Phytochemical Characterization, and Antioxidant and Antimicrobial Properties of Agitated Cultures of Three Rue Species: Ruta chalepensis, Ruta corsica, and Ruta graveolens
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Cultures
2.2. High-Performance Liquid Chromatography Analyses
2.3. Antioxidant Activity
2.3.1. Free Radical Scavenging Activity
2.3.2. Reducing Power Assay
2.3.3. Ferrous Ions (Fe2+) Chelating Activity
2.4. Antimicrobial Activity
2.5. Statistical Analysis
3. Results and Discussion
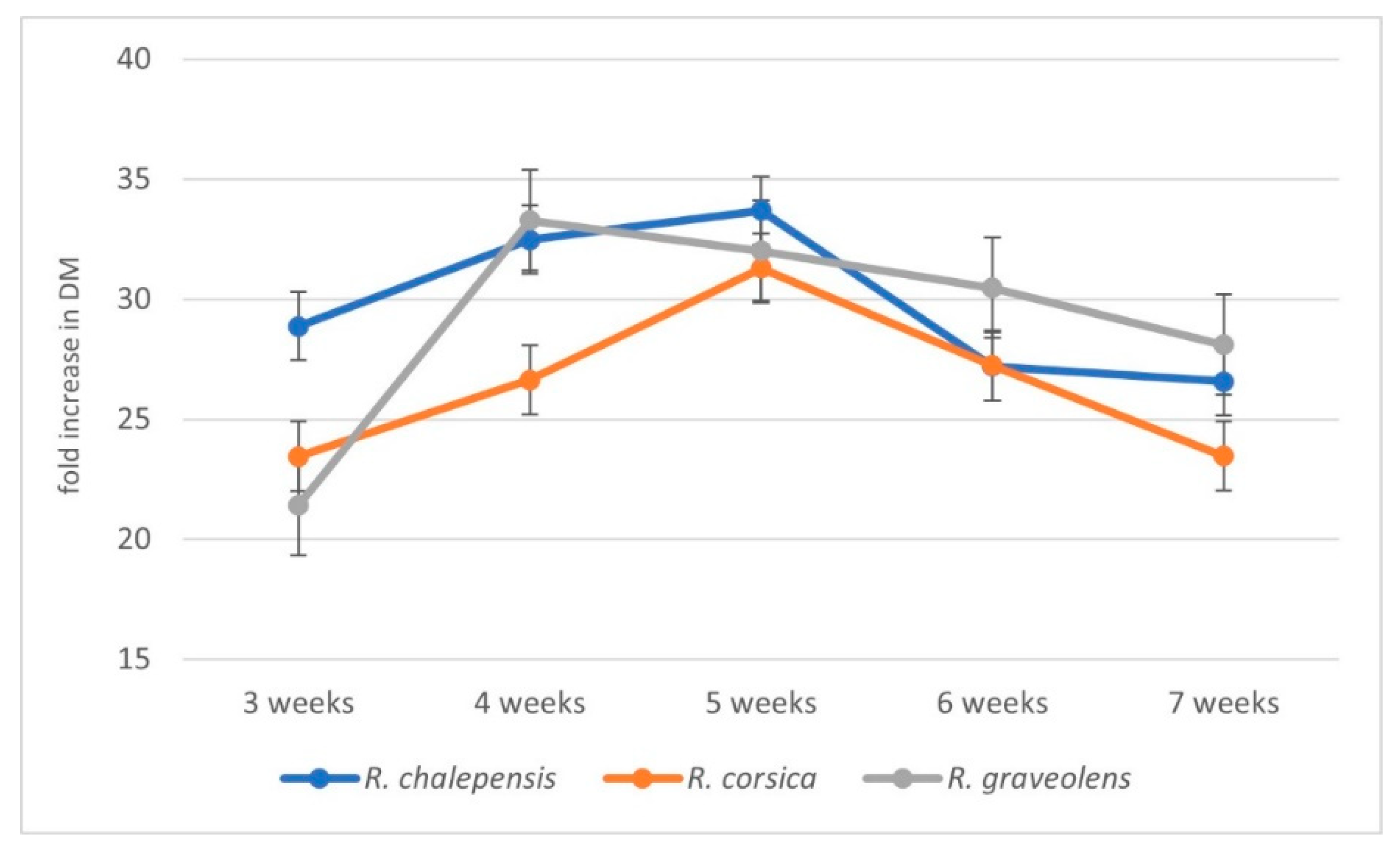
3.1. Biomass Increments
3.2. Accumulation of Bioactive Metabolites
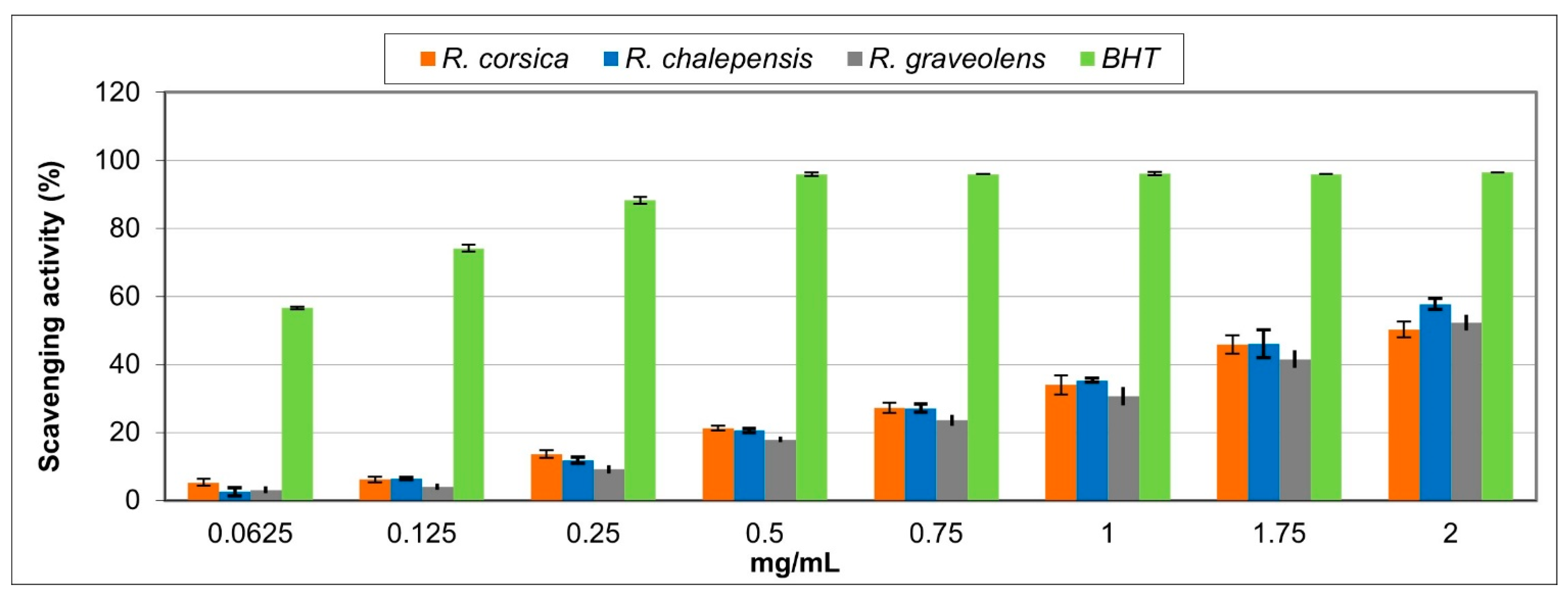
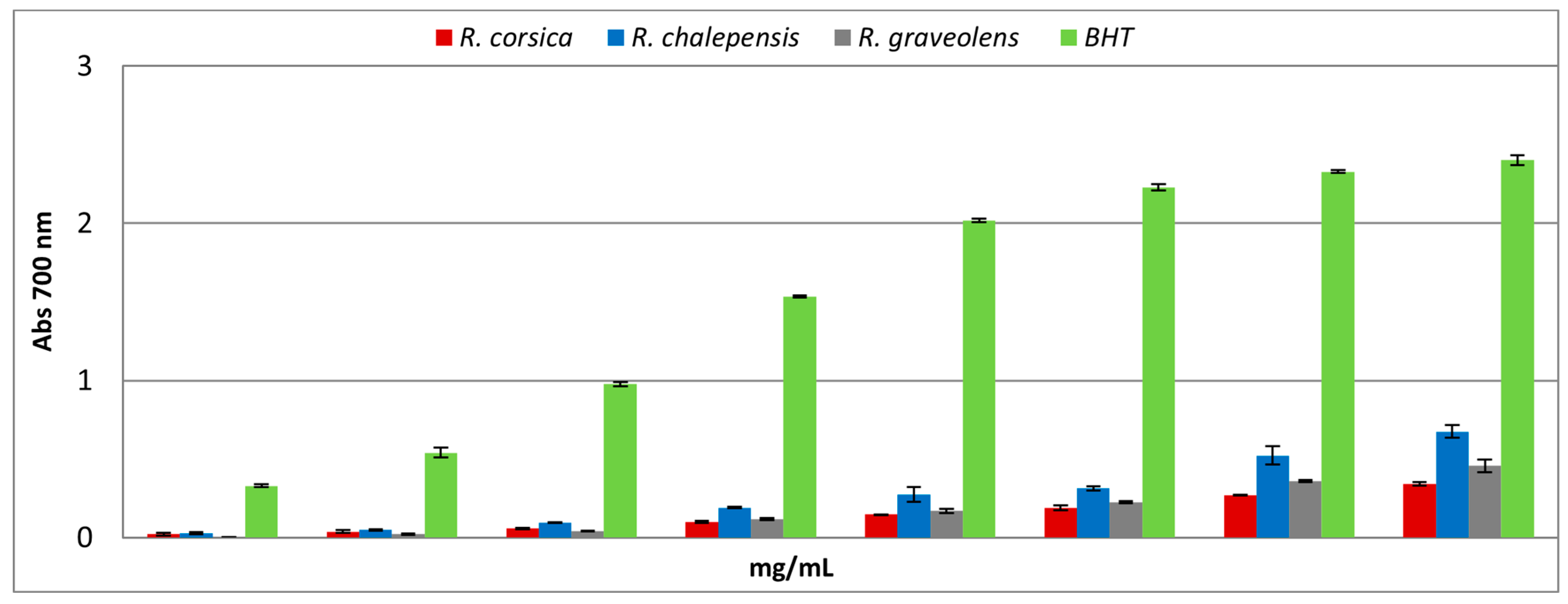
3.3. Antioxidant Activity
3.4. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engler, A. Syllabus Der Pflanzenfamilien, 12th ed.; Gebruder Borntraeger Verlag: Berlin, Germany, 1964. [Google Scholar]
- Meloni, M.; Dettori, C.A.; Reid, A.; Bacchetta, G.; Hugot, L.; Conti, E. High genetic diversity and presence of genetic structure characterise the endemics Ruta corsica and Ruta lamarmorae (Rutaceae). Caryologia 2020, 73, 11–26. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A. Flora Europea; University Press Cambridge: Cambridge, UK, 1968; p. 2. [Google Scholar]
- Hammiche, V.; Azzouz, M. Les rues: Ethnobotanique, phytopharmacologie et toxicité. Phytotherapie 2013, 11, 22–30. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Khoshkam, R. Phytochemistry and pharmacological properties of Ruta graveolens L. J. Med. Plants Res. 2012, 6, 3942–3949. [Google Scholar] [CrossRef]
