Brain Oxygen Perfusion and Oxidative Stress Biomarkers in Fetuses with Congenital Heart Disease—A Retrospective, Case-Control Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
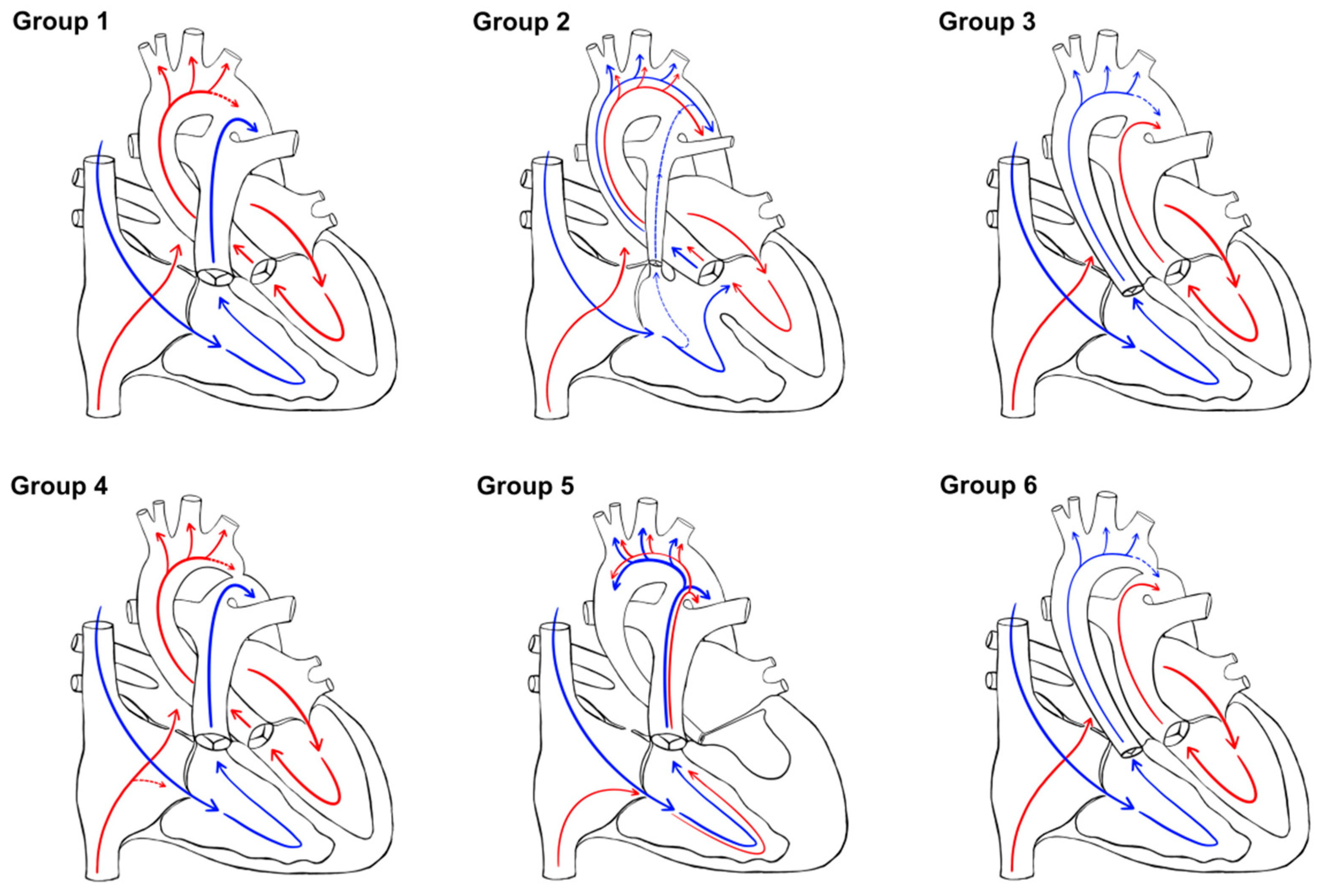
2.2. CHD Classification
- Group 1: normal oxygen with normal aortic flow (no obstruction) (CHD without significant intracardiac shunt)
- Group 2: mixed oxygen with normal aortic flow (no obstruction) (CHD with intracardiac shunt)
- Group 3: low oxygen with normal aortic flow (no obstruction) (TGA physiology without significant intracardiac shunt)
- Group 4: normal oxygen with obstructed aortic flow (CHD without significant intracardiac shunt)
- Group 5: mixed oxygen with obstructed aortic flow (CHD with intracardiac shunt, mainly HLHS)
- Group 6: low oxygen with obstructed perfusion (TGA physiology without significant intracardiac shunt).
