Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment
Abstract
1. Introduction
2. Diabetes Mellitus and Insulin Resistance
3. Diabetes Mellitus and Insulin Resistance Preventive Mechanism by Edible Mushroom
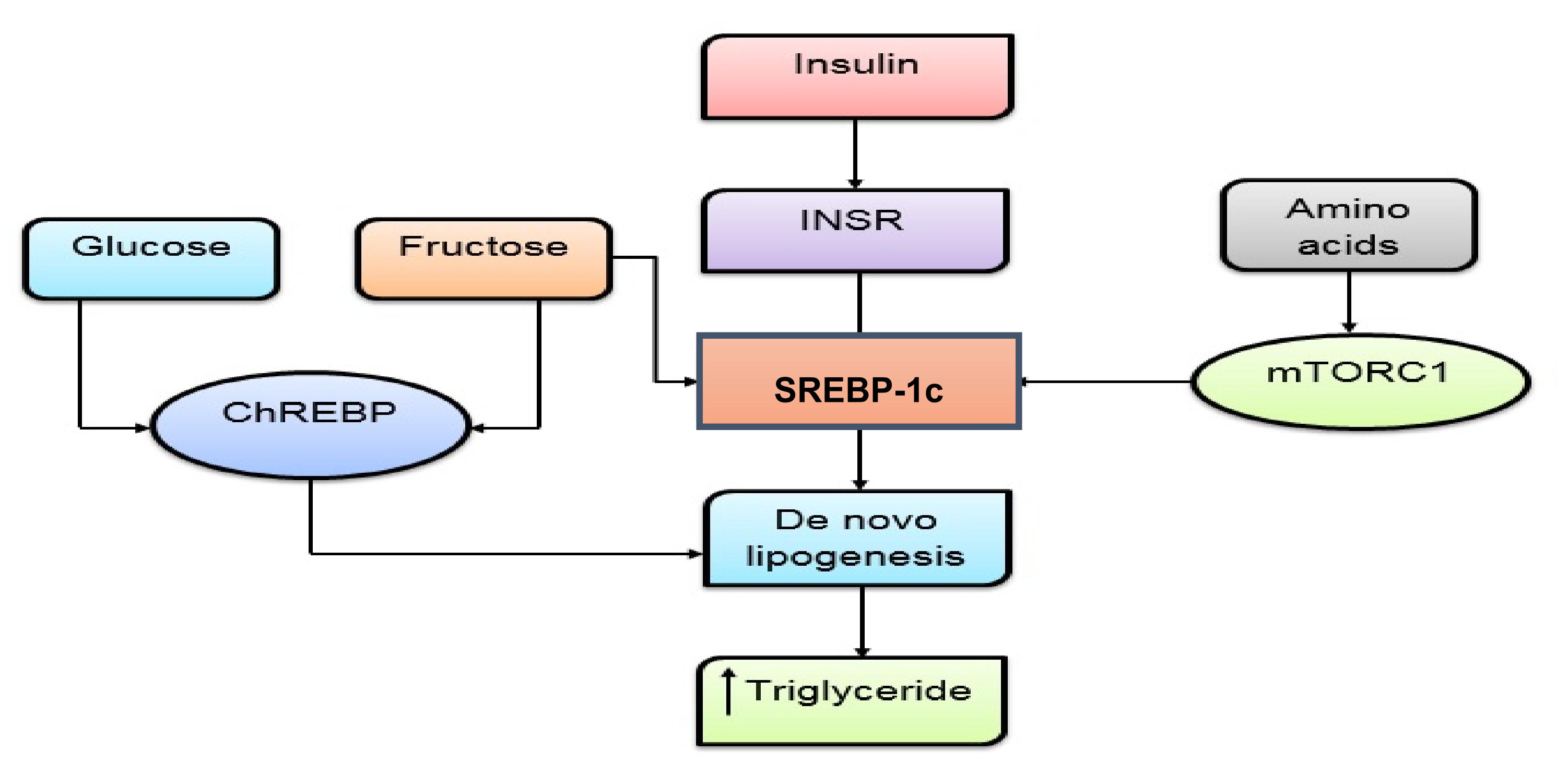
3.1. Blood Glucose-Lowering Effect of Polysaccharide
3.1.1. Inhibition of Glucose Absorption
3.1.2. Maintains Pancreatic ß Cells Activity
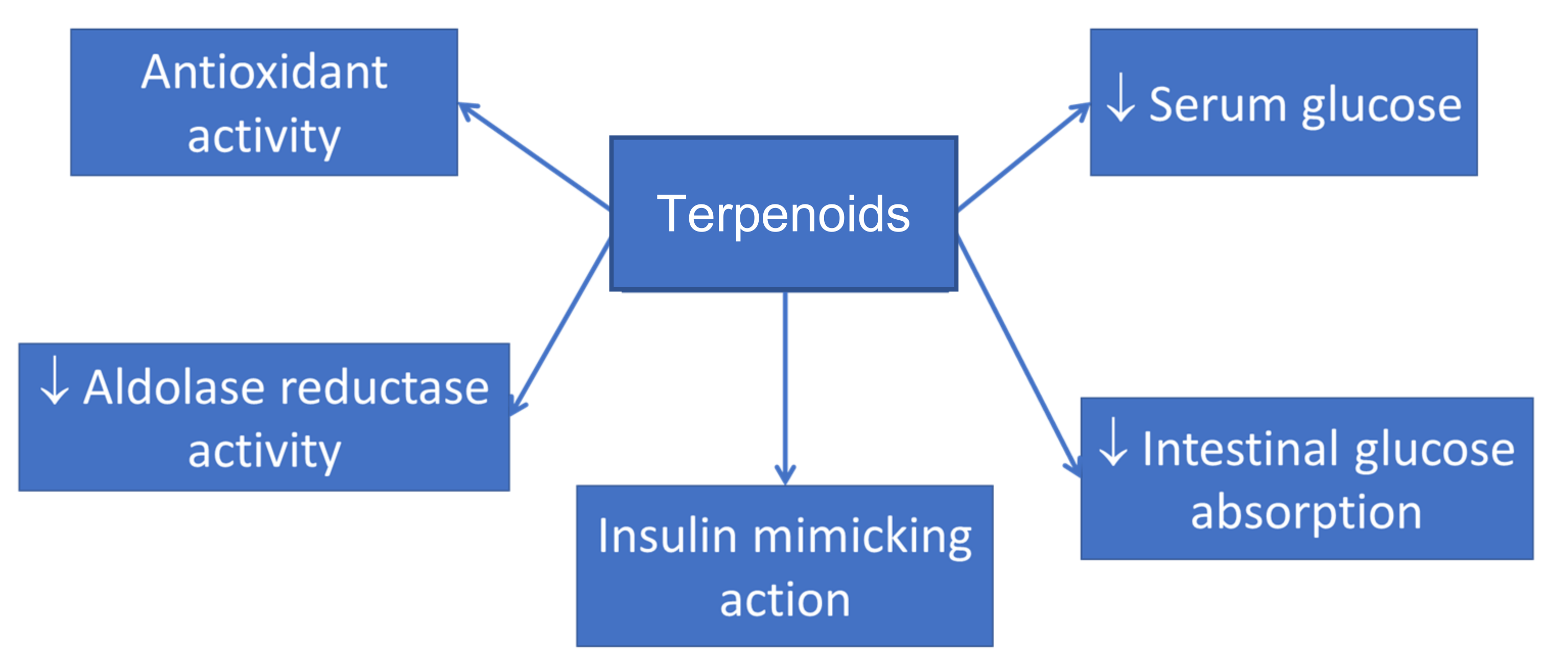
3.2. Blood Glucose-Lowering Effect of Terpenoids
3.3. Role of Vitamin D in Blood Glucose Regulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ukwuru, M.; Muritala, A.; Eze, L. Edible and non-edible wild mushrooms: Nutrition, toxicity and strategies for recognition. J. Clin. Nutr. Metab. 2018, 2, 9. [Google Scholar]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and algae as sources of medicinal and other biologically active compounds: A review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef] [PubMed]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Tagkouli, D.; Bekiaris, G.; Kaliora, A.; Tsiaka, T.; Tsiantas, K.; Chatzipavlidis, I.; Zoumpoulakis, P.; Kalogeropoulos, N.; Zervakis, G.I. Enhancing the nutritional and functional properties of Pleurotus citrinopileatus mushrooms through the exploitation of winery and olive mill wastes. Food Chem. 2022, 370, 131022. [Google Scholar] [CrossRef]
- Yadav, D.R. Edible Mushrooms. 2010. Available online: https://www.researchgate.net/publication/322210506_EDIBLE_MUSHROOMS?channel=doi&linkId=5a4bbf790f7e9b8284c2ded5&showFulltext=true (accessed on 19 January 2022).
