Non-Thermal Atmospheric Plasma for Microbial Decontamination and Removal of Hazardous Chemicals: An Overview in the Circular Economy Context with Data for Test Applications of Microwave Plasma Torch
Abstract
1. Introduction
2. Low-Temperature Non-Equilibrium Plasma for Microbial Decontamination
2.1. A Brief State of the Art
- Very diverse biological targets—for example, the interaction of plasma with prokaryotic and eukaryotic cells is probably based on different mechanisms [52]; Gram-negative and Gram-positive bacteria also have a different sensitivity to plasma treatment [53]. Cell organization in biofilms assumes a new high hierarchical level of interaction with external impacts. Bacterial metabolic status and cell morphology are important factors for inactivation efficiency [54];
- Many active plasma components with different effects on biological objects—UV affects the dimerization of thymine bases in DNA; charges particles rupture the cell membranes; reactive species of oxygen and nitrogen have a strong oxidative effect also on bacterial membranes [55], and on viral ability to bind with host receptors and to enter into host cells [51];
- Physical and chemical properties of plasma depend on many factors: the type of configuration, mode of operation, environment (air, water, surfaces), and gases used can play a significant role in the presence, proportion, and concentration of plasma inactivation agents and reactive species. It seems that the application and efficiency of plasma depend on these specific characteristics of the devices, making it challenging to study the exact mode of action and mechanism of interaction [53].
2.2. Case Study—Bactericidal Effect of Cold Argon Plasma on Surfaces and in Liquids
2.2.1. Characterization of the Plasma Generation System
2.2.2. Experimental Design
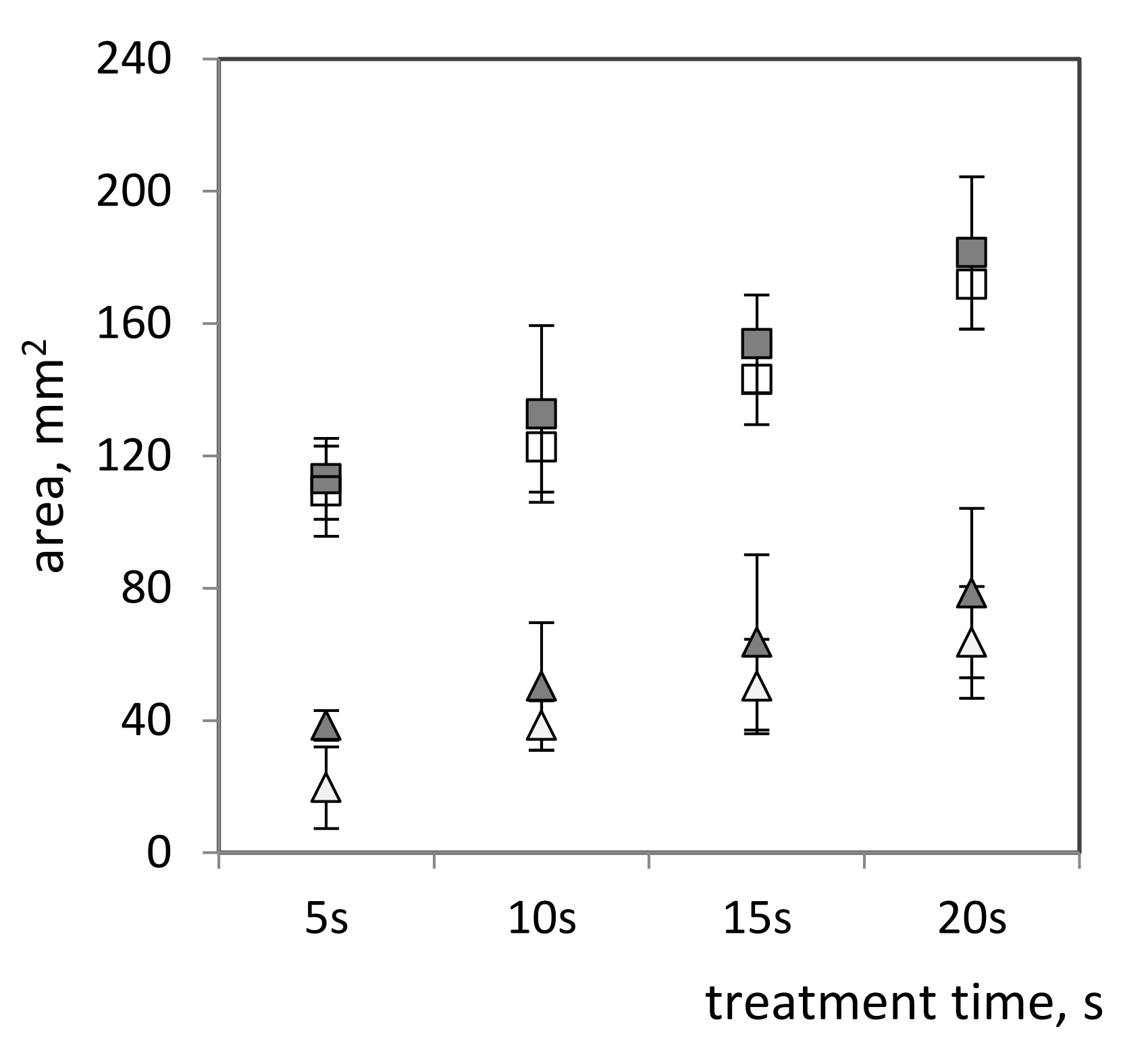
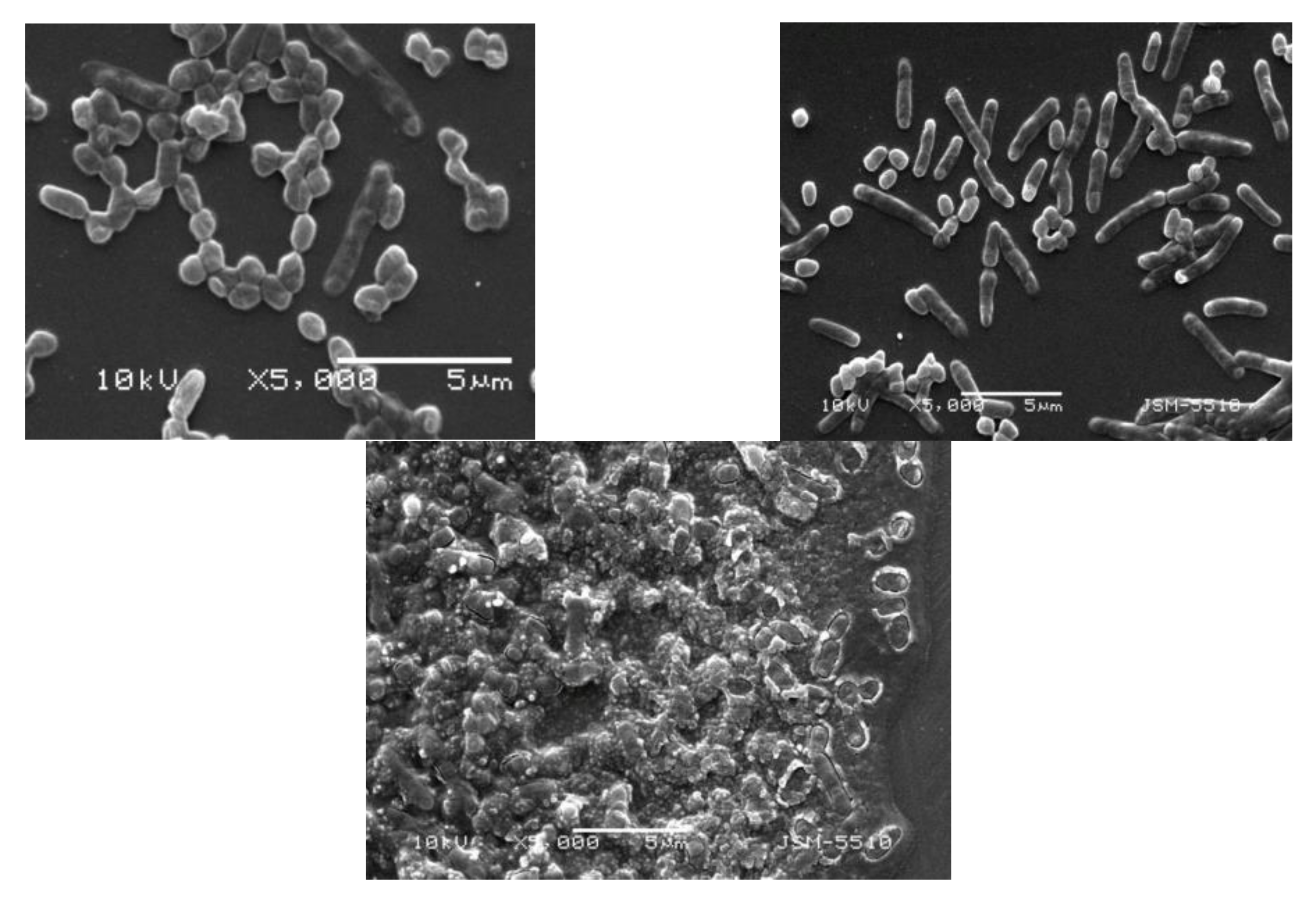
2.2.3. Plasma Treatment of Bacterial Films on Surfaces
2.2.4. Plasma Decontamination of Liquids
3. Low-Temperature, Non-Equilibrium Plasma for Removal of Hazardous Chemicals
3.1. A Brief State of the Art
3.2. Case Study—Tretment of Organic Pollutants
