Reduction in O-GlcNAcylation Mitigates the Severity of Inflammatory Response in Cerulein-Induced Acute Pancreatitis in a Mouse Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and In Vivo Glucose Homeostasis Assessment
2.2. Pancreatitis Induction
2.3. Histological Analysis
2.4. Serum Analysis and Western Blotting
2.5. RT-qPCR
2.6. Statistical Analysis
3. Results
3.1. Body Weight, Pancreas Weight, and Non-Fasted Blood Glucose Was Not Affected by Reduced Pancreatic OGT
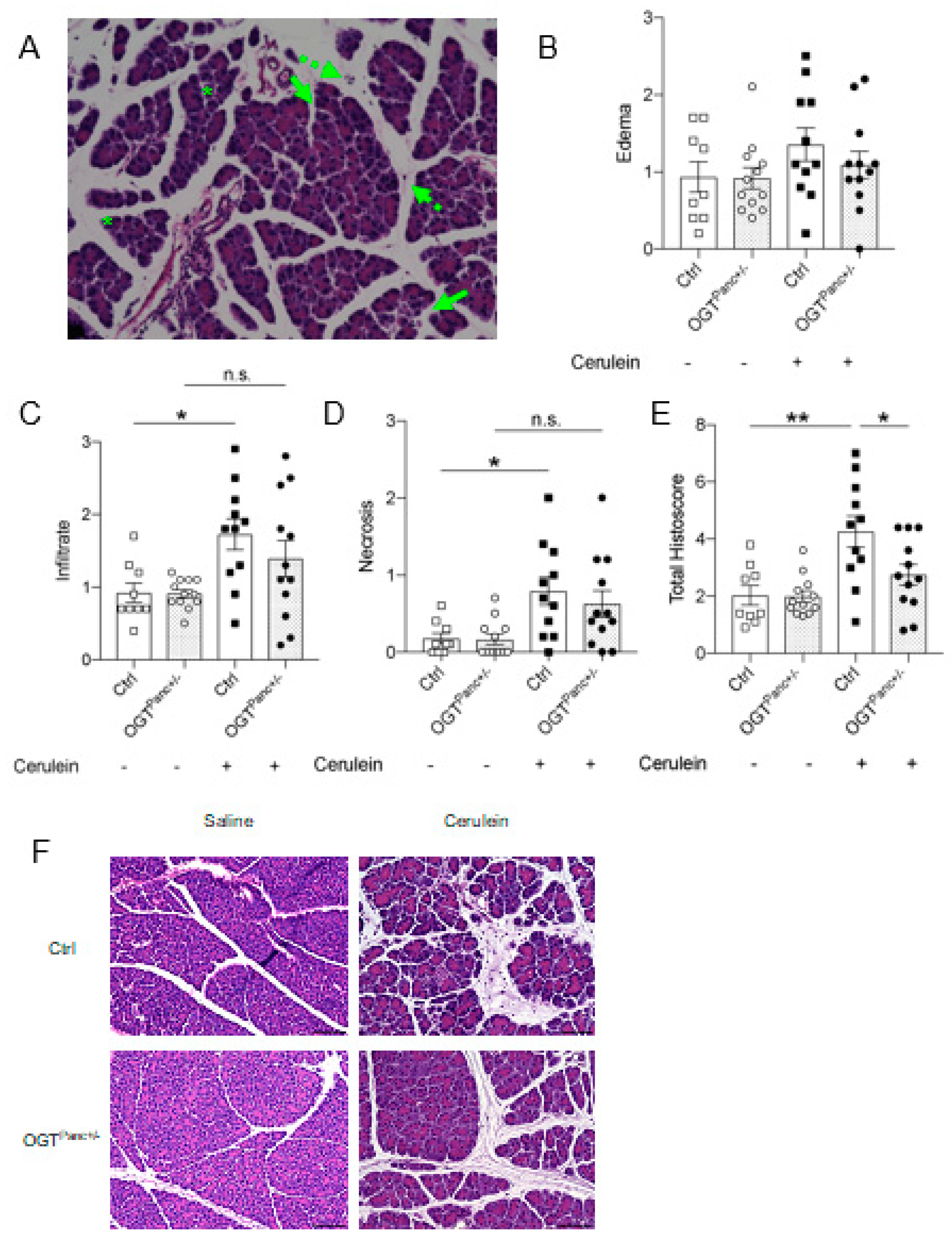
3.2. Mice with Reduced Pancreatic OGT Had Lower Histological Severity of Acute Pancreatitis
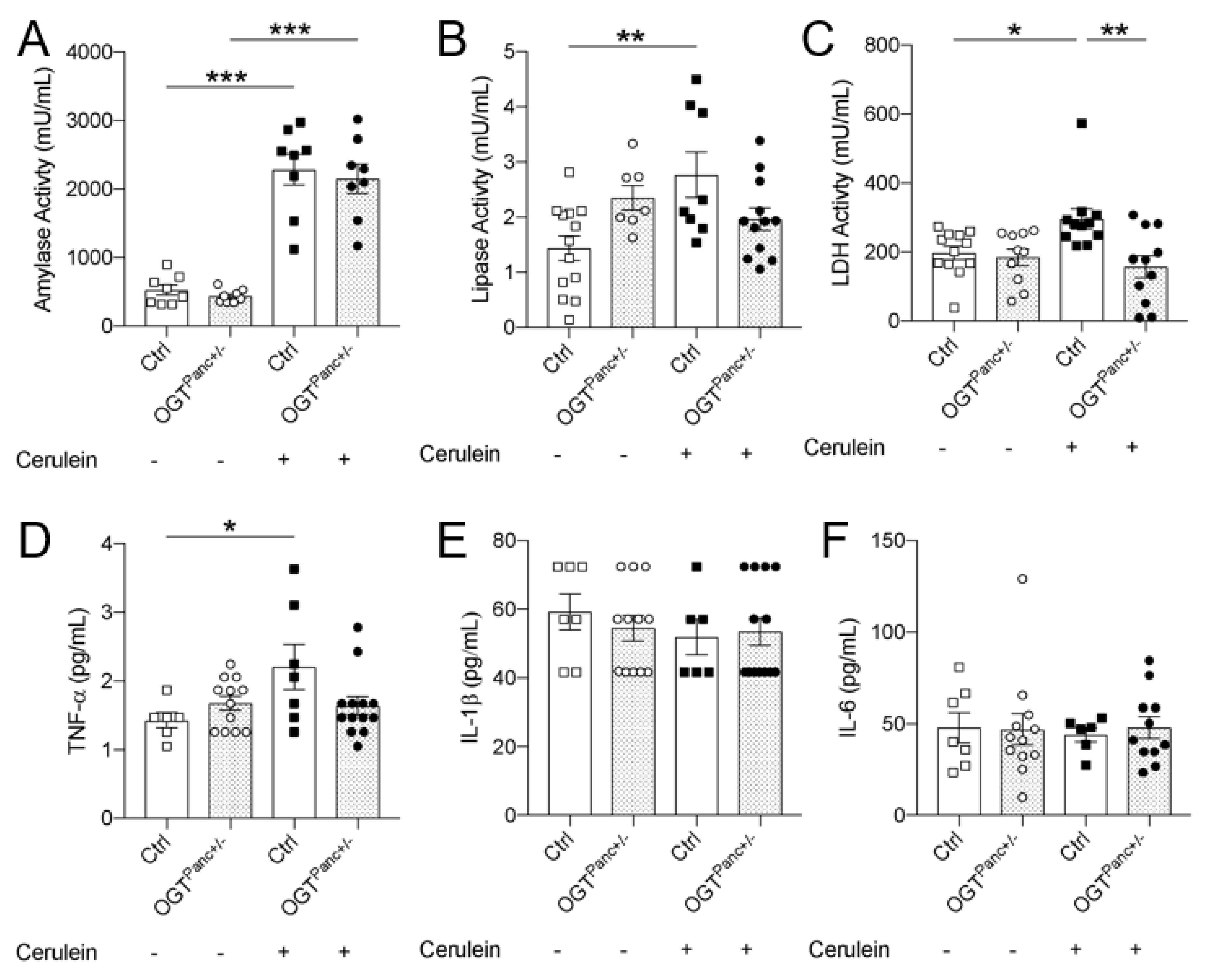
3.3. Lipase and LDH Was Not Elevated with Pancreatitis Induction in Mice with Reduced Pancreatic OGT
3.4. OGTPanc+/− Mice Did Not Exhibit an Increase in Serum TNF-α with Induction of Pancreatitis
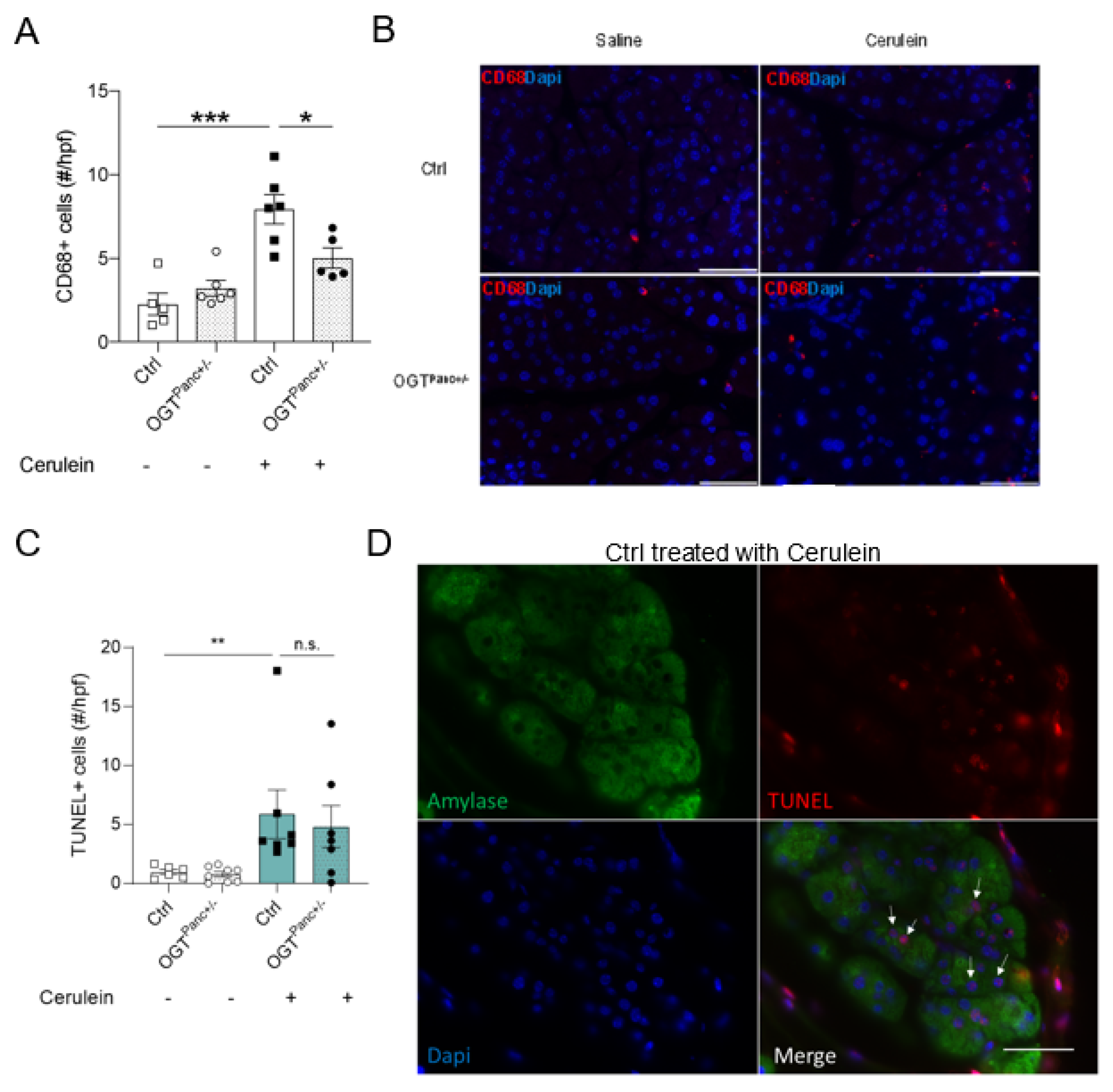
3.5. Mice with Reduced Pancreatic OGT Had Similar Levels of Cell Death and without a Significant Increase in Macrophage Infiltrate following Cerulein Treatment
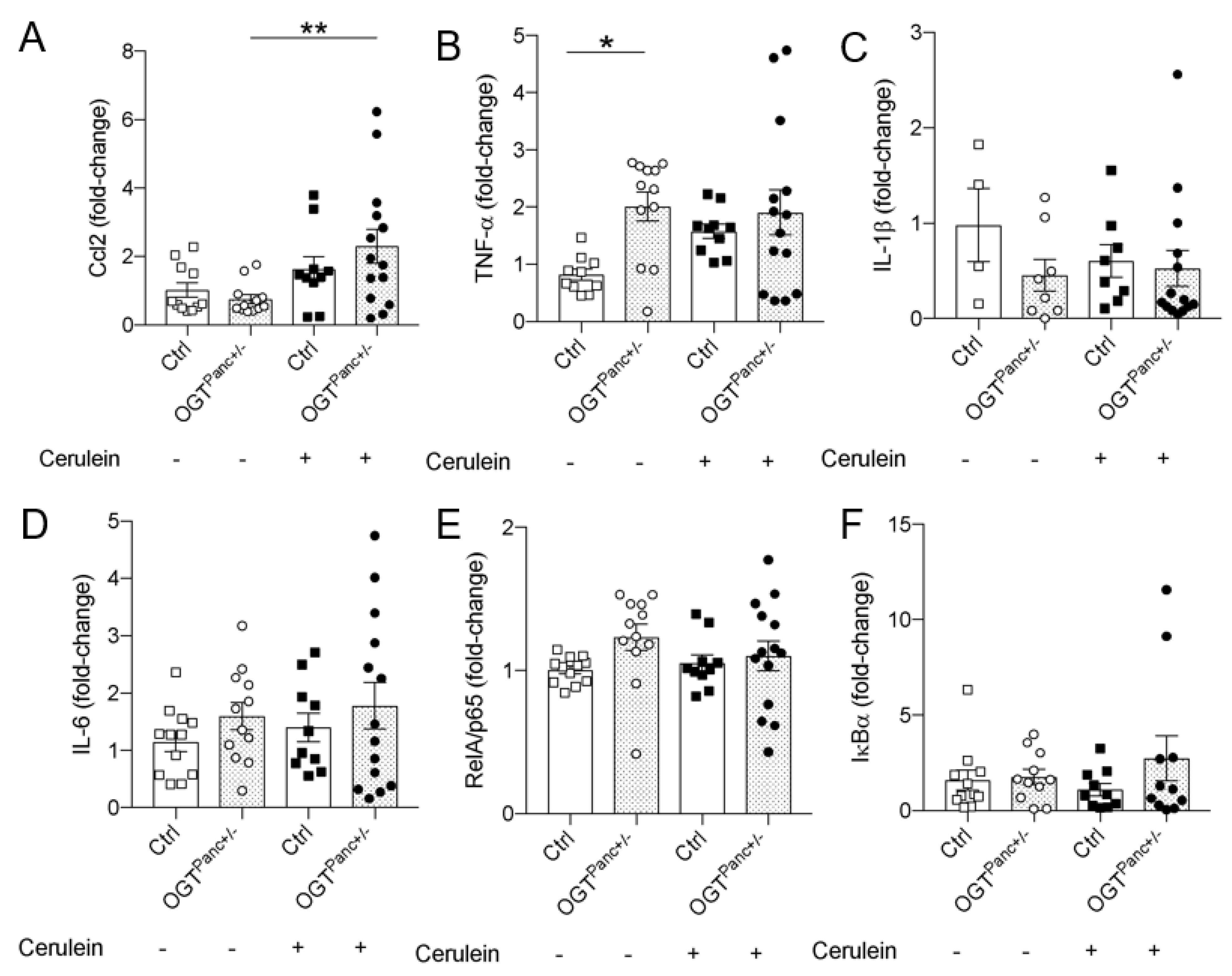
3.6. Ccl2 mRNA Was Elevated in Cerulein Treated OGTPanc+/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Spanier, B.; Dijkgraaf, M.; Bruno, M. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: An update. Best Pr. Res. Clin. Gastroenterol. 2008, 22, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, B.; Saluja, A.K.; Lerch, M.M.; Bhagat, L.; Bhatia, M.; Lee, H.S.; Steer, M.L. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am. J. Physiol. 1998, 275, G352–G362. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Lowe, M.E. Human Pancreatic Digestive Enzymes. Am. J. Dig. Dis. 2007, 52, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, F.S.; Thrower, E. The Acinar Cell and Early Pancreatitis Responses. Clin. Gastroenterol. Hepatol. 2009, 7 (Suppl. 11), S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O. Ca2+ signaling in pancreatic acinar cells: Physiology and pathophysiology. Braz. J. Med. Biol. Res. 2009, 42, 9–16. [Google Scholar] [CrossRef]
- Biczo, G.; Vegh, E.T.; Shalbueva, N.; Mareninova, O.A.; Elperin, J.; Lotshaw, E.; Gretler, S.; Lugea, A.; Malla, S.R.; Dawson, D.; et al. Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models. Gastroenterology 2018, 154, 689–703. [Google Scholar] [CrossRef]
- Saluja, A.; Dudeja, V.; Dawra, R.; Sah, R.P. Early Intra-Acinar Events in Pathogenesis of Pancreatitis. Gastroenterology 2019, 156, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Halangk, W.; Lerch, M.M.; Brandt-Nedelev, B.; Roth, W.; Ruthenbuerger, M.; Reinheckel, T.; Domschke, W.; Lippert, H.; Peters, C.; Deussing, J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Investig. 2000, 106, 773–781. [Google Scholar] [CrossRef]
- Dawra, R.; Sah, R.P.; Dudeja, V.; Rishi, L.; Talukdar, R.; Garg, P.; Saluja, A.K. Intra-acinar Trypsinogen Activation Mediates Early Stages of Pancreatic Injury but Not Inflammation in Mice With Acute Pancreatitis. Gastroenterology 2011, 141, 2210–2217.e2. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, R.; Sareen, A.; Zhu, H.; Yuan, Z.; Dixit, A.; Cheema, H.; George, J.; Barlass, U.; Sah, R.; Garg, S.K.; et al. Release of Cathepsin B in Cytosol Causes Cell Death in Acute Pancreatitis. Gastroenterology 2016, 151, 747–758.