- Kostova, I.; Ivanova, A.; Mikhova, B.; Klaiber, I. Alkaloids and Coumarins from Ruta graveolens. Mon. Für Chem. 1999, 130, 703–707. [Google Scholar] [CrossRef]
- Smolarz, H.D.; Sokołowska-Woźniak, A.; Zgórka, G. Phenolic acids from herb of Ruta graveolens L. Acta Pol. Pharm. Drugs Res. 1997, 54, 161–163. [Google Scholar]
- Ben Hadj Fredj, B.; Marzouk, B.; Chraief, I. Analysis of Tunisian Ruta graveolens L. oils from Jemmel. J. Food Agric. Environ. 2007, 5, 52–55. [Google Scholar]
- Giresha, A.S.; Anitha, M.G.; Dharmappa, K.K. (2015). Phytochemical composition, antioxidant and in-vitro anti-inflammatory activity of ethanol extract of Ruta graveolens L. leaves. Int J Pharm Pharm Sci 2015, 7, 272–276. [Google Scholar]
- Pollio, A.; De Natale, A.; Appetiti, E.; Aliotta, G.; Touwaide, A. Continuity and change in the Mediterranean medical tradition: Ruta spp. (rutaceae) in Hippocratic medicine and present practices. J. Ethnopharmacol. 2008, 116, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Roelandts, R. Photo(chemo) therapy for vitiligo. Photodermatol. Photoimmunol. Photomed. 2003, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. Psoralen-ultraviolet A endures as one of the most powerful treatments in dermatology: Reinforcement of this ‘triple-product therapy’ by the 2016 British guidelines. Br. J. Dermatol. 2016, 174, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, K.; Savci, S. Phytochemical studies on Ruta chalepensis (Lam.) Lamarck. Nat. Prod. Res. 2005, 19, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Kacem, M.; Kacem, I.; Simon, G.; Ben Mansour, A.; Chaabouni, S.; Elfeki, A.; Bouaziz, M. Phytochemicals and biological activities of Ruta chalepensis L. growing in Tunisia. Food Biosci. 2015, 12, 73–83. [Google Scholar] [CrossRef]
