Magnolol Ameliorates Cisplatin-Induced Acute Kidney Injury with Activation of Nrf2-Associated Antioxidant Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Biochemical Analysis
2.3. Histological Analysis and Immunohistochemistry (IHC)
2.4. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining
2.5. Western Blotting
2.6. Quantitative Polymerase Chain Reaction (qPCR)
2.7. Statistical Analysis
3. Results
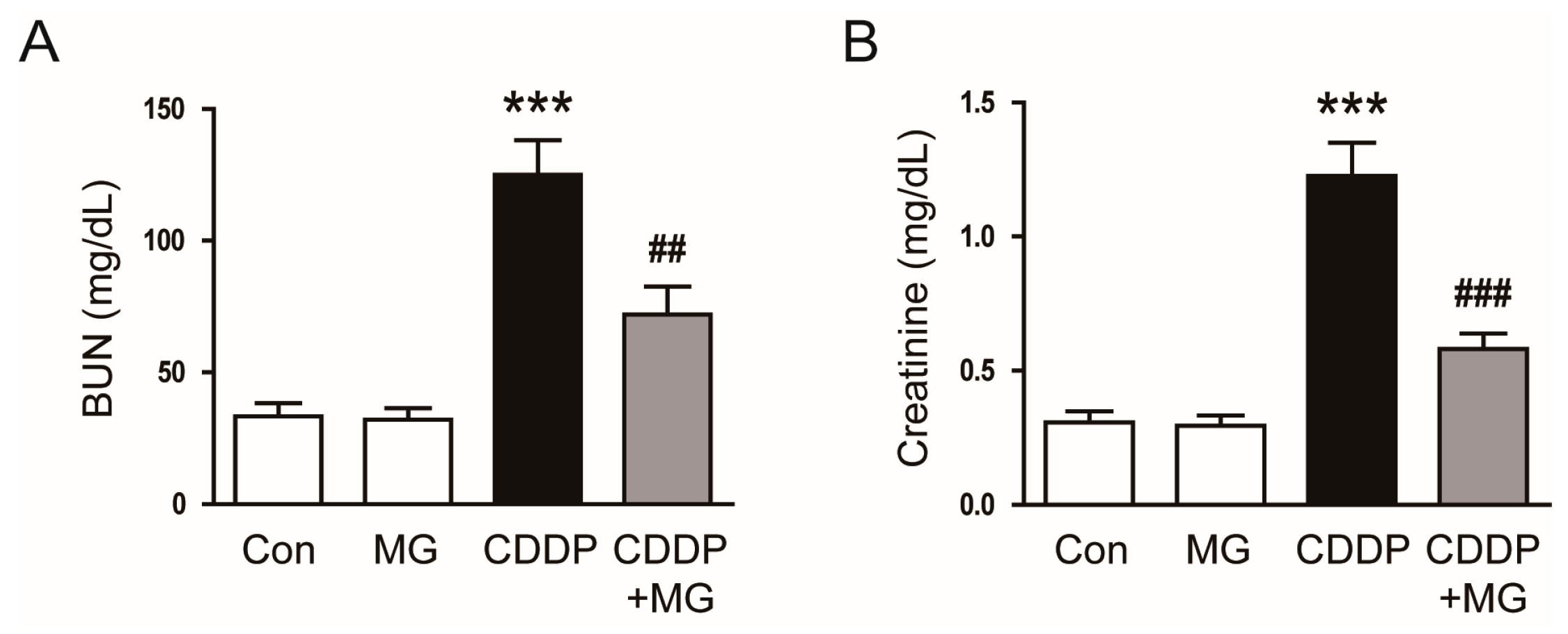
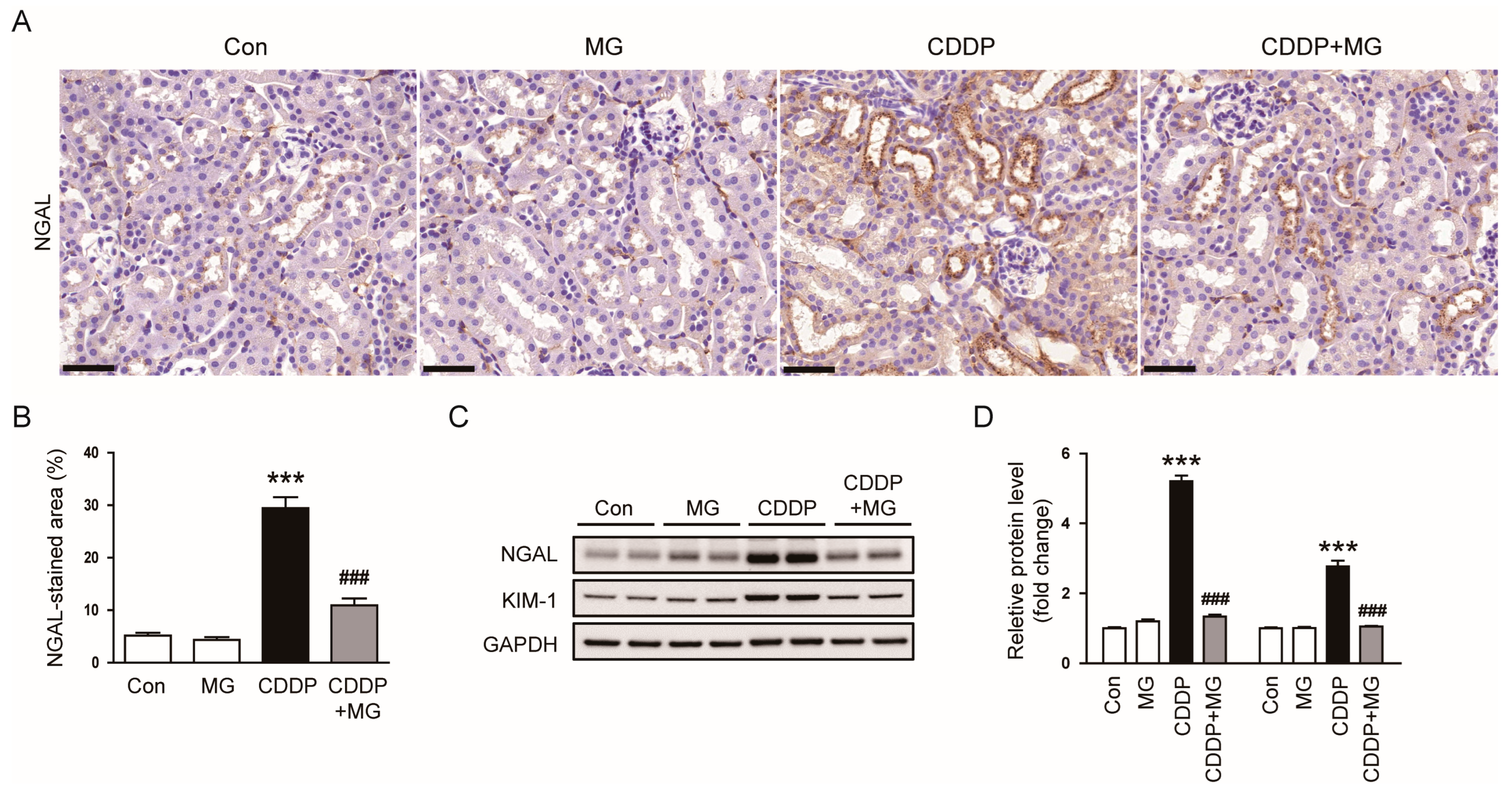
