A Novel Role of Galectin-3 and Thyroglobulin in Prognosis and Differentiation of Different Stages of Thyroid Cancer and Elucidation of the Potential Contribution of Bcl-2, IL-8 and TNF-α
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Ethical Approval and Informed Consent Statement
2.2. Sampling and Subject Criteria
2.3. Complete Blood Count (CBC) Test
2.4. Quantitative Real-Time PCR (qRT-PCR) of Bcl-2 and Bax
2.5. Estimation of Serum TG Level
2.6. Estimation of Serum IL-8 Levels
2.7. Estimation of Serum TNF-α Level
2.8. Estimation of Serum Gal-3 Levels
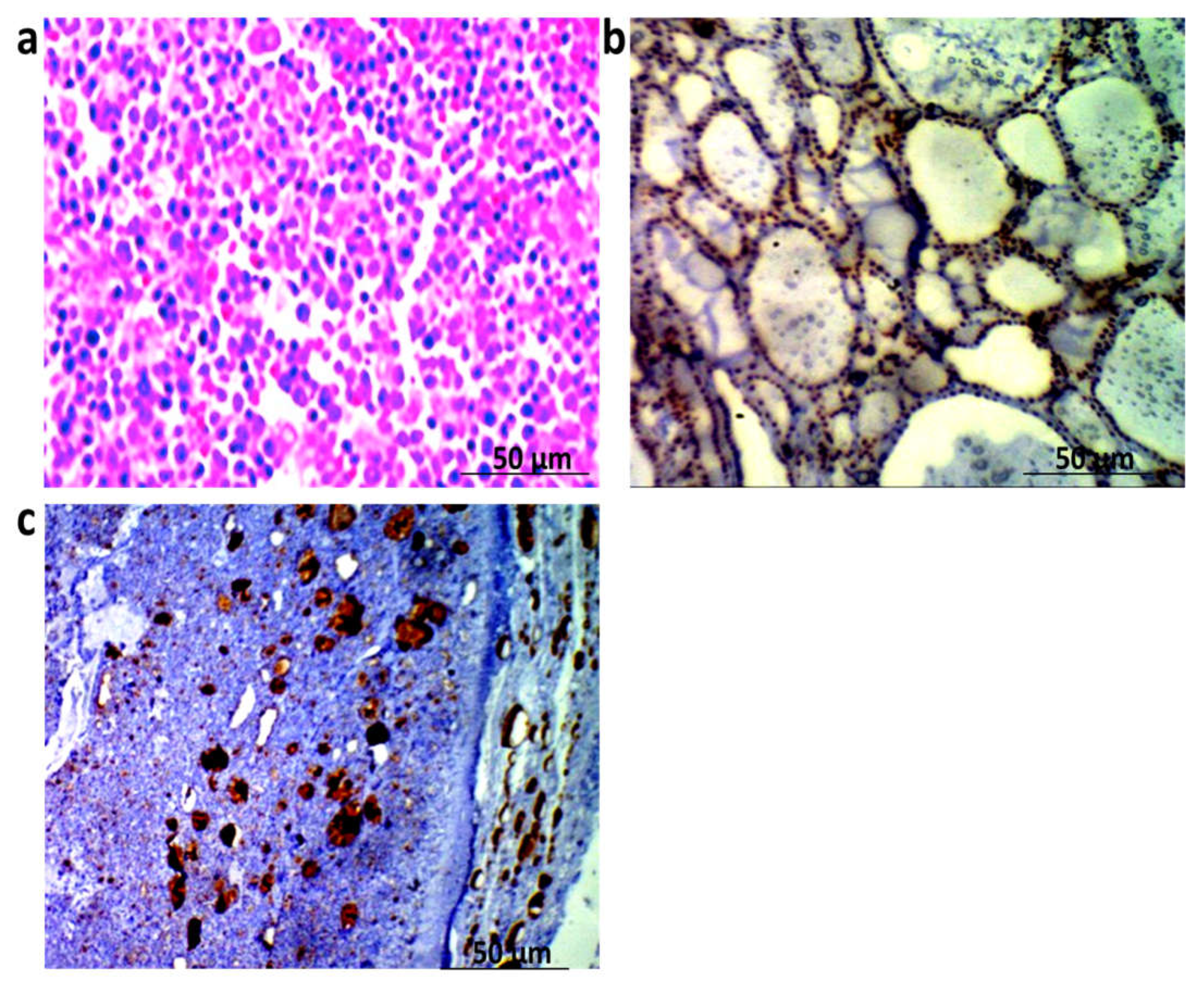
2.9. Immunohistochemistry for Determination of TG in Thyroid Tissues
2.10. Histopathological Analysis
2.11. Statistical Methods
3. Results
3.1. Demographical Data and Hematological Parameters
3.2. The Gene Expressions of Bcl-2 and Bax
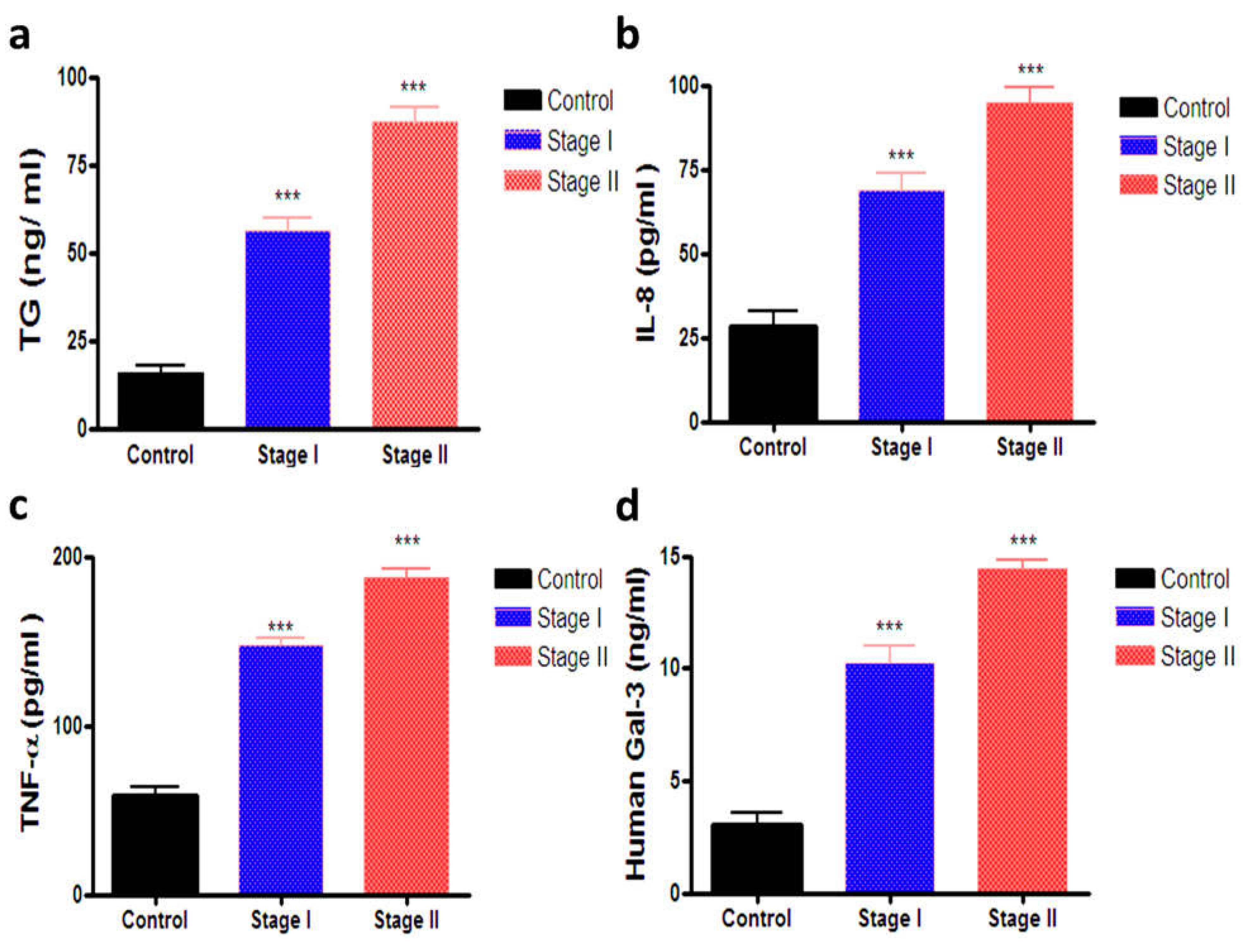
3.3. Estimation of Serum TG, IL-8, TNF-α and Gal-3
3.4. Histopathological and Immunohistochemical Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Xia, X.; Kim, G.D.; Liu, Y.; Xue, Z.; Zhang, L.; Shu, Y.; Yang, T.; Chen, Y.; Zhang, S.; et al. Characterizing dedifferentiation of thyroid cancer by integrated analysis. Sci. Adv. 2021, 7, eabf3657. [Google Scholar] [CrossRef]
- Gillanders, S.; O’Neill, J. Prognostic markers in well differentiated papillary and follicular thyroid cancer (WDTC). Eur. J. Surg. Oncol. 2018, 44, 286–296. [Google Scholar] [CrossRef]
- Mamedov, U.; Khodjaeva, D. Modern Diagnostic Approachкetreatment of Thyroid Cancer. Int. J. Dev. Public Policy 2021, 1, 101–105. [Google Scholar]
- Spencer, C.A.; LoPresti, J.S. Technology Insight: Measuring thyroglobulin and thyroglobulin autoantibody in patients with differentiated thyroid cancer. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 223–233. [Google Scholar] [CrossRef]
- Zahra, H.O.; Omran, G.A.; Gewely, A.G.; Eldehn, A.F.; Abdo, W.; Elmahallawy, E.K.; Okda, T.M. Prognostic Value of Serum Thyroglobulin and Anti-Thyroglobulin Antibody in Thyroid Carcinoma Patients following Thyroidectomy. Diagnostics 2021, 11, 2080. [Google Scholar] [CrossRef]
