | −1.90 | −2.47 * | −2.65 ** |
References
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Hisano, M.; Searle, E.B.; Chen, H.Y.H. Biodiversity as a Solution to Mitigate Climate Change Impacts on the Functioning of Forest Ecosystems. Biol. Rev. 2018, 93, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.; Kahle, H.P.; von Wilpert, K.; Spiecker, H. Resilience of Norway Spruce (Picea abies (L.) Karst) Growth to Changing Climatic Conditions in Southwest Germany. For. Ecol. Manag. 2014, 315, 12–21. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate Change and Forests of the Future: Managing in the Face of Uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef]
- Spiecker, H. Tree Rings and Forest Management in Europe. Dendrochronologia 2002, 20, 191–202. [Google Scholar] [CrossRef]
- Seim, A.; Omurova, G.; Azisov, E.; Musuraliev, K.; Aliev, K.; Tulyaganov, T.; Nikolyai, L.; Botman, E.; Helle, G.; Linan, I.D. Climate Change Increases Drought Stress of Juniper Trees in the Mountains of Central Asia. PLoS ONE 2016, 11, e0153888. [Google Scholar] [CrossRef] [PubMed]
- Katouzian, A.-R.; Sari, A.; Macher, J.N.; Weiss, M.; Saboori, A.; Leese, F.; Weigand, A.M. Drastic Underestimation of Amphipod Biodiversity in the Endangered Irano-Anatolian and Caucasus Biodiversity Hotspots. Sci. Rep. 2016, 6, 22507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Granda, E.; Rossatto, D.R.; Camarero, J.J.; Voltas, J.; Valladares, F. Growth and Carbon Isotopes of Mediterranean Trees Reveal Contrasting Responses to Increased Carbon Dioxide and Drought. Oecologia 2014, 174, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Erfanifard, Y.; Feghhi, J.; Zobeiri, M.; Namiranian, M. Spatial Pattern Analysis in Persian Oak (Quercus brantii Var. Persica) Forests on B&W Aerial Photographs. Environ. Monit. Assess. 2009, 150, 251. [Google Scholar] [CrossRef]
- Pourhashemi, M.; Mohajer, M.R.M.; Zobeiri, M.; Amiri, G.Z.; Panahi, P. Identification of Forest Vegetation Units in Support of Government Management Objectives in Zagros Forests, Iran. Scand. J. For. Res. 2004, 19, 72–77. [Google Scholar] [CrossRef]
- Khanhasani, M.; Sagheb-Talebi, K.; Akhavan, R.; Vardanyan, Z. The Effect of Environmental Factors on Distribution of Three Oak Species (Q. brantii Lindl., Q. libani Oliv. and Q. infectoria Oliv.) in Northern Zagros Forests. Iran. J. For. Poplar Res. 2015, 23, 549–561. [Google Scholar]