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible mushrooms as functional ingredients for development of healthier and more sustainable muscle foods: A flexitarian approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.P.; Barh, A.; Bairwa, R.; Annepu, S.K.; Thakur, B.; Kamal, S. Enoki mushroom (Flammulina velutipes (Curtis) Singer) breeding. In Advances in Plant Breeding Strategies: Vegetable Crops; Springer: Cham, Switzerland, 2021; pp. 423–441. [Google Scholar]
- Tiane, C.; Finimundy, T.; José, A.; Dillon, P.; Antônio, J.; Henriques, J.A.; Ely, M. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr. Sci. 2014, 5, 1095–1105. [Google Scholar] [CrossRef]
- Song, Q.; Teng, A.G.; Zhu, Z. Chemical structure and inhibition on α-glucosidase of a novel polysaccharide from Hypsizygus marmoreus. J. Mol. Struct. 2020, 1211, 128110. [Google Scholar] [CrossRef]
- Kleftaki, S.A.; Simati, S.; Amerikanou, C.; Gioxari, A.; Tzavara, C.; Zervakis, G.I.; Kalogeropoulos, N.; Kokkinos, A.; Kaliora, A.C. Pleurotus eryngii improves postprandial glycaemia, hunger and fullness perception, and enhances ghrelin suppression in people with metabolically unhealthy obesity. Pharmacol. Res. 2022, 175, 105979. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Polysaccharides in Grifola frondosa mushroom and their health promoting properties: A review. Int. J. Biol. Macromol. 2017, 101, 910–921. [Google Scholar] [CrossRef]
- Diyabalanage, T.; Mulabagal, V.; Mills, G.; Dewitt, D.; Nair, M. Health-beneficial qualities of the edible mushroom, Agrocybe aegerita. Food Chem. 2008, 108, 97–102. [Google Scholar] [CrossRef]
- Rahman, M.; Akter, R. Diabetes ameliorating effect of mushrooms. J. Nov. Physiother. 2021, 2, 9–13. [Google Scholar]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Clericuzio, M.; Armijos, C.; Vidari, G. Bioactive phytochemical constituents of wild edible mushrooms from southeast Asia. Molecules 2020, 25, 1972. [Google Scholar] [CrossRef] [PubMed]
- Ogbole, O.O.; Noleto-Dias, C.; Kamdem, R.S.T.; Akinleye, T.E.; Nkumah, A.; Ward, J.L.; Beale, M.H. γ-Glutamyl-β-phenylethylamine, a novel α-glucosidase and α-amylase inhibitory compound from Termitomyces robustus, an edible Nigerian mushroom. Nat. Prod. Res. 2021, 1–11. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Tung, Y.T.; Pan, C.H.; Chien, Y.W.; Huang, H.Y. Edible mushrooms: Novel medicinal agents to combat metabolic syndrome and associated diseases. Curr. Pharm. Des. 2020, 26, 4970–4981. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yan, J.; Yang, L.; Meng, Y.; Wang, N.; He, C.; Fan, Y.; Zhou, Y. Alkali-soluble polysaccharides from mushroom fruiting bodies improve insulin resistance. Int. J. Biol. Macromol. 2019, 126, 466–474. [Google Scholar] [CrossRef]
- Dasgupta, A.; Acharya, K. Mushrooms: An emerging resource for therapeutic terpenoids. 3 Biotech 2019, 9, 369. [Google Scholar] [CrossRef]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Gabir, M.M.; Hanson, R.L.; Dabelea, D.; Imperatore, G.; Roumain, J.; Bennett, P.H.; Knowler, W.C. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care 2000, 23, 1108–1112. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Mohamed, M.; Ahmad, I.; Kamal, M.A.; Donnelly, R.; Idris, I.; Gan, S.H. Efficacy and safety of pioglitazone monotherapy in type 2 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Sci. Rep. 2019, 9, 5389. [Google Scholar] [CrossRef]
- Vitak, T.; Yurkiv, B.; Wasser, S.; Nevo, E.; Sybirna, N. Effect of medicinal mushrooms on blood cells under conditions of diabetes mellitus. World J. Diabetes 2017, 8, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Ramchandran, A.; Jayawardena, R.; Shrivastava, U.; Snehalatha, C. Diabetes in South Asians. Diabet. Med. 2014, 31, 1153–1162. [Google Scholar] [CrossRef]
- Kosiborod, M.; Gomes, M.B.; Nicolucci, A.; Pocock, S.; Rathmann, W.; Shestakova, M.V.; Watada, H.; Shimomura, I.; Chen, H.; Cid-Ruzafa, J.; et al. Vascular complications in patients with type 2 diabetes: Prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc. Diabetol. 2018, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Vannacci, A.; Rastogi, S. Insulin resistance, type 1 and type 2 diabetes, and related complications 2017. J. Diabetes Res. 2017, 2017, 1478294. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Rastogi, S.; Vannacci, A. Insulin resistance, type 1 and type 2 diabetes, and related complications 2015. J. Diabetes Res. 2015, 2015, 234135. [Google Scholar] [CrossRef]
