Non-Thermal Plasma Treatment Coupled with a Photocatalyst for Antimicrobial Performance of Ihram Cotton Fabric
Abstract
1. Introduction
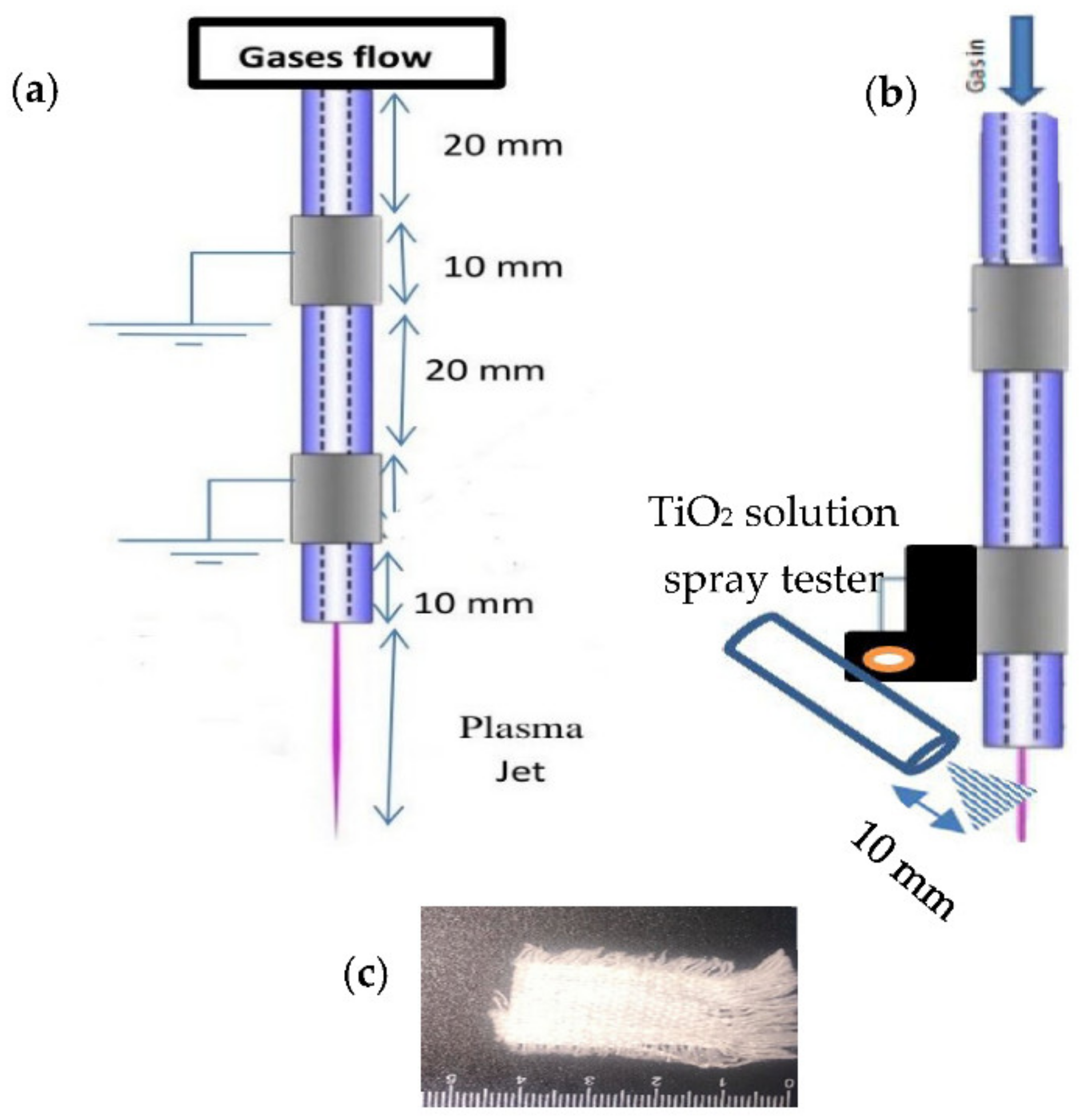
2. Experimental Setup and Procedures