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cai, Y.; Chen, M.; Gao, L.; Shen, Y.; Huang, Z. OGT-mediated O-GlcNAcylation promotes NF-kappaB activation and inflammation in acute pancreatitis. Inflamm. Res. 2015, 64, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Bimboese, P.; Gibson, C.J.; Schmidt, S.; Xiang, W.; Ehrlich, B.E. Isoform-specific Regulation of the Inositol 1,4,5-Trisphosphate Receptor by O-Linked Glycosylation. J. Biol. Chem. 2011, 286, 15688–15697. [Google Scholar] [CrossRef] [PubMed]
- Cividini, F.; Scott, B.T.; Dai, A.; Han, W.; Suarez, J.; Diaz-Juarez, J.; Dillmann, W.H. O-GlcNAcylation of 8-Oxoguanine DNA Glycosylase (Ogg1) Impairs Oxidative Mitochondrial DNA Lesion Repair in Diabetic Hearts. J. Biol. Chem. 2016, 291, 26515–26528. [Google Scholar] [CrossRef] [PubMed]
- Chatham, J.C.; Zhang, J.; Wende, A.R. Role of O-Linked N-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology. Physiol. Rev. 2021, 101, 427–493. [Google Scholar] [CrossRef]
- Lima, V.V.; Giachini, F.R.; Hardy, D.M.; Webb, R.C.; Tostes, R.C. O-GlcNAcylation: A novel pathway contributing to the effects of endothelin in the vasculature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R236–R250. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Men, L.; Du, J. O-GlcNAcylation in immunity and inflammation: An intricate system (Review). Int. J. Mol. Med. 2019, 44, 363–374. [Google Scholar] [CrossRef]
- Gurel, Z.; Sheibani, N. O-Linked β-. Clin. Sci. 2018, 132, 185–198. [Google Scholar] [CrossRef]
- Raman, V.S.; Loar, R.; Renukuntla, V.S.; Hassan, K.V.; Fishman, D.S.; Gilger, M.A.; Heptulla, R.A. Hyperglycemia and Diabetes Mellitus in Children with Pancreatitis. J. Pediatr. 2011, 158, 612–616.e1. [Google Scholar] [CrossRef]