- Mejri, J.; Abderrabba, M.; Mejri, M. Chemical composition of the essential oil of Ruta chalepensis L: Influence of drying, hydro-distillation duration and plant parts. Ind. Crop. Prod. 2010, 32, 671–673. [Google Scholar] [CrossRef]
- Bertrand, C.; Fabre, N.; Moulis, C. A new coumarin glucoside, coumarins and alkaloids from Ruta corsica roots. Fitoterapia 2004, 75, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; Fabre, N.; Moulis, C.; Bessiere, J.-M. Composition of the Essentials Oil of Ruta Corsica DC. J. Essent. Oil Res. 2003, 15, 98–99. [Google Scholar] [CrossRef]
- Koblovská, R.; Mackova, Z.; Vitkova, M.; Kokoska, L.; Klejdus, B.; Lapcik, O. Isoflavones in the Rutaceae family: Twenty selected representatives of the genera Citrus, Fortunella, Poncirus, Ruta and Severinia. Phytochem. Anal. 2008, 19, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Chołoniewska, M.; Gomółka, E. Accumulation of furanocoumarins in Ruta graveolens L. shoot culture. Biotechnol. Lett. 2001, 23, 543–545. [Google Scholar] [CrossRef]
- Ekiert, H.; Czygan, F.-C. Accumulation of biologically active furanocoumarins in agitated cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) Gams. Die Pharm. 2005, 60, 623–626. [Google Scholar]
- Ekiert, H.; Abou-Mandour, A.A.; Czygan, F.C. Accumulation of biologically active furanocoumarins in Ruta graveolens ssp. divaricata (Tenore) Gams in vitro culture. Die Pharm. 2005, 60, 66–68. [Google Scholar]