3. Results and Discussion
3.1. Reynolds Number
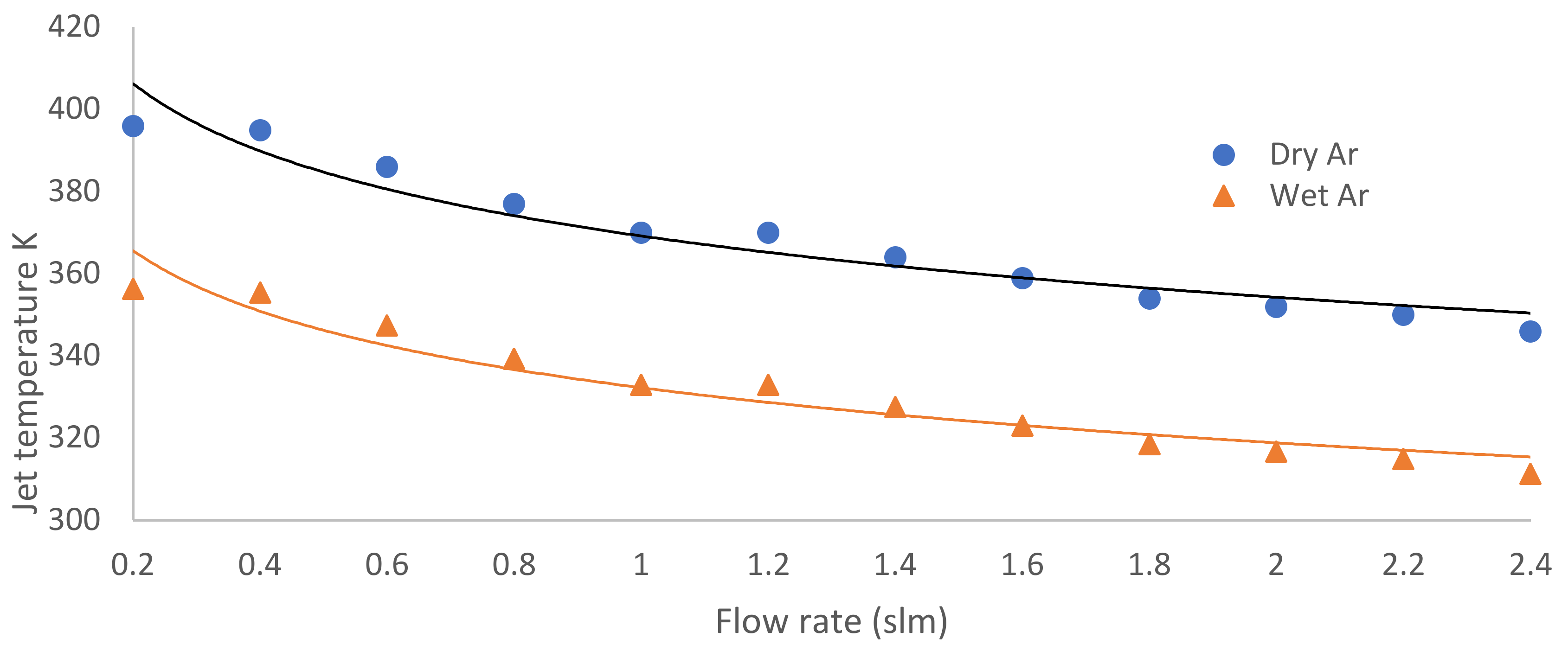
3.2. Jet Temperature and Heat Impacting the Bacterial Colonies
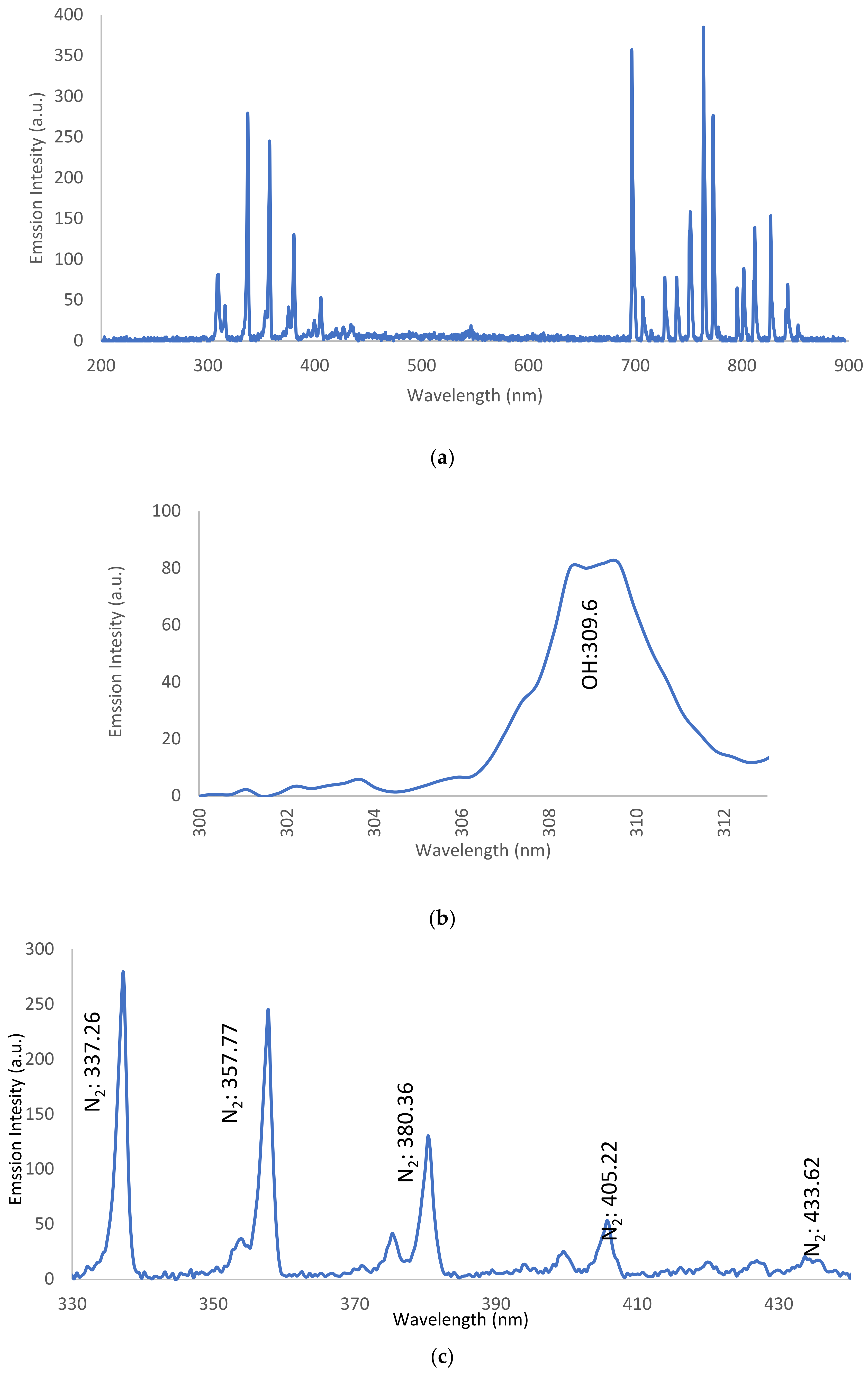
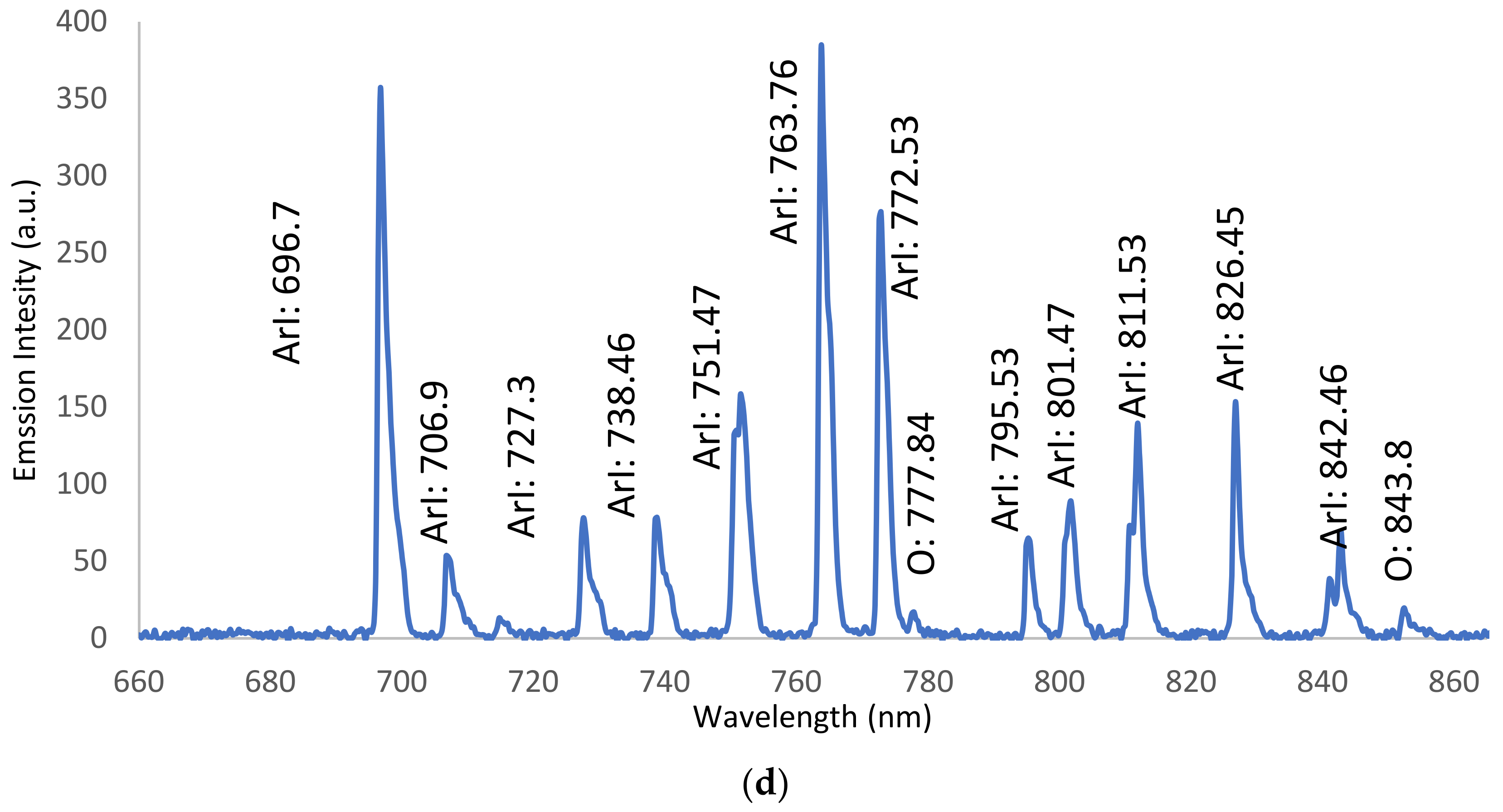