- Shenoy, S.; Cody, D.; Rickett, A.; Swift, P. Acute Pancreatitis and its Association with Diabetes Mellitus in Children. J. Pediatr. Endocrinol. Metab. 2004, 17, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Levine, Z.G.; Walker, S. The Biochemistry of O-GlcNAc Transferase: Which Functions Make It Essential in Mammalian Cells? Annu. Rev. Biochem. 2016, 85, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Phoomak, C.; Vaeteewoottacharn, K.; Sawanyawisuth, K.; Seubwai, W.; Wongkham, C.; Silsirivanit, A.; Wongkham, S. Mechanistic insights of O-GlcNAcylation that promote progression of cholangiocarcinoma cells via nuclear translocation of NF-kappaB. Sci. Rep. 2016, 6, 27853. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.S.; Lee, S.; Min, K.Y.; Choi, W.S.; You, J.S. Hexosamine Biosynthetic Pathway-Derived O-GlcNAcylation Is Critical for RANKL-Mediated Osteoclast Differentiation. Int. J. Mol. Sci. 2021, 22, 8888. [Google Scholar] [CrossRef]
- Yang, X.; Yao, L.; Fu, X.; Mukherjee, R.; Xia, Q.; Jakubowska, M.A.; Huang, W. Experimental Acute Pancreatitis Models: History, Current Status, and Role in Translational Research. Front. Physiol. 2020, 11, 614591. [Google Scholar] [CrossRef]
- Baumann, D.; Wong, A.; Akhaphong, B.; Jo, S.; Pritchard, S.; Mohan, R.; Alejandro, E.U. Role of nutrient-driven O-GlcNAc-posttranslational modification in pancreatic exocrine and endocrine islet development. Development 2020, 147, dev186643. [Google Scholar] [CrossRef]
- Gannon, M.; Herrera, P.L.; Wright, C.V. Mosaic Cre-mediated recombination in pancreas using the pdx-1 enhancer/promoter. Genesis 2000, 26, 143–144. [Google Scholar] [CrossRef]