2.3. Standards
2.4. AF Sample Preparation and Analysis
2.5. Statistical Analysis
3. Results
3.1. Population of the Study
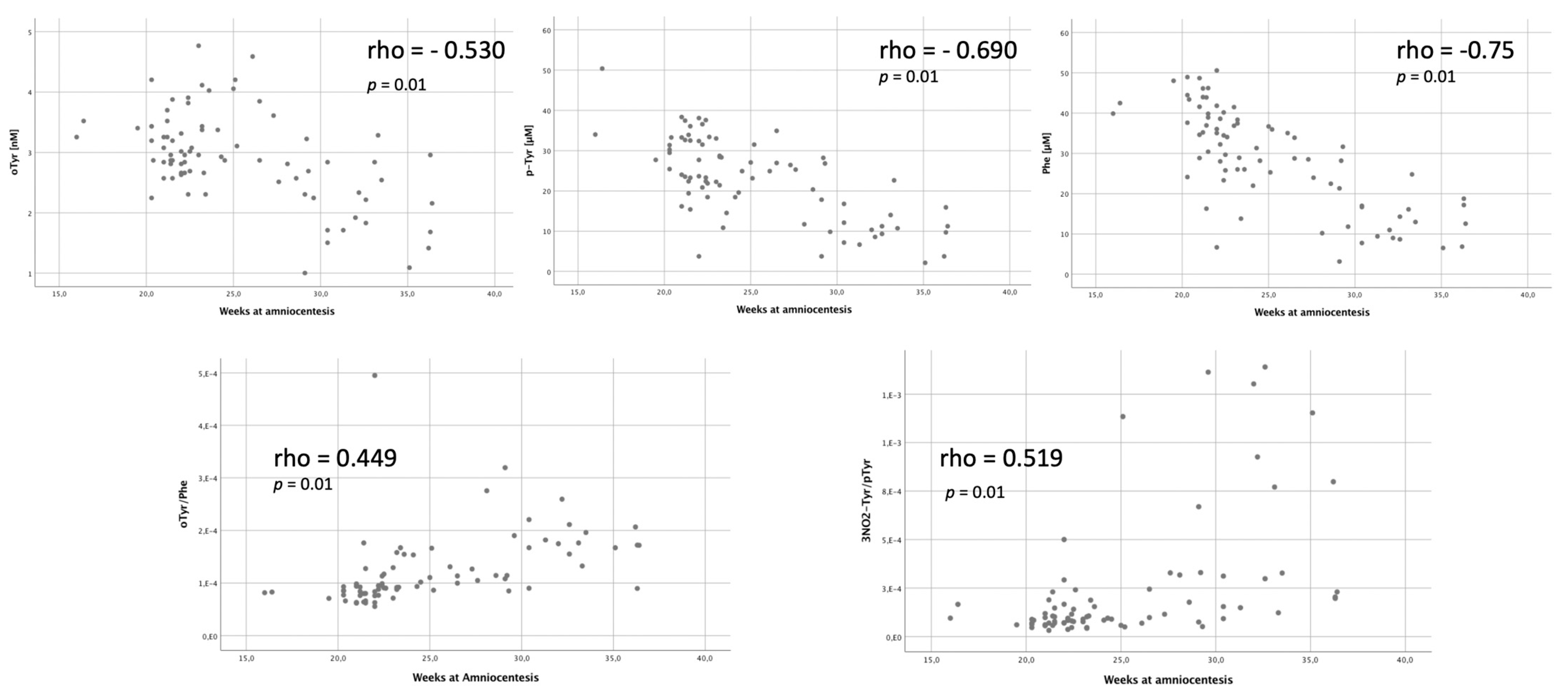
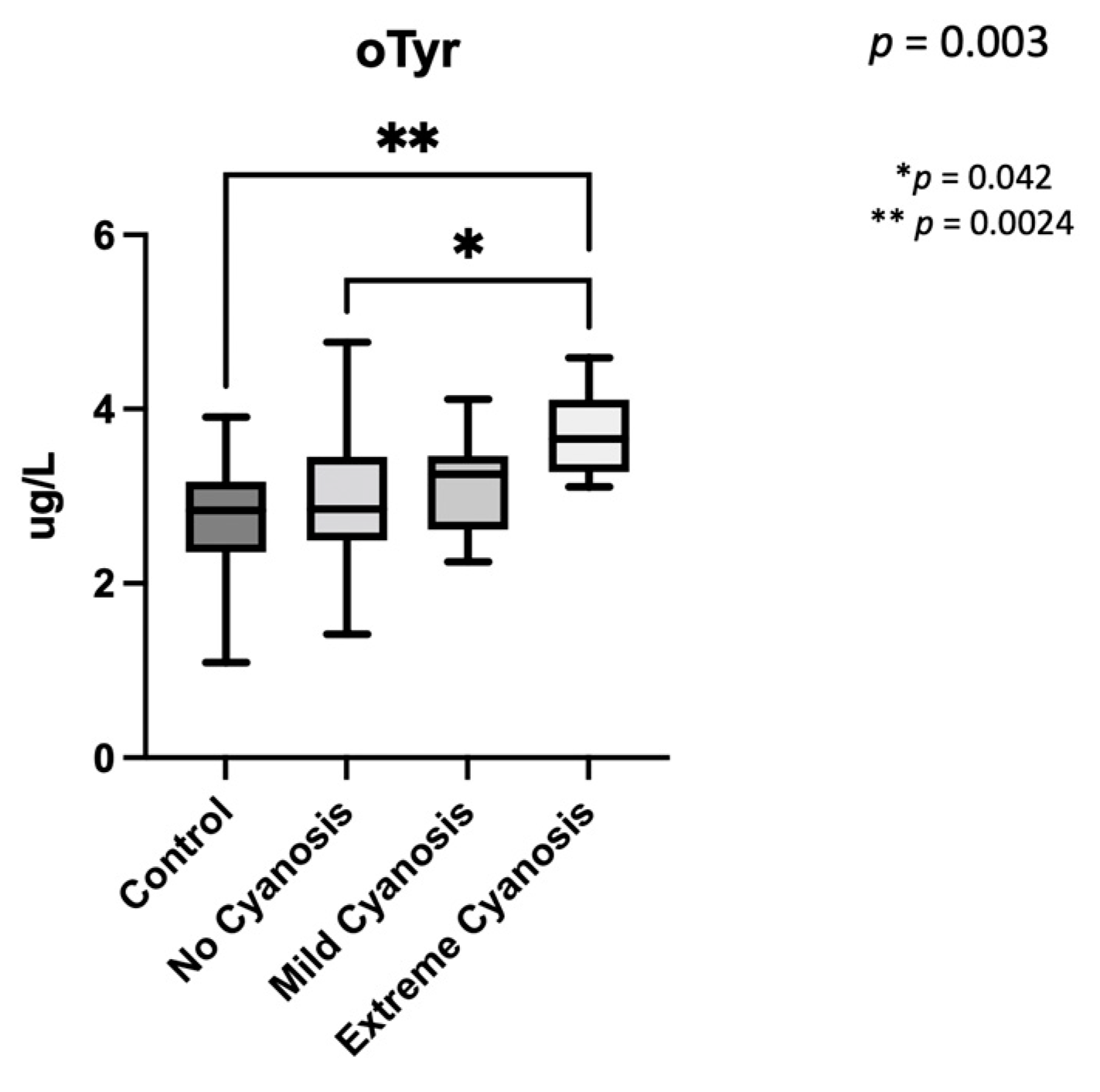
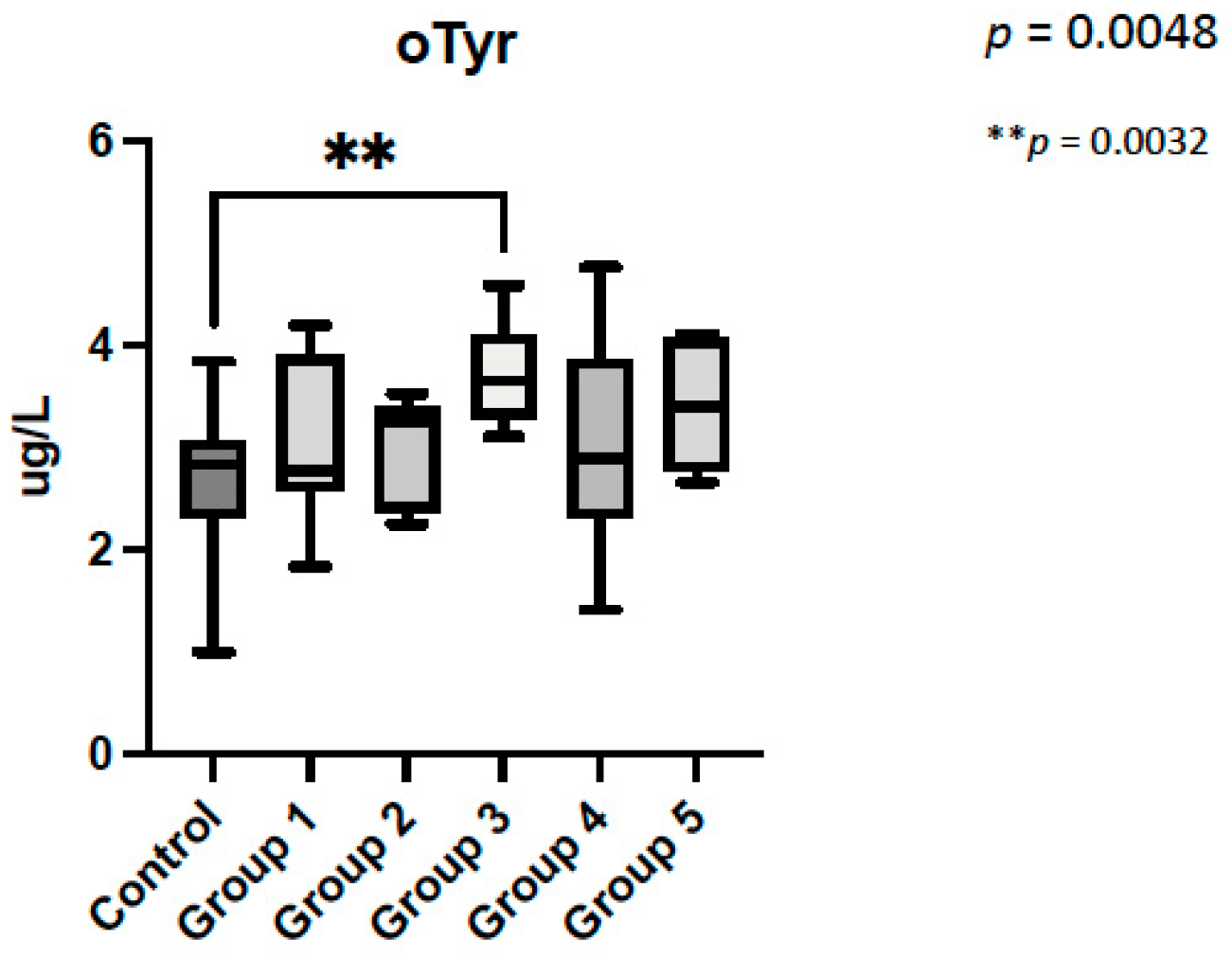
3.2. Amniotic Fluid Analysis
4. Discussion
4.1. Fetal Brain Perfusion and Oxidative Stress
4.2. Fetal Brain Perfusion and Oxidative Stress in CHD
4.3. Oxidative Stress Biomarkers Are Increased in CHD with Low Brain Oxygenation
4.4. Head Circumference Is Lower in CHD Patients
4.5. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, J.I.E.; Kaplan, S. The Incidence of Congenital Heart Disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [Green Version]
- Gilboa, S.M.; Salemi, J.L.; Nembhard, W.N.; Fixler, D.E.; Correa, A. Mortality Resulting from Congenital Heart Disease among Children and Adults in the United States, 1999 to 2006. Circulation 2010, 122, 2254–2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaynor, J.W.; Stopp, C.; Wypij, D.; Andropoulos, D.B.; Atallah, J.; Atz, A.M.; Beca, J.; Donofrio, M.T.; Duncan, K.; Ghanayem, N.S.; et al. Neurodevelopmental Outcomes after Cardiac Surgery in Infancy. Pediatrics 2015, 135, 816–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuillen, P.S.; Goff, D.A.; Licht, D.J. Effects of Congenital Heart Disease on Brain Development. Prog. Pediatr. Cardiol. 2010, 29, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaynor, J.W.; Stopp, C.; Wypij, D.; Andropoulos, D.B.; Atallah, J.; Atz, A.M.; Beca, J.; Donofrio, M.T.; Duncan, K.; Ghanayem, N.S.; et al. Impact of Operative and Postoperative Factors on Neurodevelopmental Outcomes After Cardiac Operations. Ann. Thorac. Surg. 2016, 102, 843–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scallan, M.J.H. Brain Injury in Children with Congenital Heart Disease. Paediatr. Anesth. 2003, 13, 284–293. [Google Scholar] [CrossRef]
