Diagnostic and Therapeutic Potential for HNP-1, HBD-1 and HBD-4 in Pregnant Women with COVID-19
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
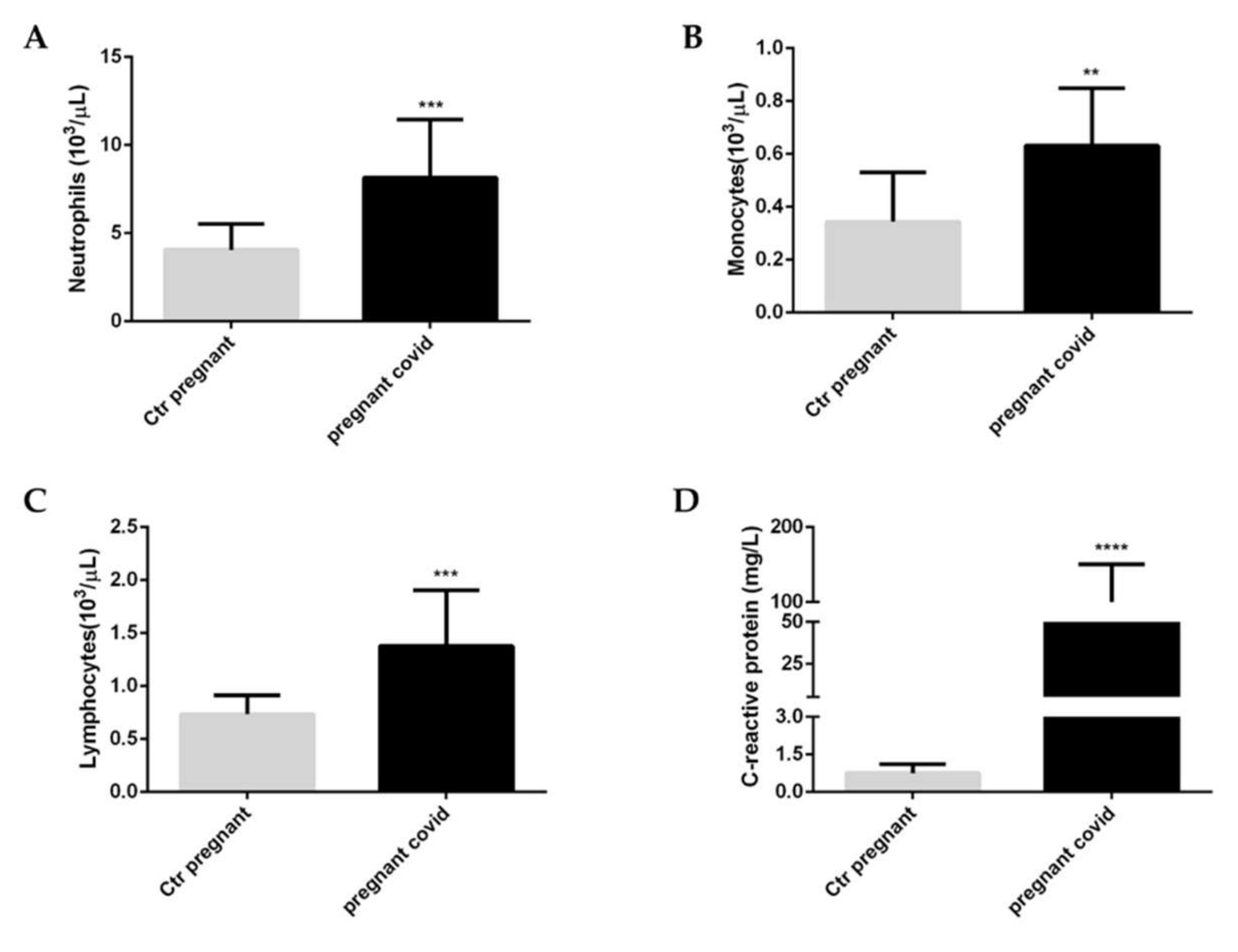
2.2. Effects of COVID-19 on the White Blood Cells and C-Reactive Protein in Pregnant Women
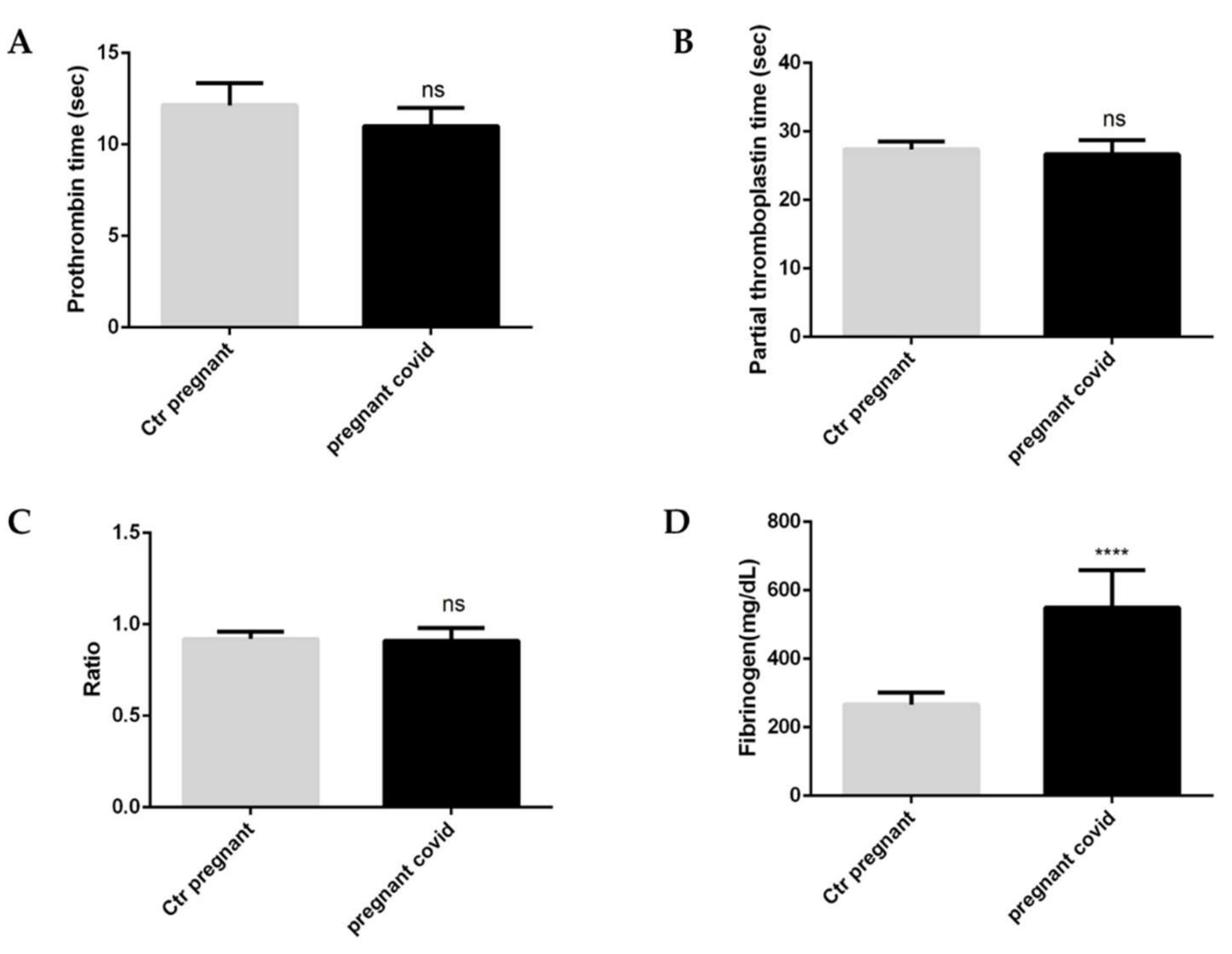
2.3. Evaluation of Coagulation Parameters in Pregnant Women with COVID-19
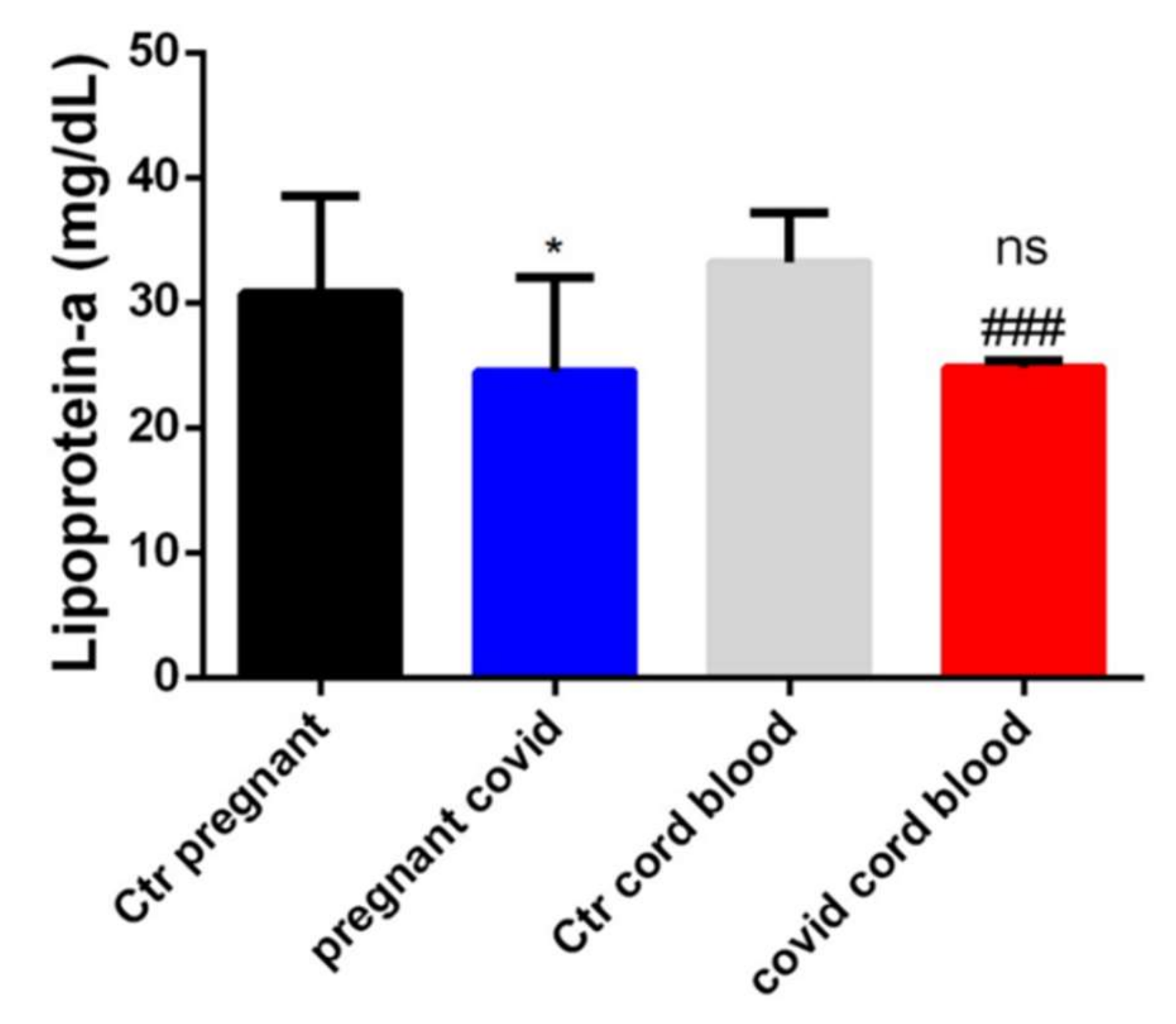
2.4. Dosage of Lipoprotein-a in Serum and Cord Blood of Mothers Affected by COVID-19
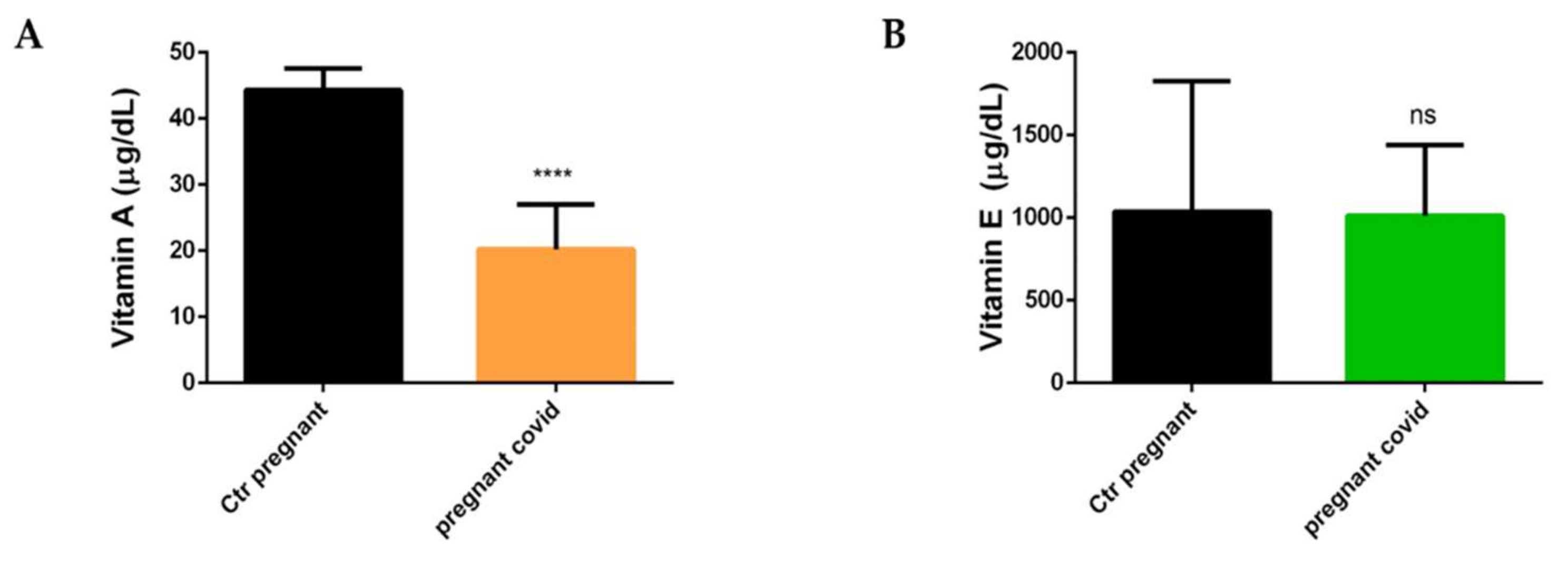
2.5. Measurements of Vitamin A and E in Pregnant Women with COVID-19
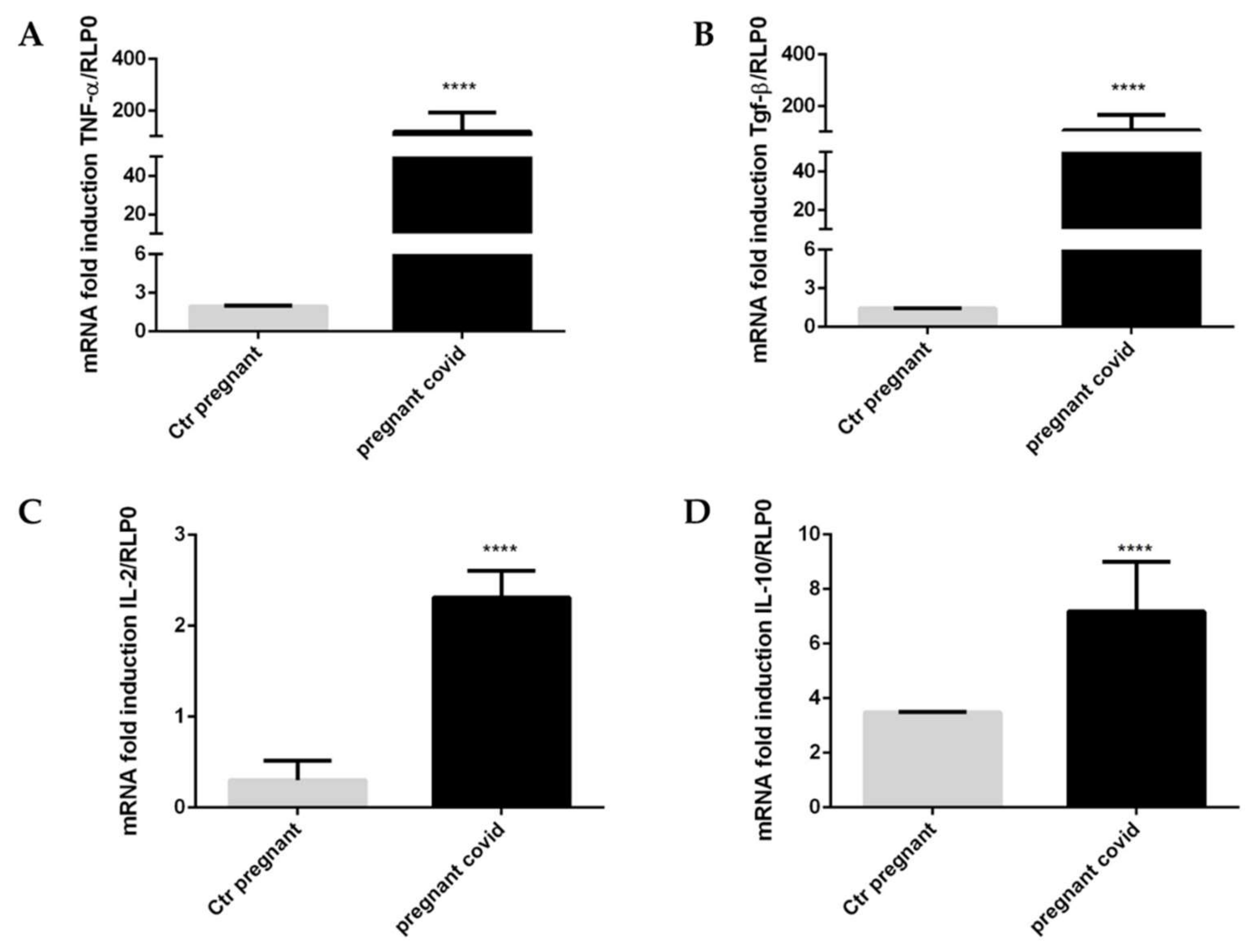
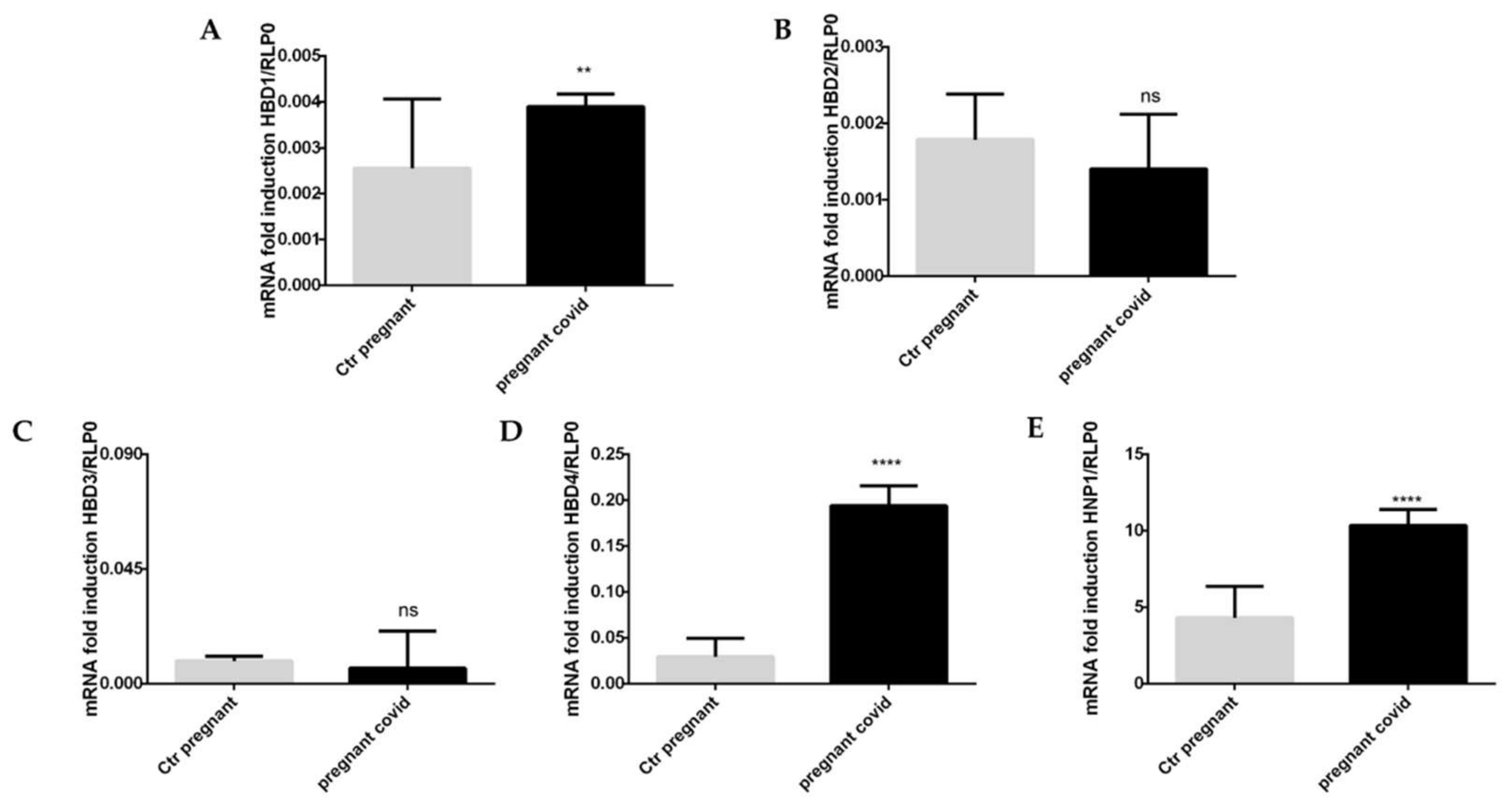
2.6. Assessment of COVID-19 on the Gene Expression of Cytokines and Defensins in Pregnant Women
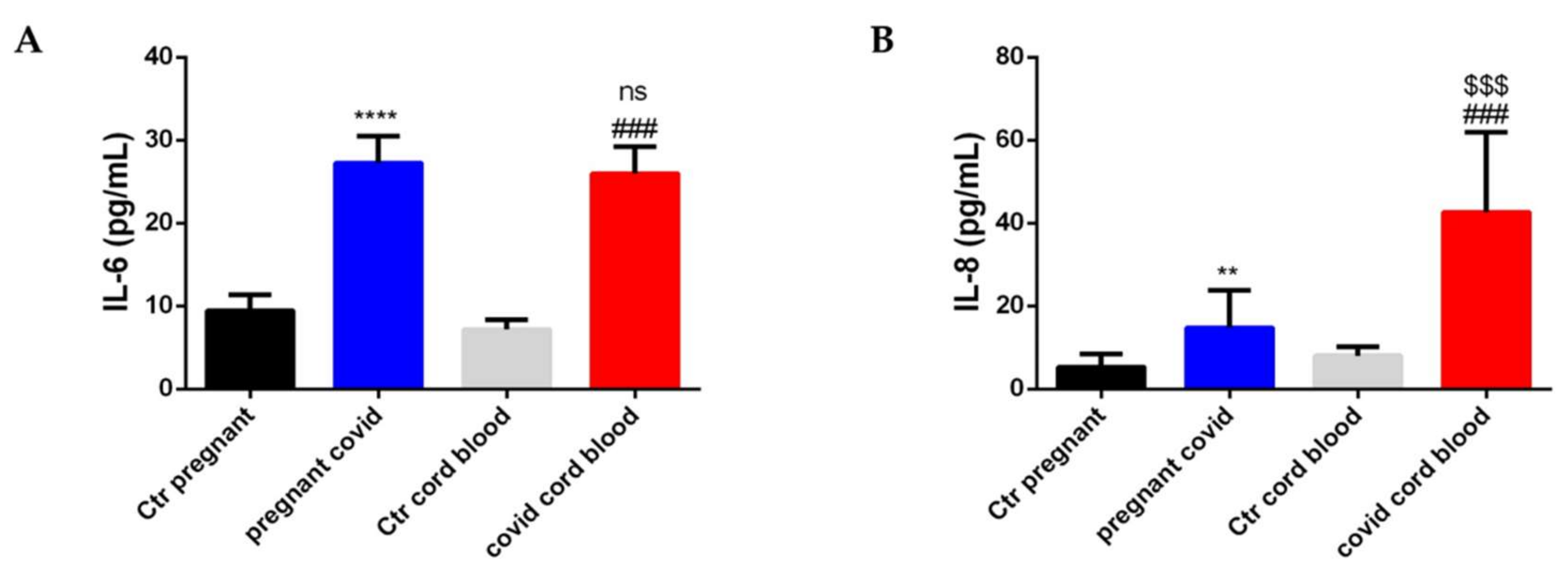
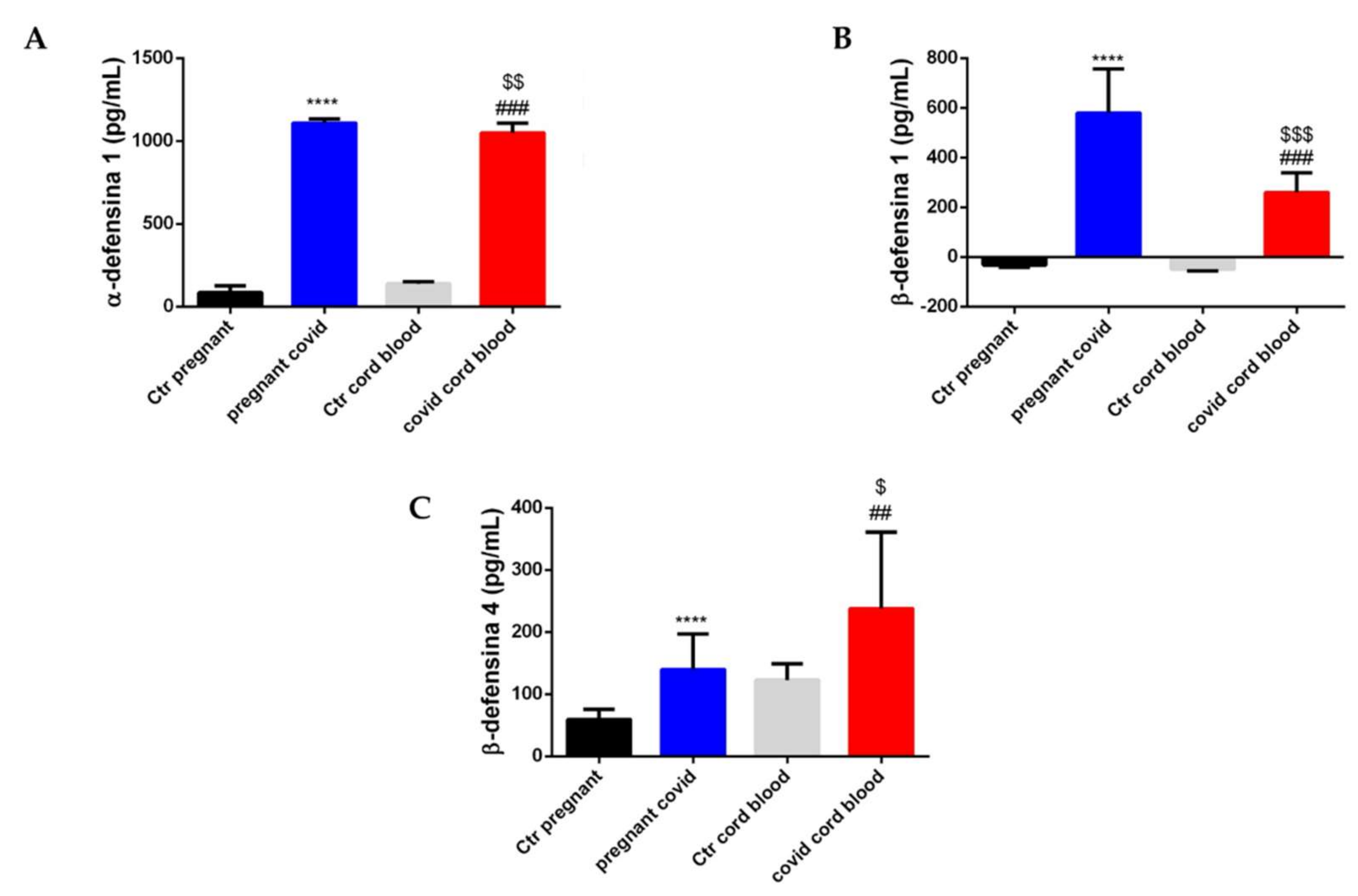
2.7. Estimation of Cytokine and Defensin Levels in Serum and Cord Blood of Mothers Affected by COVID-19
2.8. Physical Characteristics of the Newborns
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Study Design and Study Population
4.3. Samples Collection
4.4. Biochemical Determinations
4.5. Elisa Assay
4.6. RNA Extraction and cDNA Synthesis
4.7. Gene Expression by Real-Time qPCR
4.8. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen, S.F.; Ho, Y.C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Scudiero, O.; Lombardo, B.; Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Gentile, L.; Moscarella, E.; Amodio, F.; Ranieri, A.; et al. Exercise, Immune System, Nutrition, Respiratory and Cardiovascular Diseases during COVID-19: A Complex Combination. Int. J. Environ. Res. Public Health 2021, 18, 904. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2020, 137, 155323. [Google Scholar] [CrossRef]