- Bahmani, M.; Forouzan, S.H.; Fazeli-Moghadam, E.; Rafieian-Kopaei, M.; Adineh, A.; Saberianpour, S.H. Oak (Quercus branti): An Overview. J. Chem. Pharm. Res. 2015, 7, 634–639. [Google Scholar]
- Talebi, K.S.; Sajedi, T.; Pourhashemi, M. Forests of Iran: A Treasure from the Past, a Hope for the Future; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Radmehr, A.; Soosani, J.; Balapour, S.; Ghalebahmani, S.M.; Sepahvand, A. Effects of Climate Variables (Temperature and Precipitation) on the Width of Rings-Growth in Persian Coppice Oak in the Central Zagros (Case Study: Khoramabad). J. Wood For. Sci. Technol. 2015, 22, 93–110. [Google Scholar]
- Parvaneh, B.; Valipour, M. Investigation on Effects of Climatic Variables on Zagros Oak Q. Brantii Lindl Tree Rings: A Case Study of Shurab Park (Western Iran). World Appl. Sci. J. 2012, 17, 626–630. [Google Scholar]
- Zarean, H.; Yazdanpanah, H.; Movahedi, S.; Jalilvand, H.; Momeni, M.; Yarali, N. Chronological Study of Quercus Persica Growth Ring Response to Climatic Variables of Precipitation and Temperature in Zagros Forests (a Case Study of Dena Region). J. Appl. Environ. Biol. Sci. 2014, 4, 247–255. [Google Scholar]
- Nasseri, K.S.; Poursartip, L.; Moradi, M.; Soosani, J. Dynamic Effects of Climate Variables (Temperature and Precipitation) on the Annual Diameter Growth of Iranian Oak (Quercus brantii Lindl.). For. Res. Dev. 2016, 2, 63–71. [Google Scholar]
- Fayyaz, P.; Zolfaghari, R.; Balapour, S. Age-Dependent Response to Extreme Mediterranean Climate in Annual Rings of Brant’s Oak (Quercus brantii Lindl.). Dendrobiology 2017, 78, 32–39. [Google Scholar] [CrossRef][Green Version]
- Ahmadi, R.; Kiadaliri, H.; Mataji, A.; Kafaki, S. Annual Ring Analysis of Persian Oak (Quercus brantii) to Determine Periods of Stress and Tensions on Zagros Forests (Case Study: Forests of Ilam County). J. Biodivers. Environ. Sci. 2014, 5, 378–384. [Google Scholar]
- Azizi, G.; Arsalani, M.; Bräuning, A.; Moghimi, E. Precipitation Variations in the Central Zagros Mountains (Iran) since AD 1840 Based on Oak Tree Rings. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 386, 96–103. [Google Scholar] [CrossRef]
- Jalilvand, H.; Zarean, H.; Yazdanpanah, H.; Movahedi, S.; Momeni, M.; Yarali, N. The Reconstruction of the Annual Rainfall of over a Century through from Quercus Persica Tree Rings in Zagros Forests (Case Study of Dena Region). J. Geogr. Plan. 2016, 20, 65–86. [Google Scholar]