- Khalil, A.; Suff, N.; Thilaganathan, B.; Hurrell, A.; Cooper, D.; Carvalho, J.S. Brain Abnormalities and Neurodevelopmental Delay in Congenital Heart Disease: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2014, 43, 14–24. [Google Scholar] [CrossRef]
- Peyvandi, S.; Lim, J.M.; Marini, D.; Xu, D.; Reddy, V.M.; Barkovich, A.J.; Miller, S.; McQuillen, P.; Seed, M. Fetal Brain Growth and Risk of Postnatal White Matter Injury in Critical Congenital Heart Disease. J. Thorac. Cardiovasc. Surg. 2021, 162, 1007–1014.e1. [Google Scholar] [CrossRef]
- Masoller, N.; Sanz-Cortés, M.; Crispi, F.; Gõmez, O.; Bennasar, M.; Egaña-Ugrinovic, G.; Bargallõ, N.; Martínez, J.M.; Gratacõs, E. Mid-Gestation Brain Doppler and Head Biometry in Fetuses with Congenital Heart Disease Predict Abnormal Brain Development at Birth. Ultrasound Obstet. Gynecol. 2016, 47, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Jansen, F.A.R.; van Zwet, E.W.; Rijlaarsdam, M.E.B.; Pajkrt, E.; van Velzen, C.L.; Zuurveen, H.R.; Kragt, A.; Bax, C.L.; Clur, S.-A.B.; van Lith, J.M.M.; et al. Head Growth in Fetuses with Isolated Congenital Heart Defects: Lack of Influence of Aortic Arch Flow and Ascending Aorta Oxygen Saturation. Ultrasound Obstet. Gynecol. 2016, 48, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Donofrio, M.T.; Bremer, Y.A.; Schieken, R.M.; Gennings, C.; Morton, L.D.; Eidem, B.W.; Cetta, F.; Falkensammer, C.B.; Huhta, J.C.; Kleinman, C.S. Autoregulation of Cerebral Blood Flow in Fetuses with Congenital Heart Disease: The Brain Sparing Effect. Pediatr. Cardiol. 2003, 24, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial Mutations and Mitoepigenetics: Focus on Regulation of Oxidative Stress-Induced Responses in Breast Cancers. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Liu, Y.; Wang, S.; Zhang, H.; Fang, X.; Zhang, J.; Fan, L.; Zheng, B.; Roman, R.J.; Wang, Z.; et al. Molecular Sciences Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 2074. [Google Scholar] [CrossRef] [PubMed]