3.2.1. Characterization of the Plasma Generation System
3.2.2. Experimental Design
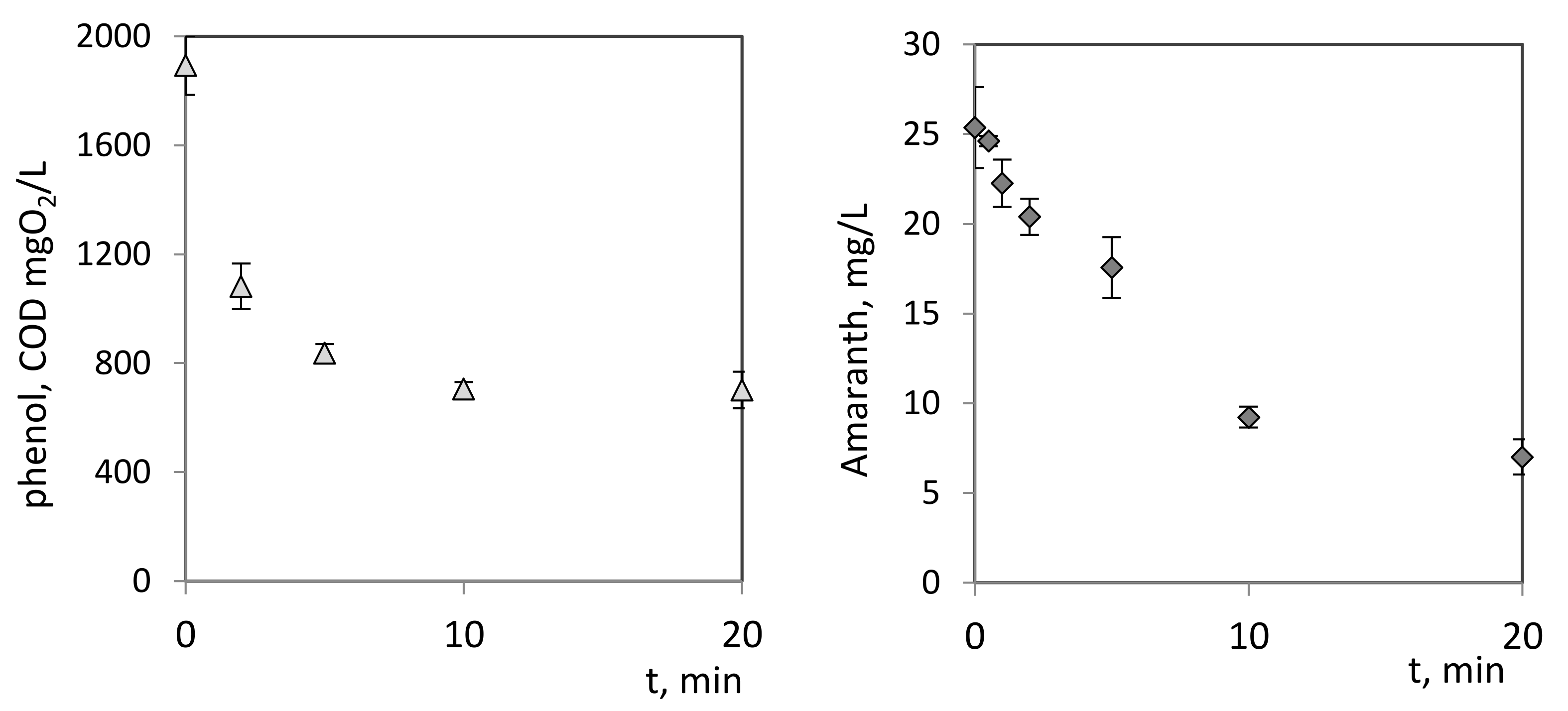
3.2.3. Reduction of Pollutant Concentration after Plasma Treatment
4. Summary of Cold Atmospheric Plasma Potential for Microbial Decontamination and Chemical Removal with Future Perspectives in the Circular Economy Context
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos, A.; Rouboa, A. Life cycle thinking of plasma gasification as a waste-to-energy tool: Review on environmental, economic and social aspects. Renew. Sustain. Energy Rev. 2022, 153, 111762. [Google Scholar] [CrossRef]
- Tian, H.; Wang, X.; Tong, Y.W. Chapter 9—Sustainability assessment: Focusing on different technologies recovering energy from waste. In Waste-to-Energy, 1st ed.; Ren, J., Ed.; Academic Press: Amsterdam, The Netherlands, 2020; pp. 235–264. [Google Scholar] [CrossRef]
- Leading the way to a global circular economy—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/31079d7e-3a96-11eb-b27b-01aa75ed71a1 (accessed on 31 January 2022).
