Gallic Acid as a Non-Selective Inhibitor of α/β-Hydrolase Fold Enzymes Involved in the Inflammatory Process: The Two Sides of the Same Coin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Venoms, Animals, and Reagents
2.2. GA Identification
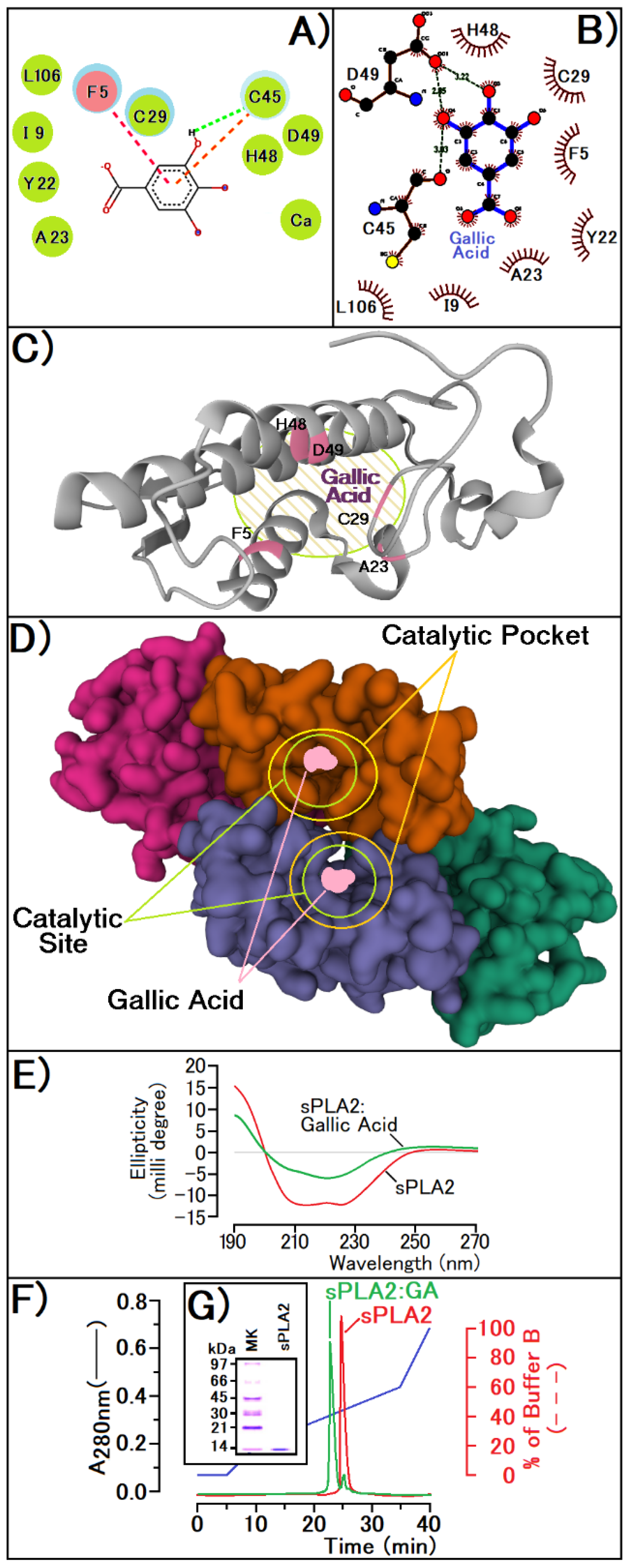
2.3. sPLA2 Purification, GA Incubation, and Circular Dichroism Spectroscopy
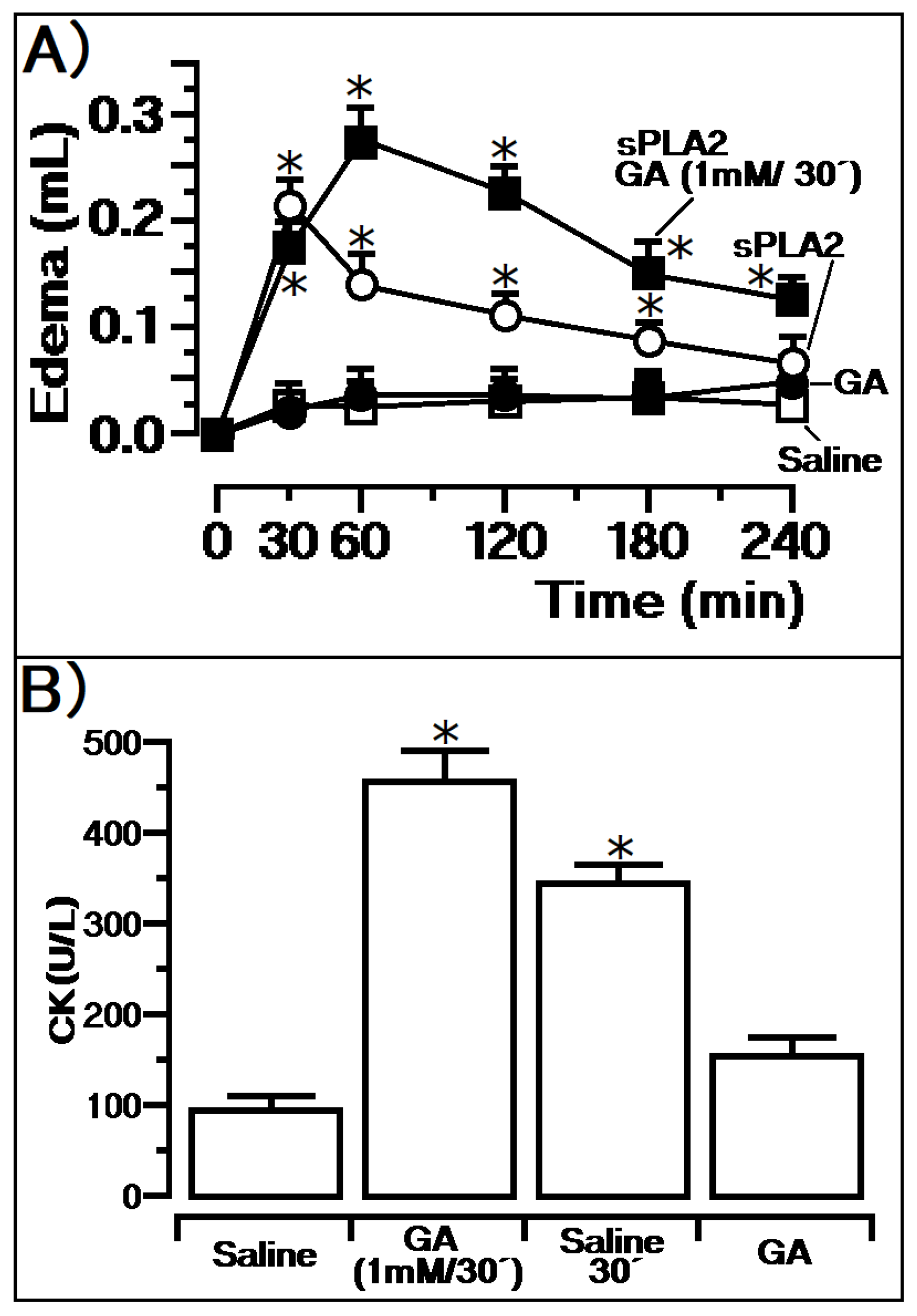
2.4. Enzymatic and Pharmacological Activity (Edema and Myonecrosis)
2.5. Molecular Modeling (Docking) and Enzymatic Inhibition
2.6. Statistical Analyses
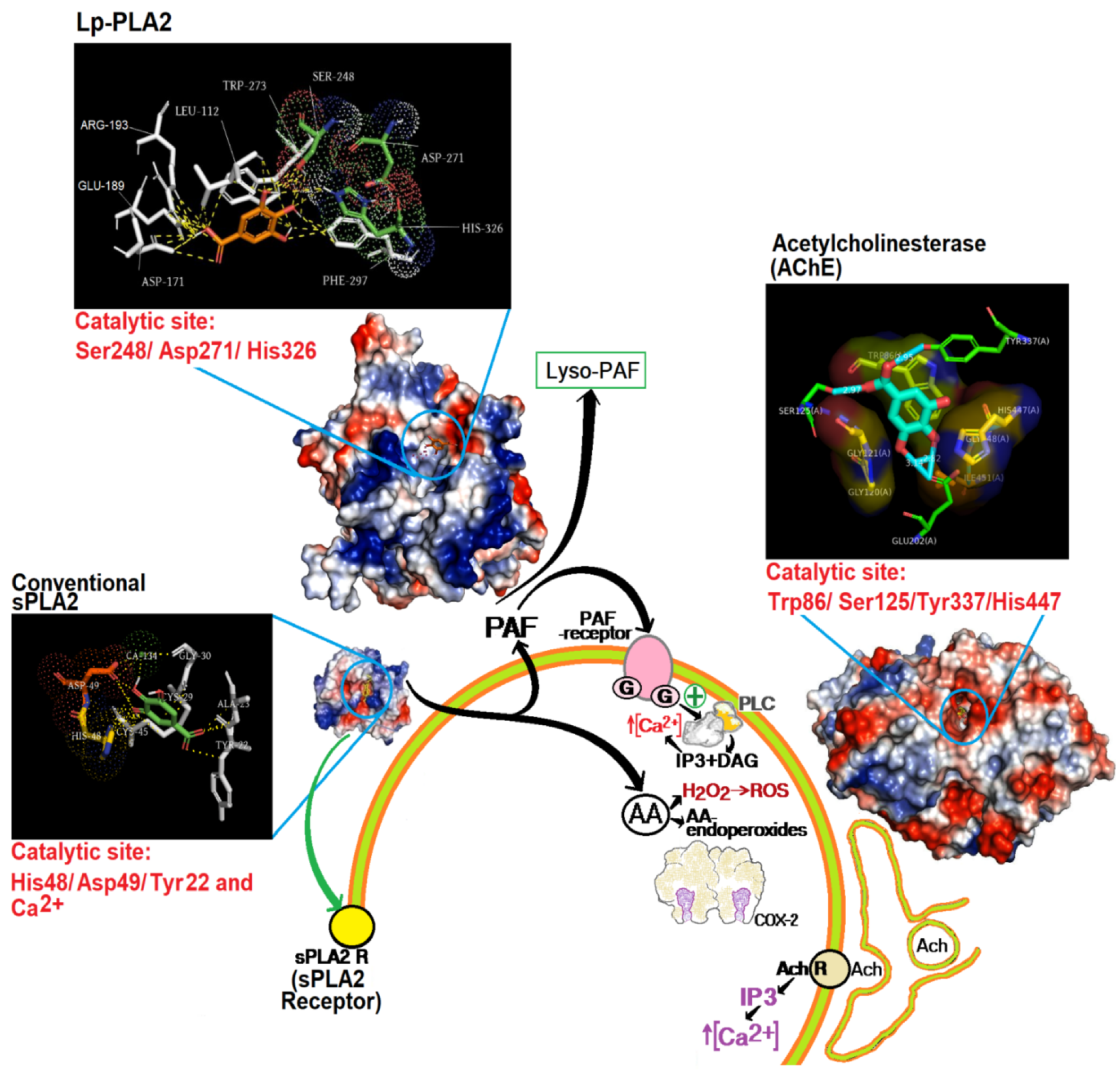
3. Results
Previous Injection of GA and Its Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 2019. [Google Scholar] [CrossRef] [Green Version]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540. [Google Scholar] [CrossRef]
- McArthur, C.; Orlando, P.; Banks, P.B.; Brown, J.S. The foraging tightrope between predation risk and plant toxins: A matter of concentration. Funct. Ecol. 2011, 26, 74–83. [Google Scholar] [CrossRef]
- Punia, A.; Chauhan, N.S.; Singh, D.; Kesavan, A.K.; Kaur, S.; Sohal, S.K. Effect of gallic acid on the larvae of Spodoptera litura and its parasitoid Bracon Hebetor. Sci. Rep. 2021, 11, 531. [Google Scholar] [CrossRef]
- Lenfant, N.; Hotelier, T.; Bourne, Y.; Marchot, P.; Chatonnet, A. Proteins with an alpha/beta hydrolase fold: Relationships between subfamilies in an ever-growing superfamily. Chem. Biol. Interact. 2013, 203, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.L.; Buchholz, P.C.F.; Pleiss, J. The modular structure of α/β-hydrolases. FEBS J. 2020, 287, 5. [Google Scholar] [CrossRef] [PubMed]
