Anti-SARS-CoV-2 Omicron Antibodies Isolated from a SARS-CoV-2 Delta Semi-Immune Phage Display Library
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immune VH Repertoire
2.2. Assemblage with Four Synthetic VL Fragments
2.3. Phage Display scFv Library Construction
2.4. Expression and Purification of SARS-CoV-2 RBD Recombinant Proteins
2.5. Phage-Antibody Selection
2.6. Expression and Specific Binding to RBD-WT and RBD-DT
2.7. Competition with P5E1-A6 and hACE2 for Binding RBD
2.8. hIgG1 Conversion
2.9. Developability Assessment
2.10. Surface Plasmon Resonance (SPR)
2.11. Control Antibodies
3. Results
3.1. Selection of Unique RBD-Positive Clones
3.2. Sequence Patterns and Binding Profile of the Selected scFvs
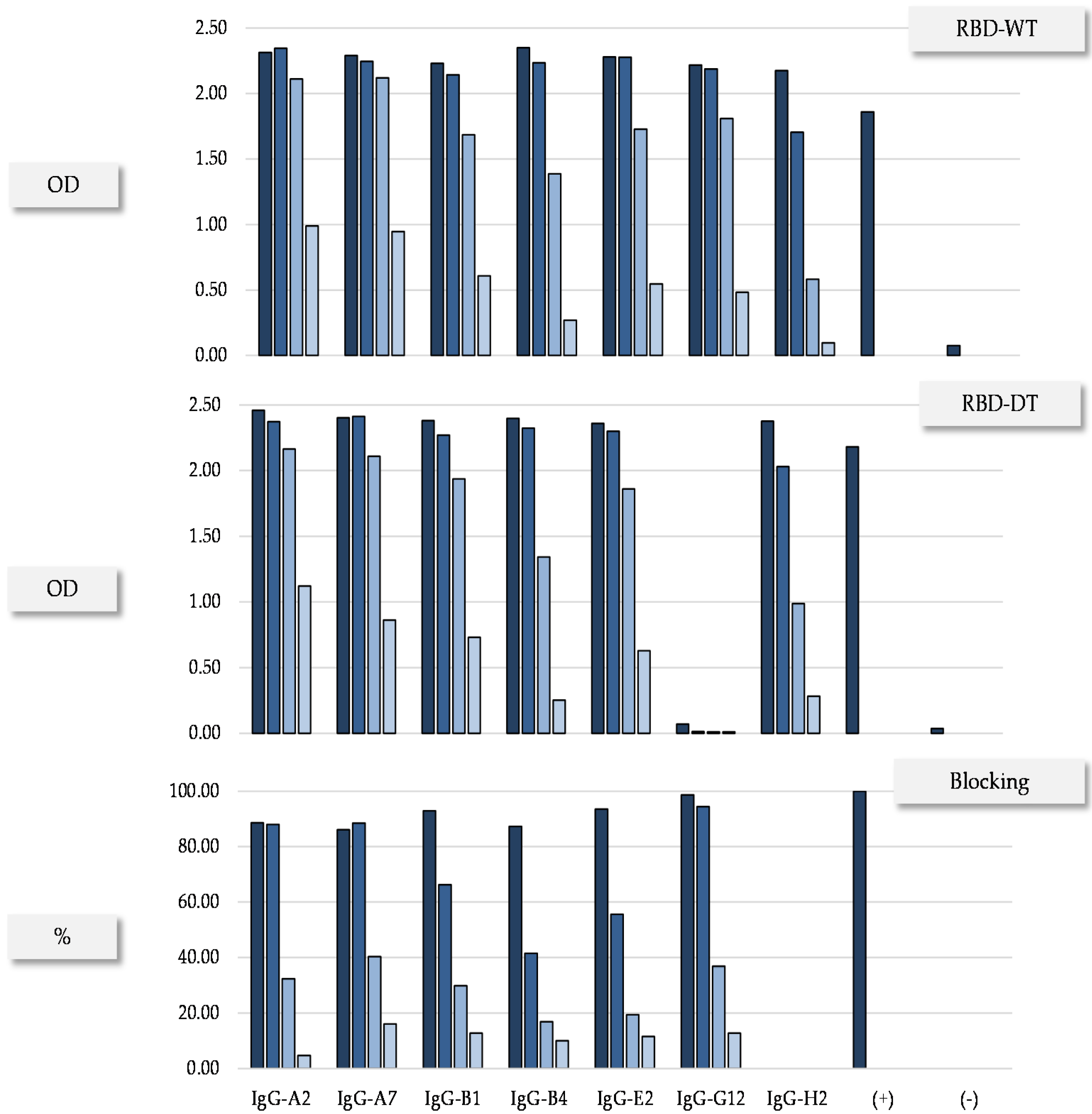
3.3. Conversion to hIgG1 and RBD Omicron Binding
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raj, R. Analysis of non-structural proteins, NSPs of SARS-CoV-2 as targets for computational drug designing. Biochem. Biophys. Rep. 2020, 25, 100847. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Genet. 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Nampoothiri, M. Structural Proteins in Severe Acute Respiratory Syndrome Coronavirus-2. Arch. Med Res. 2020, 51, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Kumari, S.; Pandey, B.; Mistry, H.; Bihani, S.C.; Das, A.; Prashar, V.; Gupta, G.D.; Panicker, L.; Kumar, M. Structural insights into SARS-CoV-2 proteins. J. Mol. Biol. 2020, 433, 166725. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Piccoli, L.; Park, Y.-J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef]
- Zimmer, C.J.; Wee, S.; Kristoffersen, M. Coronavirus Vaccine Tracker. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed on 3 January 2022).