- Lockridge, A.; Jo, S.; Gustafson, E.; Damberg, N.; Mohan, R.; Olson, M.; Alejandro, E.U. Islet O-GlcNAcylation Is Required for Lipid Potentiation of Insulin Secretion through SERCA2. Cell Rep. 2020, 31, 107609. [Google Scholar] [CrossRef]
- Lerch, M.M.; Gorelick, F.S. Models of acute and chronic pancreatitis. Gastroenterology 2013, 144, 1180–1193. [Google Scholar] [CrossRef]
- Moreno, C.; Nicaise, C.; Gustot, T.; Quertinmont, E.; Nagy, N.; Parmentier, M.; Louis, H.; Devière, J. Chemokine receptor CCR5 deficiency exacerbates cerulein-induced acute pancreatitis in mice. Am. J. Physiol. Liver Physiol. 2006, 291, G1089–G1099. [Google Scholar] [CrossRef]
- Maher, M.M.; Dessouky, B.A.M. Simplified Early Predictors of Severe Acute Pancreatitis: A Prospective Study. Gastroenterol. Res. 2010, 3, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jochheim, L.S.; Odysseos, G.; Hidalgo-Sastre, A.; Zhong, S.; Staufer, L.M.; Kroiss, M.; Kabacaoglu, D.; Lange, S.; Engleitner, T.; Hartmann, D.; et al. The neuropeptide receptor subunit RAMP1 constrains the innate immune response during acute pancreatitis in mice. Pancreatology 2019, 19, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xiong, J.; Zhang, Y.; Peng, T.; Huang, M.; Lin, Y.; Guo, Y.; Wu, H.; Wang, C. Serum lactate dehydrogenase is predictive of persistent organ failure in acute pancreatitis. J. Crit. Care 2017, 41, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Sendler, M.; Weiss, F.-U.; Golchert, J.; Homuth, G.; Brandt, C.V.D.; Mahajan, U.M.; Partecke, L.-I.; Döring, P.; Gukovsky, I.; Gukovskaya, A.; et al. Cathepsin B-Mediated Activation of Trypsinogen in Endocytosing Macrophages Increases Severity of Pancreatitis in Mice. Gastroenterology 2018, 154, 704–718.e10. [Google Scholar] [CrossRef]