- Van Tilborg, E.; Heijnen, C.J.; Benders, M.J.; van Bel, F.; Fleiss, B.; Gressens, P.; Nijboer, C.H. Impaired Oligodendrocyte Maturation in Preterm Infants: Potential Therapeutic Targets. Prog. Neurobiol. 2016, 136, 28–49. [Google Scholar] [CrossRef]
- Volpe, J.J.; Kinney, H.C.; Jensen, F.E.; Rosenberg, P.A. The Developing Oligodendrocyte: Key Cellular Target in Brain Injury in the Premature Infant. Int. J. Dev. Neurosci. 2011, 29, 423–440. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.P.; McQuillen, P.S.; Hamrick, S.; Xu, D.; Glidden, D.V.; Charlton, N.; Karl, T.; Azakie, A.; Ferriero, D.M.; Barkovich, A.J.; et al. Abnormal Brain Development in Newborns with Congenital Heart Disease. N. Engl. J. Med. 2007, 357, 1928–1938. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic Fluid: Not Just Fetal Urine Anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.-K.J.; Shan, S.J.; Winsor, E.J.; Diamandis, E.P. Proteomics Analysis of Human Amniotic Fluid. Mol. Cell. Proteom. 2007, 6, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Ashina, M.; Kido, T.; Kyono, Y.; Yoshida, A.; Suga, S.; Nakasone, R.; Abe, S.; Tanimura, K.; Nozu, K.; Fujioka, K. Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants. Int. J. Environ. Res. Public Health 2021, 18, 10726. [Google Scholar] [CrossRef]
- Matayatsuk, C.; Poljak, A.; Bustamante, S.; Smythe, G.A.; Kalpravidh, R.W.; Sirankapracha, P.; Fucharoen, S.; Wilairat, P. Quantitative Determination of Ortho- and Meta-Tyrosine as Biomarkers of Protein Oxidative Damage in β-Thalassemia. Redox Rep. 2007, 12, 219–228. [Google Scholar] [CrossRef]
