1. Introduction
We are currently witnessing a constant increase in the amount of municipal waste generated as a result of the growing production and consumption in all sectors of the economy [
1]. Part of this municipal waste is recycled, part is stored at waste landfills, which comprise waste-type-specific installations, and part is combusted in specialist waste incineration plants, which use high-temperature methods like plasma gasification [
2,
3,
4,
5]. Numerous countries in the world operate waste-to-energy facilities, although municipal waste incineration remains a socially controversial practice. One of the major problems related to the management of municipal waste is its illegal combustion in household furnaces [
6]. A large part of households in Poland (CEE) is still heated with hard coal, which is combined with various types of municipal waste [
7]. This practice results in high concentrations of air pollutants, which include PM
2.5 and PM
10 particles or sulphur (SO
2) and nitrogen (NOx) compounds [
8,
9]. Waste incineration in household furnaces does not bring any significant benefits, not even energy-related ones. When burnt in household furnaces, the material displays a low energy content, in contrast to its combustion in specialist furnaces. Furthermore, installations intended for waste incineration only or furnaces used in the industrial power sector (in power plants or combined heat and power plants—CHP) are equipped with systems that maximally prevent the emissions of pollutants, especially gases and dusts, into the atmosphere [
10,
11,
12,
13]. Furnaces in domestic boiler houses do not have dedusting, desulphurisation, or denitrogenation systems.
Another problem would be residues remaining after the combustion of various types of alternative fuels (i.e., coal, wood, peat, and others) combined with different fractions of municipal waste [
6,
14,
15]. It often happens that these residues, in the form of ash, enter the municipal waste stream collected by municipal enterprises. A large part of this material, especially in rural areas, is disposed of onto arable land, despite its negative impact on soils or the groundwater environment [
16,
17]. Ashes from waste incineration, power, or CHP plants are most often used as various additives to concrete mixes [
5,
18,
19] or stored, having been properly secured as they can easily be air borne by winds. The process of eolic transportation of the finest particulate fractions may have very negative consequences, especially environmental ones, in the form of an adverse impact on the proper development of plants, such as decreasing their assimilation [
10,
20,
21,
22].
The estimated number of households heated with solid fuels in Poland is over 3 million, which results in the generation of 2.5 million tons of ashes annually [
23]. Ashes from household furnaces are a problematic type of waste due to the fact that not all communes (administrative units responsible for municipal engineering) provide for the special collection of such waste.
An important legal matter related to the management of ashes generated in household furnaces is their classification in accordance with the applicable waste catalogue. Initially, when communes started to collect household ashes, the latter were classified in the same group as waste generated by power plants or other power-related facilities. However, under the currently applicable legal regulations [
24], ashes from household furnaces are classified as municipal waste. The reason for this change was the properties and chemical composition of ashes vary greatly and depend on the type of fuel or fuel mixes, as well as other additives burnt in household furnaces [
6,
11,
12,
25,
26]. Additives such as municipal waste significantly determine the composition and properties of the ash generated. The main components of ashes are silicon oxide (SiO
2), iron oxide (Fe
2O
3), and aluminum oxide (Al
2O
3). The structure of the ash obtained depends mainly on the primary material used and the combustion temperature. Ashes are predominantly fine particulate products [
20,
27]. However, they are characterised by a certain degree of size variability, making their fraction classification quite broad, from a psammite fraction to fine-grained pelite particulates. The size of ash particles is immensely important when assessing their impact on the plant and soil environment [
10,
22,
26]. As demonstrated in previous studies, the sand and silt fractions are of major importance, as they determine the dustiness and filtrating properties of ashes [
20,
28,
29]. Another important property of ashes is their solubility and high susceptibility of their components, especially the toxic ones, to leaching [
11,
15,
27,
30,
31]. The latter contain potentially toxic elements (PTEs), which mainly include heavy metals (i.e., Al, Cd, Cr, Fe, Ni, Pb, and Zn) and metalloids (As). Their excessive amounts may be toxic, or even carcinogenic, to living organisms. At the biochemical level, PTEs in excessive concentrations compete with basic metabolites, disrupt the process of major ion exchange, damage cellular membranes, and react with phosphate groups in ADP and ATP. This pertains to all three trophic levels: producers, consumers, and decomposers [
26,
27].