- Arsalani, M.; Pourtahmasi, K.; Azizi, G.; Bräuning, A.; Mohammadi, H. Tree-Ring Based December–February Precipitation Reconstruction in the Southern Zagros Mountains, Iran. Dendrochronologia 2018, 49, 45–56. [Google Scholar] [CrossRef]
- Olfat, A.M.; Pourtahmasi, K. Anatomical Characters in Three Oak Species (Q. libani, Q. brantii and Q. infectoria) from Iranian Zagros Mountains. Aust. J. Basic Appl. Sci. 2010, 4, 3230–3237. [Google Scholar]
- Naji, H.; Taher Pour, M. The Effect of Simulated Dust Storm on Wood Development and Leaf Stomata in Quercus brantii L. Desert 2019, 24, 43–49. [Google Scholar]
- Bordbar, K.; Sagheb-Talebi, K.H.; Hamzehpour, M.; Joukar, L.; Pakparvar, M.; Abbasi, A.R. Impact of Environmental Factors on Distribution and Some Quantitative Characteristics of Manna Oak (Quercus brantii Lindl.) in Fars Province. Iran. J. For. Poplar Res. 2010, 18, 390–404. [Google Scholar]
- Safari, A.; Shabanian, N.; Heidari, R.H.; Erfanifard, S.Y.; Pourreza, M. Spatial Pattern of Manna Oak Trees (Quercus brantii Lindl.) in Bayangan Forests of Kermanshah. Iran. J. For. Poplar Res. 2010, 18, 596–608. [Google Scholar]
- Taghvayipour, E.; Salehi, A.; Askari, Y. Distribution Pattern of Declined Brant’s Oak (Quercus brantii Lindl.) Trees in Forests of Tol-e Ghah, Yasuj. Iran. J. For. Poplar Res. 2016, 24, 53–64. [Google Scholar]
- Arsalani, M.; Azizi, G.; Bräuning, A. Dendroclimatic Reconstruction of May–June Maximum Temperatures in the Central Zagros Mountains, Western Iran. Int. J. Climatol. 2015, 35, 408–416. [Google Scholar] [CrossRef]
- Goodarzi, M.; Pourhashemi, M.; Azizi, Z. Investigation on Zagros Forests Cover Changes under the Recent Droughts Using Satellite Imagery. J. For. Sci. 2019, 65, 9–17. [Google Scholar] [CrossRef]
- Habibi, M. Investigating the Impact of Climate Changes on Qualitative and Quantitative Growth of Oak Trees (Case Study: Central Zagros). Open J. Ecol. 2016, 6, 358–366. [Google Scholar] [CrossRef]
- Salehi, A.; Eriksson, L.O. A Model for Management of Mixed Coppice Stands in Semiarid Persian Oak Forests. Math. Comput. For. Nat. Resour. Sci. 2010, 2, 20–29. [Google Scholar]
- Ostakh, E.; Soosani, J.; Pilehvar, B.; Poursartip, L.; Musavi, S. Investigation on Climate Variables (Temperature and Precipitation) Effects on Annual Width Rings of Pinus Brutia in Lorestan Province. Ecol. Iran. For. 2014, 2, 19–27. [Google Scholar]
- Vannoppen, A.; Kint, V.; Ponette, Q.; Verheyen, K.; Muys, B. Tree Species Diversity Impacts Average Radial Growth of Beech and Oak Trees in Belgium, Not Their Long-Term Growth Trend. For. Ecosyst. 2019, 6, 10. [Google Scholar] [CrossRef]