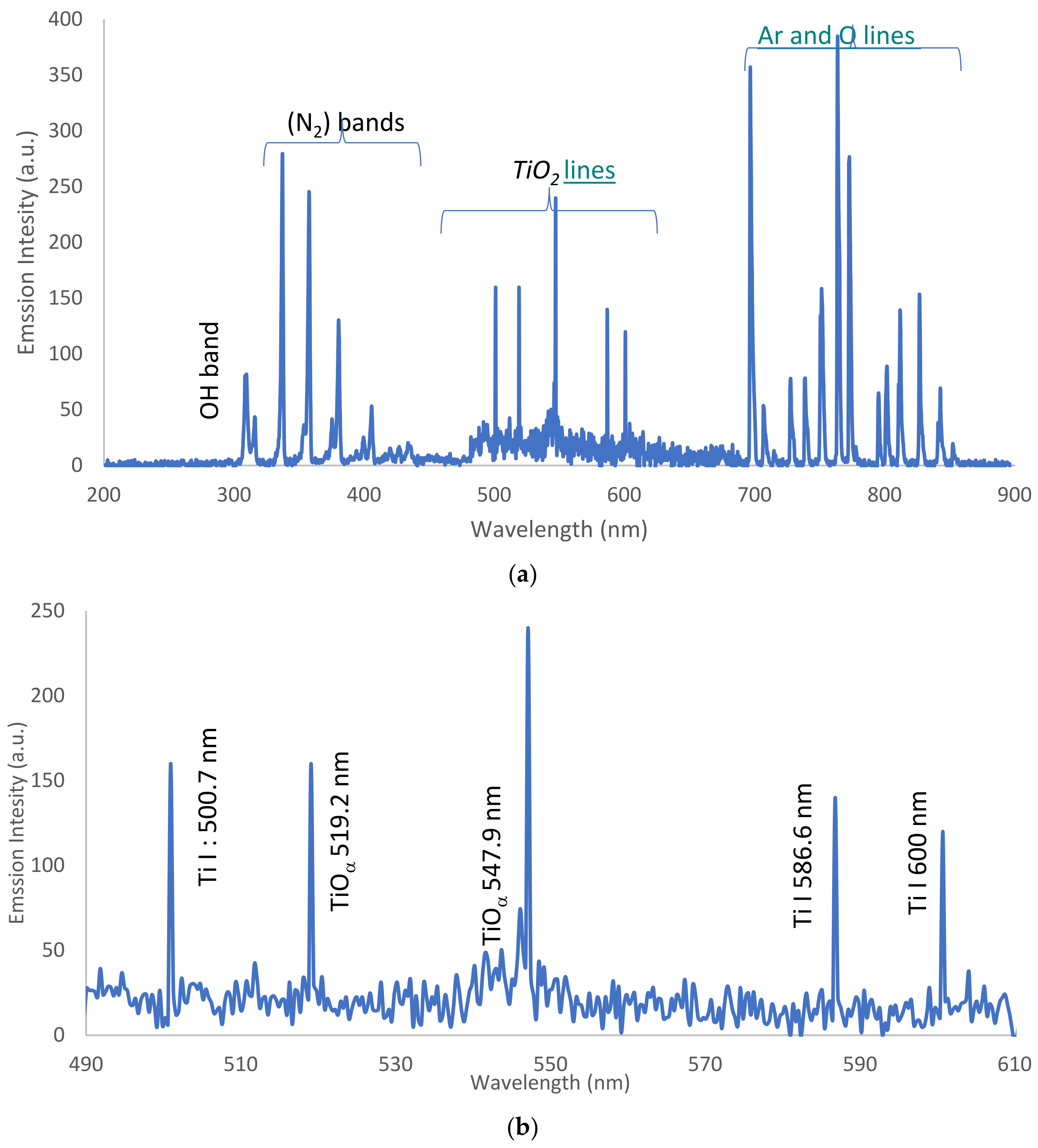
3.3. Optical Emission Spectroscopy (OES)
3.3.1. OES for Dry Ar-APPJ Discharge
3.3.2. OES for Wet Ar-APPJ Discharge
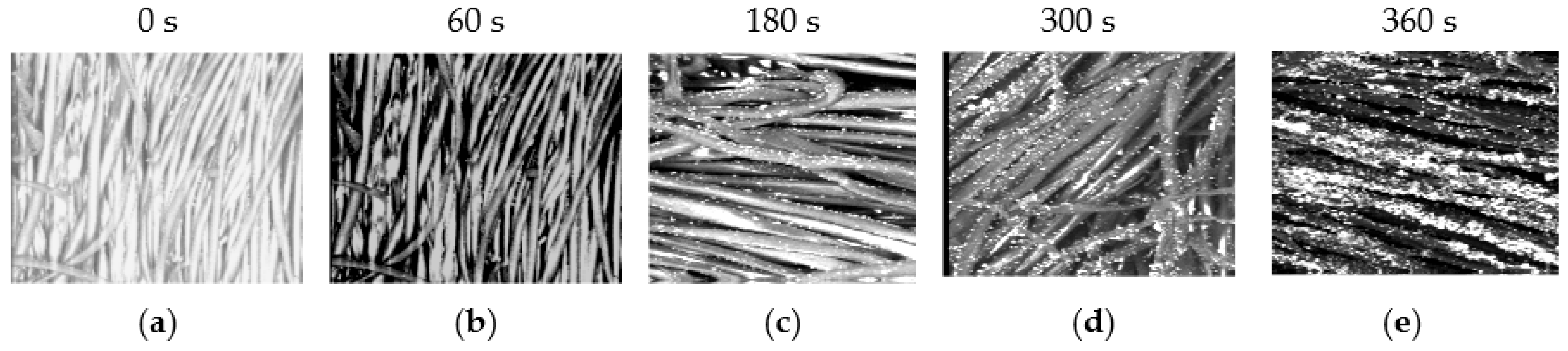