- Taylor, R. Insulin resistance and type 2 diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, L.; Duvnjak, M. The metabolic syndrome—an ongoing story. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60 (Suppl. 7), 19–24. [Google Scholar]
- Ndisang, J.F.; Rastogi, S.; Vannacci, A. Insulin resistance, type 1 and type 2 diabetes, and related complications: Current status and future perspective. J. Diabetes Res. 2014, 2014, 276475. [Google Scholar] [CrossRef]
- Montoya, L.; Herrera, M.; Bandala, V.M.; Ramos, A. Two new species and a new record of yellow Cantharellus from tropical Quercus forests in eastern Mexico with the proposal of a new name for the replacement of Craterellus confluens. MycoKeys 2021, 80, 91. [Google Scholar] [CrossRef]
- Hermawan, R.; Putra, I. Calvatia rugosa: Epigeous Puffball mushroom reported from West Java. Sci. Educ. Appl. J. 2021, 3, 1. [Google Scholar] [CrossRef]
- Nowakowski, P.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Borawska, M.H.; Socha, K. The two faces of Coprinus comatus- functional properties and potential hazards. Phytother. Res. 2020, 34, 2932–2944. [Google Scholar] [CrossRef] [PubMed]
- Starzycki, M.; Paukszta, D.; Starzycka, E. Vitro growth of oyster mushroom (pleurotus ostreatus) mycelium on composites filled with rapeseed. Phytopathologia 2014, 65, 33–37. [Google Scholar]
- Frank, J.; Siegel, N.; Schwarz, C.; Araki, B.; Vellinga, E. Xerocomellus (Boletaceae) in western North America. Fungal Syst. Evol. 2020, 6, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.; Ezzat el ghwas, D.; Gundoju, N.; Tiruveedhula, S.; Akram, M.; Daba, G. Chicken of the woods Laetiporus Sulphureus and Schizophyllum Commune treasure of medicinal mushrooms. J. Microbiol. Biotechnol. 2021, 6, 1–7. [Google Scholar] [CrossRef]
- Dissanayake, A.A.; Zhang, C.R.; Mills, G.L.; Nair, M.G. Cultivated maitake mushroom demonstrated functional food quality as determined by in vitro bioassays. J. Funct. Foods 2018, 44, 79–85. [Google Scholar] [CrossRef]
- Ekowati, N.; Yuniati, N.I.; Hernayanti, H.; Ratnaningtyas, N.I. Antidiabetic potentials of button mushroom (Agaricus bisporus) on alloxan-induced diabetic rats. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 655–662. [Google Scholar] [CrossRef]
- Halama, M.; Pech, P.; Shiryaev, A.G. Contribution to the knowledge of Ramariopsis subarctica (Clavariaceae, Basidiomycota). Pol. Bot. J. 2017, 62, 123–133. [Google Scholar] [CrossRef]
- Masaphy, S.; Zabari, L.; Goldberg, D.; Jander-Shagug, G. The complexity of Morchella systematics: A case of the yellow morel from Israel. Fungi 2010, 3, 14–18. [Google Scholar]
- Wu, D.; Yang, S.; Tang, C.; Liu, Y.; Li, Q.; Zhang, H.; Cui, F.; Yang, Y. Structural properties and macrophage activation of cell wall polysaccharides from the fruiting bodies of Hericium erinaceus. Polymers 2018, 10, 850. [Google Scholar] [CrossRef]
- Bao, D.; Gong, M.; Zheng, H.; Chen, M.; Zhang, L.; Wang, H.; Jiang, J.; Wu, L.; Zhu, Y.; Zhu, G.; et al. Sequencing and comparative analysis of the straw mushroom (Volvariella volvacea) genome. PLoS ONE 2013, 8, e58294. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Tsai, S.-Y. Differences in the moisture capacity and thermal stability of Tremella fuciformis polysaccharides obtained by various drying processes. Molecules 2019, 24, 2856. [Google Scholar] [CrossRef] [PubMed]
- Senthilarasu, G. The lentinoid fungi (Lentinus and Panus) from Western Ghats, India. IMA Fungus 2015, 6, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Chakraborty, U.; Barman, S.; Roy, S. Effect of different substrates and casing materials on growth and yield of Calocybe indica (P&C) in North Bengal, India. J. Appl. Nat. Sci. 2016, 8, 683–690. [Google Scholar]
- Karun, N.; Sharma, B.; Sridhar, K. Biodiversity of macrofungi in Yenepoya Campus, Southwest India. Microb. Biosyst. 2018, 3, 1–11. [Google Scholar] [CrossRef][Green Version]
- Adhikari, M.K.; Watanabe, K.; Parajuli, G. A new variety of Pholiota microspora (Agaricales) from Nepal. Biodiversitas J. Biol. Divers. 2014, 15, 101–103. [Google Scholar] [CrossRef]
- Hu, Y.-N.; Sung, T.-J.; Chou, C.-H.; Liu, K.-L.; Hsieh, L.-P.; Hsieh, C.-W. Characterization and antioxidant activities of yellow Strain Flammulina velutipes (Jinhua Mushroom) polysaccharides and their effects on ROS content in L929 cell. Antioxidants 2019, 8, 298. [Google Scholar] [CrossRef]