- Motta, R.; Nola, P. Growth Trends and Dynamics in Sub-alpine Forest Stands in the Varaita Valley (Piedmont, Italy) and Their Relationships with Human Activities and Global Change. J. Veg. Sci. 2001, 12, 219–230. [Google Scholar] [CrossRef]
- Daniels, L.D.; Maertens, T.B.; Stan, A.B.; McCloskey, S.P.J.; Cochrane, J.D.; Gray, R.W. Direct and Indirect Impacts of Climate Change on Forests: Three Case Studies from British Columbia. Can. J. Plant Pathol. 2011, 33, 108–116. [Google Scholar] [CrossRef]
- Mausolf, K.; Härdtle, W.; Jansen, K.; Delory, B.M.; Hertel, D.; Leuschner, C.; Temperton, V.M.; von Oheimb, G.; Fichtner, A. Legacy Effects of Land-Use Modulate Tree Growth Responses to Climate Extremes. Oecologia 2018, 187, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vallejo, C.; Navarro-Cerrillo, R.M. Contrasting Response to Drought and Climate of Planted and Natural Pinus Pinaster Aiton Forests in Southern Spain. Forests 2019, 10, 603. [Google Scholar] [CrossRef]
- Mausolf, K.; Wilm, P.; Härdtle, W.; Jansen, K.; Schuldt, B.; Sturm, K.; von Oheimb, G.; Hertel, D.; Leuschner, C.; Fichtner, A. Higher Drought Sensitivity of Radial Growth of European Beech in Managed than in Unmanaged Forests. Sci. Total Environ. 2018, 642, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Valipour, A.; Plieninger, T.; Shakeri, Z.; Ghazanfari, H.; Namiranian, M.; Lexer, M.J. Traditional Silvopastoral Management and Its Effects on Forest Stand Structure in Northern Zagros, Iran. For. Ecol. Manag. 2014, 327, 221–230. [Google Scholar] [CrossRef]
- Zabiholahii, S.; Haidari, M.; Namiranian, N.; Shabanian, N. Effect of Traditional Forest Management Practices in Havare Khol Pattern on Forest Structure (Case Study: Kurdistan Province, Northern Zagros Forest). IOSR J. Pharm. Biol. Sci. 2012, 5, 42–47. [Google Scholar] [CrossRef]
- Baillie, M.G.L.; Pilcher, J.R. A Simple Crossdating Program for Tree-Ring Research. Tree-Ring Bull. 1973, 33, 7–14. [Google Scholar]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating Crossdating Accuracy: A Manual and Tutorial for the Computer Program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R.; Krusic, P.J. Program ARSTAN: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Lamont-Doherty Earth Observatory, Columbia University: Palisades, NY, USA, 2005. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Cooley, W.W.; Lohnes, P.R. Multivariate Data Analysis; John Wiley and Sons: New York, NY, USA, 1971. [Google Scholar]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Camarero, J.J.; Manzanedo, R.D.; Sanchez-Salguero, R.; Navarro-Cerrillo, R.M. Growth Response to Climate and Drought Change along an Aridity Gradient in the Southernmost Pinus Nigra Relict Forests. Ann. For. Sci. 2013, 70, 769–780. [Google Scholar] [CrossRef]