- Ekiert, H.; Szewczyk, A.; Kus, A. Free phenolic acids in Ruta graveolens L. in vitro culture. Pharmazie 2009, 64, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Piekoszewska, A.; Muszyńska, B.; Baczyńska, S. Accumulation of p-coumaric acid and other bioactive phenolic acids in in vitro culture of Ruta graveolens ssp. divaricata (Tenore) Gams. MIR 2014, 26, 24–30. [Google Scholar]
- Szopa, A.; Ekiert, H.; Szewczyk, A.; Fugas, E. Production of bioactive phenolic acids and furanocoumarins in in vitro cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) Gams. under different light conditions. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 110, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Baumert, A.; Gröger, D.; Kuzovkina, I.N.; Reisch, J. Secondary metabolites produced by callus cultures of various Ruta species. Plant Cell Tissue Organ Cult. 1992, 28, 159–162. [Google Scholar] [CrossRef]
- Fischer, H.; Römer, A.; Ulbrich, B.; Arens, H. A New Biscoumarin Glucoside Ester fromRuta ChalepensisCell Cultures. Planta Medica 1988, 54, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Linsmaier, E.M.; Skoog, F. Organic Growth Factor Requirements of Tobacco Tissue Cultures. Physiol. Plant 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Maślanka, A.; Szewczyk, A.; Muszyńska, B. Physiologically Active Compounds in Four Species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, M.; Morishita, H.; Iwahashi, H.; Toda, S.; Shirataki, Y.; Kimura, M.; Kido, R. Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. Phytochemistry 1994, 36, 579–583. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Standard M27-A3; Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. CLSI: Wayne, PA, USA, 2008.
- Standard M07-Ed11; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. CLSI: Wayne, PA, USA, 2018.