- Laneri, S.; Brancaccio, M.; Mennitti, C.; De Biasi, M.G.; Pero, M.E.; Pisanelli, G.; Scudiero, O.; Pero, R. Antimicrobial Peptides and Physical Activity: A Great Hope against COVID 19. Microorganisms 2021, 9, 1415. [Google Scholar] [CrossRef]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human antimicrobial peptides as therapeutics for viral infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef] [Green Version]
- Doss, M.; White, M.R.; Tecle, T.; Hartshorn, K.L. Human defensins and LL-37 in mucosalimmunity. J. Leukoc. Biol. 2010, 87, 79–92. [Google Scholar] [CrossRef]
- Pero, R.; Brancaccio, M.; Laneri, S.; Biasi, M.G.; Lombardo, B.; Scudiero, O. A Novel View of Human Helicobacter pylori Infections: Interplay between Microbiota and Beta-Defensins. Biomolecules 2019, 9, 237. [Google Scholar] [CrossRef] [Green Version]
- Pero, R.; Angrisano, T.; Brancaccio, M.; Falanga, A.; Lombardi, L.; Natale, F.; Laneri, S.; Lombardo, B.; Galdiero, S.; Scudiero, O. Beta-defensins and analogs in Helicobacter pylori infections: mRNA expression levels, DNA methylation, and antibacterial activity. PLoS ONE 2019, 14, e0222295. [Google Scholar] [CrossRef] [Green Version]
- Pero, R.; Brancaccio, M.; Mennitti, C.; Gentile, L.; Franco, A.; Laneri, S.; De Biasi, M.G.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. HNP-1 and HBD-1 as Biomarkers for the Immune Systems of Elite Basketball Athletes. Antibiotics 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Brancaccio, M.; Mennitti, C.; Laneri, S.; Lombardo, B.; De Biasi, M.G.; De Gregorio, E.