- Samanta, U.; Bahnson, B.J. Crystal Structure of Human Plasma Platelet-activating Factor Acetylhydrolase. J. Biol. Chem. 2008, 283, 31617–31624. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Hariprasad, G. Structural Modeling of Wild and Mutant Forms of Human Plasma Platelet Activating Factor-Acetyl Hydrolase Enzyme. J. Inflamm. Res. 2020, 13, 1125–1139. [Google Scholar] [CrossRef]
- Cousin, X.; Hotelier, T.; Giles, K.; Lievin, P.; Toutant, J.P.; Chatonnet, A. The alpha/beta fold family of proteins database and the cholinesterase gene server ESTHER. Nucleic Acids Res. 1997, 25, 1125–1139. [Google Scholar] [CrossRef] [Green Version]
- Bourne, Y.; Marchot, P. Hot Spots for Protein Partnerships at the Surface of Cholinesterases and Related α/β Hydrolase Fold Proteins or Domains-A Structural Perspective. Molecules 2017, 23, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessen, A. Phospholipase A2 enzymes: Structural diversity in lipid messenger metabolismo. Structure 2000, 8, R15–R22. [Google Scholar] [CrossRef] [Green Version]
- Costa, T.R.; Francisco, A.F.; Cardoso, F.F.; Moreira-Dill, L.S.; Fernades, C.A.H.; Gomes, A.A.S.; Guimarães, C.L.S.; Marcussi, S.; Pereira, P.S.; Oliveira, H.C.; et al. Gallic acid anti-myotoxic activity and mechanism of action, a snake venom phospholipase A 2 toxin inhibitor, isolated from the medicinal plant Anacardium humile. Int. J. Biol Macromol. 2021, 185, 494–512. [Google Scholar] [CrossRef] [PubMed]
- Kankara, I.A.; Abdullahi, I.; Paulina, G.A. Ethnomedicinal plants: A source of phytochemical compounds against snake venom PLA2s activity. J. Pharmacogn. Phytochem. 2020, 9, 1270–1275. [Google Scholar]