- Marino, A.; Nostro, A.; Mandras, N.; Roana, J.; Ginestra, G.; Miceli, N.; Taviano, M.F.; Gelmini, F.; Beretta, G.; Tullio, V. Evaluation of antimicrobial activity of the hydrolate of Coridothymus capitatus (L.) Reichenb. fil. (Lamiaceae) alone and in combination with antimicrobial agents. BMC Complement. Med. Ther. 2020, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Kisiel, W. Coumarins and alkaloids in shoot culture of Ruta graveolens L. Acta Soc. Bot. Pol. 1997, 66, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Milesi, S.; Massot, B.; Gontier, E.; Bourgaud, F.; Guckert, A. Ruta graveolens L.: A promising species for the production of furanocoumarins. Plant Sci. 2001, 161, 189–199. [Google Scholar] [CrossRef]
- Adamska-Szewczyk, A.; Głowniak, K.; Baj, T. Furochinoline alkaloids in plants from Rutaceae family—A review. Curr. Issues Pharm. Med. Sci. 2016, 29, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Gali, L.; Bedjou, F. Antioxidant and anticholinesterase effects of the ethanol extract, ethanol extract fractions and total alkaloids from the cultivated Ruta chalepensis. S. Afr. J. Bot. 2018, 120, 163–169. [Google Scholar] [CrossRef]
- Fakhfakh, N.; Zouari, S.; Zouari, M.; Loussayef, C.; Zouari, N. Chemical composition of volatile compounds and antioxidant activities of essential oil, aqueous and ethanol extracts of wild Tunisian Ruta chalepensis L. (Rutaceae). J. Med. Plants Res. 2012, 6, 593–600. [Google Scholar]
- Ereifej, K.I.; Feng, H.; Rababah, T.; Almajwal, A.; Alu’Datt, M.; Gammoh, S.I.; Oweis, L.I. Chemical Composition, Phenolics, Anthocyanins Concentration and Antioxidant Activity of Ten Wild Edible Plants. Food Nutr. Sci. 2015, 06, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Rached, W.; Benamar, H.; Bennaceur, M.; Marouf, A. Screening of the Antioxidant Potential of Some Algerian Indigenous Plants. J. Biol. Sci. 2010, 10, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Falco, T.; Bonesi, M.; Sicari, V.; Tundis, R.; Bruno, M. Ruta chalepensis L. (Rutaceae) leaf extract: Chemical composition, antioxidant and hypoglicaemic activities. Nat. Prod. Res. 2018, 32, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, S.M.; Saleem, M.S.; Al-humaidi, J.G. Phytochemical contents and biological evaluation of Ruta chalepensis L. growing in Saudi Arabia. Saudi Pharm. J. 2018, 26, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Khadhri, A.; Bouali, I.; Belkhir, S.; Mokded, R.; Smiti, S.; Falé, P.; Araújo, M.E.M.; Serralheiro, M.L.M. In vitro digestion, antioxidant and antiacetylcholinesterase activities of two species of Ruta: Ruta chalepensis and Ruta montana. Pharm. Biol. 2017, 55, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, M.; Jerković, I.; Suknović, D.; Rajs, B.B.; Aladić, K.; Šubarić, D.; Jokić, S. Screening