- Zechner, D.; Sempert, K.; Genz, B.; Timm, F.; Bürtin, F.; Kroemer, T.; Butschkau, A.; Kuhla, A.; Vollmar, B. Impact of hyperglycemia and acute pancreatitis on the receptor for advanced glycation endproducts. Int. J. Clin. Exp. Pathol. 2013, 6, 2021–2029. [Google Scholar]
- Zechner, D.; Spitzner, M.; Bobrowski, A.; Knapp, N.; Kuhla, A.; Vollmar, B. Diabetes aggravates acute pancreatitis and inhibits pancreas regeneration in mice. Diabetologia 2012, 55, 1526–1534. [Google Scholar] [CrossRef]
- Hart, G.W. Nutrient regulation of immunity: O-GlcNAcylation regulates stimulus-specific NF-κB-dependent transcription. Sci. Signal. 2013, 6, pe26. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Clark, P.M.; Mason, D.E.; Peters, E.C.; Hsieh-Wilson, L.C.; Baltimore, D. Activation of the transcriptional function of the NF-κB protein c-Rel by O-GlcNAc glycosylation. Sci. Signal. 2013, 6, ra75. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Bozadjieva, N.; Kumusoglu, D.; Abdulhamid, S.; Levine, H.; Haataja, L.; Bernal-Mizrachi, E. Disruption of O-linked N-Acetylglucosamine Signaling Induces ER Stress and beta Cell Failure. Cell Rep. 2015, 13, 2527–2538. [Google Scholar] [CrossRef]
- Mohan, R.; Jo, S.; Lockridge, A.; Ferrington, D.A.; Murray, K.; Eschenlauer, A.; Bernal-Mizrachi, E.; Fujitani, Y.; Alejandro, E.U. OGT Regulates Mitochondrial Biogenesis and Function via Diabetes Susceptibility Gene Pdx1. Diabetes 2021, 70, 2608–2625. [Google Scholar] [CrossRef]
