Effectiveness of Low Copper-Containing Chemicals against Olive Leaf Spot Disease Caused by Venturia oleaginea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trial and Experimental Design
- (1)
- Copper complexed with lignosulphonic acid and gluconic acid (Copper complexed with LS and G acid) (Disper Cu Max®-Eden Modern Agriculture S.L-Spain), which contains 14% Cu and was applied at the dose of 150 g/100 L of water;
- (2)
- Self-defense inducer (Disper Broton GS®-Eden Modern Agriculture S.L-Spain) which contains 3.5% complexed Cu as well as 1.5% complexed Mn, 1% complexed Zn, 10% nitrogen (Ureic N); it was applied at the dose of 300 g/100 L of water;
- (3)
- Copper hydroxide (Fugoran®-Agri-Estrella-Mexico), which contains 70% Cu and was applied at the dose of 150 g/100 L of water;
- (4)
- Dodine (Syllit 544 SC), which was applied at the dose of 165 mL/100 L of water.
- (5)
- Control (untreated trees).
2.2. Defoliation and Infection Evaluations
2.3. Agronomic Evaluations
2.4. Statistical Analysis
3. Results
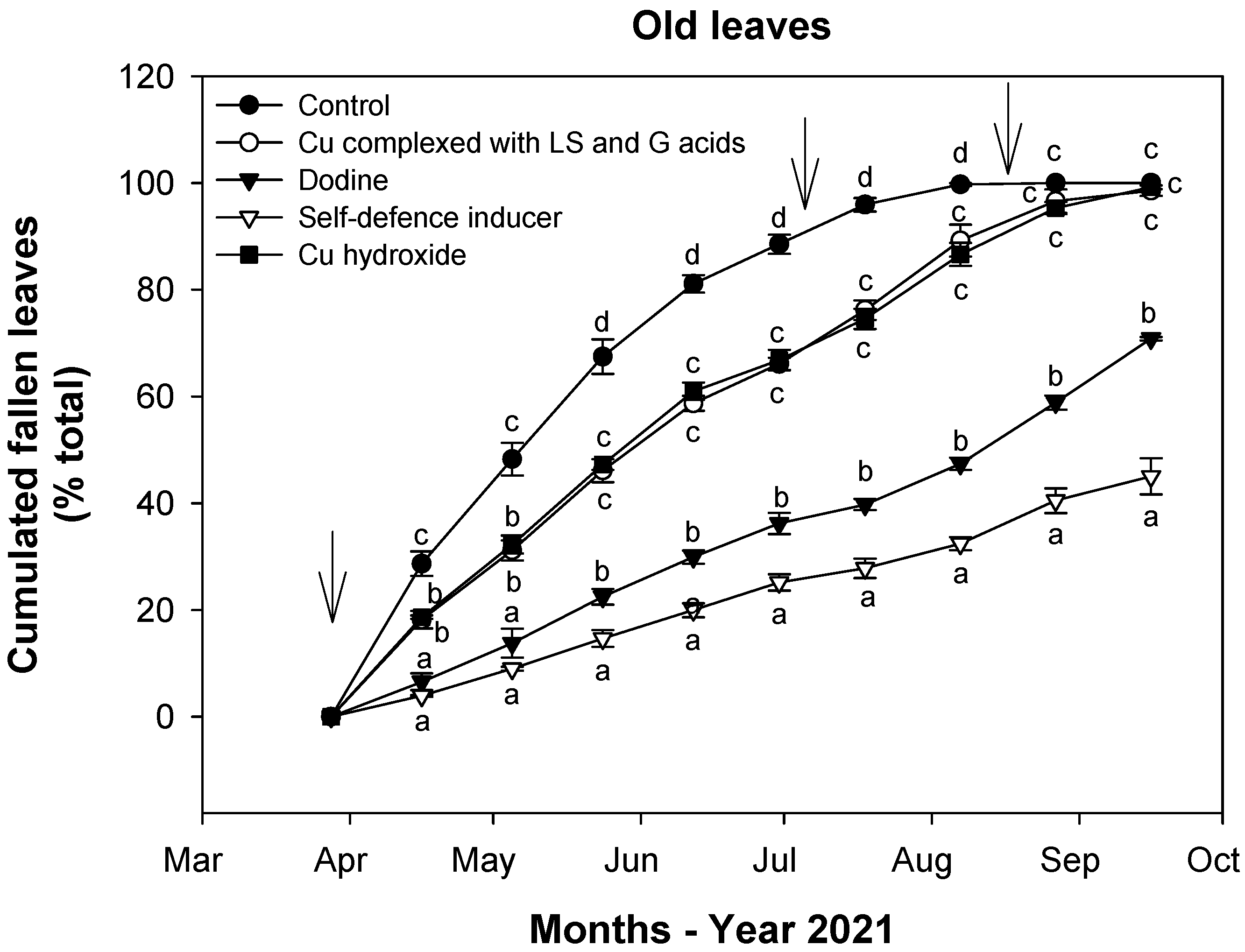
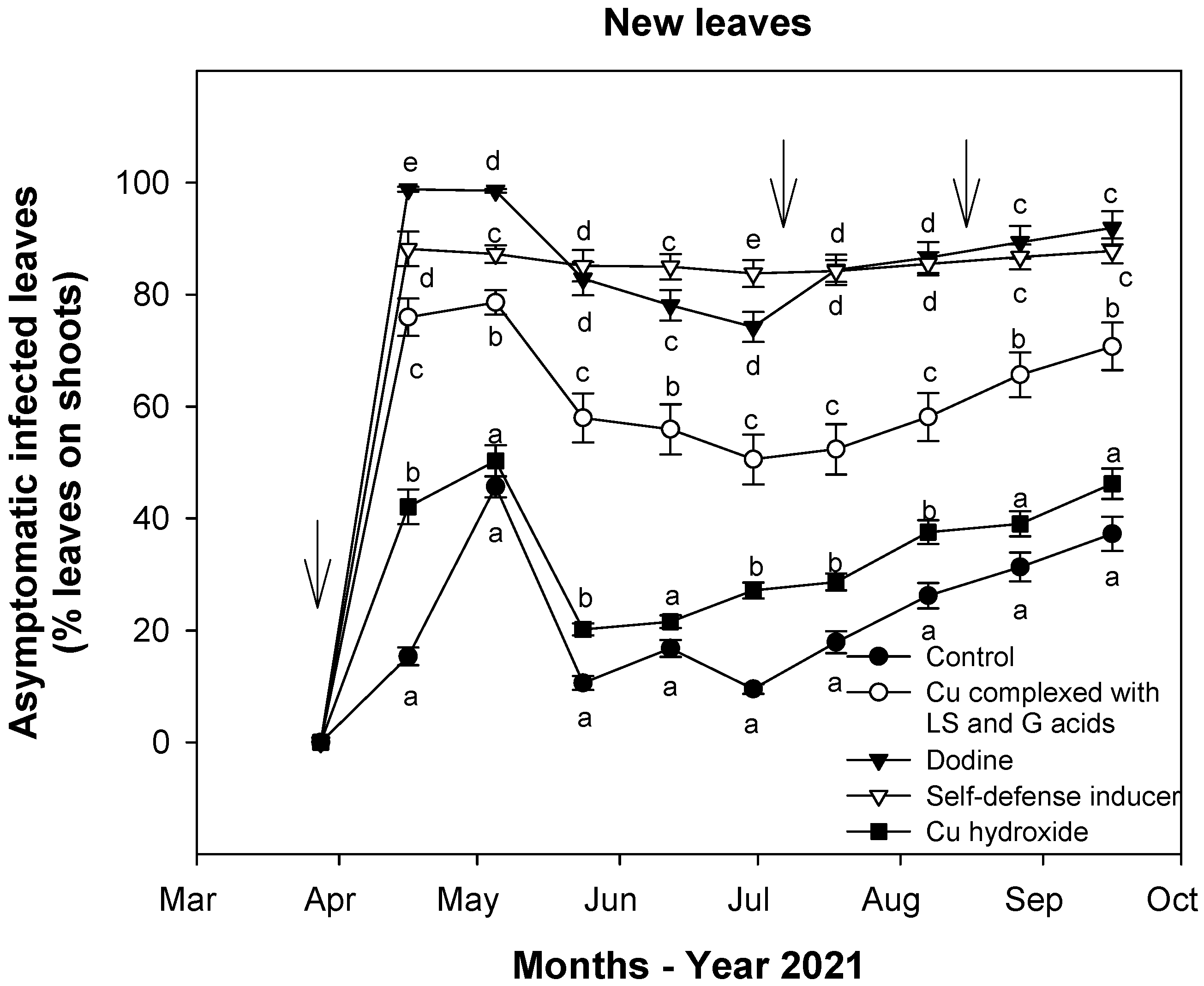
3.1. Effect of Treatments on Defoliation