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. Human Defensins: A Novel Approach in the Fight against Skin Colonizing Staphylococcus aureus. Antibiotics 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudryashova, E.; Zani, A.; Vilmen, G.; Sharma, A.; Lu, W.; Yount, J.S.; Kudryashov, D.S. Inhibition of SARS-CoV-2 Infection by Human Defensin HNP1 and Retrocyclin RC-101. J. Mol. Biol. 2021, 434, 167225. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Ren, Y.; Gao, S.; Zhang, L. High level of defensin alpha 5 in intestine may explain the low incidence of diarrhoea in COVID-19 patients. Eur. J. Gastroenterol. Hepatol. 2020, 34, e3–e4. [Google Scholar] [CrossRef]
- Zhang, L.; Ghosh, S.K.; Basavarajappa, S.C.; Muller-Greven, J.; Penfield, J.; Breweer, A.; Ramakrishnan, P.; Buck, M.; Weinberg, A. Molecular dynamics simulations and functional studies reveal that hBD-2 binds SARS-CoV-2 spike RBD and blocks viral entry into ACE2 expressing cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kammerer, U.; von Wolff, M.; Markert, U.R. Immunology of human endometrium. Immunobiology 2004, 209, 569–574. [Google Scholar] [CrossRef]
- Thellin, O.; Coumans, B.; Zorzi, W.; Igout, A.; Heinen, E. Tolerance to the foeto-placental ‘graft’: Ten ways to support a child for nine months. Curr. Opin. Immunol. 2000, 12, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Hein, M.; Valore, E.V.; Helmig, R.B.; Uldbjerg, N.; Ganz, T. Antimicrobial factors in the cervical mucus plug. Am. J. Obstet. Gynecol. 2002, 187, 137–144. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, I.; Lehrer, R.I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996, 396, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Soto, E.; Espinoza, J.; Nien, J.K.; Kusanovic, J.P.; Erez, O.; Richani, K.; Santolaya-Forgas, J.; Chief, R.R. Human beta-defensin-2: A natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J. Matern. Fetal Neonatal Med. 2007, 20, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.K.; Gerken, T.A.; Schneider, K.M.; Feng, Z.; McCormick, T.S.; Weinberg, A. Quantification of human beta-defensin-2 and 3 in body fluids: Application forstudies of innate immunity. Clin. Chem. 2007, 53, 757–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.R.; Krause, A.; Schulz, S.; Rodriguez-Jimenez, F.J.; Kluver, E.; Adermann, K.; Forssmann, U.; Frimpong-Boateng, A.; Bals, R.; Forssmann, W.G. Human beta-defensin 4: A novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001, 15, 1819–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Nagase, T.; Makita, R.; Fukuhara, S.; Tomita, T.; Tominaga, T.; Kurihara, H.; Ouchi, Y. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J. Immunol. 2002, 169, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef] [Green Version]
- Erol, S.A.; Tanacan, A.; Anuk, A.T.; Tokalioglu, E.O.; Biriken, D.; Keskin, H.L.; Moraloglu, O.T.; Yazihan, N.; Sahin, D. Evaluation of maternal serum afamin and vitamin E levels in pregnant women with COVID-19 and its association with composite adverse perinatal outcomes. J. Med. Virol. 2021, 93, 2350–2358. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Vano, M.; Gargiulo, B.; Caiazza, M.; Amodio, F.; Coto, I.; D’Alicandro, G.; et al. The Biological Role of Vitamins in Athletes’ Muscle, Heart and Microbiota. Int. J. Environ. Res. Public Health 2022, 19, 1249. [Google Scholar] [CrossRef]
- Nawsherwan Khan, S.; Zeb, F.; Shoaib, M.; Nabi, G.; Ul Haq, I.; Xu, K.; Li, H. Selected Micronutrients: An Option to Boost Immunity against COVID-19 and Prevent Adverse Pregnancy Outcomes in Pregnant Women: A Narrative Review. Iran. J. Public Health 2020, 49, 2032–2043. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 202: Gestational hypertension and preeclampsia. Obs. Gynecol. 2019, 133, 1. [Google Scholar]