- Chakraborty, S.; Mallajosyula, V.; Tato, C.M.; Tan, G.S.; Wang, T.T. SARS-CoV-2 vaccines in advanced clinical trials: Where do we stand? Adv. Drug Deliv. Rev. 2021, 172, 314–338. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clin. Pr. 2021, 11, 778–784. [Google Scholar] [CrossRef]
- Callaway, E.; Ledford, H. How bad is Omicron? What scientists know so far. Nature 2021, 600, 197–199. [Google Scholar] [CrossRef]
- He, X.; Hong, W.; Pan, X.; Lu, G.; Wei, X. SARS-CoV-2 Omicron variant: Characteristics and prevention. MedComm 2021, 2, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Edara, V.V.; Norwood, C.; Floyd, K.; Lai, L.; Davis-Gardner, M.E.; Hudson, W.H.; Mantus, G.; Nyhoff, L.E.; Adelman, M.W.; Fineman, R.; et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 2021, 29, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- FDA Authorizes REGEN-COV Monoclonal Antibody Therapy for Post-Exposure Prophylaxis (Prevention) for COVID-19. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-regen-cov-monoclonal-antibody-therapy-post-exposure-prophylaxis-prevention-covid-19 (accessed on 10 October 2021).
- FDA Authorizes Bamlanivimab And Etesevimab Monoclonal Antibody Therapy for Post-Exposure Prophylaxis (Prevention) for COVID-19. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-bamlanivimab-and-etesevimab-monoclonal-antibody-therapy-post-exposure-prophylaxis (accessed on 16 September 2021).
- Coronavirus (COVID-19) Update: FDA Authorizes New Long-Acting Monoclonal Antibodies for Pre-exposure Prevention of COVID-19 in Certain Individuals. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-long-acting-monoclonal-antibodies-pre-exposure (accessed on 8 December 2021).