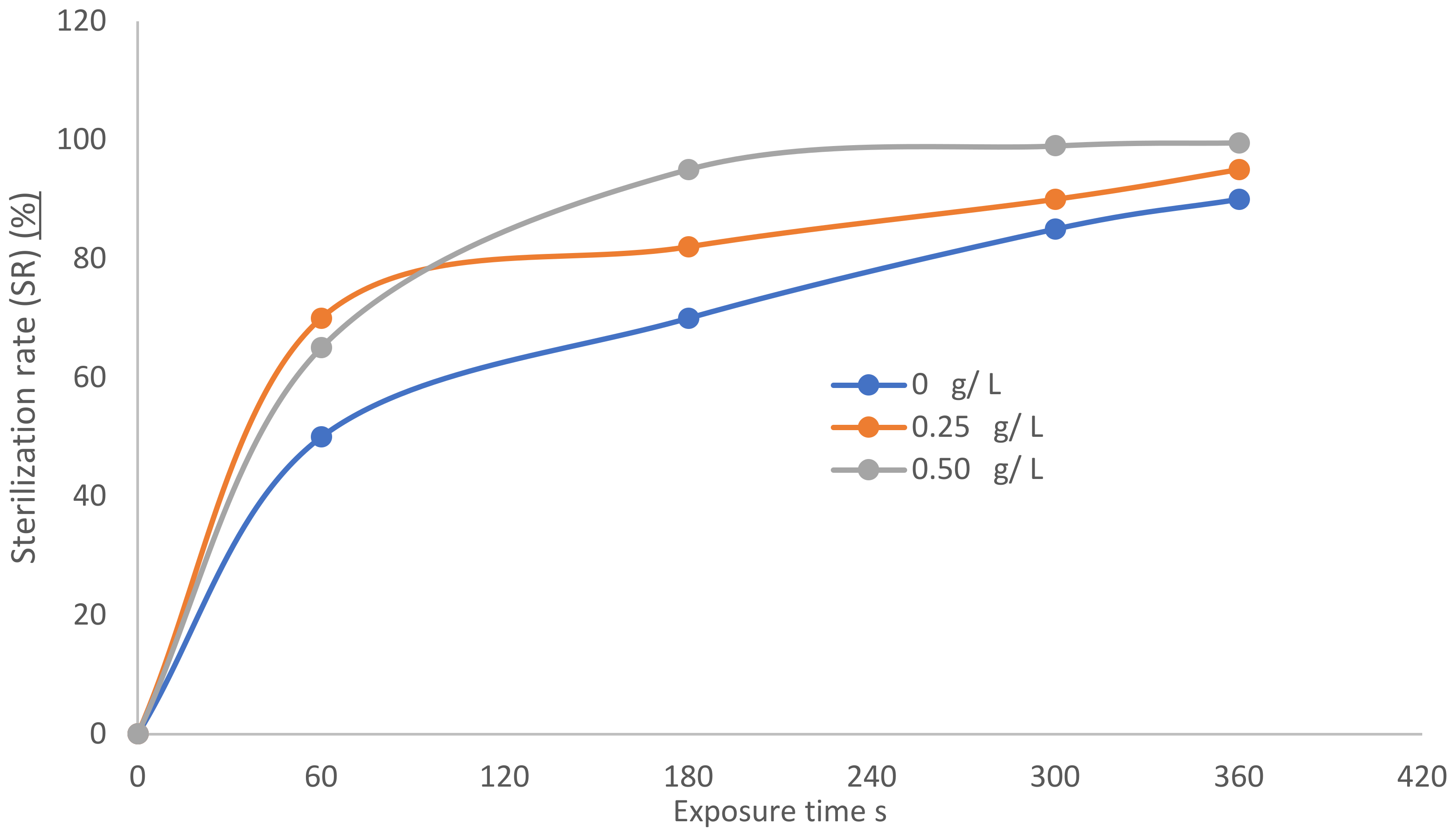
3.4. Antibacterial Treatment of Cotton Fabric Samples
- (i)
- The first period comprises a moderate inactivation process within 60 s, before the dotted line, with SR ranging from 50 to 65% for an exposure time ranging from 0 to 60 s. ∆ N increases with increasing exposure time The first phase represents the limited penetration depth of the plasma jet discharge due to radicals, electrons, and positive ions [48,49,50].