- Tanacan, A.; Yazihan, N.; Erol, S.A.; Anuk, A.T.; Yetiskin, F.D.Y.; Biriken, D.; Ozgu-Erdinc, A.S.; Keskin, H.L.; Tekin, O.M.; Sahin, D. The impact of COVID-19 infection on the cytokine profile of pregnant women: A prospective case-control study. Cytokine 2021, 140, 155431. [Google Scholar] [CrossRef]
- Matsunaga, S.; Takai, Y.; Seki, H. Fibrinogen for the management of critical obstetric hemorrhage. J. Obs. Gynaecol. Res. 2019, 45, 13–21. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.G.; et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009, 4, 412–423. [Google Scholar]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos Maia, S.; Rolland Souza, A.S.; Costa Caminha, M.D.F.; Lins da Silva, S.; Callou Cruz, R.D.S.B.L.; Carvalho dos Santos, C.; Batista Filho, M. Vitamin A and Pregnancy: A Narrative Review. Nutrients 2019, 11, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, 157–165. [Google Scholar]
- Timircan, M.; Bratosin, F.; Vidican, I.; Suciu, O.; Tirnea, L.; Avram, V.; Marincu, I. Exploring Pregnancy Outcomes Associated with SARS-CoV-2 Infection. Medicina 2021, 8, 796. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef] [Green Version]
- Robertson, S.A.; Care, A.S.; Skinner, R.J. Interleukin 10 Regulates Inflammatory Cytokine Synthesis to Protect Against Lipopolysaccharide-Induced Abortion and Fetal Growth Restriction in Mice. Biol. Reprod. 