- Mohan, R.; Jo, S.; Da Sol Chung, E.; Oribamise, E.; Lockridge, A.; Abrahante-Lloréns, J.E.; Alejandro, E.U. Pancreatic beta-Cell O-GlcNAc Transferase Overexpression Increases Susceptibility to Metabolic Stressors in Female Mice. Cells 2021, 10, 2801. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, L.; Issad, T. O-GlcNAcylation and Inflammation: A Vast Territory to Explore. Front. Endocrinol. 2014, 5, 235. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Luan, H.H.; Zhang, B.; Zhang, K.; Nam, J.H.; Li, Z.; Fu, M.; Munk, A.; Zhang, D.; et al. OGT suppresses S6K1-mediated macrophage inflammation and metabolic disturbance. Proc. Natl. Acad. Sci. USA 2020, 117, 16616–16625. [Google Scholar] [CrossRef] [PubMed]
- James, L.R.; Tang, D.; Ingram, A.; Ly, H.; Thai, K.; Cai, L.; Scholey, J.W. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB- dependent promoter activation. Diabetes 2002, 51, 1146–1156. [Google Scholar] [CrossRef]
- Yang, W.H.; Park, S.Y.; Nam, H.W.; Kim, D.H.; Kang, J.G.; Kang, E.S.; Kim, Y.S.; Cho, J.W. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc. Natl. Acad. Sci. USA 2008, 105, 17345–17350. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.F.; Wamsley, J.J.; Kumar, M.; Li, D.; Gray, L.G.; Hart, G.W.; Mayo, M.W. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-κB acetylation and transcription. Proc. Natl. Acad. Sci. USA 2012, 109, 16888–16893. [Google Scholar] [CrossRef]
- Golks, A.; Tran, T.T.; Goetschy, J.F. Guerini D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 2007, 26, 4368–4379. [Google Scholar] [CrossRef]
- Zou, L.; Yang, S.; Hu, S.; Chaudry, I.H.; Marchase, R.B.; Chatham, J.C. The protective effects of PUGNAc on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels. Shock 2007, 27, 402–408. [Google Scholar] [CrossRef]