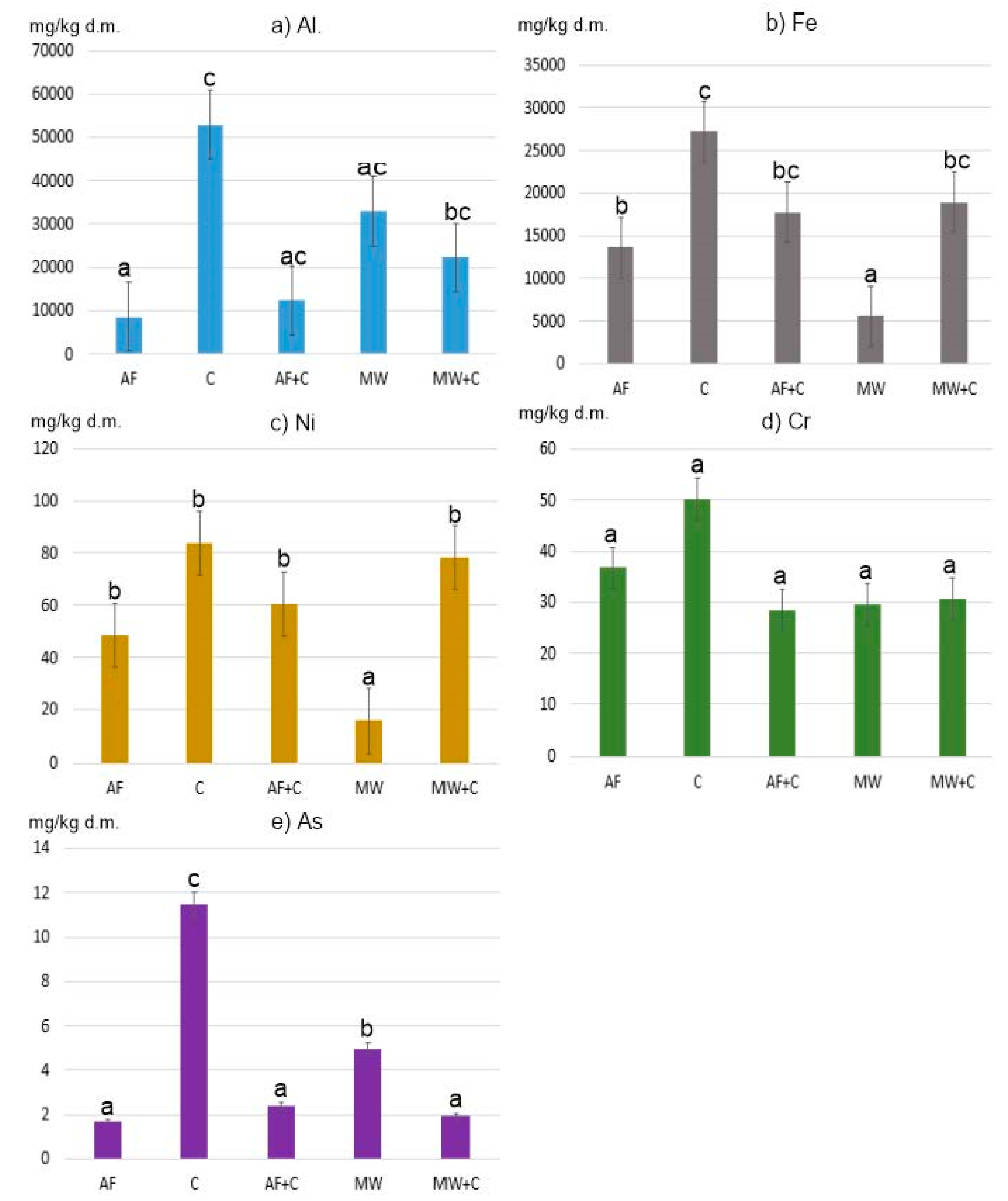
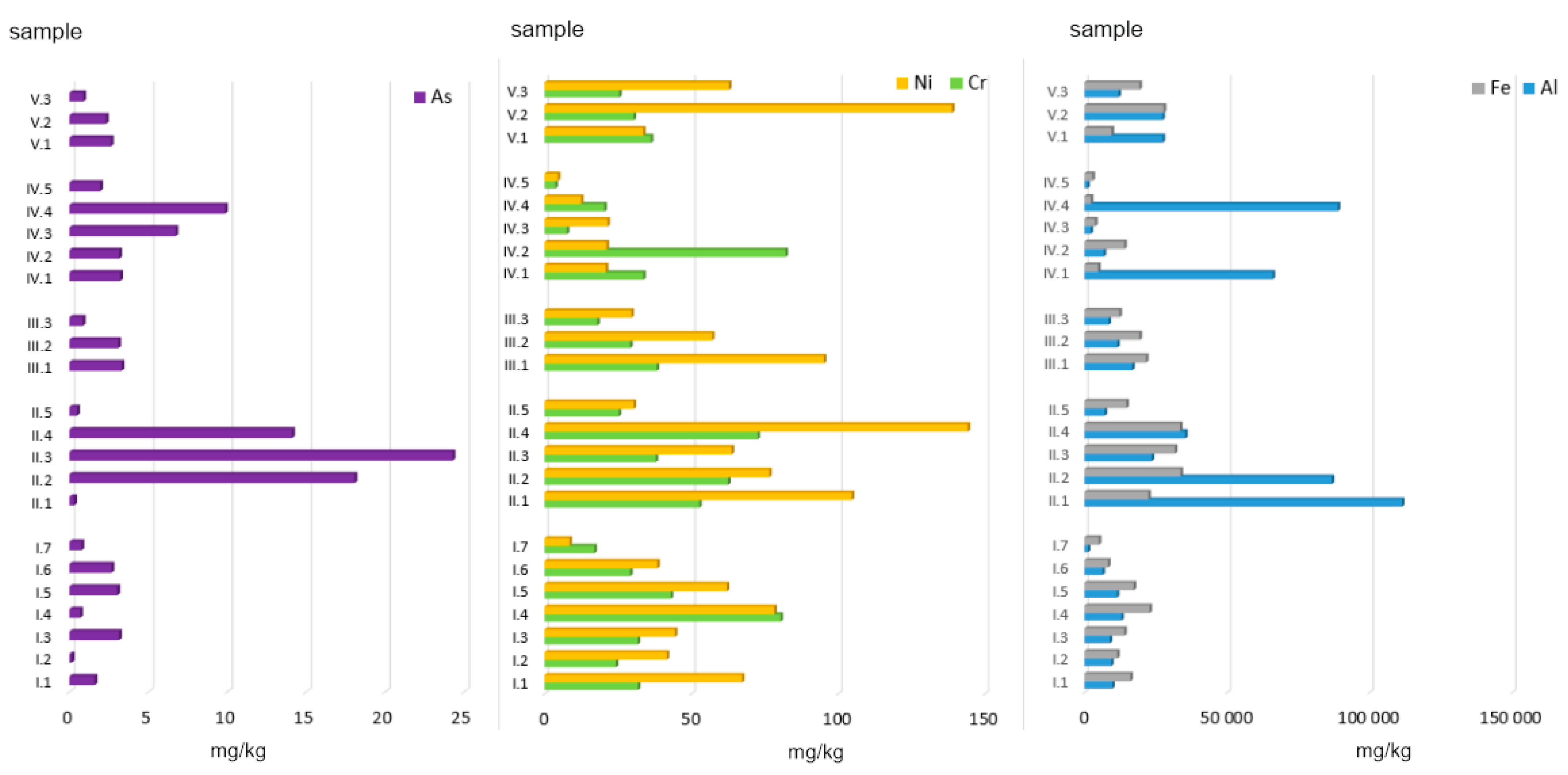
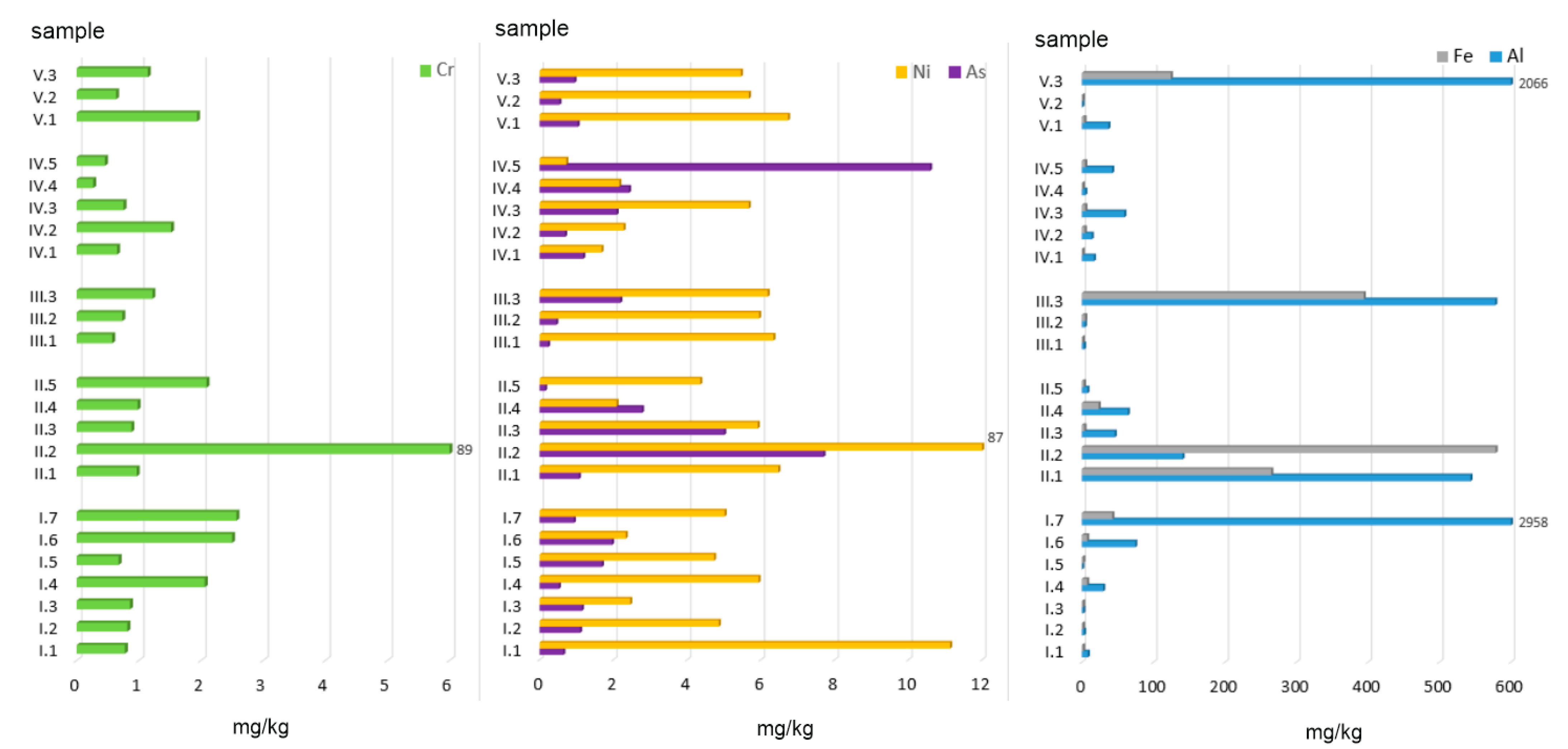
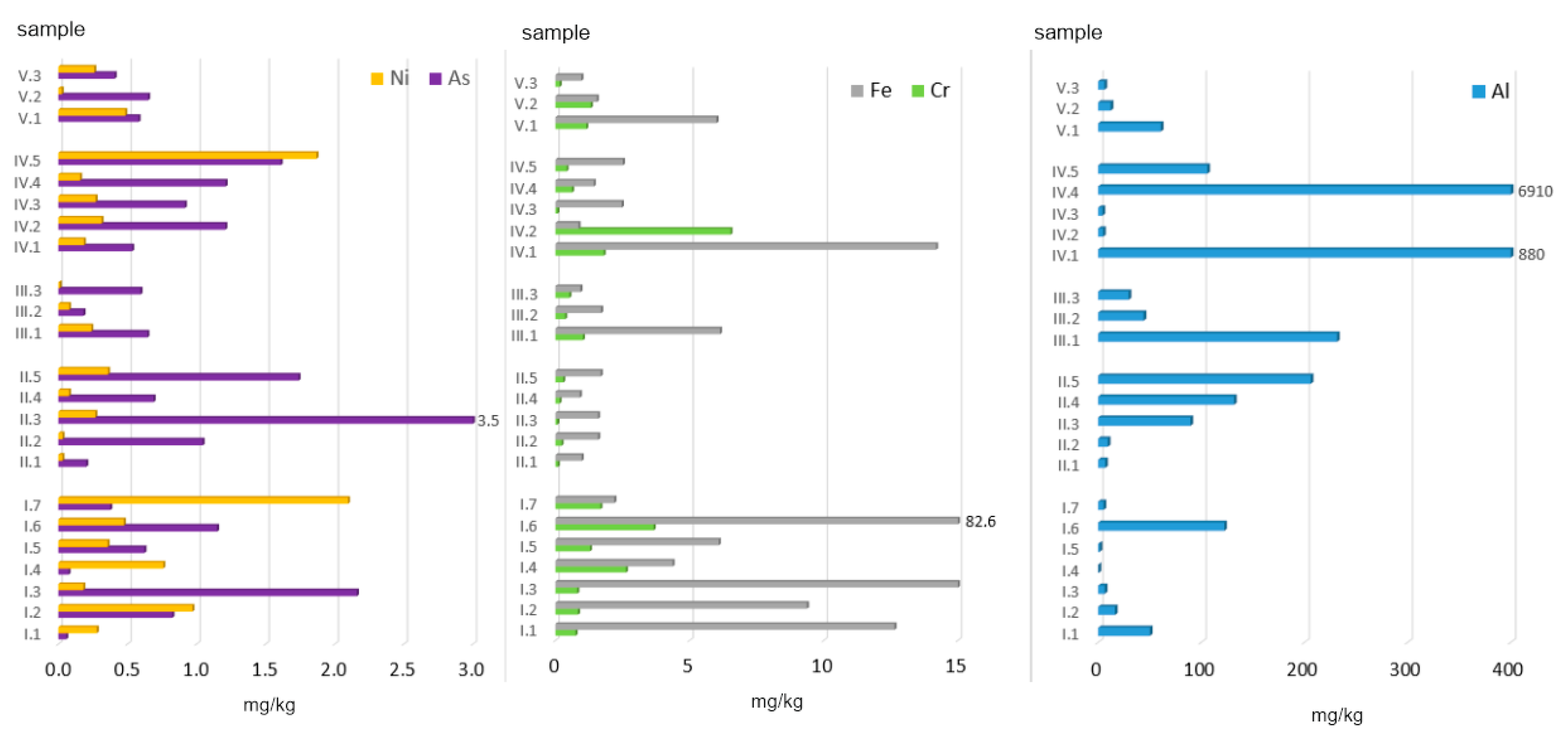
Given the above-mentioned facts, this paper presents an assessment of the environmental risk related to the presence of Potentially Toxic Elements (PTEs: Al, As, Cr, Fe, and Ni) in ashes generated from the combustion of conventional and alternative fuels combined with various fractions of municipal waste in household furnaces. Based on the mixes prepared, the following parameters were determined:
- (i)
the total content of PTEs in ashes,
- (ii)
the amount of PTEs that were bioaccessible to producers,
- (iii)
PTE fractions present in the material analysed,
- (iv)
environmental risk (Risk Assessment Code—RAC—and modified Risk Assessment Code—mRAC) and Ecological Risk index (ERI) related to the PTEs’ susceptibility of being easily leached from ashes.