- (ii)
- The second period is after the dotted line, with a rapid inactivation process within 300 s and an SR ranging from 90 to 99.5% for an exposure time of 60 to 360 s. There exists a higher etching rate and better inactivation effect than in the first period, representing the second and third phases. After adding TiO2 concentrations of 0.25 and 0.5 g/L, increases within two phases, 2 and 3. These phases deal with residual live cells that were not sufficiently inactivated during phase 1 [51,52]:
- (iii)
- This means that SR can be written:(SR)dry Ar < (SR)Wet Ar 0.25 g/L TiO2 < (SR)Wet Ar 0.25 g/L TiO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasser, E. Fundamentals of Gaseous Ionization and Plasma Electronics; Wiley-Interscience: New York, NY, USA, 1971. [Google Scholar]
- Raizer, Y.P. Gas Discharge Physics; Allen, J.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Galaly, A.R.; van Oost, G. Environmental and economic vision of plasma treatment of waste in Makkah. Plasma Sci. Technol. 2017, 19, 105503. [Google Scholar] [CrossRef][Green Version]
- Galaly, A.R. 16th Treatment of Wastes by Plasma Gasification in Makkah. 2016, pp. 293–319. Available online: https://drive.uqu.edu.sa/_/hajj/files/multaqa/143716.pdf (accessed on 25 May 2016).
- Galaly, A.R.; van Oost, G. Fast inactivation of microbes and degradation of organic compounds dissolved in water by thermal plasma. Plasma Sci. Technol. 2018, 20, 085504. [Google Scholar] [CrossRef]
- Galaly, A.R.; van Oost, G. Environmental and economic aspects of the plasma treatment of scrap tires to produce syngas and synthetic fuels in Saudi Arabia. IEEE Trans. Plasma Sci. 2021, 49, 522–534. [Google Scholar] [CrossRef]
- Galaly, A.R. Nano-coating process for Si [1 0 0] wafer using Atmospheric Pressure Plasma Jet (APPJ). J. Mod. Phys. 2012, 3, 1031. [Google Scholar] [CrossRef]
- Galaly, A.R.; Zahran, H.H. Inactivation of bacteria using combined effects of magnetic field, low pressure and ultra-low frequency plasma discharges (ULFP). J. Phys. Conf. Ser. 2013, 431, 012014. [Google Scholar] [CrossRef]
- Galaly, A.R. Gap mesh wire control on nano-particles growth. J. Mod. Phys. 2015, 6, 1162–1170. [Google Scholar] [CrossRef]
- Chen, Z.; Garcia, G., Jr.; Arumugaswami, V.; Wirz, R.E. Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys. Fluids 2020, 32, 111702. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Agrawal, A. Likelihood of survival of coronavirus in a respiratory droplet deposited on a solid surface. Phys. Fluids 2020, 32, 061704. [Google Scholar] [CrossRef]
- Asghar, A.H.; Galaly, A.R. The influence of different plasma cell discharges on the performance quality of surgical gown samples. Materials 2021, 14, 4329. [Google Scholar] [CrossRef]
- Rashidi, A.; Moussavipourgharbi, H.; Mirjalili, M.; Ghoranneviss, M. Effect of low-temperature plasma treatment on surface modification of cotton and polyester fabrics. Indian J. Fibre Text. Res. 2004, 29, 74–78. [Google Scholar]
- Morent, R.; Geyter, N.; Leys, C. Measuring the wicking behavior of textiles by the combination of a horizontal wicking experiment and image processing. Rev. Sci. Instrum. 2006, 77, 093502. [Google Scholar] [CrossRef]
- Kogoma, M.; Kusano, M.; Kusano, Y. Generation and Applications of Atmospheric Pressure Plasmas; Nova Science: New York, NY, USA, 2011. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Jelil, R.A. A review of low-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Linh, N.N.; Ghimire, B.; Pengkit, A.; Sornsakdanuphap, J.; Lee, S.-J.; Choi, E.H. Plasma and nanomaterials: Fabrication and biomedical applications. Nanomaterials 2019, 9, 98. [Google Scholar] [CrossRef]