- Zou, L.; Yang, S.; Champattanachai, V.; Hu, S.; Chaudry, I.H.; Marchase, R.B.; Chatham, J.C. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-{kappa}B signaling. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H515–H523. [Google Scholar] [CrossRef]
- Hilgers, R.H.; Xing, D.; Gong, K.; Chen, Y.F.; Chatham, J.C.; Oparil, S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H513–H522. [Google Scholar] [CrossRef]
- Xing, D.; Gong, K.; Feng, W.; Nozell, S.E.; Chen, Y.F.; Chatham, J.C.; Oparil, S. O-GlcNAc modification of NFκB p65 inhibits TNF-α-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS ONE 2011, 6, e24021. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, M.; Avula, N.; Wong, A.; Beetch, M.; Jo, S.; Alejandro, E.U. Reduction in O-GlcNAcylation Mitigates the Severity of Inflammatory Response in Cerulein-Induced Acute Pancreatitis in a Mouse Model. Biology 2022, 11, 347. https://doi.org/10.3390/biology11030347
Moore M, Avula N, Wong A, Beetch M, Jo S, Alejandro EU. Reduction in O-GlcNAcylation Mitigates the Severity of Inflammatory Response in Cerulein-Induced Acute Pancreatitis in a Mouse Model. Biology. 2022; 11(3):347. https://doi.org/10.3390/biology11030347
Chicago/Turabian StyleMoore, Mackenzie, Nandini Avula, Alicia Wong, Megan Beetch, Seokwon Jo, and Emilyn U. Alejandro. 2022. "Reduction in O-GlcNAcylation Mitigates the Severity of Inflammatory Response in Cerulein-Induced Acute Pancreatitis in a Mouse Model" Biology 11, no. 3: 347. https://doi.org/10.3390/biology11030347
APA StyleMoore, M., Avula, N., Wong, A., Beetch, M., Jo, S., & Alejandro, E. U. (2022). Reduction in O-GlcNAcylation Mitigates the Severity of Inflammatory Response in Cerulein-Induced Acute Pancreatitis in a Mouse Model. Biology, 11(3), 347. https://doi.org/10.3390/biology11030347