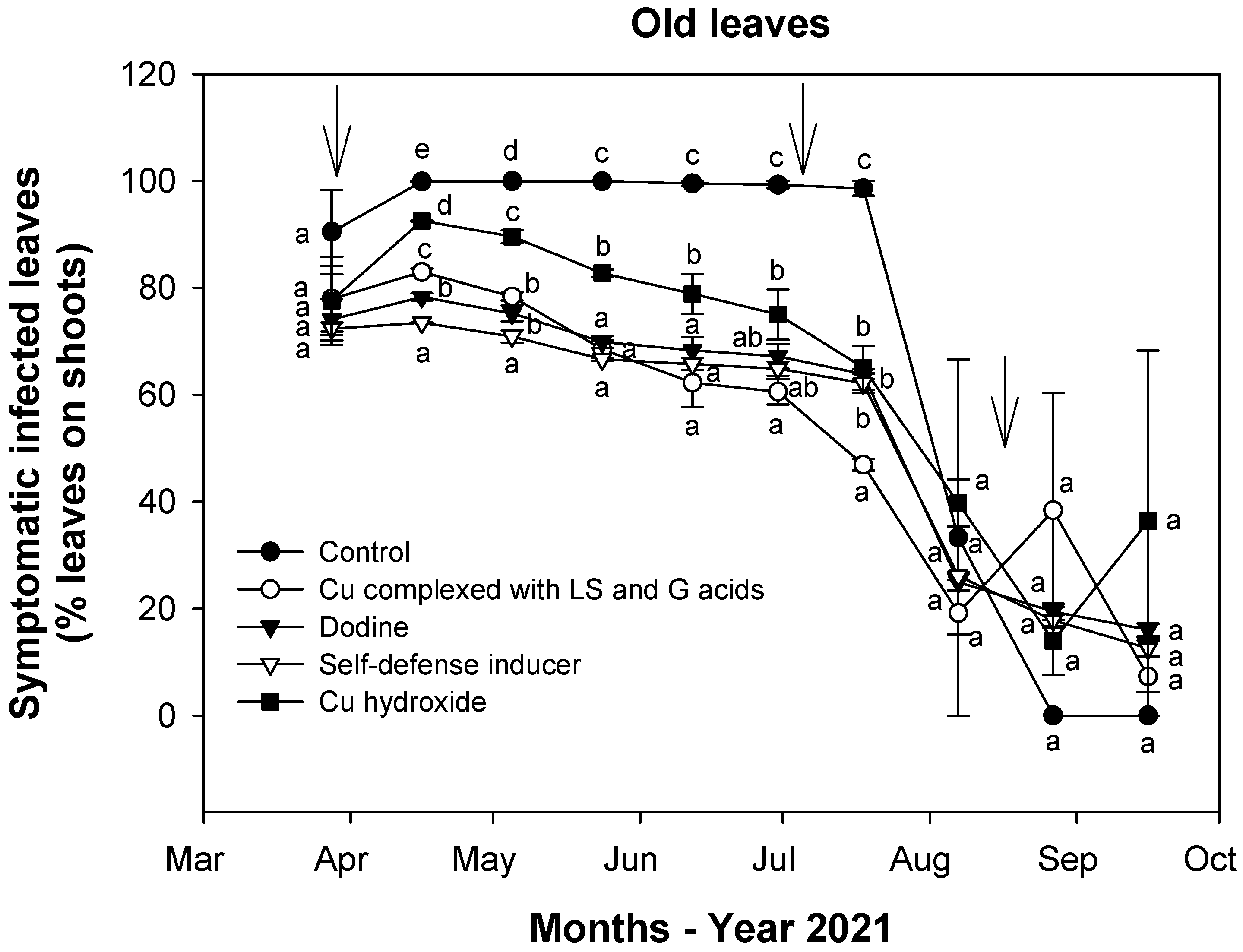
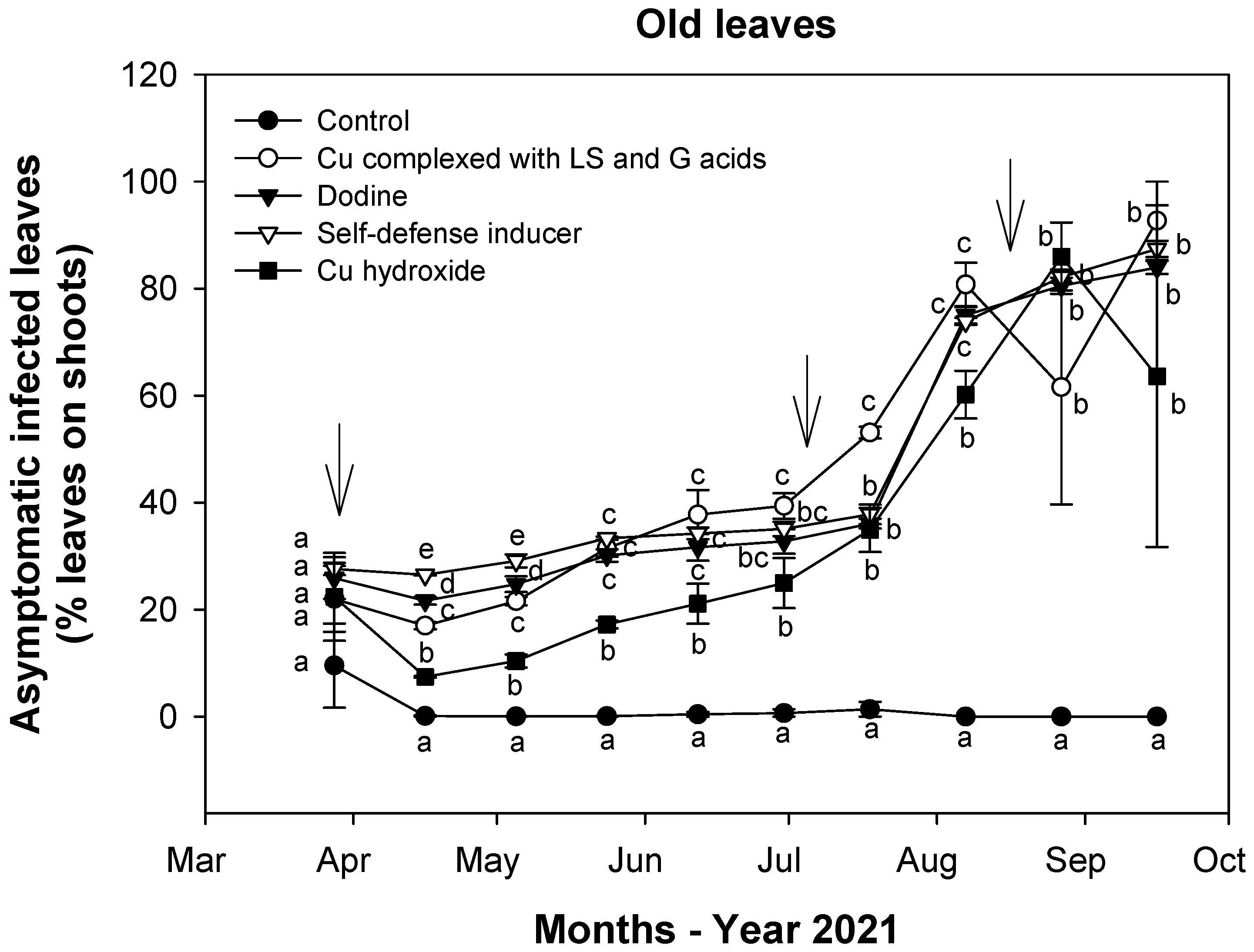
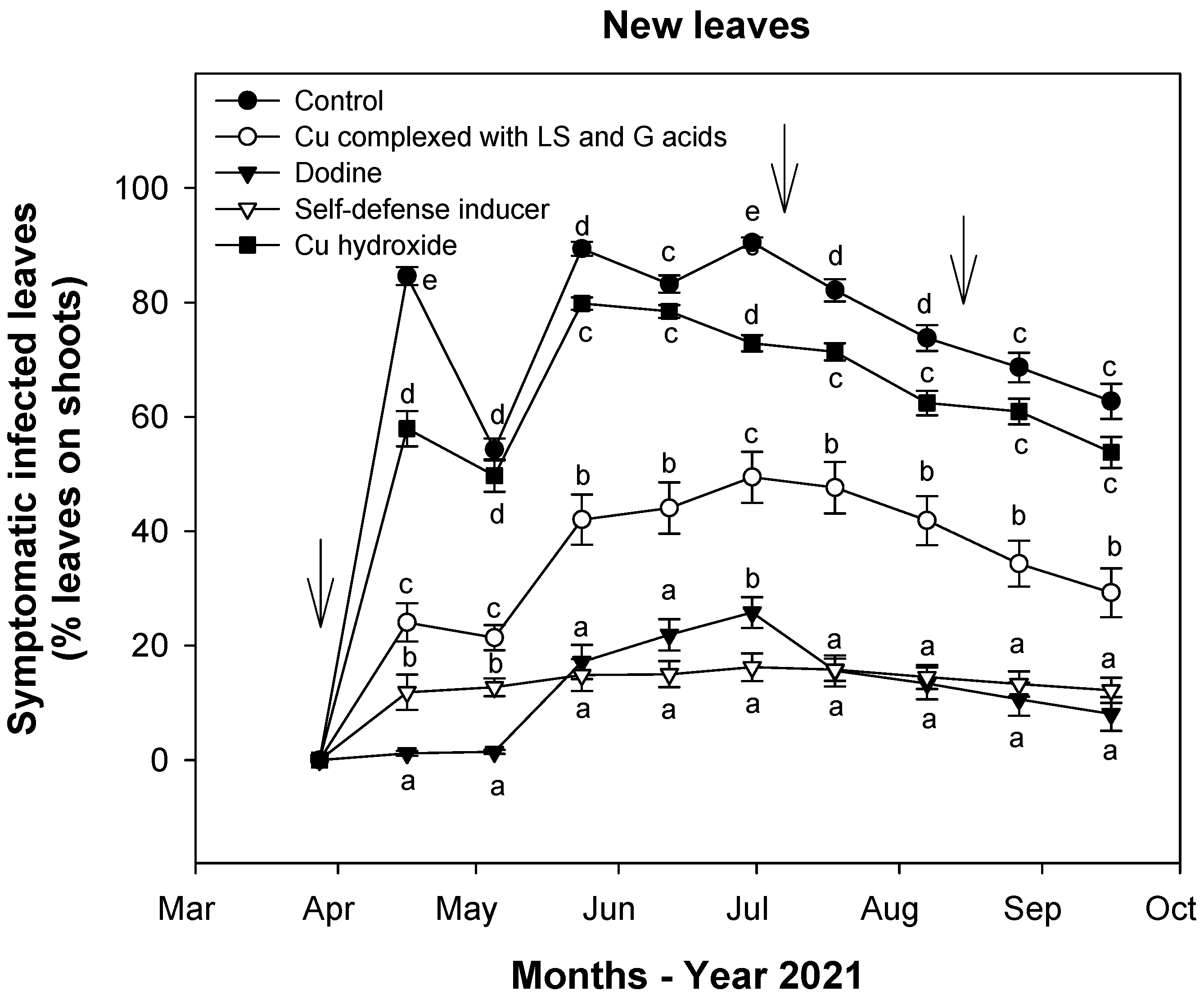
3.2. Effect of Treatments on Fungal Infections
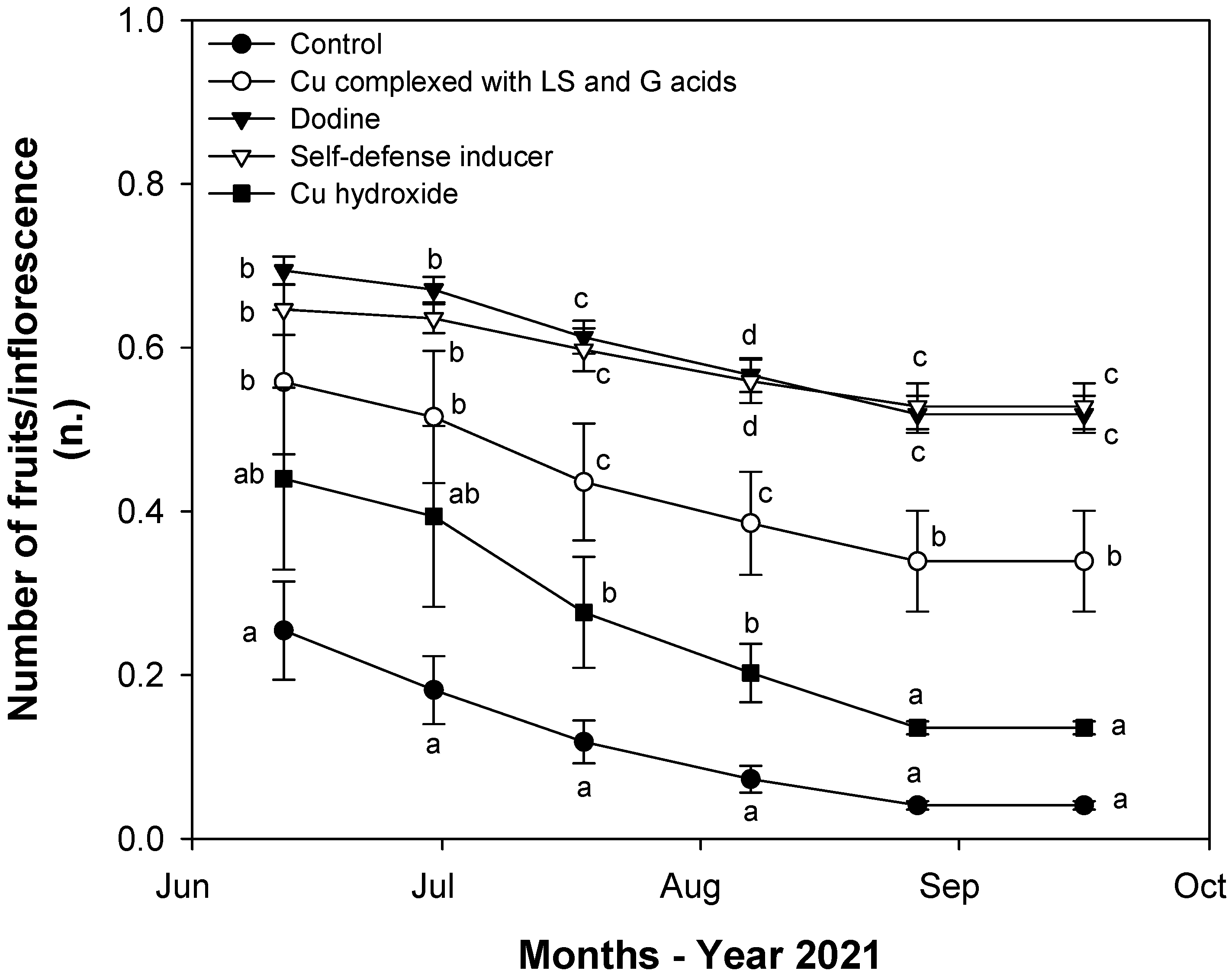
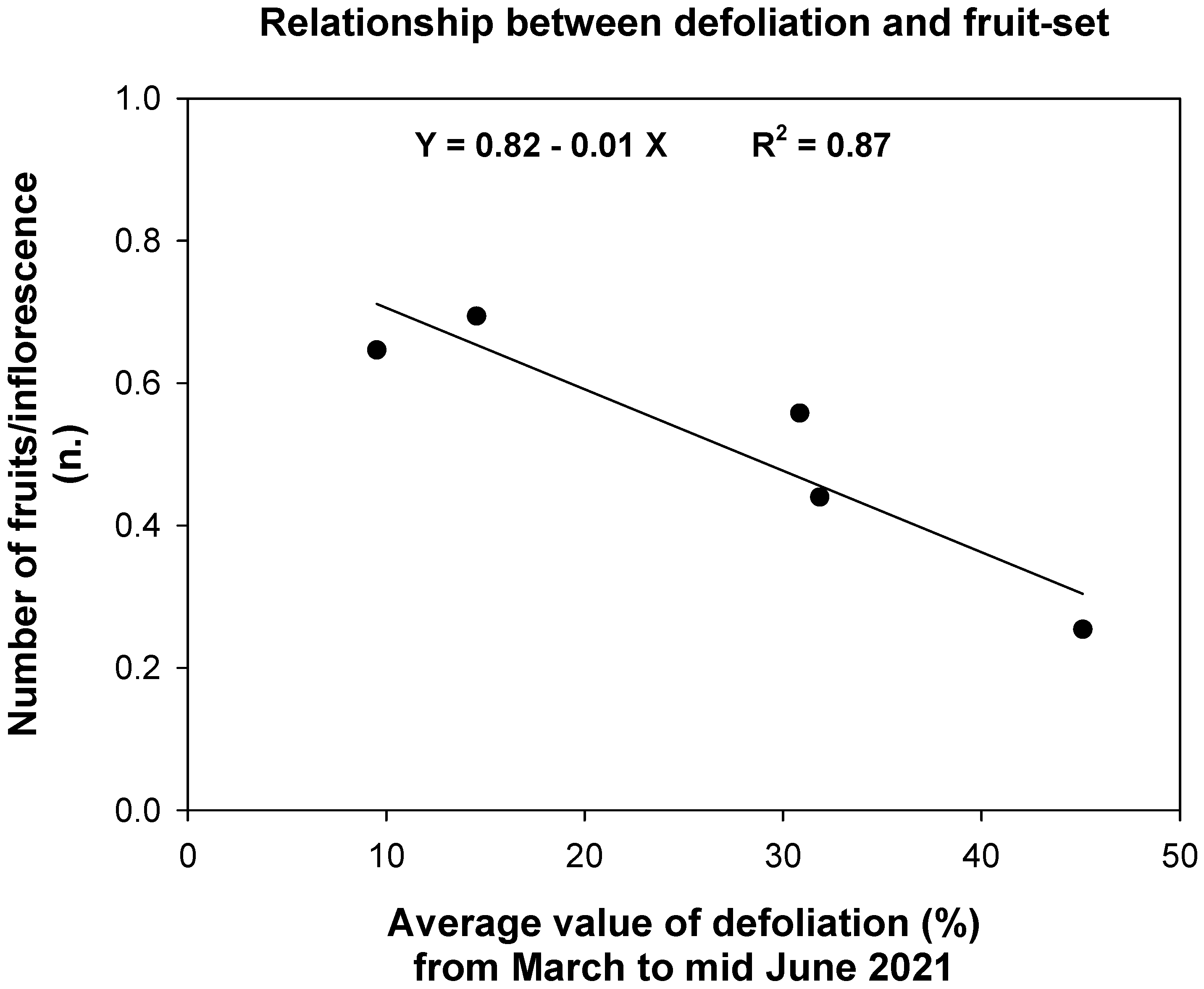
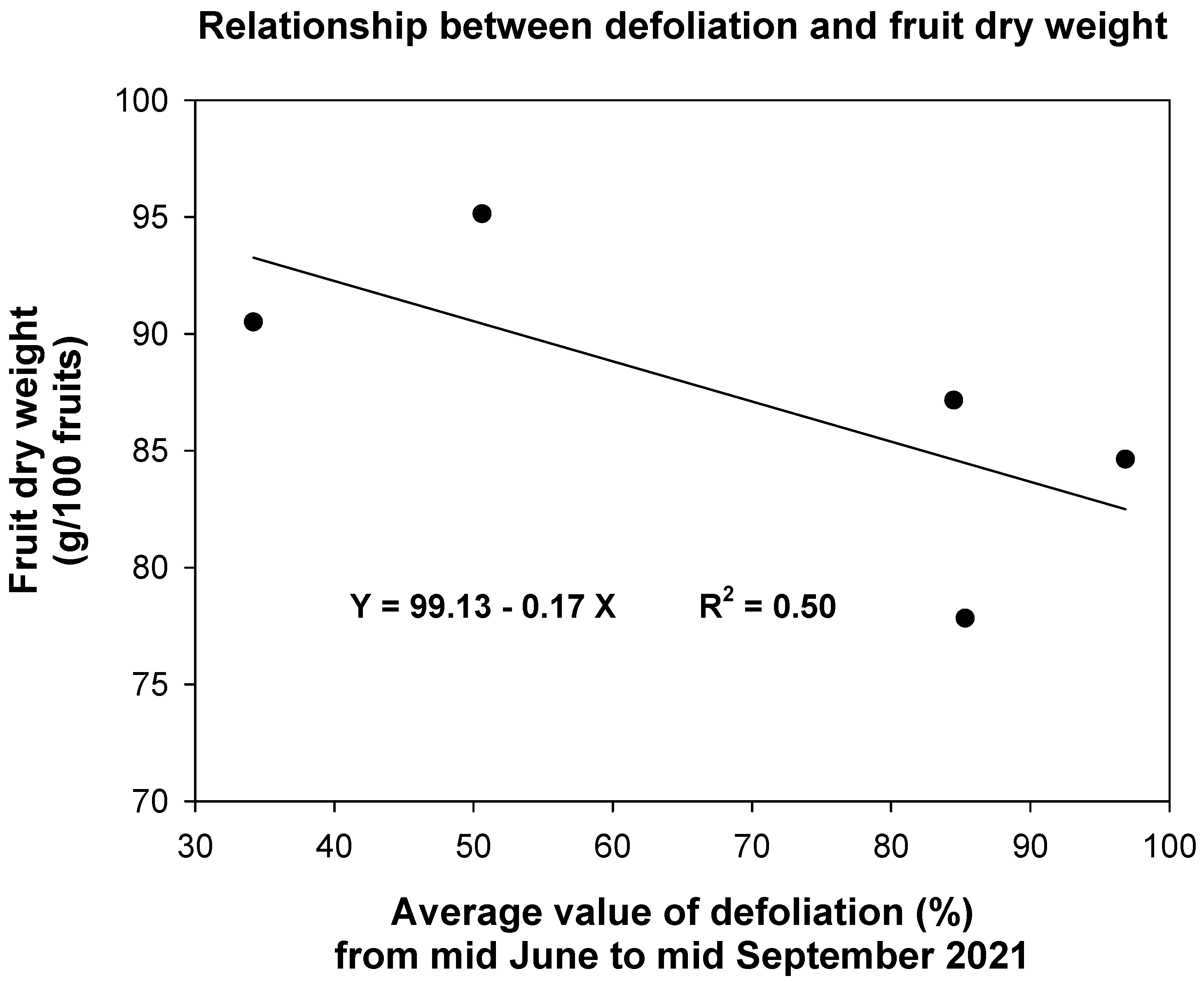
3.3. Effects of Fungal Infections on Fruit-Set and Fruit Dry Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. FAO (Food and Agricultural Organization of the United Nations). 2020. Available online: http://www.fao.org/faostat/en/#home (accessed on 30 December 2021).