- Deb, P.; Molla, M.A.; Saif-Ur-Rahman, K. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf. Health 2021, 3, 87–91. [Google Scholar] [CrossRef]
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 Variants of Concern. Yonsei Med. J. 2021, 62, 961–968. [Google Scholar] [CrossRef]
- Kannan, S.R.; Spratt, A.N.; Sharma, K.; Chand, H.S.; Byrareddy, S.N.; Singh, K. Omicron SARS-CoV-2 variant: Unique features and their impact on pre-existing antibodies. J. Autoimmun. 2021, 126, 102779. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Makdasi, E.; Alcalay, R.; Mechaly, A.; Levy, Y.; Bercovich-Kinori, A.; Zauberman, A.; Tamir, H.; Yahalom-Ronen, Y.; Israeli, M.; et al. A panel of human neutralizing mAbs targeting SARS-CoV-2 spike at multiple epitopes. Nat. Commun. 2020, 11, 4303. [Google Scholar] [CrossRef]
- Valadon, P.; Pérez-Tapia, S.M.; Nelson, R.S.; Guzmán-Bringas, O.U.; Arrieta-Oliva, H.I.; Gómez-Castellano, K.M.; Pohl, M.A.; Almagro, J.C. ALTHEA Gold Libraries™: Antibody libraries for therapeutic antibody discovery. mAbs 2019, 11, 516–531. [Google Scholar] [CrossRef] [Green Version]
- Teplyakov, A.; Obmolova, G.; Malia, T.J.; Luo, J.; Muzammil, S.; Sweet, R.; Almagro, J.; Gilliland, G.L. Structural diversity in a human antibody germline library. mAbs 2016, 8, 1045–1063. [Google Scholar] [CrossRef] [Green Version]
- Finlay, W.J.J.; Almagro, J.C. Natural and man-made V-gene repertoires for antibody discovery. Front. Immunol. 2012, 3, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almagro, J.C.; Pedraza-Escalona, M.; Arrieta, H.I.; Pérez-Tapia, S.M. Phage Display Libraries for Antibody Therapeutic Discovery and Development. Antibodies 2019, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Sandoval, R.; Nieto-Patlán, A.; Carballo-Uicab, G.; Montes-Luna, A.; Jiménez-Martínez, M.C.; Vallejo-Castillo, L.; González-González, E.; Arrieta-Oliva, H.I.; Gómez-Castellano, K.; Guzmán-Bringas, O.U.; et al. Development and Evaluation of a Set of Spike and Receptor Binding Domain-Based Enzyme-Linked Immunosorbent Assays for SARS-CoV-2 Serological Testing. Diagnostics 2021, 11, 1506. [Google Scholar] [CrossRef] [PubMed]
- Perdomo-Abúndez, F.C.; Vallejo-Castillo, L.; Vázquez-Leyva, S.; López-Morales, C.A.; Velasco-Velázquez, M.; Pavón, L.; Pérez-Tapia, S.M.; Medina-Rivero, E. Development and validation of a mass spectrometric method to determine the identity of rituximab based on its microheterogeneity profile. J. Chromatogr. B 2020, 1139, 121885. [Google Scholar] [CrossRef] [PubMed]