- Cascant-Vilaplana, M.M.; Albiach-Delgado, A.; Camprubí-Camprubí, M.; Pérez-Cruz, M.; Gómez, O.; Arráez, M.; López-Nogueroles, M.; Kuligowski, J.; Vento, M. A UPLC-MS/MS Method for the Determination of Oxidative Stress Biomarkers in Amniotic Fluid. Free Radic. Biol. Med. 2021, 179, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International Standards for Newborn Weight, Length, and Head Circumference by Gestational Age and Sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Hadley, S.; Vazquez, D.C.; Abad, M.L.; Congiu, S.; Lushchencov, D.; Camprubí, M.C.; Sanchez-De-Toledo, J. Oxidative Stress Response in Children Undergoing Cardiac Surgery: Utility of the Clearance of Isoprostanes. PLoS ONE 2021, 16, e0250124. [Google Scholar] [CrossRef] [PubMed]
- Everwijn, S.M.P.; Namburete, A.I.L.; van Geloven, N.; Jansen, F.A.R.; Papageorghiou, A.T.; Teunissen, A.K.; Rozendaal, L.; Blom, N.; van Lith, J.M.; Haak, M.C. The Association between Flow and Oxygenation and Cortical Development in Fetuses with Congenital Heart Defects Using a Brain-Age Prediction Algorithm. Prenat. Diagn. 2021, 41, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Masoller, N.; Martínez, J.M.; Gómez, O.; Bennasar, M.; Crispi, F.; Sanz-Cortés, M.; Egaña-Ugrinovic, G.; Bartrons, J.; Puerto, B.; Gratacós, E. Evidence of Second-Trimester Changes in Head Biometry and Brain Perfusion in Fetuses with Congenital Heart Disease. Ultrasound Obstet. Gynecol. 2014, 44, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Masoller, N.; Sanz-Cortés, M.; Crispi, F.; Gómez, O.; Bennasar, M.; Egaña-Ugrinovic, G.; Bargalló, N.; Martínez, J.M.; Gratacós, E. Severity of Fetal Brain Abnormalities in Congenital Heart Disease in Relation to the Main Expected Pattern of in Utero Brain Blood Supply. Fetal. Diagn. Ther. 2016, 39, 269–278. [Google Scholar] [CrossRef]