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Velenturf, A.P.M.; Purnell, P. Principles for a sustainable circular economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Growth within: A circular economy vision for a competitive Europe McKinsey. Available online: https://www.mckinsey.com/business-functions/sustainability/our-insights/growth-within-a-circular-economy-vision-for-a-competitive-europe (accessed on 31 January 2022).
- van Dam, K.; Simeone, L.; Keskin, D.; Baldassarre, B.; Niero, M.; Morelli, N. Circular Economy in Industrial Design Research: A Review. Sustainability 2020, 12, 10279. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Liu, N.; Xu, L.; Han, L.; Huang, G.; Ciric, L. Microbiological safety and antibiotic resistance risks at a sustainable farm under large-scale open-air composting and composting toilet systems. J. Hazard. Mater. 2021, 401, 123391. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.M.R.; Ma, H.; Xu, B.; Devi, S.; Siddique, M.A.B.; Stanley, S.L.; Bhandari, B.; Zhu, J. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020, 97, 417–432. [Google Scholar] [CrossRef]
- Zhou, R.; Rezaeimotlagh, A.; Zhou, R.; Zhang, T.; Wang, P.; Hong, J.; Soltani, B.; Mai-Prochnow, A.; Liao, X.; Ding, T.; et al. In-package plasma: From reactive chemistry to innovative food preservation technologies. Trends Food Sci. Technol. 2022, 120, 59–74. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Advances in emerging technologies for the decontamination of the food contact surfaces. Food Res. Int. 2022, 151, 110865. [Google Scholar] [CrossRef]
- Murugesan, P.; Evanjalin Monica, V.; Moses, J.A.; Anandharamakrishnan, C. Water decontamination using non-thermal plasma: Concepts, applications, and prospects. J. Environ. Chem. Eng. 2020, 8, 104377. [Google Scholar] [CrossRef]
- Hippler, R.; Kersten, H.; Schmidt, M.; Schoenbach, K.H. (Eds.) Low Temperature Plasmas: Fundamentals, Technologies and Techniques, 2nd ed.; Wiley-VCH: Berlin, Germany, 2008; p. 945. [Google Scholar]
- Marinova, P.; Benova, E.; Todorova, Y.; Topalova, Y.; Yotinov, I.; Atanasova, M.; Krcma, F. Surface-wave-sustained plasma torch for water treatment. J. Phys. Conf. Ser. 2018, 982. [Google Scholar] [CrossRef]
- Bogdanov, T.; Tsonev, I.; Marinova, P.; Benova, E.; Rusanov, K.; Rusanova, M.; Atanassov, I.; Kozáková, Z.; Krčma, F. Microwave Plasma Torch Generated in Argon for Small Berries Surface Treatment. Appl. Sci. 2018, 8, 1870. [Google Scholar] [CrossRef]
- Benova, E.; Marinova, P.; Tafradjiiska-Hadjiolova, R.; Sabit, Z.; Bakalov, D.; Valchev, N.; Traikov, L.; Hikov, T.; Tsonev, I.; Bogdanov, T. Characteristics of 2.45 GHz Surface-Wave-Sustained Argon Discharge for Bio-Medical Applications. Appl. Sci. 2022, 12, 969. [Google Scholar] [CrossRef]
- Choi, E.H.; Uhm, H.S.; Kaushik, N.K. Plasma bioscience and its application to medicine. AAPPS Bull. 2021, 31, 10. [Google Scholar] [CrossRef]
- Patinglag, L.; Melling, L.M.; Whitehead, K.A.; Sawtell, D.; Iles, A.; Shaw, K.J. Non-thermal plasma-based inactivation of bacteria in water using a microfluidic reactor. Water Res. 2021, 201, 117321. [Google Scholar] [CrossRef]
- Lin, C.M.; Herianto, S.; Chen, H.L.; Chiu, Y.C.; Hou, C.Y. The application of a novel non-thermal plasma device with double rotary plasma jets for inactivation of Salmonella Enteritidis on shell eggs and its effects on sensory properties. Int. J. Food Microbiol. 2021, 355, 109332. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold plasma: Background, applications and current trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Attri, P.; Yusupov, M.; Park, J.H.; Lingamdinne, L.P.; Koduru, J.R.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Mechanism and comparison of needle-type non-thermal direct and indirect atmospheric pressure plasma jets on the degradation of dyes. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Sarangapani, C.; Patange, A.; Bourke, P.; Keener, K.; Cullen, P.J. Recent Advances in the Application of Cold Plasma Technology in Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Benova, E.; Atanasova, M.; Bogdanov, T.; Marinova, P.; Krčma, F.; Mazankova, V.; Dostal, L. Microwave Plasma Torch at a Water Surface. Plasma Med. 2016, 6, 59–65. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Xu, M.; Chen, H.; Lu, X.; Ostrikov, K. Cold atmospheric pressure plasmas in dermatology: Sources, reactive agents, and therapeutic effects. Plasma Process. Polym. 2020, 17, 1900218. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection, sterilization, and antisepsis: An overview. Am. J. Infect. Control 2016, 44, e1–e6. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Von Woedtke, T. Plasma medicine—current state of research and medical application. Plasma Phys. Control. Fusion 2016, 59, 014031. [Google Scholar] [CrossRef]
- Wiegand, C.; Fink, S.; Hipler, U.C.; Beier, O.; Horn, K.; Pfuch, A.; Schimanski, A.; Grünler, B. Cold atmospheric pressure plasmas exhibit antimicrobial properties against critical bacteria and yeast species. J. Wound Care 2017, 26, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Low-Temperature Plasmas for Medicine? IEEE Trans. Plasma Sci. 2009, 37, 714–725. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold atmospheric plasma: Charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Laroussi, M.; Mendis, D.A.; Rosenberg, M. Plasma Interaction With Microbes. New J. Phys. 2003, 5, 41–42. [Google Scholar] [CrossRef]
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- Min, S.C.; Roh, S.H.; Niemira, B.A.; Sites, J.E.; Boyd, G.; Lacombe, A. Dielectric barrier discharge atmospheric cold plasma inhibits Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Tulane virus in Romaine lettuce. Int. J. Food Microbiol. 2016, 237, 114–120. [Google Scholar] [CrossRef]
- Modic, M.; McLeod, N.P.; Sutton, J.M.; Walsh, J.L. Cold atmospheric pressure plasma elimination of clinically important single- and mixed-species biofilms. Int. J. Antimicrob. Agents 2017, 49, 375–378. [Google Scholar] [CrossRef]
- Takamatsu, T.; Kawate, A.; Uehara, K.; Oshita, T.; Miyahara, H.; Dobrynin, D.; Fridman, G.; Fridman, A.; Okino, A. Bacterial Inactivation in Liquids Using Multi-Gas Plasmas. Plasma Med. 2012, 2, 237–248. [Google Scholar] [CrossRef]
- Timmons, C.; Pai, K.; Jacob, J.; Zhang, G.; Ma, L.M. Inactivation of Salmonella enterica, Shiga toxin-producing Escherichia coli, and Listeria monocytogenes by a novel surface discharge cold plasma design. Food Control 2018, 84, 455–462. [Google Scholar] [CrossRef]
- Van Gils, C.A.J.; Hofmann, S.; Boekema, B.K.H.L.; Brandenburg, R.; Bruggeman, P.J. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 175203. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Yuan, L.; Surendhiran, D.; Lin, L. Sequential effect of phages and cold nitrogen plasma against Escherichia coli O157:H7 biofilms on different vegetables. Int. J. Food Microbiol. 2018, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Niveditha, A.; Pandiselvam, R.; Prasath, V.A.; Singh, S.K.; Gul, K.; Kothakota, A. Application of cold plasma and ozone technology for decontamination of Escherichia coli in foods—A review. Food Control 2021, 130, 108338. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Chen, Z.; Garcia, G.; Arumugaswami, V.; Wirz, R.E. Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys. Fluids 2020, 32, 111702. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Nayak, G.; Rendine, N.; Wigdahl, B.; Krebs, F.C.; Bruggeman, P.J.; Miller, V. Non-Thermal Plasma as a Novel Strategy for Treating or Preventing Viral Infection and Associated Disease. Front. Phys. 2021, 9, 683118. [Google Scholar] [CrossRef]