- Asghar, A.H.; Ahmed, O.B.; Galaly, A.R. Inactivation of E. coli using atmospheric pressure plasma jet with dry and wet argon discharges. Membranes 2021, 11, 46. [Google Scholar] [CrossRef]
- Asghar, A.; Galaly, A.R. The Effect of oxygen admixture with argon discharges on the impact parameters of atmospheric pressure plasma jet characteristics. Appl. Sci. 2021, 11, 6870. [Google Scholar] [CrossRef]
- Mei, B.; Sanchez, M.D.; Reinecke, T.; Kaluza, S.; Xia, W.; Muhler, M. The synthesis of Nb-doped TiO2 nanoparticles by spray drying: An efficient and scalable method. J. Mater. Chem. 2011, 21, 11781–11790. [Google Scholar] [CrossRef]
- Stevens, N.; Priest, C.I.; Sedev, R.; Ralston, J. Wettability of photoresponsive titanium dioxide surfaces. Langmuir 2003, 19, 3272–3275. [Google Scholar] [CrossRef]
- Lee, K.-N.; Paek, K.-H.; Ju, W.-T.; Lee, Y.-H. Sterilization of bacteria, yeast, and bacterial endospores by atmospheric-pressure cold plasma using helium and oxygen. J. Microbiol. 2006, 44, 269–275. [Google Scholar] [PubMed]
- Mdgan, M.; Martinko, J.; Parker, J. Biology of Microorganisms, 10th ed.; Pearson Education Publishers: London, UK, 2002; pp. 696–707. [Google Scholar]
- Hugo, W.B.; Ayliffe, G.; Russell, A.D. Principles and Practice of Disinfection, Preservation and Sterilization, 3rd ed.; Blackwell Science: Oxford, UK, 1999. [Google Scholar]
- Galaly, A.R.; Ahmed, O.B.; Asghar, H.A. Antibacterial effects of combined non-thermal plasma and photocatalytic treatment of culture media in the laminar flow mode. Phys. Fluids 2021, 33, 043604. [Google Scholar] [CrossRef]
- Jin, D.J.; Uhm, H.S.; Cho, G. Influence of the gas-flow Reynolds number on a plasma column in a glass tube. Phys. Plasmas 2013, 20, 083513. [Google Scholar] [CrossRef]
- Al-rawaf, A.F.; Fuliful, F.K.; Khalaf, M.K.; Oudah, H.K. Studying the non-thermal plasma jet characteristics and application on bacterial decontamination. J. Theor. Appl. Phys. 2018, 12, 45–51. [Google Scholar] [CrossRef]
- Nwankire, C.E.; Law, V.J.; Nindrayog, A.; Twomey, B.; Niemi, K.; Milosavljevic, V.; Graham, W.G.; Dowling, D.P. Electrical, thermal and optical diagnostics of an atmospheric plasma jet system. Plasma Chem. Plasma Process. 2010, 30, 537–552. [Google Scholar] [CrossRef]
- Law, V.J.; Milosavljevic, V.; O’Connor, N.; Lalor, J.F.; Daniels, S. Handheld flyback driven coaxial dielectric barrier discharge: Development and characterization. Rev. Sci. Instrum. 2008, 79, 094707. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.-A.H.; Aljuhani, M.M.; Almarashi, J.Q.M.; Alhazime, A.A. The effect of a second grounded electrode on the atmospheric pressure argon plasma jet. Plasma Res. Express 2020, 2, 015011. [Google Scholar] [CrossRef]
- Liu, L.; John, B.; Yeung, K.L.; Si, G. Non-UV based germicidal activity of metal-doped TiO2 coating on solid surfaces. J. Environ. Sci. 2007, 19, 745–750. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial activity of TiO2 photocatalyst alone or in coatings on E. coli: The Influence of Methodological Aspects. Coatings 2014, 4, 670–686. [Google Scholar] [CrossRef]
- Rincón, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended–fixed) TiO2 concentration. Appl. Catal. B Environ. 2003, 44, 263–284. [Google Scholar] [CrossRef]
- NIST Atomic Spectra Database. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 21 August 2020).