of six medicinal plant extracts obtained by two conventional methods and supercritical CO2 extraction targeted on coumarin content, 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity and total phenols content. Molecules 2017, 22, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlović, D.R.; Vukelic, M.; Najman, S.; Kostic, M.; Zlatkovic, B.; Mihajilov-Krstev, T.; Kitic, D. Assessment of polyphenol content, in vitro antioxidant, antimicrobial and toxic potentials of wild growing and cultured rue. J. Appl. Bot. Food Qual. 2014, 181, 175–181. [Google Scholar] [CrossRef]
- Coimbra, A.T.; Ferreira, S.; Duarte, A.P. Genus Ruta: A natural source of high value products with biological and pharmacological properties. J. Ethnopharmacol. 2020, 260, 113076. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Skalicka-Woźniak, K.S.; Khan, M.T.H.; Orhan, I.E.; Sener, B.; Głowniak, K. An in vitro and in silico approach to cholinesterase inhibitory and antioxidant effects of the methanol extract, furanocoumarin fraction, and major coumarins of Angelica officinalis L. fruits. Phytochem. Lett. 2011, 4, 462–467. [Google Scholar] [CrossRef]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef] [Green Version]
- Smyth, T.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins. Int. J. Antimicrob. Agents 2009, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Mahizan, N.A.; Yang, S.-K.; Moo, C.L.; Song, A.A.-L.; Chong, C.-M.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Alzoreky, N.S.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, F.; Smyth, T.J.P.; Ramachandran, V.; Smyth, W. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aniszewski, T. Biology of Alkaloids. In Alkaloids, 2nd ed.; Elsevier: Helsinki, Finland, 2015. [Google Scholar]
- Murugan, N.; Srinivasan, R.; Murugan, A.; Kim, M.; Natarajan, D. Glycosmis pentaphylla (Rutaceae): A Natural Candidate for the Isolation of Potential Bioactive Arborine and Skimmianine Compounds for Controlling Multidrug-Resistant Staphylococcus aureus. Front. Public Health 2020, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Luca, S.V.; Skiba, A.; Chinou, I.; Marcourt, L.; Wolfender, J.L.; Skalicka-Wozniak, K. Isolation and Antimicrobial Activity of Coumarin Derivatives from Fruits of Peucedanum luxurians Tamasha. Molecules 2018, 23, 1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Accumulated Compounds | Growth Period | R. chalepensis | R. corsica | R. graveolens |
|---|---|---|---|---|
| bergapten | 3 weeks | 91.537 ± 11.656 ace | 89.049 ± 13.492 ace | 55.391 ± 3.004 be |
| 4 weeks | 112.803 ± 8.821 adl | 135.548 ± 10.760 cdghl | 78.183 ± 6.148 abe | |