- Chicatto, J.; Castamann, V.; Helm, C.; Tavares, L. Optimization of the production process of enzymatic activity of Lentinula edodes (Berk.) pegler in holocelulases. Nat. Resour. 2014, 5, 241–255. [Google Scholar] [CrossRef]
- Shah, S.R.; Ukaegbu, C.I.; Hamid, H.A.; Alara, O.R. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellatus (white and brown var.) extracted with different solvents. J. Food Meas. Charact. 2018, 12, 1947–1961. [Google Scholar] [CrossRef]
- Nagalakshmi, M.; Krishnakumari, S.; Kathiravan, S. Comparitive study of various substrate supplements in the growth and yield of Agrocybe aegerita, black poplar mushroom. World J. Pharm. Res. 2014, 3, 487–496. [Google Scholar]
- Rahmad, N.; Al-Obaidi, J.; Mohd Nor Rashid, N.; Zean, N.; Yuswan, M.H.; Shaharuddin, N.; Mohd Jamil, N.A.; Mohd Saleh, N. Comparative proteomic analysis of different developmental stages of the edible mushroom Termitomyces heimii. Biol. Res. 2014, 47, 30. [Google Scholar] [CrossRef] [PubMed]
- Ogbole, O.O.; Nkumah, A.O.; Linus, A.U.; Falade, M.O. Molecular identification, in vivo and in vitro activities of Calvatia gigantea (macro-fungus) as an antidiabetic agent. Mycology 2019, 10, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Kong, D.; Cai, W.; Zhang, J.; Jia, L. Characterization and anti-diabetic nephropathic ability of mycelium polysaccharides from Coprinus comatus. Carbohydr. Polym. 2021, 251, 117081. [Google Scholar] [CrossRef] [PubMed]
- Asrafuzzaman, M.; Rahman, M.; Mandal, M.; Marjuque, M.; Bhowmik, D.; Begum, R.; Hassan, M.Z.; Faruque, M. Oyster mushroom functions as an anti-hyperglycaemic through phosphorylation of AMPK and increased expression of GLUT4 in type 2 diabetic model rats. J. Taibah Univ. Med. Sci. 2018, 13, 465–471. [Google Scholar] [CrossRef]
- Ali Sangi, S.; Bawadekji, A.; Al Ali, M. Comparative effects of metformin, Pleurotus ostreatus, Nigella sativa, and Zingiber officinale on the streptozotocin-induced diabetes mellitus in rats. Pharmacogn. Mag. 2018, 14, 268–273. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L.; Fan, Y.; Yan, P.; Li, S.; Zhou, X. The effect of boletus polysaccharides on diabetic hepatopathy in rats. Chem.-Biol. Interact. 2019, 308, 61–69. [Google Scholar] [CrossRef]
- Xiao, C.; Jiao, C.; Xie, Y.; Ye, L.; Li, Q.; Wu, Q. Grifola frondosa GF5000 improves insulin resistance by modulation the composition of gut microbiota in diabetic rats. J. Funct. Foods 2021, 77, 104313. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.J.; Lim, K.T. Mushroom-derived bioactive molecules as immunotherapeutic agents: A review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef]
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the health effects and bioactive components in Agaricus bisporus mushrooms: A scoping review. J. Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Li, J.; Liu, Y.; Park, H.; Yang, L. Recent advances on bioactive ingredients of Morchella esculenta. Appl. Biochem. Biotechnol. 2021, 193, 4197–4213. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Hu, C.; Wang, J.; Zhang, J.; Ren, Z.; Song, X.; Jia, L. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice. Sci. Rep. 2017, 7, 10847. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Huang, C.-H.; Lin, W.-K.; Chang, S.H.; Tsai, G.-J. Evaluation of the hypoglycaemic and antioxidant effects of submerged Ganoderma lucidum cultures in type 2 diabetic rats. Mycology 2021, 12, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zeb, M.; Lee, C.H. Medicinal properties and bioactive compounds from wild mushrooms native to North America. Molecules 2021, 26, 251. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Advances in the extraction, purification, structural-property relationships and bioactive molecular mechanism of Flammulina velutipes polysaccharides: A review. Int. J. Biol. Macromol. 2021, 167, 528–538. [Google Scholar] [CrossRef]
- Song, X.; Fu, H.; Chen, W. Effects of Flammulina velutipes polysaccharides on quality improvement of fermented milk and antihyperlipidemic on streptozotocin-induced mice. J. Funct. Foods 2021, 87, 104834. [Google Scholar] [CrossRef]
- Rivera, O.A.; Albarracín, W.; Lares, M. Bioactive components of shiitake (Lentinula edodes Berk. Pegler) and its impact on health. Arch. Venez. De Farmacol. Y Ter. 2017, 36, 67–71. [Google Scholar]