- Menzen, T.; Friess, W. High-Throughput Melting-Temperature Analysis of a Monoclonal Antibody by Differential Scanning Fluorimetry in the Presence of Surfactants. J. Pharm. Sci. 2013, 102, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef]
- Wu, X.; Li, N.; Wang, G.; Liu, W.; Yu, J.; Cao, G.; Wang, J.; Chen, Y.; Ma, J.; Wu, J.; et al. Tolerability, Safety, Pharmacokinetics, and Immunogenicity of a Novel SARS-CoV-2 Neutralizing Antibody, Etesevimab, in Chinese Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, First-in-Human Phase 1 Study. Antimicrob. Agents Chemother. 2021, 65, e00350-21. [Google Scholar] [CrossRef]
- Harper, M.; Lema, F.; Boulot, G.; Poljak, R.J. Antigen specificity and cross-reactivity of monoclonal anti-lysozyme antibodies. Mol. Immunol. 1987, 24, 97–108. [Google Scholar] [CrossRef]
- Briney, B.S.; Willis, J.R.; Crowe, J.E., Jr. Human Peripheral Blood Antibodies with Long HCDR3s Are Established Primarily at Original Recombination Using a Limited Subset of Germline Genes. PLoS ONE 2012, 7, e36750. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef]
- Chen, F.; Tzarum, N.; Wilson, I.A.; Law, M. VH1-69 antiviral broadly neutralizing antibodies: Genetics, structures, and relevance to rational vaccine design. Curr. Opin. Virol. 2019, 34, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Lerner, R.A. Rare antibodies from combinatorial libraries suggests an S.O.S. component of the human immunological repertoire. Mol. BioSyst. 2011, 7, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, G.L.; Luo, J.; Vafa, O.; Almagro, J.C. Leveraging SBDD in Protein Therapeutic Development: Antibody Engineering. Struct.-Based Drug Discov. 2011, 841, 321–349. [Google Scholar] [CrossRef]
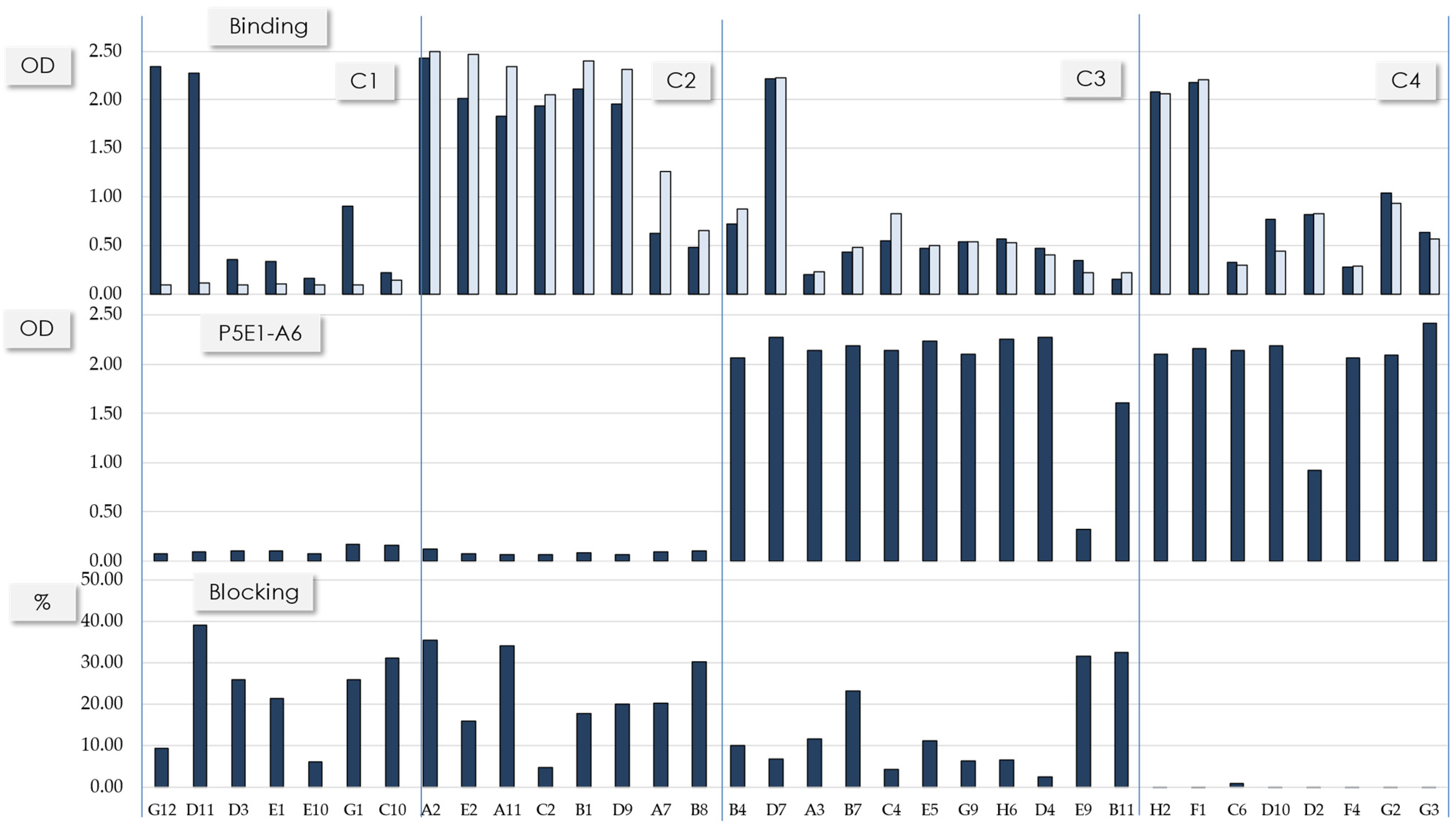


| Cluster | scFv | Frequency | VL Scaffold | IGHV Germline Gene | HCDR3 Length (aa) |
|---|---|---|---|---|---|
| 1 | G12 | 1 | 1-39 | 1-69 | 22 |
| 1 | D11 | 1 | 1-39 | 1-69 | 22 |
| 1 | D3 | 1 | 3-11 | 1-69 | 22 |
| 1 | E1 | 1 | ND | 1-69 | 22 |
| 1 | E10 | 1 | 3-20 | 1-69 | 22 |
| 1 | G1 | 1 | 3-20 | 1-69 | 22 |
| 1 | C10 | 1 | 4-01 | 1-69 | 22 |
| 2 | A2 | 2 | 1-39 | 3-53 | 11 |
| 2 | E2 | 1 | 1-39 | 3-53 | 11 |