- Prpić, M.; Franceschi, M.; Romić, M.; Jukić, T.; Kusić, Z. Thyroglobulin as a Tumor Marker in Differentiated Thyroid Cancer–Clinical Considerations. Acta Clin. Croat. 2018, 57, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Eyler, C.E.; Rich, J.N. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008, 26, 2839–2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauerle, K.T.; Schweppe, R.E.; Haugen, B.R. Inhibition of nuclear factor-kappa B differentially affects thyroid cancer cell growth, apoptosis, and invasion. Mol. Cancer 2010, 9, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; Khalil, M.G.; Abd El-latif, D.M.; Okda, T.; Abdelaziz, A.I.; Kamal, M.M.; Wahid, A. The influence of vinpocetine alone or in combination with Epigallocatechin-3-gallate, Coenzyme COQ10, Vitamin E and Selenium as a potential neuroprotective combination against aluminium-induced Alzheimer’s disease in Wistar Albino Rats. Arch. Gerontol. Geriatr. 2022, 98, 104557. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.; Kunicka, J.; Kratz, A. The ADVIA 2120 hematology system: Flow cytometry-based analysis of blood and body fluids in the routine hematology laboratory. Lab. Hematol. 2005, 11, 47–61. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hahn, H.P.; Bundock, E.A.; Hornick, J.L. Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics. Am. J. Clin. Pathol. 2006, 125, 203–208. [Google Scholar] [CrossRef]
- Park, S.; Jeon, M.J.; Oh, H.-S.; Lee, Y.-M.; Sung, T.-Y.; Han, M.; Han, J.M.; Kim, T.Y.; Chung, K.-W.; Kim, W.B.; et al. Changes in serum thyroglobulin levels after lobectomy in patients with low-risk papillary thyroid cancer. Thyroid 2018, 28, 997–1003. [Google Scholar] [CrossRef]
- Li, J.; Vasilyeva, E.; Wiseman, S.M. Beyond immunohistochemistry and immunocytochemistry: A current perspective on galectin-3 and thyroid cancer. Expert Rev. Anticancer. Ther. 2019, 19, 1017–1027. [Google Scholar] [CrossRef]
- Makki, F.M.; Taylor, S.M.; Shahnavaz, A.; Leslie, A.; Gallant, J.; Douglas, S.; Teh, E.; Trites, J.; Bullock, M.; Inglis, K.; et al. Serum biomarkers of papillary thyroid cancer. J. Otolaryngol.-Head Neck Surg. 2013, 42, 16. [Google Scholar] [CrossRef] [Green Version]