- Rugini, E.; Baldoni, L.; Muleo, R.; Sebastiani, L. The Olive Tree Genome; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kleef, F.; Salman, M. Antifungal Effect of Ambrosia artemisiifolia L. Extract and Chemical Fungicide Against Spilocaea oleagina Causing Olive Leaf Spot. Arab. J. Sci. Eng. 2022, 43, 113–117. [Google Scholar] [CrossRef]
- Jazairi, L. The Road to Olive Farming: Challenge to Developing the Economy of Olive Oil in the West Bank; Oxfam International: Oxford, UK, 2010. [Google Scholar]
- MIFTAH. Olive trees More Than Just a Tree in Palestine. The Palestinian Initiative for the Promotion of Global Dialogue and Democracy. Available online: http://www.miftah.org/Doc/Factsheets/Miftah/English/factsheet-OliveTrees.pdf (accessed on 30 January 2021).
- Salman, M.; Hawamda, A.A.; Al-Ashqar Amarni, A.; Rahil, M.; Hajjeh, H.; Natsheh, B.; Abuamsha, R. Evaluation of the in-cidence and severity of olive leaf spot caused by Spilocaea oleagina on olive trees in Palestine. Am. J. Plant Sci. 2011, 2, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.; Roldán, R.A.; Álvarez, B.; Canale, M.; Couanon, W.; Gkisakis, V.; Milonas, P.; Nigro, F.; Nobre, T.; Pascual, S.R.; et al. Report of European Innovation Partnership for Agricultural Productivity and Sustainability Focus Group: Pests and Diseases of the Olive Tree; European Innovation Partnership for Agricultural productivity and Sustainability: Lisbon, Portugal, 2020. [Google Scholar]
- Azeri, T. Research on olive leaf spot, olive knot and verticillium wilt of olive in Turkey 1. EPPO Bull. 1993, 23, 437–440. [Google Scholar] [CrossRef]
- Obanor, F.O.; Jaspers, M.V.; Jones, E.E.; Walter, M. Greenhouse and field evaluation of fungicides for control of olive leaf spot in New Zealand. Crop Prot. 2008, 27, 1335–1342. [Google Scholar] [CrossRef]
- Obanor, F.O.; Walter, M.; Jones, E.E.; Jaspers, M.V. Efficacy of systemic acquired resistance inducers in olive leaf spot management. Australas. Plant Pathol. 2013, 42, 163–168. [Google Scholar] [CrossRef]
- Salman, M. Biological control of Spilocaea oleagina, the causal agent of olive leaf spot disease, using antagonistic bacteria. J. Plant Pathol. 2017, 99, 741–744. [Google Scholar]
- Almadi, L.; Jarrar, S.; Sbaihat, L.; Issa, T.; Tucci, M.; Buonaurio, R.; Famiani, F. Effects of peacock eye disease (Venturia oleaginea) on olive leaves and fruiting and evaluation of controlling it using fungicides with no or low content of copper. Agriculture 2022. submitted. [Google Scholar]
- D’Ascenzo, D.; Crivelli, L.; Camillo, L.D. New defence strategies against olive leaf spot in central Italy. In Proceedings of the “Giornate Fitopatologiche”, Chianciano Terme, Siena, Italy, 18–21 March 2014; Second Volume, pp. 139–146. [Google Scholar]
- Almadi, L.; Frioni, T.; Paoletti, A.; Cinosi, N.; Moretti, C.; Buonaurio, R.; Famiani, F. Dodine as an efficient alternative to copper for the control of peacock eye disease in highly infected olive trees. Agriculture 2022. submitted. [Google Scholar]
- Belfiore, T.; Perri, E.; Scalercio, S.; Iannotta, N.; Tocci, C. A Systemic Fungicide (Tetraconazole) against the Olive Leaf Spot, Spilocaea oleagina, in Italy. Acta Hortic. 2014, 1057, 185–189. [Google Scholar] [CrossRef]
- Iannotta, N.; Monardo, D.; Perri, L. Effects of Different Treatments against Spilocaea oleagina (Cast.) Hugh. Acta Hortic. 2002, 586, 741–744. [Google Scholar] [CrossRef]
- Ntasiou, P.; Kaldeli Kerou, A.; Karamanidou, T.; Vlachou, A.; Tziros, G.T.; Tsouknidas, A.; Karaoglanidis, G.S. Synthesis and Characterization of Novel Copper Nanoparticles for the Control of Leaf Spot and Anthracnose Diseases of Olive. Nanomaterials 2021, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Silva, K.; Roca-Castillo, L.; Benlloch-González, M.; Fernández-Escobar, R. Silicon Reduces the Incidence of Venturia oleaginea (Castagne) Rossman & Crous in Potted Olive Plants. HortScience 2019, 54, 1962–1966. [Google Scholar]
- Agosteo, G.; Schena, L. Olive leaf spot. Olive Dis. Disord. 2011, 2, 143–176. [Google Scholar]
- Graniti, A. Olive scab: A review. EPPO Bull. 1993, 23, 377–384. [Google Scholar] [CrossRef]
- Vitanovic, E. Use of Cu Fungicides in Vineyards and Olive Groves. In Fungicides for Plant and Animal Diseases; Dhanasekaran, D., Ed.; InTech: Rijeka, Croatia, 2010; pp. 279–298. Available online: http://www.intechopen.com/books/fungicides-for-plant-and-animal-diseases/use-of-cu-fungicides-in-vineyardsand-olive-groves (accessed on 1 February 2022)ISBN 978-953-307-804-5.