- Assunção, P.I.D.; Conceição, E.C.; Borges, L.L.; de Paula, J.A.M. Development and Validation of a HPLC-UV Method for the Evaluation of Ellagic Acid in Liquid Extracts of Eugenia uniflora L. (Myrtaceae) Leaves and Its Ultrasound-Assisted Extraction Optimization. Evid. -Based Complementary. Altern. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrim, C.A.; de Oliveira, S.C.B.; Diz Filho, E.B.S.; Fonseca, F.V.; Baldissera, L.; Antunes, E.; Ximenes, R.M.; Monteiro, H.S.A.; Rabello, M.M.; Hernades, M.Z.; et al. Quercetin as an inhibitor of snake venom secretory phospholipase A2. Chem. -Biol. Interact. 2011, 189, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Toyama, M.H.; Gaeta, H.H.; Pinho, M.V.T.; Ferreira, M.J.P.; Romoff, P.; Matioli, F.F.; Magro, A.J.; Fontes, M.R.M.; Toyama, M.H. An Evaluation of 3-Rhamnosylquercetin, a Glycosylated Form of Quercetin, against the Myotoxic and Edematogenic Effects of sPLA2 from Crotalus durissus terrificus. BioMed Res. Int. 2014, 2014, 341270. [Google Scholar] [CrossRef] [Green Version]
- Samad, N.; Javed, A. Therapeutic Effects of Gallic Acid: Current Scenario. J. Phytochem. Biochem. 2019, 2, 113. [Google Scholar]
- Simmons, M.A. Platelet Activating Factor. In xPharm: The Comprehensive Pharmacology Reference; NEOUCOM: Rootstown, OH, USA, 2007; pp. 1–3. [Google Scholar]
- Marathe, G.K.; Chaithra, V.H.; Ke, L.Y.; Chen, C.H. Effect of acyl and alkyl analogs of platelet-activating factor on inflammatory signaling. Prostaglandins Other Lipid Mediat. 2020, 151, 106478. [Google Scholar] [CrossRef]
- Toyama, M.H.; Costa, C.R.C.; Belchor, M.N.; Novaes, D.P.; Oliveira, M.A.; Ie, R.; Gaeta, H.H.; Toyama, D.O. Edema Induced by sPLA2 from Crotalus Durissus Terrificus Involves PLC and PKC Signaling, Activation of cPLA2 and Oxidative Stress; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Toyama, M.H.; Costa, C.R.C.; Belchor, M.N.; Junior, A.B.D.S.; Moraes, L.L.F.D.; Silva, A.R.D.S.; Oliveira, M.A.D. Evaluation of Thiol-dependent Enzymes on the Pharmacological Effects Induced by the Catalytically Active PLA2 from Bothrops jararacussu. Preprints 2021, 0012. [Google Scholar] [CrossRef]