- Hamivand, Z.; Haddadi, G.; Fardid, R. Expression of Bax and Bcl2 genes in peripheral blood lymphocytes of patients with differentiated thyroid cancer. J. Med. Phys. 2018, 43, 41–45. [Google Scholar] [PubMed]
- Spanos, S.; Rice, S.; Karagiannis, P.; Taylor, D.; Becker, D.; Winston, R.; Hardy, K. Caspase activity and expression of cell death genes during development of human preimplantation embryos. Reproduction 2002, 124, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Li, Y.; Gao, Z.; Wang, R. Antitumor activity of Koningic acid in thyroid cancer by inhibiting cellular glycolysis. Endocrine 2022, 75, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Okda, T.M.; Abd-Εlghaffar, S.K.; Katary, M.A.; Abd-Alhaseeb, M.M. Chemopreventive and anticancer activities of indomethacin and vitamin D combination on colorectal cancer induced by 1,2-dimethylhydrazine in rats. Biomed. Rep. 2021, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Okda, T.M.; Katry, M.A.; Ragab, N.M.; Shalkami, A.-G.S. Phytic acid potentiates oxaliplatin effects in colorectal cancer induced by 1, 2-DMH: The role of miR-224 and miR-200a. Contemp. Oncol. 2021, 25, 118. [Google Scholar]
- Gonzalez-Aparicio, M.; Alfaro, C. Influence of Interleukin-8 and Neutrophil Extracellular Trap (NET) Formation in the Tumor Microenvironment: Is There a Pathogenic Role? J. Immunol. Res. 2019, 2019, 6252138. [Google Scholar] [CrossRef] [Green Version]
- Fakhry, N.; Gowily, A.; Okda, T.; Houssen, M. Serum soluble toll-like receptor 2 and 4 as diagnostic and prognostic biomarkers for non-Hodgkin lymphoma. Contemp. Oncol. 2020, 24, 157–162. [Google Scholar] [CrossRef]
- Bauerle, K.T.; Schweppe, R.E.; Lund, G.; Kotnis, G.; Deep, G.; Agarwal, R.; Pozdeyev, N.; Wood, W.M.; Haugen, B.R. Nuclear Factor κB–Dependent Regulation of Angiogenesis, and Metastasis in an In Vivo Model of Thyroid Cancer Is Associated With Secreted Interleukin-8. J. Clin. Endocrinol. Metab. 2014, 99, E1436–E1444. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, C.; Sanmamed, M.F.; Rodríguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef]