| 2 | A11 | 1 | 3-11 | 3-53 | 11 |
| 2 | C2 | 1 | 1-39 | 3-53 | 11 |
| 2 | B1 | 1 | 1-39 | 3-53 | 12 |
| 2 | D9 | 1 | 1-39 | 3-53 | 12 |
| 2 | A7 | 3 | 3-20 | 1-24 | 13 |
| 2 | B8 | 1 | 3-20 | 1-24 | 13 |
| 3 | B4 | 1 | 1-39 | 3-53 | 11 |
| 3 | D7 | 1 | 1-39 | 1-46 | 15a |
| 3 | A3 | 8 | 3-20 | 1-46 | 15b |
| 3 | B7 | 3 | 3-20 | 1-46 | 15b |
| 3 | C4 | 3 | 3-20 | 1-46 | 15b |
| 3 | E5 | 1 | 3-20 | 1-46 | 15b |
| 3 | G9 | 1 | 3-20 | 1-46 | 15b |
| 3 | H6 | 2 | 4-01 | 1-46 | 15b |
| 3 | D4 | 5 | 4-01 | 1-46 | 15b |
| 3 | E9 | 1 | 3-20 | 3-23 | 17 |
| 3 | B11 | 1 | 3-20 | 3-9 | 19 |
| 4 | H2 | 1 | 1-39 | 1-46 | 15a |
| 4 | F1 | 1 | 1-39 | 1-46 | 15a |
| 4 | C6 | 1 | 3-20 | 1-46 | 15b |
| 4 | D10 | 1 | 3-20 | 1-46 | 15b |
| 4 | D2 | 1 | 1-39 | 1-46 | 15b |
| 4 | F4 | 1 | 3-20 | 1-46 | 15b |
| 4 | G2 | 1 | 3-20 | 1-46 | 15b |
| 4 | G3 | 1 | 3-20 | 1-46 | 15b |
| IgG | Monomer a (%) | SDS-PAGE b | Tm c (°C) | Expression Yield d (mg/L) | |
|---|---|---|---|---|---|
| NR (kDa) | R (kDa) | ||||
| A2 | 100 | 140 | 49/25 | 71.3 | 19.92 |
| A7 | 100 | 148 | 52/25 | 68.5 (81.8) | 24.76 |
| B1 | 100 | 158 | 48/25 | 71.9 | 15.82 |
| G12 | 100 | 176 | 50/25 | 71.1 | 19.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Salazar, I.; Gómez-Castellano, K.M.; González-González, E.; Gamboa-Suasnavart, R.; Rodríguez-Luna, S.D.; Santiago-Casas, G.; Cortés-Paniagua, M.I.; Pérez-Tapia, S.M.; Almagro, J.C. Anti-SARS-CoV-2 Omicron Antibodies Isolated from a SARS-CoV-2 Delta Semi-Immune Phage Display Library. Antibodies 2022, 11, 13. https://doi.org/10.3390/antib11010013
Mendoza-Salazar I, Gómez-Castellano KM, González-González E, Gamboa-Suasnavart R, Rodríguez-Luna SD, Santiago-Casas G, Cortés-Paniagua MI, Pérez-Tapia SM, Almagro JC. Anti-SARS-CoV-2 Omicron Antibodies Isolated from a SARS-CoV-2 Delta Semi-Immune Phage Display Library. Antibodies. 2022; 11(1):13. https://doi.org/10.3390/antib11010013
Chicago/Turabian StyleMendoza-Salazar, Ivette, Keyla M. Gómez-Castellano, Edith González-González, Ramsés Gamboa-Suasnavart, Stefany D. Rodríguez-Luna, Giovanni Santiago-Casas, María I. Cortés-Paniagua, Sonia M. Pérez-Tapia, and Juan C. Almagro. 2022. "Anti-SARS-CoV-2 Omicron Antibodies Isolated from a SARS-CoV-2 Delta Semi-Immune Phage Display Library" Antibodies 11, no. 1: 13. https://doi.org/10.3390/antib11010013