- Teixeira, C.; Fernandes, C.M.; Leiguez, E.; Chudzinski-Tavassi, A.M. Inflammation Induced by Platelet-Activating Viperid Snake Venoms: Perspectives on Thromboinflammation. Front. Immunol. 2019, 10, 2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva Junior, I.A.; Andrade, L.N.S.; Jancar, S.; Chammas, R. Platelet activating factor receptor antagonists improve the efficacy of experimental chemo- and radiotherapy. Clinics 2018, 73, e792s. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Baird, A.W.; Parsons, M.J.; Fan, K.; Skerrett-Byrne, D.A.; Nair, P.M.; Makanyengo, S.; Chen, J.; Neal, R.; Goggins, B.J.; et al. Platelet activating factor receptor acts to limit colitis-induced liver inflammation. FASEB J. 2020, 34, 7718–7732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Wang, K.; Shen, J. Lipoprotein-associated phospholipase A2: The story continues. Med. Res. Rev. 2020, 40, 79–134. [Google Scholar] [CrossRef]
- Fang, L.; Pan, Y.; Muzyka, J.L.; Zhan, C.G. Active site gating and substrate specificity of butyrylcholinesterase and acetylcholinesterase: Insights from molecular dynamics simulations. J. Phys. Chem. B 2011, 115, 8797–8805. [Google Scholar] [CrossRef] [Green Version]
- Camara, P.R.S.; Esquisatto, L.C.M.; Camargo, E.A.; Toyama, M.H.; Marangoni, S.; Ribela, M.T.C.; De Nucci, G.; Antunes, E. Inflammatory oedema induced by phospholipases A2 isolated from Crotalus durissus sp. in rat dorsal skin: A role for mast cells and sensory C-fibers. Toxicon 2003, 41, 823–829. [Google Scholar] [CrossRef]