- Kirkpatrick, M.J.; Dodet, B.; Odic, E. Atmospheric pressure humid argon DBD plasma for the application of sterilization—Measurement and simulation of hydrogen, oxygen, and hydrogen peroxide formation. Int. J. Plasma Environ. Sci. Technol. 2007, 1, 96–101. [Google Scholar]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Fakhouri, H.; Salem, D.B.; Carton, O.; Pulpytel, J.; Arefi-Khonsari, F. Highly efficient photocatalytic TiO2 coatings deposited by open air atmospheric pressure plasma jet with aerosolized TTIP precursor. J. Phys. D Appl. Phys. 2014, 47, 265301. [Google Scholar] [CrossRef]
- Kutlu, N.; Oturak, H. The optical and crystallite characterization of bilayer TiO2 films coated on different ITO layers. Open Chem. J. 2018, 16, 1275. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef]
- Laroussi, M.; Mendis, D.A.; Rosenberg, M. Plasma interaction with microbes. New J. Phys. 2003, 5, 41. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Proc. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Ouyang, K.; Dai, K.; Walker, S.L.; Huang, Q.; Yin, X.; Cai, P. Efficient photocatalytic disinfection of Escherichia coli O157:H7 using C70-TiO2 hybrid under visible light irradiation. Sci. Rep. 2016, 6, 25702. [Google Scholar] [CrossRef]
- Pal, A.; Min, X.; Yu, L.E.; Pehkonen, S.O.; Ray, M.B. Photocatalytic inactivation of bioaerosols by TiO2 coated membrane. Int. J. Chem. React. Eng. 2005, 3, 14. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli: Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Gumy, D.; Morais, C.; Bowen, P.; Pulgarin, C.; Giraldo, S.; Hajdu, R.; Kiwi, J. Catalytic activity of commercial of TiO2 powders for the abatement of the bacteria (E. coli) under solar simulated light: Influence of the isoelectric point. Appl. Catal. B Environ. 2006, 63, 76–84. [Google Scholar] [CrossRef]
- Park, H.-J.; Nguyen, T.T.M.; Yoon, J.; Lee, C. Role of reactive oxygen species in Escherichia coli inactivation by cupric ion. Environ. Sci. Technol. 2012, 46, 11299–11304. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Y.; An, T.; Li, G.; Yip, H.Y.; Yu, J.C.; Wong, P.K. Visible-light-driven photocatalytic inactivation of E. coli K-12 by bismuth vanadate nanotubes: Bactericidal performance and mechanism. Environ. Sci. Technol. 2012, 46, 4599–4606. [Google Scholar] [CrossRef]
- Deng, X.; Nikiforov, A.Y.; Coenye, T.; Cools, P.; Aziz, G.; Morent, R.; Geyter, N.D.; Leys, C. Antimicrobial nano-silver non-woven polyethylene terephthalate fabric via an atmospheric pressure plasma deposition process. Sci. Rep. 2015, 5, 10138. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Value | Unit |
|---|---|---|
| Ar flow rate | 2.4 | Slm |
| Re | 2819 | |
| Jet temperature for dry Ar | 346 | K |
| Jet temperature for wet Ar | 311 | K |
| for dry argon | 230 | mW |
| for wet argon | 77 | mW |
| Frequency | 25 | kHz |
| Applied voltage | 11.2 | kV |
| Power | 2.34 | W |
| Jet length | 11.5 | mm |
| Jet width | 1.5 | mm |
| Energy | 96 | m J |
| Species | Wavelength (nm) | Transition |
|---|---|---|
| OH band | 309.6 | A2∑+ − X2∏ |
| N2 bands | 3.15.37, 337.26, 357.77, and 380.36 | C3Πu − B3Πg |
| Ar lines | 696.7, 706.9, 727.3, 738.46, 751.47, 763.76, 772.53, 795.53, 801.47, 811.53, and 826.45 | 3s23p5(2P°3/2)4p |
| (O) lines | 777.84 and 843.8 | 3s23p5(2P°3/2)4s |
| Species | Wavelength (nm) |
|---|---|
| atomic lines, Ti I | 500.7, 586.6, and 600 |
| molecular lines, TiOα | 547.9 and 519.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galaly, A.R.; Dawood, N. Non-Thermal Plasma Treatment Coupled with a Photocatalyst for Antimicrobial Performance of Ihram Cotton Fabric. Nanomaterials 2022, 12, 1004. https://doi.org/10.3390/nano12061004
Galaly AR, Dawood N. Non-Thermal Plasma Treatment Coupled with a Photocatalyst for Antimicrobial Performance of Ihram Cotton Fabric. Nanomaterials. 2022; 12(6):1004. https://doi.org/10.3390/nano12061004
Chicago/Turabian StyleGalaly, Ahmed Rida, and Nagia Dawood. 2022. "Non-Thermal Plasma Treatment Coupled with a Photocatalyst for Antimicrobial Performance of Ihram Cotton Fabric" Nanomaterials 12, no. 6: 1004. https://doi.org/10.3390/nano12061004
APA StyleGalaly, A. R., & Dawood, N. (2022). Non-Thermal Plasma Treatment Coupled with a Photocatalyst for Antimicrobial Performance of Ihram Cotton Fabric. Nanomaterials, 12(6), 1004. https://doi.org/10.3390/nano12061004