| 5 weeks | 184.083 ± 10.970 fhjk | 149.363 ± 6.694 dghjl | 162.236 ± 9.359 dfghj | |
| 6 weeks | 281.388 ± 8.330 i | 165.047 ± 7.075 fghk | 186.603 ± 2.905 fhjk | |
| 7 weeks | 130.150 ± 2.211 cdgl | 174.666 ± 12.736 fghjk | 67.990 ± 12.442 abe | |
| isoimperatorin | 3 weeks | 15.128 ± 1.156 abh | 23.696 ± 4.974 abcd | 14.611 ± 1.951 abdh |
| 4 weeks | 20.944 ± 2.184 abcd | 32.090 ± 10.191 bcdef | 23.299 ± 1.807 abcd | |
| 5 weeks | 28.573 ± 3.600 bcde | 36.684 ± 2.145 cdefg | 22.003 ± 4.661 abcd | |
| 6 weeks | 43.126 ± 1.183 cefg | 46.771 ± 1.435 efg | 23.931 ± 1.068 abcd | |
| 7 weeks | 22.742 ± 2.426 abcd | 42.981 ± 4.047 cefg | 6.610 ± 1.690 ah | |
| isopimpinellin | 3 weeks | 37.867 ± 9.467 ab | 43.184 ± 1.851 abd | 92.809 ± 1.865 c |
| 4 weeks | 31.680 ± 1.116 ab | 47.130 ± 1.859 abd | 76.459 ± 2.762 c | |
| 5 weeks | 40.157 ± 2.292 ab | 57.455 ± 1.945 bd | 145.251 ± 7.313 de | |
| 6 weeks | 77.140 ± 9.462 c | 46.760 ± 1.668 abd | 138.345 ± 4.211 e | |
| 7 weeks | 36.063 ± 5.455 ab | 44.033 ± 10.052 abd | 84.774 ± 8.748 c | |
| psoralen | 3 weeks | 122.970 ± 15.264 abcdg | 98.344 ± 0.902 abcgi | 70.121 ± 9.557 abcgih |
| 4 weeks | 177.943 ± 27.752 ade | 246.241 ± 51.342 def | 100.141 ± 9.547 abcgi | |
| 5 weeks | 268.823 ± 6.085 ef | 256.226 ± 37.091 ef | 105.269 ± 5.043 abcg | |
| 6 weeks | 98.766 ± 3.088 abcgi | 278.699 ± 26.560 ef | 129.266 ± 3.940 abcdg | |
| 7 weeks | 24.047 ± 1.514 chi | 179.779 ± 23.994 ade | 37.421 ± 22.530 bchi | |
| xanthotoxin | 3 weeks | 349.997 ± 54.203 abdehijk | 279.242 ± 44.171 abcj | 228.435 ± 11.563 bcjk |
| 4 weeks | 404.187 ± 42.136 adeghj | 397.999 ± 19.047 adehj | 385.553 ± 24.113 adehj | |
| 5 weeks | 509.827 ± 14.507 fgh | 375.924 ± 54.065 adehj | 385.480 ± 18.961 dfgh | |
| 6 weeks | 365.223 ± 20.883 abdehj | 340.774 ± 10.539 abdehj | 428.302 ± 19.060 adefgh | |
| 7 weeks | 101.527 ± 2.700 ik | 319.537 ± 27.264 abcdej | 171.908 ± 45.753 cik | |
| Total coumarins | 3 weeks | 617.498 ± 28.808 abf | 533.515 ± 32.612 abc | 461.368 ± 9.025 bcm |
| 4 weeks | 747.557 ± 59.484 defl | 849.022 ± 85.331 dehij | 663.636 ± 32.960 adfl | |
| 5 weeks | 1031.463 ± 21.196 gi | 875.651 ± 40.408 ehijl | 917.230 ± 32.550 eghijl | |
| 6 weeks | 865.643 ± 26.639 ehijl | 878.051 ± 40.412 ehij | 906.447 ± 15.819 ehij | |
| 7 weeks | 314.530 ± 5.806 kl | 760.995 ± 40.617 defhl | 368.703 ± 26.781 ckm | |
| γ-fagarine | 3 weeks | 41.203 ± 9.563 acfhil | 83.388 ± 5.113 bdjk | 47.164 ± 5.480 acdfhil |
| 4 weeks | 65.350 ± 5.152 bcdhik | 133.804 ± 7.766 eg | 34.663 ± 0.728 acfhl | |
| 5 weeks | 78.810 ± 6.455 bdjk | 114.682 ± 10.950 egj | 54.517 ± 3.776 acdhi | |
| 6 weeks | 58.341 ± 2.812 acdhik | 97.303 ± 6.759 bgj | 53.649 ± 0.803 acdfhi | |