2007, 76, 738–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szarka, A.; Rigò, J., Jr.; Lazar, R.; Beko, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Dai, F.; Yuan, M.; Zheng, Y.; Liu, S.; Deng, Z.; Tan, W.; Chen, L.; Zhang, Q.; Zhao, X.; et al. Role of Transforming Growth Factor-β1 in Regulating Fetal-Maternal Immune Tolerance in Normal and Pathological Pregnancy. Front Immunol. 2021, 12, 689181. [Google Scholar] [CrossRef]
- Feng, Y.; Pan, X.; Huang, N.; Feng, Y.; Wu, Q.; Wang, B. The human beta-defensins expression in female genital tract and pregnancy-related tissues. Sichuan Da Xue Xue Bao Yi Xue Ban 2003, 34, 217–219. [Google Scholar]
- Ryan, L.K.; Diamond, G. Modulation of Human β-Defensin-1 Production by Viruses. Viruses 2017, 9, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okazaki, T.; Ota, Y.; Yuki, N.; Hayashida, A.; Shoda, A.; Nishikawa, M.; Oshima, K.; Fukasawa, I.; Watanabe, H.; Inaba, N. Plasma levels of alpha-defensins 1–3 are an indicator of neutrophil activation in pregnant and post-partum women. J. Obs. Gynaecol. Res. 2007, 5, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin 6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bönig, H.; Packeisen, J.; Röhne, B.; Hempel, L.; Hannen, M.; Klein-Vehne, A.; Burdach, S.; Körholz, D. Interaction between interleukin 10 and interleukin 6 in human B-cell differentiation. Immunol. Investig. 1998, 27, 267–280. [Google Scholar] [CrossRef]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Hayward, A.R. The human fetus and newborn: Development of the immune response. Birth Defects Orig. Artic. Ser. 1983, 19, 289–294. [Google Scholar]
- McGovern, N.; Shin, A.; Low, G.; Low, D.; Duan, K.; Yao, L.J.; Msallam, R.; Low, I.; Shadan, N.B.; Sumatoh, H.R.; et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 2017, 546, 662–666. [Google Scholar] [CrossRef]