The novel aspect to this study is the parametrisation of the assessment of the environmental risk stemming from the presence of PTEs in ashes and their improper handling, such as their deposition on arable land or storage at municipal waste landfills unfit for this type of waste.
2. Materials and Methods
The study material was comprised of 23 samples of ashes generated as a result of the co-incineration of primary material mixes. The latter were composed of conventional fuels (C), alternative fuels (AF), and selected fractions of municipal waste (MW) collected from the grate of a Keller 10 kW furnace (approx. 80% AFUE efficiency, maximum working pressure 0.25 MPa). The primary material mixes were selected in a way that most accurately reflected the fuels and additives used in household furnaces.
The furnace charge was comprised of fuel mixes with an addition of single or mixed fractions of municipal waste (
Table 1). Prior to being placed in the furnace, the primary material was fragmented (about 2–5 cm fragments) to facilitate combustion. The wood used in the experiment was freshly cut.
The primary material was incinerated in April 2019 under identical conditions for each lot. The hearth and ash collector were thoroughly cleaned after combusting each portion of the material. The exact procedure of obtaining ash samples has been described previously [
31].
The 23 ash samples were divided into 5 groups (
Table 1):
- −
group I (AF, n = 7) was comprised of alternative fuels from biomass. This group included such materials as: wood of acacia (Acacia Mill.), wood of ash (Fraxinus), wood of black elderberry (Sambucus nigra L.), wood of willows (Salix L.), wood of acacia + elderberry + ash + willows, straw, and nuts.
- −
group II (C, n = 5) was comprised of conventional fuels, whose chemical composition was dominated by carbon. This group included 3 types of hard coal: hard coal no. 1, hard coal no. 2, hard coal no. 3, and coal pellets. The three types of hard coal were obtained from different coal storage sites located in various regions of Poland. Coal no. 1 was sourced from Małopolskie Province, coal no. 2 was purchased from a coal storage site in Mazowieckie Province, and coal no. 3 was obtained from Podkarpackie Province. This group also included peat due to the high content of C > 50 wt.%.
- −
group III (AF + C, n = 3) was comprised of alternative fuels mixed with conventional fuels. This group included samples of hard coal no. 1 mixed with wood: ash, willow, and acacia.
- −
group IV (MW, n = 5) was comprised of 5 types of municipal waste fractions. This group included such types of waste as: paper and cardboard, plywood, sawdust, plastic-coated paper cartons, and used disposable diapers.
- −
group V (C + MW, n = 3) was comprised of material obtained after incinerating hard coal mixed with municipal waste. This group included ashes generated from combusting hard coal no. 1 combined with textiles, mixed municipal waste, and plastic.
The exact procedure of ash sample preparation has been described by Kicińska and Caba [
31]. The fuels were incinerated according to the following steps:
- −
a portion of the primary material was placed in the furnace chamber;
- −
the material underwent incomplete combustion, initiated with a gas burner;
- −
the furnace and ash were cooled down;
- −
the ash was collected into a container;
- −
the coarse fraction was separated using a sieve;
- −
a sample of the ash was collected and placed in a tightly sealed plastic bag;
- −
the chamber and bottom hopper were cleaned mechanically and with compressed air before the incineration of the next portion of fuel.
The primary material cobusted and cooled down naturally. In each experiment, the air supply was the same. In the case of mixes, the fuel and waste materials were used in equal proportions, 1:1 v/v. The incineration conditions were to reflect those found in household furnaces. As most of household furnaces do not feature any built-in measurement system, the furnace used for the experiments was not equipped with one either.
2.1. Physicochemical Analyses
In the preliminary stage of laboratory testing, each ash sample was dried at 105 °C for 2 h to determine the constant weight, and then secured against moisture.
2.1.1. pH
The pH of the aqueous extracts of ashes was analysed in accordance with the [
32] PN-EN 12176:2004 standard, using a pH meter (CPC-401), which is an electronic voltmeter indicating the pH of a solution based on the electromotive force measurement. The pH measurement was repeated three times for each extract (1:2.5 solid phase/solution ratio), with the suspension being previously stirred with a glass rod. Each time, the pH electrode was rinsed with distilled water, and any excessive liquid was removed with blotting paper. The results obtained were averaged.