- Merlin, M.; Perot, T.; Perret, S.; Korboulewsky, N.; Vallet, P. Effects of Stand Composition and Tree Size on Resistance and Resilience to Drought in Sessile Oak and Scots Pine. For. Ecol. Manag. 2015, 339, 22–33. [Google Scholar] [CrossRef]
- Zimmermann, J.; Hauck, M.; Dulamsuren, C.; Leuschner, C. Climate Warming-Related Growth Decline Affects Fagus Sylvatica, but Not Other Broad-Leaved Tree Species in Central European Mixed Forests. Ecosystems 2015, 18, 560–572. [Google Scholar] [CrossRef]
- Gou, X.; Deng, Y.; Gao, L.; Chen, F.; Cook, E.; Yang, M.; Zhang, F. Millennium Tree-ring Reconstruction of Drought Variability in the Eastern Qilian Mountains, Northwest China. Clim. Dyn. 2015, 45, 1761–1770. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Xu, R.; Liu, J. Drought Severity Change in China during 1961–2012 Indicated by SPI and SPEI. Nat. Hazards 2015, 75, 2437–2451. [Google Scholar] [CrossRef]
- Venegas-González, A.; Juñent, F.R.; Gutiérrez, A.G.; Tomazello Filho, M. Recent Radial Growth Decline in Response to Increased Drought Conditions in the Northernmost Nothofagus Populations from South America. For. Ecol. Manag. 2018, 409, 94–104. [Google Scholar] [CrossRef]
- Camarero, J.J.; Sangüesa-Barreda, G.; Vergarechea, M. Prior Height, Growth, and Wood Anatomy Differently Predispose to Drought-Induced Dieback in Two Mediterranean Oak Speciesk. Ann. For. Sci. 2016, 73, 341–351. [Google Scholar] [CrossRef]
- Arsalani, M.; Grießinger, J.; Pourtahmasi, K.; Bräuning, A. Multi-Centennial Reconstruction of Drought Events in South-Western Iran Using Tree Rings of Mediterranean Cypress (Cupressus sempervirens L.). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 567, 110296. [Google Scholar] [CrossRef]
- Mann, H.B. Nonparametric Tests against Trend. Econom. J. Econom. Soc. 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R Package for the Numerical Calibration of Proxy-climate Relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Zandebasiri, M.; Soosani, J.; Pourhashemi, M. Evaluating Existing Strategies in Environmental Crisis of Zagros Forests of Iran. Appl. Ecol. Environ. Res. 2017, 15, 621–632. [Google Scholar] [CrossRef]