- Xi, C.; Zhang, G.-Q.; Sun, Z.-K.; Song, H.-J.; Shen, C.-T.; Chen, X.-Y.; Sun, J.-W.; Qiu, Z.-L.; Luo, Q.-Y. Interleukins in thyroid cancer: From basic researches to applications in clinical practice. Front. Immunol. 2020, 11, 1124. [Google Scholar] [CrossRef]
- Bär, E.; Whitney, P.G.; Moor, K.; Sousa, C.R.e.; LeibundGut-Landmann, S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 2014, 40, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Wang, Q.; Tian, Y.; Xiong, S.; Li, G.; Xu, L. Expressions of IL-17 and TNF-α in patients with Hashimoto’s disease combined with thyroid cancer before and after surgery and their relationship with prognosis. Clin. Transl. Oncol. 2019, 22, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, G.; Radcenco, A.L.; Evaristo, J.; Monnerat, G. Novel strategies for clinical investigation and biomarker discovery: A guide to applied metabolomics. Horm. Mol. Biol. Clin. Investig. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, R.W.; Wharton, K.A.; Quigley, C.; Lowe, A. The Use of Immunohistochemistry for Biomarker Assessment—Can It Compete with Other Technologies? Toxicol. Pathol. 2011, 39, 988–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawes, D.; Shi, S.-R.; Dabbs, D.J.; Taylor, C.R.; Cote, R.J. Immunohistochemistry. Mod. Surg. Pathol. 2009, 1, 48–70. [Google Scholar] [CrossRef]
- Kim, S.-W.; Roh, J.; Park, C.-S. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef] [Green Version]


| Primer | Sequence (5′-3′) Direction |
|---|---|
| Bcl-2 | F: 5′CATCGCCCTGTGGATGACTG 3′ |
| R: 5′GGCCATATAGTTCCACAAAGGC 3′ | |
| Bax | F: 5′GTCTCCGGCGAATTGGAGAT 3′ |
| R: 5′ACCCGGAAGAAGACCTCTCG 3′ | |
| Gapdh | F: 5′ GTATCGGACGCCTGGTTAC 3′ |
| R: 5′ CTTGCCGTGGGTAGAGTCAT 3′ |
| Data | Control | Stage 1 | Stage II |
|---|---|---|---|
| Sex | Male | Male | Male |
| Age | 42.27 ± 5.43 | 39.98 ± 7.18 | 43.88 ± 5.95 |
| History of thyroid cancer | None | None | None |
| Types of thyroid carcinoma | ------- | 77.4% Papillary 22.6% Follicular | 81.2% Papillary 18.8% Follicular |
| Biochemical Indices | Control | Stage 1 | Stage II |
|---|---|---|---|
| Hb (g/dl) | 12.28 ± 0.90 | 11.32 ± 0.63 * | 11.01 ± 0.92 * |
| RBCs × 106 | 5.79 ± 1.23 | 4.67 ± 2.03 * | 4.09 ± 1.93 * |