- Laurino, L.F.; Viroel, F.J.M.; Caetano, E.; Spim, S.; Pickler, T.B.; Rosa-Castro, R.M.; Vasconcelos, E.A.; Jozala, A.F.; Hataka, A.; Grotto, D.; et al. Lentinus edodes exposure before and after fetus implantation: Materno-fetal development in rats with gestational diabetes mellitus. Nutrients 2019, 11, 2720. [Google Scholar] [CrossRef]
- Ugbogu, E.A.; Akubugwo, E.; Ude, V.; Okezie, E.; Nduka, O.; Ibeh, C.; Onyero, O. Safety evaluation of aqueous extract of Termitomyces robustus (Agaricomycetes) in Wistar Rats. Int. J. Med. Mushrooms 2018, 21, 193–203. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Shyur, L.-F.; Varga, V.; Chen, C.-M.; Mu, S.-C.; Chang, Y.-C.; Li, S.-C. Extract of white sweet potato tuber against TNF-α-induced insulin resistance by activating the PI3K/Akt pathway in C2C12 myotubes. Bot. Stud. 2021, 62, 7. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.J.; Kiviniemi, A.M.; Lepojärvi, E.S.; Tulppo, M.; Piira, O.-P.; Kenttä, T.; Perkiömäki, J.S.; Ukkola, O.H.; Myerburg, R.J.; Huikuri, H.V. Type 2 diabetes and coronary artery disease: Preserved ejection fraction and sudden cardiac death. Heart Rhythm. 2018, 15, 1450–1456. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Aramabašić Jovanović, J.; Mihailović, M.; Uskoković, A.; Grdović, N.; Dinić, S.; Vidaković, M. The effects of major mushroom bioactive compounds on mechanisms that control blood glucose level. J. Fungi 2021, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.M.; Tamura, K.; Déjean, G.; Abbott, D.W.; Brumer, H. Polysaccharide utilization loci: Fueling microbial communities. J. Bacteriol. 2017, 199, e00860-16. [Google Scholar] [CrossRef]
- Dubey, S.K.; Chaturvedi, V.K.; Mishra, D.; Bajpeyee, A.; Tiwari, A.; Singh, M.P. Role of edible mushroom as a potent therapeutics for the diabetes and obesity. 3 Biotech 2019, 9, 450. [Google Scholar] [CrossRef]
- Kou, L.; Du, M.; Liu, P.; Zhang, B.; Zhang, Y.; Yang, P.; Shang, M.; Wang, X. Anti-diabetic and anti-nephritic activities of Grifola frondosa mycelium polysaccharides in diet-streptozotocin-induced diabetic rats via modulation on oxidative stress. Appl. Biochem. Biotechnol. 2019, 187, 310–322. [Google Scholar] [CrossRef]
- Ratnaningtyas, N.I.; Hernayanti, H.; Andarwanti, S.; Ekowati, N.; Purwanti, E.S.; Sukmawati, D. Effects of Ganoderma lucidum extract on diabetic rats. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 6. [Google Scholar] [CrossRef]
- Lin, X.; Brennan-Speranza, T.C.; Levinger, I.; Yeap, B.B. Undercarboxylated osteocalcin: Experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients 2018, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Panigrahy, S.; Bhatt, R.; Kumar, A. Targeting type II diabetes with plant terpenes: The new and promising antidiabetic therapeutics. Biologia 2020, 76, 241–254. [Google Scholar] [CrossRef]
- Kolundzic, M.; Grozdanić, N.; Stanojkovic, T.; Milenkovic, M.; Dinic, M.; Golić, N.; Kojic, M.; Kundaković, T. Antimicrobial and cytotoxic activities of the sulphur shelf medicinal mushroom laetiporus sulphureus (Agaricomycetes) from Serbia. Int. J. Med. Mushrooms 2016, 18, 469–476. [Google Scholar] [CrossRef]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.; Li, C. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211000541. [Google Scholar] [CrossRef]
- Zhu, G.; Hayashi, M.; Shimomura, N.; Yamaguchi, T.; Aimi, T. Differential expression of three α-amylase genes from the basidiomycetous fungus Pholiota microspora. Mycoscience 2017, 58, 188–191. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef]
- Sung, C.-C.; Liao, M.-T.; Lu, K.-C.; Wu, C.-C. Role of vitamin D in insulin resistance. J. Biomed. Biotechnol. 2012, 2012, 634195. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef]
- Talaei, A.; Mohamadi, M.; Adgi, Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 8. [Google Scholar] [CrossRef]
- Zakhary, C.M.; Rushdi, H.; Hamdan, J.A.; Youssef, K.N.; Khan, A.; Abdalla, M.A.; Khan, S. Protective role of vitamin D therapy in diabetes mellitus type II. Cureus 2021, 13, e17317. [Google Scholar] [CrossRef]