| 7 weeks | 42.638 ± 5.295 acfhil | 74.105 ± 10.043 bdik | 31.106 ± 4.124 acfl | |
| Isopentenyloxy-γ-fagarine | 3 weeks | 2.778 ± 0.714 aefh | 17.482 ± 1.638 b | 9.143 ± 0.503 ceg |
| 4 weeks | 2.935 ± 0.100 aefh | 26.505 ± 2.900 d | 6.311 ± 0.705 acefg | |
| 5 weeks | 4.379 ± 0.357 aefgh | 20.397 ± 1.339 b | 6.788 ± 0.027 cefg | |
| 6 weeks | 3.015 ± 1.010 aefh | 25.669 ± 0.066 d | 5.479 ± 0.100 acefg | |
| 7 weeks | 3.337 ± 0.498 aefgh | 19.931 ± 2.741 b | 1.644 ± 0.088 afh | |
| skimmianine | 3 weeks | 17.267 ± 5.937 ad | 74.520 ± 11.342 bfi | 48.836 ± 2.680 cdfgj |
| 4 weeks | 33.336 ± 2.404 acdg | 133.412 ± 1.278 e | 63.099 ± 5.734 bcfj | |
| 5 weeks | 41.127 ± 6.753 cdgj | 123.816 ± 10.221 e | 94.635 ± 5.419 hi | |
| 6 weeks | 37.608 ± 4.668 cdgj | 62.912 ± 1.729 bcfj | 85.399 ± 3.297 bhi | |
| 7 weeks | 55.535 ± 5.968 cfgj | 95.575 ± 9.810 hi | 45.517 ± 3.340 cdfgj | |
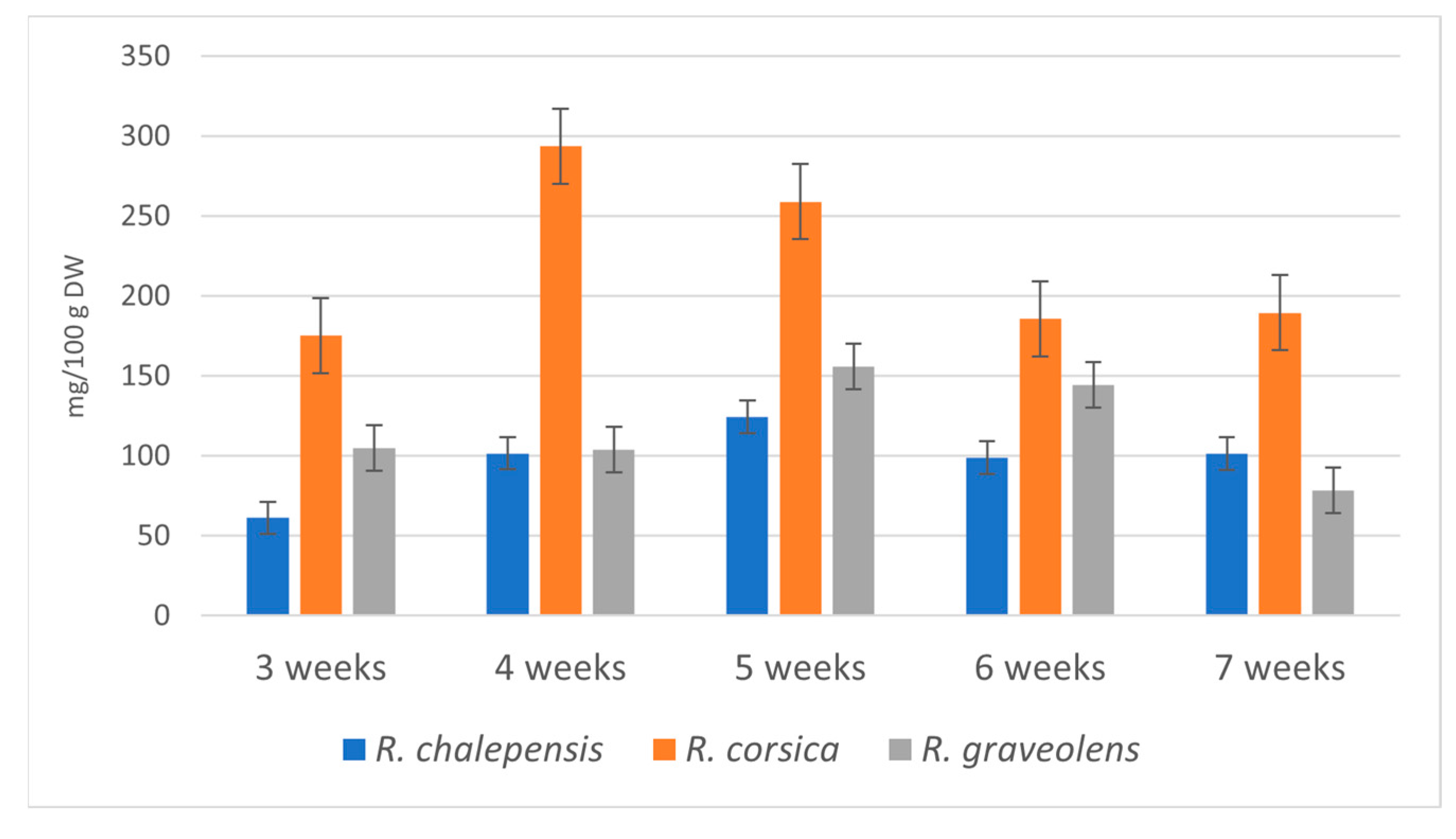
| Total alkaloids | 3 weeks | 61.247 ± 13.462 aj | 175.390 ± 9.103 bgh | 105.143 ± 6.920 cej |
| 4 weeks | 101.621 ± 4.374 cej | 293.721 ± 8.627 d | 104.074 ± 6.286 cej | |
| 5 weeks | 124.316 ± 12.860 cei | 258.895 ± 12.770 f | 155.940 ± 7.130 bgi | |
| 6 weeks | 98.964 ± 6.569 cej | 185.883 ± 5.235 bh | 144.527 ± 2.506 egi | |
| 7 weeks | 101.510 ± 5.749 cej | 189.612 ± 20.186 bh | 78.267 ± 3.789 acj |
| Extracts | DPPH TestIC50 (mg/mL) | Reducing Power AssayASE/mL | Fe2+ Chelating ActivityIC50 (mg/mL) |
|---|---|---|---|
| Ruta chalepensis | 1.665 ± 0.009 a | 15.493 ± 0.207 a | 1.452 ± 0.012 a |
| Ruta corsica | 2.032 ± 0.001 b | 20.516 ± 0.016 b | 0.736 ± 0.100 b |
| Ruta graveolens | 1.883 ± 0.007 c | 26.012 ± 0.019 c | 0.671 ± 0.013 a c |
| Standard | BHT0.065 ± 0.008 d | BHT1.131 ± 0.037 d | EDTA0.0067 ± 0.0003 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, A.; Marino, A.; Molinari, J.; Ekiert, H.; Miceli, N. Phytochemical Characterization, and Antioxidant and Antimicrobial Properties of Agitated Cultures of Three Rue Species: Ruta chalepensis, Ruta corsica, and Ruta graveolens. Antioxidants 2022, 11, 592. https://doi.org/10.3390/antiox11030592
Szewczyk A, Marino A, Molinari J, Ekiert H, Miceli N. Phytochemical Characterization, and Antioxidant and Antimicrobial Properties of Agitated Cultures of Three Rue Species: Ruta chalepensis, Ruta corsica, and Ruta graveolens. Antioxidants. 2022; 11(3):592. https://doi.org/10.3390/antiox11030592
Chicago/Turabian StyleSzewczyk, Agnieszka, Andreana Marino, Jessica Molinari, Halina Ekiert, and Natalizia Miceli. 2022. "Phytochemical Characterization, and Antioxidant and Antimicrobial Properties of Agitated Cultures of Three Rue Species: Ruta chalepensis, Ruta corsica, and Ruta graveolens" Antioxidants 11, no. 3: 592. https://doi.org/10.3390/antiox11030592