- Sartim, M.A.; Souza, C.O.S.; Diniz, C.R.A.F.; da Fonseca, V.M.B.; Sousa, L.O.; Peti, A.P.F.; Costa, T.R.; Lourenço, A.G.; Borges, M.C.; Sorgi, C.A.; et al. Crotoxin-Induced Mice Lung Impairment: Role of Nicotinic Acetylcholine Receptors and COX-Derived Prostanoids. Biomolecules 2020, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Ranawaka, U.K.; Lalloo, D.G.; de Silva, H.J. Neurotoxicity in Snakebite—The Limits of Our Knowledge. PloS. Negl. Trop. Dis. 2013, 7, e2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuefner, M.S. Secretory Phospholipase A2s in Insulin Resistance and Metabolism. Front. Endocrinol. 2021, 12, 732726. [Google Scholar] [CrossRef]
- Ponce-Soto, L.A.; Toyama, M.H.; Hyslop, S.; Novello, J.C.; Marangoni, S. Isolation and preliminary enzymatic characterization of a novel PLA2 from Crotalus durissus collilineatus venom. J. Protein Chem. 2002, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Leslie, C.L. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paloschi, M.V.; Lopes, J.A.; Boeno, C.N.; Silva, M.D.S.; Evangelista, J.R.; Pontes, A.S.; da Silva Setúbal, S.; Rego, C.M.A.; Néry, N.M.; Ferreira, A.A.; et al. Cytosolic phospholipase A2-α participates in lipid body formation and PGE2 release in human neutrophils stimulated with an L-amino acid oxidase from Calloselasma rhodostoma venom. Sci. Rep. 2020, 10, 10976. [Google Scholar] [CrossRef] [PubMed]
- Amaravani, M.; Prasad, N.K.; Ramakrishna, V. COX-2 structural analysis and docking studies with gallic acid structural analogues. SpringerPlus 2012, 1, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Chung, K.F. Platelet-Activating Factor. Encycl. Respir. Med. 2022, 22, 462–473. [Google Scholar]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the War Against Inflammation with Natural Products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef]