- Megli, C.J.; Coyne, C.B. Infections at the maternal–fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef]
- Silasi, M.; Cardenas, I.; Kwon, J.Y.; Racicot, K.; Aldo, P.; Mor, G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Perricone, C.; Triggianese, P.; Bursi, R.; Cafaro, G.; Bartoloni, E.; Chimenti, M.S.; Gerli, R.; Perricone, R. Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections. Microorganisms 2021, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, E.; Mazzocco, M.; La Torre, R.; Ligi, P.; Sali, E.; Nigro, G. Therapy or prevention of fetal infection by cytomegalovirus with immunoglobulin infusion in pregnant women with primary infection. Acta Biomed. Ateneo Parm. 2000, 71, 547–551. [Google Scholar]
- Steinmuller, D.R.; Novick, A.C.; Streem, S.B.; Graneto, D.; Swift, C. Intravenous immunoglobulin infusions for the prophylaxis of secondary cytomegalovirus infection. Transplantation 1990, 49, 68–70. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Pregnant Women with COVID-19 | Pregnant Women without COVID-19 | p Value |
|---|---|---|---|
| Maternal Age | 31.5 ± 6 years | 34 ± 4 years | 0.3348 |
| Pre-partumWweight | 67 ± 13 kg | 65 ± 11 kg | 0.5846 |
| Height | 162 ± 6 cm | 162.4 ± 5.6 cm | 0.8203 |
| Gestational Age | 39 ± 1.6 weeks | 40 ± 2 weeks | 0.0742 |
| Blood Pressure (120–80 mmHg) * | 117 ± 6.6 mmHg 77 ± 6.6 mmHg | 108 ± 12 mmHg 68 ± 10 mmHg | 0.0036 0.0010 |
| Saturation (O2) | 97 ± 1.5% | 97 ± 1.5% | >0.9999 |
| Characteristics | Children Born to COVID-Positive Mothers | Children Born to Non COVID-Positive Mothers | p Value |
|---|---|---|---|
| Weight | 3.45 ± 0.5 kg | 3.45 ± 0.3 kg | >0.9999 |
| Height | 50 ± 1.7 cm | 50.4 ± 1.3 cm | 0.3856 |
| Head Circumferences | 35 ± 1.5 cm | 35 ± 1 cm | >0.9999 |
| Gene | Accession Numbers |