| Hct | 39.91 ± 4.53 | 37.81 ± 3.82 | 33.22 ± 2.77 * |
| Platelets × 103 | 248.55 ± 16.97 | 207.21 ± 21.17 * | 201.90 ± 17.51 * |
| WBCs × 103 | 7.05 ± 2.25 | 5.82 ± 1.87 * | 5.03 ± 1.33 * |
| Lymphocytes | 29.81 ± 3.85 | 37.28 ± 3.17 * | 43.75 ± 4.55 * |
| Neutrophils | 34.55 ± 4.09 | 49.85 ± 3.33 * | 47.18 ± 3.08 * |
| Biochemical Indices | Control | Stage 1 | Stage II |
|---|---|---|---|
| TG (ng/mL) | 15.64 ± 2.11 | 56.19 ± 3.59 * | 87.00 ± 4.20 * |
| IL-8 (pg/mL) | 28.54 ± 4.46 | 68.72 ± 4.95 * | 94.83 ± 4.32 * |
| TNF-α (pg/mL) | 58.41 ± 5.02 | 147.8 ± 4.27 * | 187.9 ± 4.74 * |
| Gal-3 (ng/mL) | 3.04 ± 0.57 | 10.22 ± 0.81 * | 14.46 ± 0.3378 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okda, T.M.; Atwa, G.M.K.; Eldehn, A.F.; Dahran, N.; Alsharif, K.F.; Elmahallawy, E.K. A Novel Role of Galectin-3 and Thyroglobulin in Prognosis and Differentiation of Different Stages of Thyroid Cancer and Elucidation of the Potential Contribution of Bcl-2, IL-8 and TNF-α. Biomedicines 2022, 10, 352. https://doi.org/10.3390/biomedicines10020352
Okda TM, Atwa GMK, Eldehn AF, Dahran N, Alsharif KF, Elmahallawy EK. A Novel Role of Galectin-3 and Thyroglobulin in Prognosis and Differentiation of Different Stages of Thyroid Cancer and Elucidation of the Potential Contribution of Bcl-2, IL-8 and TNF-α. Biomedicines. 2022; 10(2):352. https://doi.org/10.3390/biomedicines10020352
Chicago/Turabian StyleOkda, Tarek M., Gamal M. K. Atwa, Ahmed Fathy Eldehn, Naief Dahran, Khalaf F Alsharif, and Ehab Kotb Elmahallawy. 2022. "A Novel Role of Galectin-3 and Thyroglobulin in Prognosis and Differentiation of Different Stages of Thyroid Cancer and Elucidation of the Potential Contribution of Bcl-2, IL-8 and TNF-α" Biomedicines 10, no. 2: 352. https://doi.org/10.3390/biomedicines10020352
APA StyleOkda, T. M., Atwa, G. M. K., Eldehn, A. F., Dahran, N., Alsharif, K. F., & Elmahallawy, E. K. (2022). A Novel Role of Galectin-3 and Thyroglobulin in Prognosis and Differentiation of Different Stages of Thyroid Cancer and Elucidation of the Potential Contribution of Bcl-2, IL-8 and TNF-α. Biomedicines, 10(2), 352. https://doi.org/10.3390/biomedicines10020352