- Attarod, P.; Rostami, F.; Dolatshahi, A.; Sadeghi, S.M.M.; Amiri, G.Z.; Bayramzadeh, V. Do Changes in Meteorological Parameters and Evapotranspiration Affect Declining Oak Forests of Iran? J. For. Sci. 2016, 62, 553–561. [Google Scholar] [CrossRef]
- Enright, N.J. Principal Components Analysis of Tree-Ring/Climate Relationships in White Spruce (Picea glauca) from Schefferville, Canada. J. Biogeogr. 1984, 11, 353–361. [Google Scholar] [CrossRef]
- Liu, L.S.; Shao, X.M.; Liang, E.Y. Climate Signals from Tree Ring Chronologies of the Upper and Lower Treelines in the Dulan Region of the Northeastern Qinghai-Tibetan Plateau. J. Integr. Plant Biol. 2006, 48, 278–285. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; McRae, D.J. Spatial and Age-Dependent Tree-Ring Growth Responses of Larix Gmelinii to Climate in Northeastern China. Trees 2009, 23, 875–885. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Bernabei, M.; Di Filippo, A.; Schirone, B. Spatial and Altitudinal Bioclimatic Zones of the Italian Peninsula Identified from a Beech (Fagus sylvatica L.) Tree-Ring Network. Acta Oecologica 2005, 27, 197–210. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.; Wei, W.; Yu, S.; Zhang, T.; Shang, H.; Zhang, R.; Qin, L.; Fan, Z. Tree-Ring Recorded Hydroclimatic Change in Tienshan Mountains during the Past 500 Years. Quat. Int. 2015, 358, 35–41. [Google Scholar] [CrossRef]
- Frank, D.; Esper, J. Characterization and Climate Response Patterns of a High-Elevation, Multi-Species Tree-Ring Network in the European Alps. Dendrochronologia 2005, 22, 107–121. [Google Scholar] [CrossRef]
- Jacoby, G.C.; Cook, E.R. Past Temperature Variations Inferred from a 400-Year Tree-Ring Chronology from Yukon Territory, Canada. Arct. Alp. Res. 1981, 13, 409–418. [Google Scholar] [CrossRef]
- Peters, K.; Jacoby, G.C.; Cook, E.R. Principal Components Analysis of Tree-Ring Sites. Tree-Ring Bull. 1981, 41, 1–19. [Google Scholar]
- George, S.S. An Overview of Tree-Ring Width Records across the Northern Hemisphere. Quat. Sci. Rev. 2014, 95, 132–150. [Google Scholar] [CrossRef]
- Sheppard, P.R.; Comrie, A.C.; Packin, G.D.; Angersbach, K.; Hughes, M.K. The Climate of the US Southwest. Clim. Res. 2002, 21, 219–238. [Google Scholar] [CrossRef]
- Du, S.; Yamanaka, N.; Yamamoto, F.; Otsuki, K.; Wang, S.; Hou, Q. The Effect of Climate on Radial Growth of Quercus Liaotungensis Forest Trees in Loess Plateau, China. Dendrochronologia 2007, 25, 29–36. [Google Scholar] [CrossRef]