- Pérez-Cruz, M.; Gómez, O.; Gibert, M.; Masoller, N.; Marimon, E.; Lip-Sosa, D.; Bennassar, M.; Bonet-Carne, E.; Gómez-Roig, M.D.; Martínez-Crespo, J.M.; et al. Corpus Callosum Size by Neurosonography in Fetuses with Congenital Heart Defect and Relationship with Expected Brain Oxygen Supply Patterns. Ultrasound Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Clancy, R.R.; Mcgaurn, S.A.; Wernovsky, G.; Spray, T.L.; Norwood, W.I.; Jacobs, M.L.; Murphy, J.D.; Gaynor, J.W.; Goin, J.E. Preoperative Risk-of-Death Prediction Model in Heart Surgery with Deep Hypothermic Circulatory Arrest In The Neonate. J. Thorac. Cardiovasc. Surg. 2000, 119, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Buonocore, G.; Perrone, S.; Bracci, R. Free Radicals and Brain Damage in the Newborn. Biol. Neonate 2001, 79, 180–186. [Google Scholar]
- Perrone, S.; Tataranno, L.M.; Stazzoni, G.; Ramenghi, L.; Buonocore, G. Brain Susceptibility to Oxidative Stress in the Perinatal Period. J. Matern. Fetal. Neonatal Med. 2015, 28, 2291–2295. [Google Scholar] [CrossRef]
- Maskos, Z.; Rush, J.D.; Koppenol, W.H. The Hydroxylation of Phenylalanine and Tyrosine: A Comparison with Salicylate and Tryptophan. Arch. Biochem. Biophys. 1992, 296, 521–529. [Google Scholar] [CrossRef]
- Escobar, J.; Teramo, K.; Stefanovic, V.; Andersson, S.; Asensi, M.A.; Arduini, A.; Cubells, E.; Sastre, J.; Vento, M. Amniotic Fluid Oxidative and Nitrosative Stress Biomarkers Correlate with Fetal Chronic Hypoxia in Diabetic Pregnancies. Neonatology 2013, 103, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.N.; Adrianzen, L.; Kessler, E.C.; Andelfinger, G.; Dehaes, M.; Côté-Corriveau, G.; Trelles, M.P. Congenital Heart Disease and Neurodevelopment: Clinical Manifestations, Genetics, Mechanisms, and Implications. Can. J. Cardiol. 