- Wende, K.; Landsberg, K.; Lindequist, U.; Weltmann, K.D.; Von Woedtke, T. Distinctive activity of a nonthermal atmospheric-pressure plasma jet on eukaryotic and prokaryotic cells in a cocultivation approach of keratinocytes and microorganisms. IEEE Trans. Plasma Sci. 2010, 38, 2479–2485. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Ziuzina, D.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Cold Plasma Inactivation of Bacterial Biofilms and Reduction of Quorum Sensing Regulated Virulence Factors. PLoS ONE 2015, 10, e0138209. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Fernández, A.; Thompson, A. The inactivation of Salmonella by cold atmospheric plasma treatment. Food Res. Int. 2012, 45, 678–684. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Vaze, N.; Gangoli, S.; Vasilets, V.N.; Gutsol, A.F.; Milovanova, T.N.; Anandan, S.; Murasko, D.M.; Fridman, A.A. Rapid inactivation of airborne bacteria using atmospheric pressure dielectric barrier grating discharge. IEEE Trans. Plasma Sci. 2007, 35, 1501–1510. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Randeniya, L.K.; De Groot, G.J.J.B. Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Ito, M.; Ohta, T.; Hori, M. Plasma agriculture. J. Korean Phys. Soc. 2012, 60, 937–943. [Google Scholar] [CrossRef]
- Ito, M.; Oh, J.S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym. 2018, 15, 1700073. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018, 15, 1700174. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.G.; Sun, D.W.; Cheng, J.H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Misra, N.N.; Schlüter, O.; Cullen, P.J.; Patrick, J. Cold Plasma in Food and Agriculture: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128014899. [Google Scholar]
- Kim, T.-K.; Yong, H.-I.; Jung, S.; Kim, H.-W.; Choi, Y.-S. Technologies for the production of meat products with a low sodium chloride content and improved quality characteristic—A Review. Foods 2021, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Von Woedtke, T.; Brandenburg, R.; Von, T.; Hagen, D.; Weltmann, K.-D.; Von Dem Hagen, T. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2011, 44, 013002. [Google Scholar] [CrossRef]
- Lu, X.; Laroussi, M.; Puech, V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci. Technol. 2012, 21, 034005. [Google Scholar] [CrossRef]
- Todorova, Y.; Yotinov, I.; Topalova, Y.; Benova, E.; Marinova, P.; Tsonev, I.; Bogdanov, T. Evaluation of the effect of cold atmospheric plasma on oxygenases’ activities for application in water treatment technologies. Environ. Technol. 2018, 40, 3783–3792. [Google Scholar] [CrossRef]
- Starikovskiy, A.; Yang, Y.; Cho, Y.I.; Fridman, A. Non-equilibrium plasma in liquid water: Dynamics of generation and quenching. Plasma Sources Sci. Technol. 2011, 20, 024003. [Google Scholar] [CrossRef]
- Ermolaeva, S.A.; Varfolomeev, A.F.; Chernukha, M.Y.; Yurov, D.S.; Vasiliev, M.M.; Kaminskaya, A.A.; Moisenovich, M.M.; Romanova, J.M.; Murashev, A.N.; Selezneva, I.I.; et al. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J. Med. Microbiol. 2011, 60, 75–83. [Google Scholar] [CrossRef]
- Joshi, S.G.; Paff, M.; Friedman, G.; Fridman, G.; Fridman, A.; Brooks, A.D. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: A biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Control 2010, 38, 293–301. [Google Scholar] [CrossRef]
- Cerf, O. Tailing of survival curves of bacterial spores. J. Appl. Bacteriol. 1977, 42, 1–19. [Google Scholar] [CrossRef]
- Xiong, R.; Xie, G.; Edmondson, A.E.; Sheard, M.A. A mathematical model for bacterial inactivation. Int. J. Food Microbiol. 1999, 46, 45–55. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Gao, J. Low temperature argon plasma sterilization effect on Pseudomonas aeruginosa and its mechanisms. J. Electrostat. 2009, 4, 646–651. [Google Scholar] [CrossRef]