|---|---|
| RLP0 | NM_053275.4 |
| TNF-α | NM_000594.4 |
| TGF-β | NM_000660.7 |
| IL-2 | NM_000586.4 |
| IL-10 | NM_001382624.1 |
| HBD1 | NM_005218.4 |
| HBD2 | NM_004942.4 |
| HBD3 | NM_001081551.4 |
| HBD4 | NC_000008.11 |
| HNP1 | NM_004084.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancaccio, M.; Mennitti, C.; Calvanese, M.; Gentile, A.; Musto, R.; Gaudiello, G.; Scamardella, G.; Terracciano, D.; Frisso, G.; Pero, R.; et al. Diagnostic and Therapeutic Potential for HNP-1, HBD-1 and HBD-4 in Pregnant Women with COVID-19. Int. J. Mol. Sci. 2022, 23, 3450. https://doi.org/10.3390/ijms23073450
Brancaccio M, Mennitti C, Calvanese M, Gentile A, Musto R, Gaudiello G, Scamardella G, Terracciano D, Frisso G, Pero R, et al. Diagnostic and Therapeutic Potential for HNP-1, HBD-1 and HBD-4 in Pregnant Women with COVID-19. International Journal of Molecular Sciences. 2022; 23(7):3450. https://doi.org/10.3390/ijms23073450
Chicago/Turabian StyleBrancaccio, Mariarita, Cristina Mennitti, Mariella Calvanese, Alessandro Gentile, Roberta Musto, Giulia Gaudiello, Giulia Scamardella, Daniela Terracciano, Giulia Frisso, Raffaela Pero, and et al. 2022. "Diagnostic and Therapeutic Potential for HNP-1, HBD-1 and HBD-4 in Pregnant Women with COVID-19" International Journal of Molecular Sciences 23, no. 7: 3450. https://doi.org/10.3390/ijms23073450
APA StyleBrancaccio, M., Mennitti, C., Calvanese, M., Gentile, A., Musto, R., Gaudiello, G., Scamardella, G., Terracciano, D., Frisso, G., Pero, R., Sarno, L., Guida, M., & Scudiero, O. (2022). Diagnostic and Therapeutic Potential for HNP-1, HBD-1 and HBD-4 in Pregnant Women with COVID-19. International Journal of Molecular Sciences, 23(7), 3450. https://doi.org/10.3390/ijms23073450