- Al-Shoumer, K.A.; Al-Essa, T.M. Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J. Diabetes 2015, 6, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Urbain, P.; Singler, F.; Ihorst, G.; Biesalski, H.K.; Bertz, H. Bioavailability of vitamin D₂ from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: A randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Wenclewska, S.; Szymczak-Pajor, I.; Drzewoski, J.; Bunk, M.; Śliwińska, A. Vitamin D supplementation reduces both oxidative DNA damage and insulin resistance in the elderly with metabolic disorders. Int. J. Mol. Sci. 2019, 20, 2891. [Google Scholar] [CrossRef] [PubMed]
- Mutt, S.J.; Raza, G.S.; Mäkinen, M.J.; Keinänen-Kiukaanniemi, S.; Järvelin, M.R.; Herzig, K.H. Vitamin D deficiency induces insulin resistance and re-supplementation attenuates hepatic glucose output via the PI3K-AKT-FOXO1 mediated pathway. Mol. Nutr. Food Res. 2020, 64, e1900728. [Google Scholar] [CrossRef]
- El Hajj, C.; Walrand, S.; Helou, M.; Yammine, K. Effect of vitamin D supplementation on inflammatory markers in non-obese Lebanese patients with type 2 diabetes: A randomized controlled trial. Nutrients 2020, 12, 2033. [Google Scholar] [CrossRef]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The effects of vitamin D supplementation on metabolic and oxidative stress markers in patients with type 2 diabetes: A 6-month follow up randomized controlled study. Front. Endocrinol. 2021, 12, 610893. [Google Scholar] [CrossRef]


| S. No. | Scientific Name | Vernacular Name | Photos | Reference |
|---|---|---|---|---|
| 1 | Craterellus aureus | Cantharellus, chanterelles |  | [31] |
| 2 | Calvatia rugosa | Puffballs |  | [32] |
| 3 | Coprinus comatus | Shaggy mane |  | [33] |
| 4 | Pleurotus ostreatus | Oyster mushroom |  | [34] |
| 5 | Boletaceae Boletales | Boletes |  | [35] |
| 6 | Laetiporus sulphurous | Sulfer shelf |  | [36] |
| 7 | Grifora frondosa | Hen of the woods |  | [37] |
| 8 | Agaricus bisporus | Button mushroom |  | [38] |
| 9 | Ramariopsis subarctica | Coral fungi |  | [39] |
| 10 | Morachella esculenta | Morels |  | [40] |
| 11 | Hericium erinaceus | Bearded tooth |  | [41] |
| 12 | Volvariella volvacea | Straw mushroom |  | [42] |
| 13 | Ganoderma lucidium | Lingzhi mushroom |  | [43] |
| 14 | Tremella fuciformis | Snow fungus |  | [44] |
| 15 | Lentinus concentricus | - |  | [45] |
| 16 | Calocybe indica | Milky white mushroom |  | [46] |
| 17 | Lenzites betulina | Wood-rooting fungi |  | [47] |
| 18 | Pholiota microspora | Slippery mushroom |  | [48] |
| 19 | Flammulina velutipes | Enoki mushroom |  | [49] |
| 20 | Lentinula edodes | Shiitake mushroom |  | [50] |
| 21 | Hypsizygus tessellatus | Buna shimeji |  | [51] |
| 22 | Agrocybe aegerita | Poplar mushroom, Chestnut mushroom, Velvet pioppini |  | [52] |
| 23 | Termitomyces robustus | Termitomyces mushrooms |  | [53] |
| S. No. | Scientific Name | Compounds | Functions | Models | Mushroom Doses | Mechanism/Action | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Calvatia gigantea | 2-Pyrrolidinone, 1-Dodecene, ergosterol, hexadecane, benzeneacetic acid. | Anti-diabetic, antioxidant, anti-inflammatory. | Alloxan-induced diabetic rat. | 100, 200, and 400 mg/kg BW/day. | -Alpha-amylase inhibitory activity. | [54] |
| 2 | Coprinus comatus | Mycelium, polysaccharides. | Immunomodulatory, anti-diabetic, antioxidant, anti-cancer. | High-fat diet and STZ-induced mice. | 400 mg/kg BW/day. | -Reduce BG level, relieves oxidative stress, ameliorate DN via PI3K/Akt and Wnt-1/β-catenin pathways. | [55] |
| 3 | Pleurotus ostreatus, Pleurotus pulmonarius, and Pleurotus fossulatus | Terpenoids, heterocyclic amines, phenols, glucan, proteoglycan. | Anti-cholesterol, anti-cancer effect, anti-inflammatory, anti-diabetic. | STZ and metformin-induced rat. | 5–10% powder, 50, 80, 200, 250, 400, and 500 mg/kg BW/day extract. | -Decreases serum glucose level, alpha-amylase activity; -Increases P-AMPK and GLUT4 in muscle and adipose tissue; -Improves liver functions and maintains AST, ALT, and ALP levels. | [56,57] |
| 4 | Boletus | Tocopherol, quinic acid, hydroxy benzoic acid. | Antioxidant, anti-inflammatory, hypoglycemic. | STZ-induced rat. | 400 mg extract/kg BW/day. | -Decreases TC, TG, TNF-alpha, and NF-kB level; -Maintains MDA level; -Improves antioxidant (CAT, SOD, and GSH) and CYP7A1 levels. | [58] |