- Najafi Harsini, F.; Pourtahmasi, K.; Karimi, A. Dendrochronological Investigation of Radial Growth of Quercus infectoria in Kermanshah Oak Forests. J. For. Wood Prod. (JFWP) Iran. J. Nat. Resour. 2012, 65, 119–129. [Google Scholar]
- Vicente-Serrano, S.M.; Camarero, J.J.; Azorin-Molina, C. Diverse Responses of Forest Growth to Drought Time-scales in the Northern Hemisphere. Glob. Ecol. Biogeogr. 2014, 23, 1019–1030. [Google Scholar] [CrossRef]
- Borrelli, P.; Robinson, D.A.; Fleischer, L.R.; Lugato, E.; Ballabio, C.; Alewell, C.; Meusburger, K.; Modugno, S.; Schütt, B.; Ferro, V. An Assessment of the Global Impact of 21st Century Land Use Change on Soil Erosion. Nat. Commun. 2017, 8, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.A. Soil Productivity and Sustainability in Agroforestry Systems. In Agroforestry: A Decade of Development; Steppler, H.A., Nair, P.K.R., Eds.; International Council for Research in Agroforestry: Nairobi, Kenya, 1987; pp. 205–223. [Google Scholar]
- Ong, C.K.; Corlett, J.E.; Singh, R.P.; Black, C.R. Above and below Ground Interactions in Agroforestry Systems. For. Ecol. Manag. 1991, 45, 45–57. [Google Scholar] [CrossRef]
- Ong, C.K.; Black, C.R.; Muthuri, C.W. Modifying Forestry and Agroforestry to Increase Water Productivity in the Semi-Arid Tropics. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006, 1, 1–19. [Google Scholar] [CrossRef]
- Anderson, S.H.; Udawatta, R.P.; Seobi, T.; Garrett, H.E. Soil Water Content and Infiltration in Agroforestry Buffer Strips. Agrofor. Syst. 2009, 75, 5–16. [Google Scholar] [CrossRef]
- Fritts, H. Tree Rings and Climate; Academic Press: London, UK, 1976; ISBN 0323145280. [Google Scholar]
- Lo, Y.-H.; Blanco, J.A.; Seely, B.; Welham, C.; Kimmins, J.P.H. Relationships between Climate and Tree Radial Growth in Interior British Columbia, Canada. For. Ecol. Manag. 2010, 259, 932–942. [Google Scholar] [CrossRef]
- Forde, B.; Lorenzo, H. The Nutritional Control of Root Development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Van Wijk, M.T.; Bouten, W. Towards Understanding Tree Root Profiles: Simulating Hydrologically Optimal Strategies for Root Distribution. Hydrol. Earth Syst. Sci. 2001, 5, 629–644. [Google Scholar] [CrossRef][Green Version]
- Schenk, H.J. Vertical Vegetation Structure below Ground: Scaling from Root to Globe. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2005; Volume 66, pp. 341–373. [Google Scholar]