- Yu, H.; Perni, S.; Shi, J.J.; Wang, D.Z.; Kong, M.G.; Shama, G. Effects of cell surface loading and phase of growth in cold atmospheric gas plasma inactivation of Escherichia coli K12. J. Appl. Microbiol. 2006, 101, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Aparecida Delben, J.; Evelin Zago, C.; Tyhovych, N.; Duarte, S.; Eduardo Vergani, C. Effect of Atmospheric-Pressure Cold Plasma on Pathogenic Oral Biofilms and In Vitro Reconstituted Oral Epithelium. PLoS ONE 2016, 11, e0155427. [Google Scholar] [CrossRef]
- Helgadóttir, S.; Pandit, S.; Mokkapati, V.R.S.S.; Westerlund, F.; Apell, P.; Mijakovic, I. Vitamin C pretreatment enhances the antibacterial effect of Cold Atmospheric Plasma. Front. Cell. Infect. Microbiol. 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Dobslaw, D.; Schulz, A.; Helbich, S.; Dobslaw, C.; Engesser, K.H. VOC removal and odor abatement by a low-cost plasma enhanced biotrickling filter process. J. Environ. Chem. Eng. 2017, 5, 5501–5511. [Google Scholar] [CrossRef]
- Karatum, O.; Deshusses, M.A. A comparative study of dilute VOCs treatment in a non-thermal plasma reactor. Chem. Eng. J. 2016, 294, 308–315. [Google Scholar] [CrossRef]
- Talebizadeh, P.; Babaie, M.; Brown, R.; Rahimzadeh, H.; Ristovski, Z.; Arai, M. The role of non-thermal plasma technique in NOx treatment: A review. Renew. Sustain. Energy Rev. 2014, 40, 886–901. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef]
- Schiavon, M.; Torretta, V.; Casazza, A.; Ragazzi, M. Non-thermal Plasma as an Innovative Option for the Abatement of Volatile Organic Compounds: A Review. Water Air Soil Pollut. 2017, 228, 1–20. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, D.; Qiu, R.; Tang, Y.; Du, C. Non-thermal plasma technology for organic contaminated soil remediation: A review. Chem. Eng. J. 2017, 313, 157–170. [Google Scholar] [CrossRef]
- Ho, G.S.; Faizal, H.M.; Ani, F.N. Microwave induced plasma for solid fuels and waste processing: A review on affecting factors and performance criteria. Waste Manag. 2017, 69, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Du, C.M.; Shi, T.H.; Sun, Y.W.; Zhuang, X.F. Decolorization of Acid Orange 7 solution by gas–liquid gliding arc discharge plasma. J. Hazard. Mater. 2008, 154, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, L.R.; Van Veldhuizen, E.M.; Pemen, A.J.M.; Rutgers, W.R. Corona Above Water Reactor for Systematic Study of Aqueous Phenol Degradation. Plasma Chem. Plasma Process. 2006, 26, 3–17. [Google Scholar] [CrossRef]
- Iya-Sou, D.; Laminsi, S.; Cavadias, S.; Ognier, S. Removal of Model Pollutants in Aqueous Solution by Gliding Arc Discharge: Determination of Removal Mechanisms. Part I: Experimental Study. Plasma Chem. Plasma Process. 2009, 33, 97–113. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, X.; Liu, Y. Degradation of bisphenol A and formation of hydrogen peroxide induced by glow discharge plasma in aqueous solutions. J. Hazard. Mater. 2008, 154, 1106–1114. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Li, X.; Zhang, J.; Chen, J.; Li, X. Remove of Phenolic Compounds in Water by Low-Temperature Plasma: A Review of Current Research. J. Water Resour. Prot. 2009, 1, 99–109. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.J.; Jiang, X.Z. Phenol degradation by a nonpulsed diaphragm glow discharge in an aqueous solution. Environ. Sci. Technol. 2005, 39, 8512–8517. [Google Scholar] [CrossRef]