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Issa, T.; Almadi, L.; Jarrar, S.; Tucci, M.; Buonaurio, R.; Famiani, F. Factors affecting Venturia oleaginea infections on olive and effects of the disease on floral biology. Phytopathol. Mediterr. 2019, 58, 221–229. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Polverigiani, S.; Ali, S.; Mutawea, M.; Qutub, M.; Pierini, F.; Neri, D. Effect of complementary irrigation on yield components and alternate bearing of a traditional olive orchard in semi-arid conditions. Span. J. Agric. Res. 2016, 14, e1203. [Google Scholar] [CrossRef] [Green Version]
- Lodolini, E.M.; Polverigiani, S.; Ali, S.; Mutawea, M.; Qutub, M.; Arabasi, T.; Pierini, F.; Abed, M.; Neri, D. Oil characteristics of four Palestinian olive varieties. J. Oleo Sci. 2017, 66, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Abuamsha, R.; Abueid, M.; Hajjeh, H.; Salman, M. Evaluation of the Incidence and Severity of Olive Leaf Spot caused by Spilocaea oleagina in different olive cultivars in Palestine. J. Agric. Environ. Int. Dev. (JAEID) 2013, 107, 201–212. [Google Scholar]
- Duffy, B.; Défago, G. Macro-and microelement fertilizers influence the severity of Fusarium crown and root rot of tomato in a soilless production system. HortScience 1999, 34, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Quaglia, M.; Bocchini, M.; Orfei, B.; D’Amato, R.; Famiani, F.; Moretti, C.; Buonaurio, R. Zinc phosphate protects tomato plants against Pseudomonas syringae pv. tomato. J. Plant Dis. Prot. 2021, 128, 989–998. [Google Scholar] [CrossRef]
- Walters, D.R.; Newton, A.C.; Lyon, G.D. Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Proietti, P.; Nasini, L.; Famiani, F. Effect of different leaf-to-fruit ratios on photosynthesis and fruit growth in olive (Olea europaea L.). Photosynthetica 2006, 44, 275–285. [Google Scholar] [CrossRef]
- Teviotdale, B.; Sibbett, G. Consistent annual treatment helps future olive leaf spot control. Calif. Agric. 1995, 49, 27–32. [Google Scholar] [CrossRef] [Green Version]
| Treatment | Metallic Copper (g/ha) |
|---|---|
| Control | 0 |
| Copper complexed with LS and G acid (Disper Cu Max®) | 210 |
| Dodine | 0 |
| Self-defense inducer (Disper Broton GS®) | 105 |
| Copper hydroxide | 1050 |
| Treatment | Symptomatic Leaves (% Total) | Asymptomatic Infected Leaves (% Total) | Symptomatic + Asymptomatic Infected (% Total) |
|---|---|---|---|
| Control | 90.4 a | 9.6 a | 100.0 a |
| Copper complexed with LS and G acid | 78.0 a | 22.0 a | 100.0 a |
| Dodine | 74.1 a | 25.9 a | 100.0 a |
| Self-defense inducer | 72.4 a | 27.6 a | 100.0 a |
| Copper hydroxide | 77.6 a | 22.4 a | 100.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adawi, A.; Jarrar, S.; Almadi, L.; Alkowni, R.; Gallo, M.; D’Onghia, A.M.; Buonaurio, R.; Famiani, F. Effectiveness of Low Copper-Containing Chemicals against Olive Leaf Spot Disease Caused by Venturia oleaginea. Agriculture 2022, 12, 326. https://doi.org/10.3390/agriculture12030326
Adawi A, Jarrar S, Almadi L, Alkowni R, Gallo M, D’Onghia AM, Buonaurio R, Famiani F. Effectiveness of Low Copper-Containing Chemicals against Olive Leaf Spot Disease Caused by Venturia oleaginea. Agriculture. 2022; 12(3):326. https://doi.org/10.3390/agriculture12030326
Chicago/Turabian StyleAdawi, Amer, Samer Jarrar, Leen Almadi, Raed Alkowni, Marilita Gallo, Anna Maria D’Onghia, Roberto Buonaurio, and Franco Famiani. 2022. "Effectiveness of Low Copper-Containing Chemicals against Olive Leaf Spot Disease Caused by Venturia oleaginea" Agriculture 12, no. 3: 326. https://doi.org/10.3390/agriculture12030326
APA StyleAdawi, A., Jarrar, S., Almadi, L., Alkowni, R., Gallo, M., D’Onghia, A. M., Buonaurio, R., & Famiani, F. (2022). Effectiveness of Low Copper-Containing Chemicals against Olive Leaf Spot Disease Caused by Venturia oleaginea. Agriculture, 12(3), 326. https://doi.org/10.3390/agriculture12030326