2017, 33, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, S.; Latal, B.; Miller, S.P.; McQuillen, P.S. The Neonatal Brain in Critical Congenital Heart Disease: Insights and Future Directions. NeuroImage 2019, 185, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Howell, H.B.; Zaccario, M.; Kazmi, S.H.; Desai, P.; Sklamberg, F.E.; Mally, P. Neurodevelopmental Outcomes of Children with Congenital Heart Disease: A Review. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 100685. [Google Scholar] [CrossRef]
- Matthiesen, N.B.; Henriksen, T.B.; Gaynor, J.W.; Agergaard, P.; Bach, C.C.; Hjortdal, V.E.; Østergaard, J.R. Congenital Heart Defects and Indices of Fetal Cerebral Growth in a Nationwide Cohort of 924,422 Liveborn Infants. Circulation 2016, 133, 566–575. [Google Scholar] [CrossRef] [Green Version]


| Clinical Characteristics | Control Group (n = 44) | CHD Group (n = 41) | p Value |
|---|---|---|---|
| Age (years) | 33.1 ± 5 | 34.2 ± 5 | 0.36 |
| Amniocentesis weeks | 26.2 ± 5 | 24.20 ± 5 | 0.088 |
| Parity | 0 [0–1] | 1 [0–1] | 0.94 |
| Body mass index (kg/m2) | 25 ± 7 | 27 ± 3 | 0.61 |
| Hypothyroidism % | 0 | 20 | 0.30 |
| Preeclampsia % | 4.3 | 2.8 | 0.86 |
| Gestational diabetes % | 5.7 | 8.1 | 0.50 |
| Smoking habit % | 12.8 | 9.5 | 0.96 |
| Gestational age at birth (weeks) | 39.4 ± 1.4 | 39.4 ± 0.9 | 0.91 |
| Neonatal birth-weight (gr) | 3280 ± 559 | 3178 ± 436 | 0.42 |
| Birth-weight percentile | 51 ± 28 | 39 ± 26 | 0.12 |
| Head circumference at birth (cm) | 34.5 ± 1.6 | 33.53 ± 1.5 | 0.023 |
| Head circumference percentile | 59 ± 30 | 44 ± 31 | 0.077 |
| Head circumference z-score | 0.38 ± 1.17 | −0.29 ± 1.3 | 0.044 |
| Amniocentesis indication | Club foot: 26% Cleft palate:10% Vascular ring: 12% Short long bones: 5% Suspected CNS alterations: 23% Suspected infection: 3% Others: 21% | CHD Classification Group 1: 22% (n = 9) Group 2: 24% (n = 10) Group 3: 20% (n = 8) Group 4: 22% (n = 9) Group 5: 12% (n = 5) Group 6: 0 |