- Britt, R.D., Jr.; Locy, M.L.; Tipple, T.E.; Nelin, L.D.; Rogers, L.K. Lipopolysaccharide-induced cyclooxygenase-2 expression in mouse transformed Clara cells. Cell Physiol. Biochem. 2012, 29, 213–222. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyama, M.H.; Rogero, A.; de Moraes, L.L.F.; Fernandes, G.A.; da Cruz Costa, C.R.; Belchor, M.N.; De Carli, A.M.; de Oliveira, M.A. Gallic Acid as a Non-Selective Inhibitor of α/β-Hydrolase Fold Enzymes Involved in the Inflammatory Process: The Two Sides of the Same Coin. Pharmaceutics 2022, 14, 368. https://doi.org/10.3390/pharmaceutics14020368
Toyama MH, Rogero A, de Moraes LLF, Fernandes GA, da Cruz Costa CR, Belchor MN, De Carli AM, de Oliveira MA. Gallic Acid as a Non-Selective Inhibitor of α/β-Hydrolase Fold Enzymes Involved in the Inflammatory Process: The Two Sides of the Same Coin. Pharmaceutics. 2022; 14(2):368. https://doi.org/10.3390/pharmaceutics14020368
Chicago/Turabian StyleToyama, Marcos Hikari, Airam Rogero, Laila Lucyane Ferreira de Moraes, Gustavo Antônio Fernandes, Caroline Ramos da Cruz Costa, Mariana Novo Belchor, Agatha Manzi De Carli, and Marcos Antônio de Oliveira. 2022. "Gallic Acid as a Non-Selective Inhibitor of α/β-Hydrolase Fold Enzymes Involved in the Inflammatory Process: The Two Sides of the Same Coin" Pharmaceutics 14, no. 2: 368. https://doi.org/10.3390/pharmaceutics14020368
APA StyleToyama, M. H., Rogero, A., de Moraes, L. L. F., Fernandes, G. A., da Cruz Costa, C. R., Belchor, M. N., De Carli, A. M., & de Oliveira, M. A. (2022). Gallic Acid as a Non-Selective Inhibitor of α/β-Hydrolase Fold Enzymes Involved in the Inflammatory Process: The Two Sides of the Same Coin. Pharmaceutics, 14(2), 368. https://doi.org/10.3390/pharmaceutics14020368