- Laureano, R.G.; García-Nogales, A.; Seco, J.I.; Rodríguez, J.G.P.; Linares, J.C.; Martínez, F.; Merino, J. Growth and Maintenance Costs of Leaves and Roots in Two Populations of Quercus Ilex Native to Distinct Substrates. Plant Soil 2013, 363, 87–99. [Google Scholar] [CrossRef]
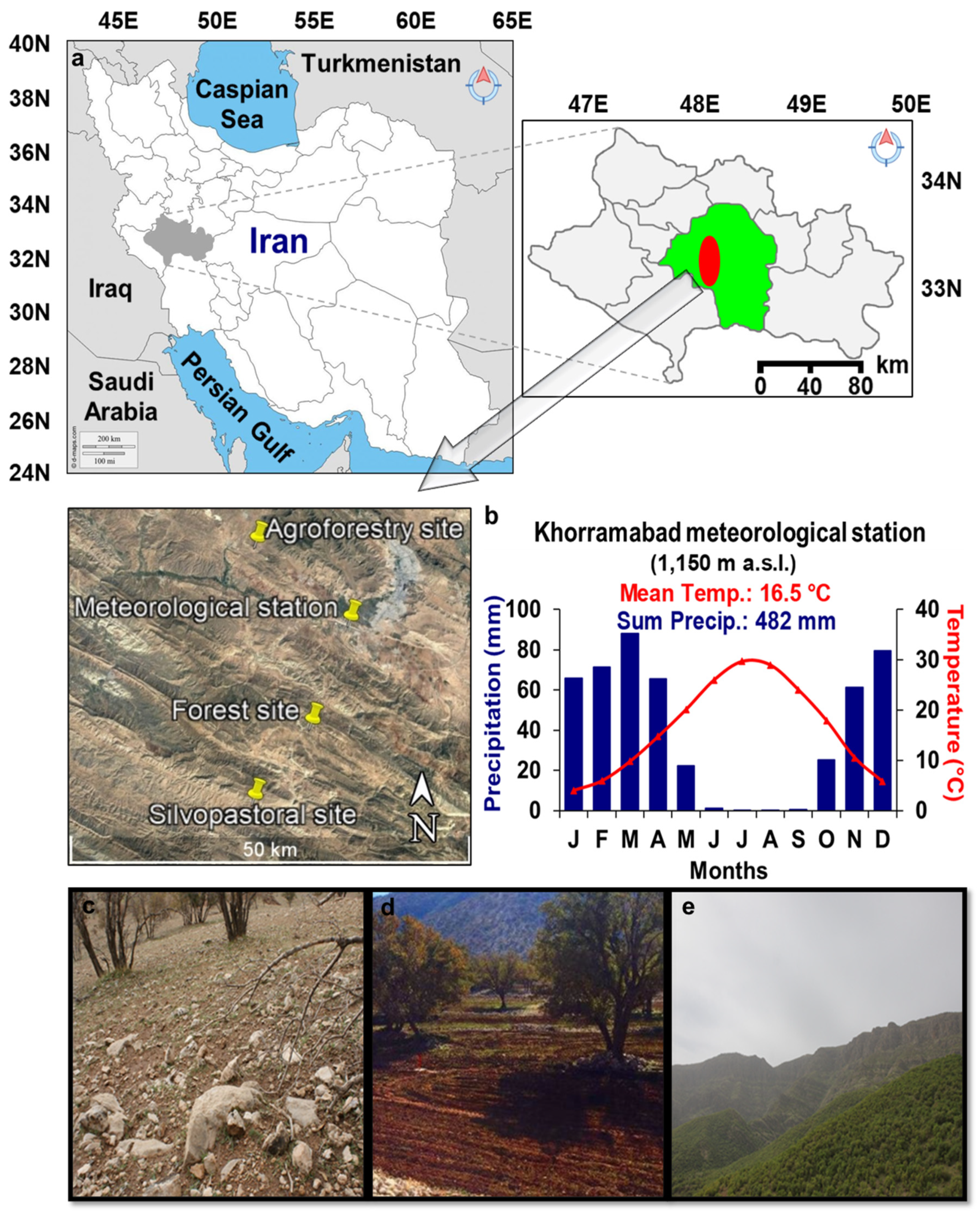
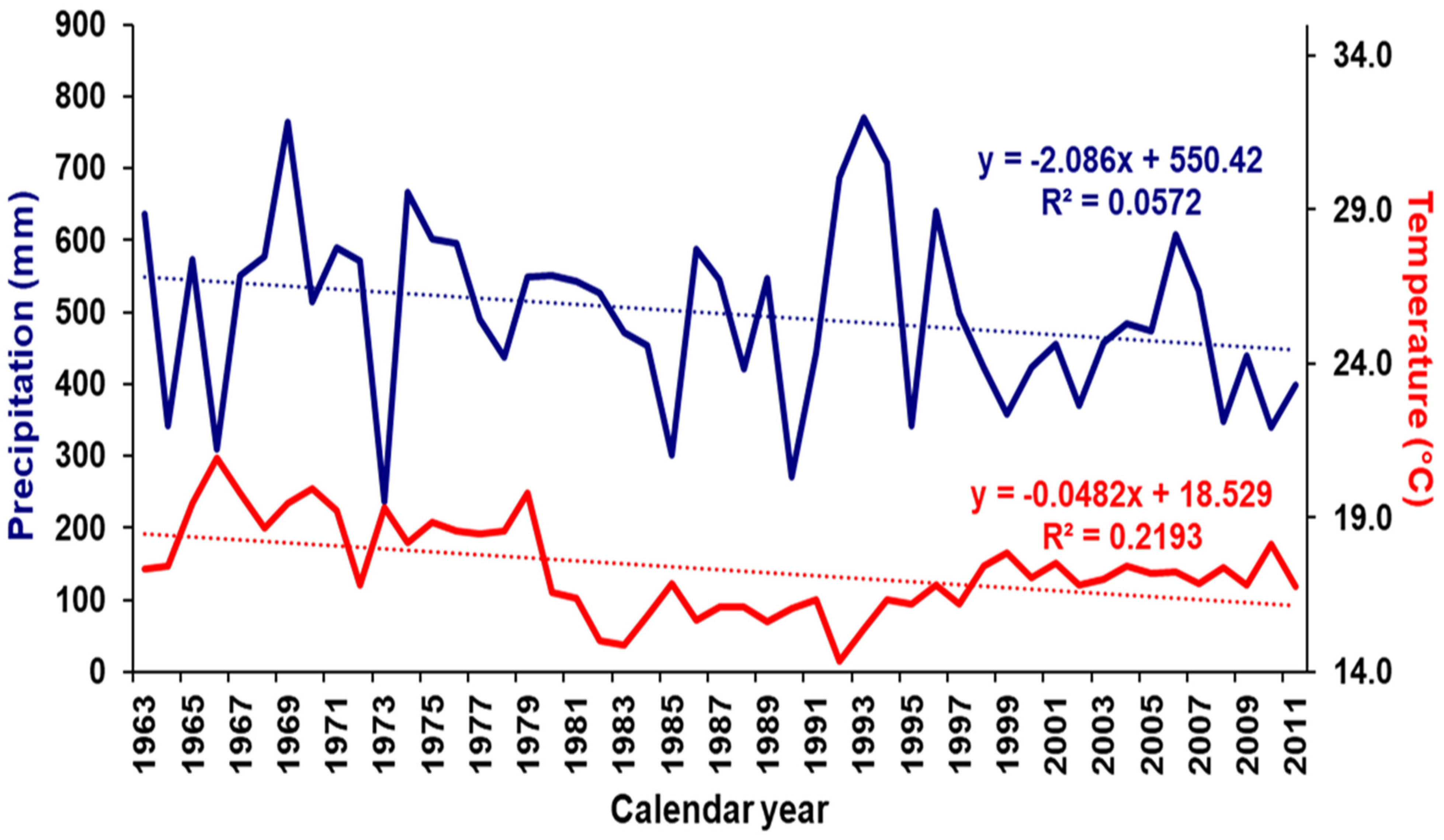
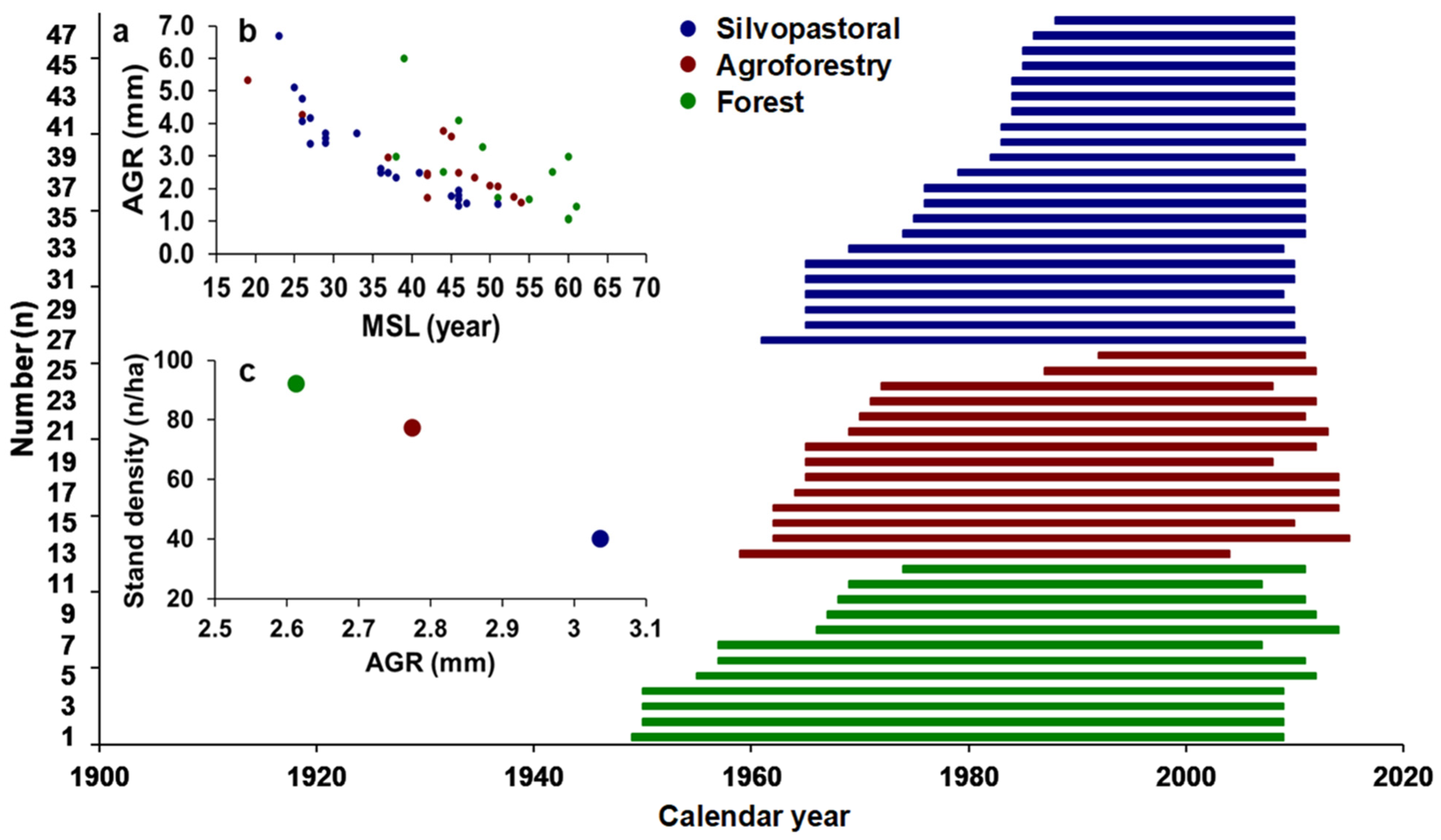
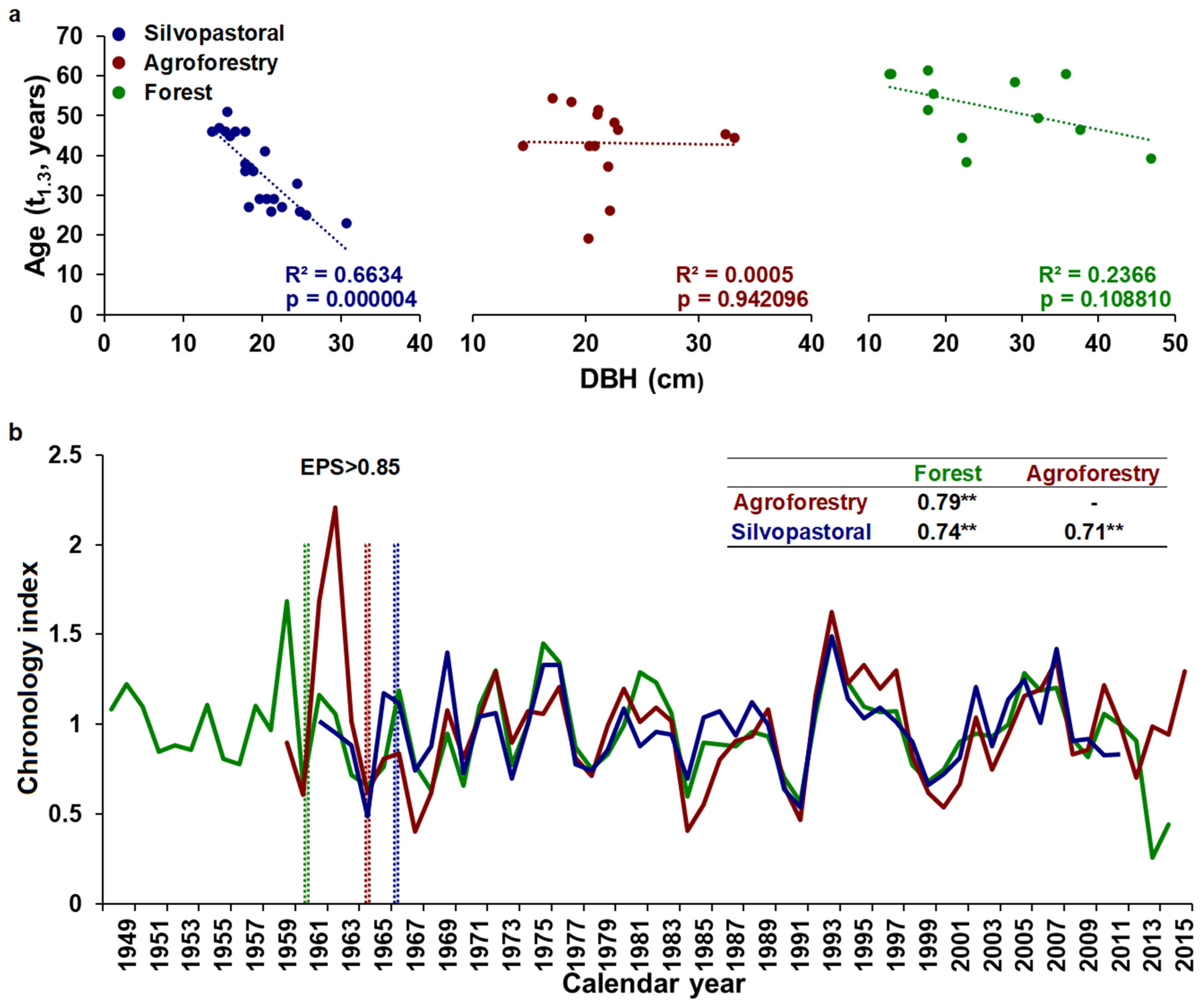
| Management Type | Site Name | Tree Density (n/ha) | Long. I | Lat. (N) | Elev. (m a.s.l) |
|---|---|---|---|---|---|
| Silvopastoral | Shahanshah | 40 | 48°09′02″ | 33°13′47″ | 1490 |
| Agroforestry | Mele-Shabanan | 77 | 48°09′16″ | 33°31′20″ | 1326 |
| Forest | Dadabad | 92 | 48°13′49″ | 33°19′07″ | 1704 |
| Management Type | Number of Trees | Number of Radii | Chronology Time Span | Length of Chronology | MSL (Years) | AGR (mm/Year) | Min AGR | Max AGR | MS | Rbar |
|---|---|---|---|---|---|---|---|---|---|---|
| Silvopastoral | 22 | 64 | 1961–2011 | 51 | 36 | 3.05 | 1.49 | 6.68 | 0.342 | 0.595 |
| Agroforestry | 14 | 40 | 1959–2015 | 57 | 43 | 2.77 | 1.57 | 5.32 | 0.361 | 0.593 |
| Forest | 12 | 37 | 1948–2014 | 67 | 52 | 2.61 | 1.05 | 6.01 | 0.325 | 0.561 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safari, E.; Moradi, H.; Seim, A.; Yousefpour, R.; Mirzakhani, M.; Tegel, W.; Soosani, J.; Kahle, H.-P. Regional Drought Conditions Control Quercus brantii Lindl. Growth within Contrasting Forest Stands in the Central Zagros Mountains, Iran. Forests 2022, 13, 495. https://doi.org/10.3390/f13040495
Safari E, Moradi H, Seim A, Yousefpour R, Mirzakhani M, Tegel W, Soosani J, Kahle H-P. Regional Drought Conditions Control Quercus brantii Lindl. Growth within Contrasting Forest Stands in the Central Zagros Mountains, Iran. Forests. 2022; 13(4):495. https://doi.org/10.3390/f13040495
Chicago/Turabian StyleSafari, Ehsan, Hossein Moradi, Andrea Seim, Rasoul Yousefpour, Mahsa Mirzakhani, Willy Tegel, Javad Soosani, and Hans-Peter Kahle. 2022. "Regional Drought Conditions Control Quercus brantii Lindl. Growth within Contrasting Forest Stands in the Central Zagros Mountains, Iran" Forests 13, no. 4: 495. https://doi.org/10.3390/f13040495
APA StyleSafari, E., Moradi, H., Seim, A., Yousefpour, R., Mirzakhani, M., Tegel, W., Soosani, J., & Kahle, H.-P. (2022). Regional Drought Conditions Control Quercus brantii Lindl. Growth within Contrasting Forest Stands in the Central Zagros Mountains, Iran. Forests, 13(4), 495. https://doi.org/10.3390/f13040495