| CHD Classification | Number of Patients |
|---|---|
Group 1
| (n = 9) 4 3 1 1 |
Group 2
| (n = 10) 1 2 2 3 2 |
Group 3
| (n = 8) 4 4 |
Group 4
| (n = 9) 3 1 2 2 1 |
Group 5
| (n = 5) 4 1 |
Group 6
| 0 |
| Control | CHD | p Value | |
|---|---|---|---|
| o-Tyr [nm] | 2.68 ± 0.64 | 3.27 ± 0.76 | 0.0003 |
| No2-Tyr [nm] | 3.71 ± 3.19 | 3.52 ± 4.4 | 0.82 |
| p-Tyr [µm] | 20.97 ± 9.8 | 25.42 ± 9.8 | 0.045 |
| Phe [µm] | 26.62 ± 13 | 31.36 ± 11.30 | 0.084 |
| o-Tyr/Phe | 0.000126 ± 0.0000618 | 0.0001241 ± 0.0000755 | 0.89 |
| 3No2-Tyr/p-Tyr | 0.0002648 ± 0.000335 | 0.0001939 ± 0.0002921 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Diaz, M.C.; Pérez-Cruz, M.; Arráez, M.; Cascant-Vilaplana, M.-M.; Albiach-Delgado, A.; Kuligowski, J.; Vento, M.; Masoller, N.; Gómez-Roig, M.D.; Gómez, O.; et al. Brain Oxygen Perfusion and Oxidative Stress Biomarkers in Fetuses with Congenital Heart Disease—A Retrospective, Case-Control Pilot Study. Antioxidants 2022, 11, 299. https://doi.org/10.3390/antiox11020299
Escobar-Diaz MC, Pérez-Cruz M, Arráez M, Cascant-Vilaplana M-M, Albiach-Delgado A, Kuligowski J, Vento M, Masoller N, Gómez-Roig MD, Gómez O, et al. Brain Oxygen Perfusion and Oxidative Stress Biomarkers in Fetuses with Congenital Heart Disease—A Retrospective, Case-Control Pilot Study. Antioxidants. 2022; 11(2):299. https://doi.org/10.3390/antiox11020299
Chicago/Turabian StyleEscobar-Diaz, Maria C., Miriam Pérez-Cruz, Miguel Arráez, Mari-Merce Cascant-Vilaplana, Abel Albiach-Delgado, Julia Kuligowski, Máximo Vento, Narcis Masoller, Maria Dolores Gómez-Roig, Olga Gómez, and et al. 2022. "Brain Oxygen Perfusion and Oxidative Stress Biomarkers in Fetuses with Congenital Heart Disease—A Retrospective, Case-Control Pilot Study" Antioxidants 11, no. 2: 299. https://doi.org/10.3390/antiox11020299