| 5 | Grifora frondosa | Grifolan polysaccharide, D-fraction, MD-fraction, polysaccharide, galactomannan, heteroglycan. | Hypoglycemic, anti-inflammatory, anti-modulatory, anti-tumor. | STZ, alloxan-induced rat and palmitate-induced C2C12 cells. | 0.5–20 µM (introduced to C2C12 cell), 112.5, 200, and 675 mg/kg BW/day extract (introduced to STZ- and alloxan-induced rat). | -Inhibits serum levels of IL-2, IL-6; -Modulates serum level of oxidant factors such as superoxide dismutase, glutathione peroxidase, catalase, malondialdehyde, and reactive oxygen species; -Increases glucose uptake and decreases ROS formation and up-regulates IRS-1, p-IRS-1, PI3K, Akt, pAkt and GLUT4 protein, and down-regulates p-JNK and p-p38 expression; -Improves insulin resistance and gut microflora content. | [59,60] |
| 6 | Agricus bisporus | Pyrogallol, hydroxybenzoic acid derivatives glavonoid. | Anti-inflammatory, anti-diabetic. | Alloxan-induced rat. | 15–30 g/day, 250, 500 and 750 mg/kg BW/day. | -Improves antioxidant (SOD) level; -Improves ALP, AST, ALT level; -Reduces hyperlipidemia. | [38,61] |
| 7 | Morachella esculenta | Polysaccharides (mannose, galactose, and glucose), phenolic compounds. | Antioxidant, anti-inflammation, immunoregulation, hypoglycemic. | - | - | - | [62] |
| 8 | Hericium erinaceus | 4-chloro-3,5-dimethoxybenzoic acid-O-arabitol ester, 2-hydroxymethyl-5-α-hydroxy-ethyl-γ-pyranone, 6-methyl-2,5-dihydroxymethyl-γ-pyranone, 4-chloro-3,5-dihydroxybenzaldehyde, 4-chloro-3,5-dihydroxybenzyl alcohol. | Immunomodulatory, hypoglycemic, antimicrobial. | STZ-treated rat. | 400–600 mg/kg BW/day. | -Reduces blood glucose, BUN, and CRT level; -Maintains ALP, ALT, and AST levels; -Improves antioxidant level (SOD, glutathione). | [63,64] |
| 9 | Ganoderma lucidium | Ganoderic acid, danoderiol, danderenic acid, lucidenic acid, ganoderma leucidum polysaccharide. | Anti-diabetic, anti-inflammatory. | Metformin-, STZ-, and high-fat-treated rat. | 1–3% freeze-dried mushroom, 25, 50, 100, 250, 500 and 1000 mg/kg BW/day extract. | -Decreases HBA1c and improves AST, ALT level. | [60,65] |
| 10 | Lenzites betulina | α-glucan, ß-glucan, ß-glucan protein, galacturonic acid. | Antioxidant, anti-hyperglycaemic, anti-inflammatory, anti-proliferative, antibacterial. | - | - | - | [66] |
| 11 | Flammulina velutipes | Flammulinolide, enokipodin, proflamin, other polysaccharide. | Anti-tumor, anti-hypertension, anti-hypercholesterolemia, hypoglycemic. | STZ-induced mice. | 400 mg/kg, 600 mg/kg, and 800 mg/kg BW/day. | -Improves PI3K/AKT pathway. | [67,68] |
| 12 | Lentinula edodes | Lentinan, eritadenina. | Anti-carcinogenic, antioxidant, hypocholesterolemic action. | STZ-treated rat (gestational diabetes). | 100 mg/kg BW/day. | -Improves maternal insulin level; -Reduces aminotransferase, aspartate aminotransferase, triglyceride, and total cholesterol level. | [69,70] |
| 13 | Termitomyces robustus | γ glutamyl-ß-phenylethylamine, tryptophan 1,4-hydroxyphenylacetic acid, hydroxyphenyl propionic acid and phenyllactic acid. | Hypoglycemic effect. | In vitro assay, Wister rat. | Crude extract 78.05 and 86.10 µg/mL. 500, 1000, and 1500 mg/kg BW/day. In vitro assay, rate acute toxicity test (10 g/kg extract) and subacute toxicity test (500, 1000, and 1500 mg/kg) BW/day. | -α-glucosidase and α-amylase inhibitory activity. | [15,71] |
| Organ Name | Functions |
|---|---|
| Pancreas | Increases insulin secretion and enhances the transformation of pro-insulin to insulin |
| Skeletal muscle | Through VDR expression maintains glucose homeostasis |
| Skin | Improves skin micro-circulations and fasten wound healing |
| Nervous system | Improves nerve conduction and shows the analgesic effect |
| Kidney | Controls urinary albuminuria |
| Retina | Defend against oxidative stress |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Chen, C.-M.; Mu, S.-C.; Yang, S.-H.; Ju, Y.-M.; Li, S.-C. Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment. Pharmaceutics 2022, 14, 436. https://doi.org/10.3390/pharmaceutics14020436
Das A, Chen C-M, Mu S-C, Yang S-H, Ju Y-M, Li S-C. Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment. Pharmaceutics. 2022; 14(2):436. https://doi.org/10.3390/pharmaceutics14020436
Chicago/Turabian StyleDas, Arpita, Chiao-Ming Chen, Shu-Chi Mu, Shu-Hui Yang, Yu-Ming Ju, and Sing-Chung Li. 2022. "Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment" Pharmaceutics 14, no. 2: 436. https://doi.org/10.3390/pharmaceutics14020436
APA StyleDas, A., Chen, C.-M., Mu, S.-C., Yang, S.-H., Ju, Y.-M., & Li, S.-C. (2022). Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment. Pharmaceutics, 14(2), 436. https://doi.org/10.3390/pharmaceutics14020436