2.1.2. Pseudo Total Content of PTEs
A single-step extraction was conducted in a concentrated mixture of acids (HCl:HNO3) at a 1:3 ratio, with the solid phase/solution ratio of 1:10. This concentrated mixture of acids breaks only some of the chemical bonds (silicate bonds may remain intact). Thus, this method is associated with a certain degree of underestimation. However, in light of the research goals of the present study and the fact that substances with a stronger effect are not found in the environment, the authors decided to use this procedure to assess the total content of PTEs. It is more accurate when determining low PTE concentrations, which was of significant importance for the environmental risk assessment. Given that aluminosilicate forms do not dissolve in the solvent used, this kind of degradation is called pseudo total content (TC). Mineralisation was conducted for 2 h in a microwave oven (SCP SCIENCE, type DigiPREP HT) at 130 °C, and then the solutions were cooled down and decanted.
2.1.3. Ion-Exchange and Carbonate-Bound Fraction—Extraction with CH3COOH
To calculate the environmental risk, the first step of the BCR (European Community Bureau of References) extraction was performed [
10,
33]. The diversity of physicochemical bonds present in the materials studied results in an increase or a decrease in the mobility of elements and can also affect their bioaccessibility. To perform the extraction, a 1 g portion was weighed out from each of the previously dried samples and then placed in PCV test tubes. Next, 40 mL of 0.11 M CH3CCOH was added to each of the tubes. The obtained suspensions were mixed for 16 h at 22 °C. The eluates were then decanted and the concentrations of the elements analysed were determined. The extraction with 0.11 M CH
3COOH allowed for calculating the percentage share of the exchange fraction easily soluble in acid (F1) relative to the total concentration of PTEs. The procedure provided metals contained at the exchange positions as well as those bound to carbonates.
2.1.4. Bioaccessible Fraction—Extraction with CaCl2
To determine the bioaccessible fraction of PTEs, extraction with a 0.01 M CaCl
2 solution was performed [
34]. This solvent was used due to its ionic strength, which is similar to the mean concentration of salts in soils. The suspensions were shaken for 2 h at 22 °C, at a 1:20 solid phase/solution ratio. The eluates were centrifuged, decanted, and filtered.
PTE concentrations (Al, As, Cr, Fe, and Ni) in the solutions analysed (aqueous, exchange-carbonate, and bioaccessible fractions) were determined in an accredited hydrogeochemical laboratory (certificate of accreditation PCA no. AB1050) at AGH University of Science and Technology. PTE measurement precision was 10%, and accuracy ranged from 95% to 109%. The control system of the analyses (QA/QC) was compliant with the PN-EN ISO 17025 standard. In each series of determinations, blank samples, duplicate samples (min. 25%), and marked samples were used. Moreover, the Standard Reference Material (NIST) no. 1633b (Trace Elements in Coal Fly Ash) was analysed, for which the differences in all the element concentrations did not exceed 4%.
The results were statistically compiled using Excel 2013 and Statistica ver.13.1 software.
2.2. Environmental and Ecological Risk (RAC, mRAC, ERI)
The environmental risk related to the presence of PTEs in ashes from household furnaces was calculated using the Risk Assessment Code (RAC). This method involves comparing the percentage of cations found in ion-exchange positions and bound to carbonates (F1 BCR fraction), with their total concentration in a given sample [
35]. RAC was calculated using Formula (1):
Values between 1–10% denoted low risk, 11–30%—medium risk, 31–50%—high risk, and over 50%—very high risk [
6,
36].
Another index analysed was the modified Risk Assessment Code (
mRAC), calculated based on formulas suggested by Håkanson [
37], which additionally includes m—the “toxic-response” factor. The calculated RAC value is multiplied by Tri—the toxic-response factor for a given substance (Tr for As equals 10, for Ni—5, and for Cr—2).
The last tool used in the study was the Ecological Risk Index (ERI), which represented the risk from the sum of metals contained in the study material analysed [
37]. It is the sum of the calculated mRAC values (in this case for As, Cr, and Ni).