- Yan, J.H.; Du, C.M.; Li, X.D.; Cheron, B.G.; Ni, M.J.; Cen, K.F. Degradation of Phenol in Aqueous Solutions by Gas–Liquid Gliding Arc Discharges. Plasma Chem. Plasma Process. 2006, 26, 31–41. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, S.; Wu, Y.; Ho, D. Non-thermal plasma technology for degradation of organic compounds in wastewater control: A critical review. J. Environ. Eng. Manag. 2007, 17, 427–433. [Google Scholar]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; David, V.; Medvedovici, A.; Bradu, C.; Parvulescu, V.I. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011, 45, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, R.; Wang, P.; Mai-Prochnow, A.; McConchie, R.; Li, W.; Zhou, R.; Thompson, E.W.; Ostrikov, K.K.; Cullen, P.J. Degradation of cefixime antibiotic in water by atmospheric plasma bubbles: Performance, degradation pathways and toxicity evaluation. Chem. Eng. J. 2021, 421, 127730. [Google Scholar] [CrossRef]
- Sanito, R.C.; Chen, Y.W.; You, S.J.; Yang, H.H.; Hsieh, Y.K.; Wang, Y.F. Hydrogen and Methane Production from Styrofoam Waste Using an Atmospheric-pressure Microwave Plasma Reactor. Aerosol Air Qual. Res. 2020, 20, 2226–2238. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Vlig, M.; Sobota, A.; Middelkoop, E.; Boekema, B.K.H.L. Safety and bactericidal efficacy of cold atmospheric plasma generated by a flexible surface Dielectric Barrier Discharge device against Pseudomonas aeruginosa in vitro and in vivo. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 37. [Google Scholar] [CrossRef]
- Cooley, C.R.; McLain, J.M.; Dupuy, S.D.; Eder, A.E.; Wintenberg, M.; Kelly-Wintenberg, K.; Wintenberg, A.; Collier, J.J.; Burke, S.J.; Karlstad, M.D. Indirect, Non-Thermal Atmospheric Plasma Promotes Bacterial Killing in vitro and Wound Disinfection in vivo Using Monogenic and Polygenic Models of Type 2 Diabetes (Without Adverse Metabolic Complications). Shock 2020, 54, 681–687. [Google Scholar] [CrossRef]



| Treated Media | Used Microorganisms | Treatment Conditions | Indicators for Assessment |
|---|---|---|---|
| Surfaces: bacterial thick films on nutrient agar plates | Pseudomonas aureofaciens AP-9 (now: chlororaphis)-aerobic, Gram-negative, motile, polar-flagellated, rod-shaped bacterium | Time: 5, 10, 15, 20 s Wave power: 17 and 20 W Bacterial densities: 90 × 105 cells/cm2 and 3 × 105 cells/cm2 |
|
| Liquids: bacterial suspensions in nutrient broth media (exponential phase of bacterial growth) |
| Time: 10, 30, 60 s Wave power: 20 W Bacterial densities: 52 × 107 cells/mL (for Pseudomonas) and 76 × 107 cells/mL (for Brevibacillus) Volume of treated suspensions: 35 mL |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, Y.; Benova, E.; Marinova, P.; Yotinov, I.; Bogdanov, T.; Topalova, Y. Non-Thermal Atmospheric Plasma for Microbial Decontamination and Removal of Hazardous Chemicals: An Overview in the Circular Economy Context with Data for Test Applications of Microwave Plasma Torch. Processes 2022, 10, 554. https://doi.org/10.3390/pr10030554
Todorova Y, Benova E, Marinova P, Yotinov I, Bogdanov T, Topalova Y. Non-Thermal Atmospheric Plasma for Microbial Decontamination and Removal of Hazardous Chemicals: An Overview in the Circular Economy Context with Data for Test Applications of Microwave Plasma Torch. Processes. 2022; 10(3):554. https://doi.org/10.3390/pr10030554
Chicago/Turabian StyleTodorova, Yovana, Evgenia Benova, Plamena Marinova, Ivaylo Yotinov, Todor Bogdanov, and Yana Topalova. 2022. "Non-Thermal Atmospheric Plasma for Microbial Decontamination and Removal of Hazardous Chemicals: An Overview in the Circular Economy Context with Data for Test Applications of Microwave Plasma Torch" Processes 10, no. 3: 554. https://doi.org/10.3390/pr10030554
APA StyleTodorova, Y., Benova, E., Marinova, P., Yotinov, I., Bogdanov, T., & Topalova, Y. (2022). Non-Thermal Atmospheric Plasma for Microbial Decontamination and Removal of Hazardous Chemicals: An Overview in the Circular Economy Context with Data for Test Applications of Microwave Plasma Torch. Processes, 10(3), 554. https://doi.org/10.3390/pr10030554