3.1. MG Attenuates CDDP-Induced Renal Dysfunction and Histological Abnormalities
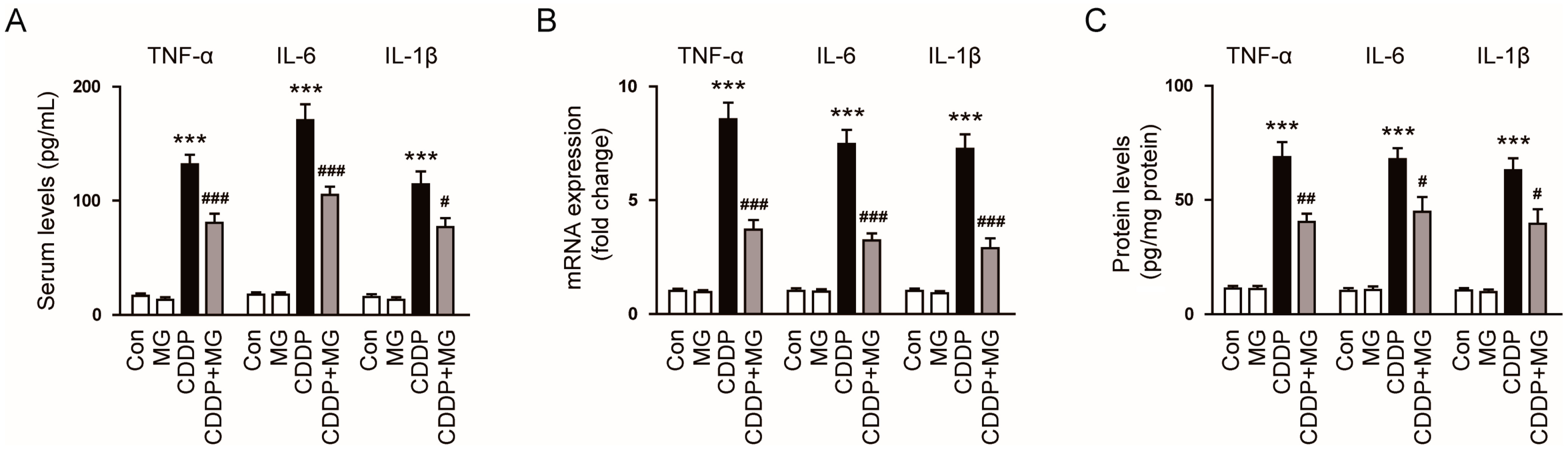
3.2. MG Reduces CDDP-Induced Inflammatory Responses
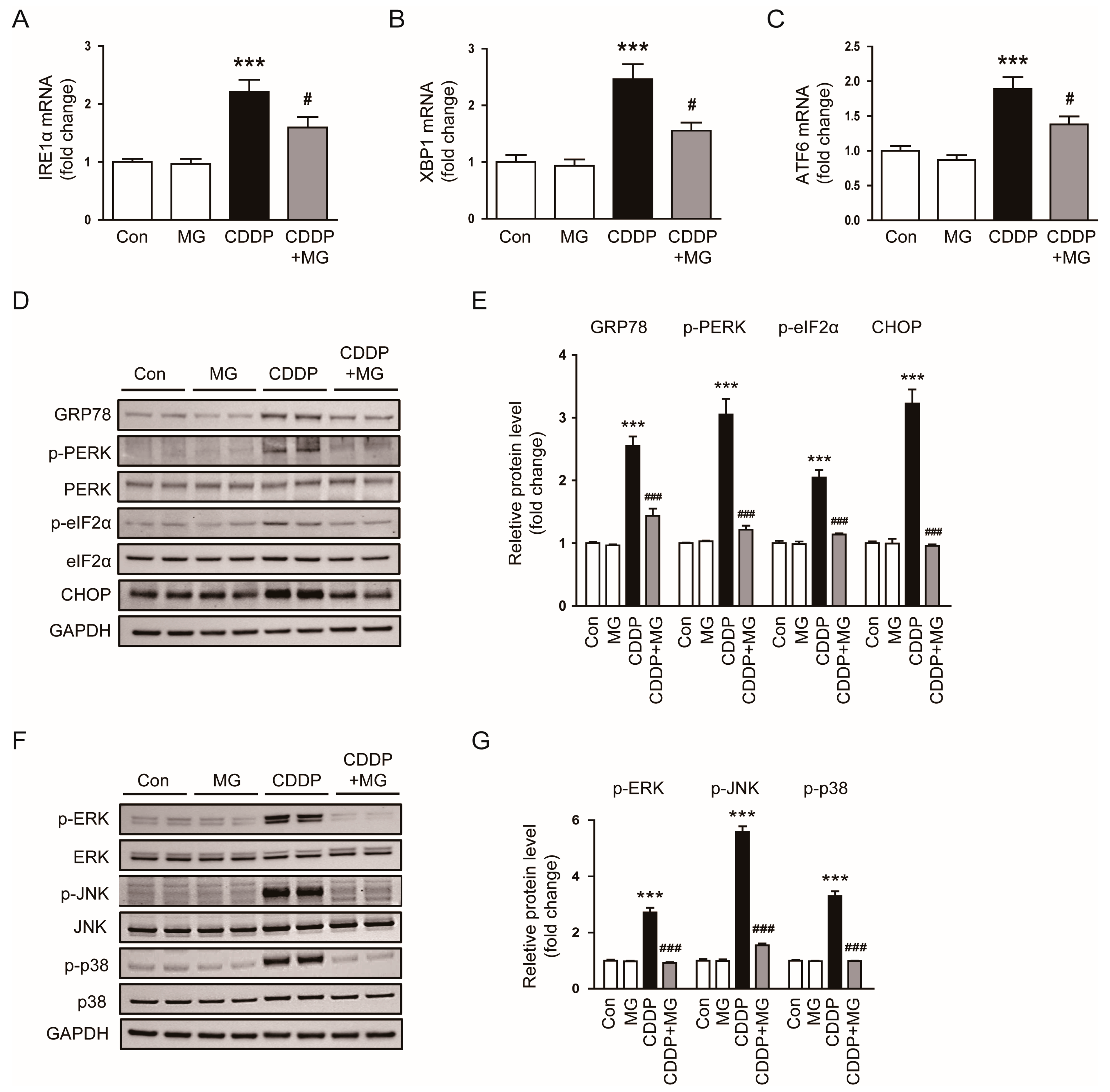
3.3. MG Suppresses CDDP-Induced Endoplasmic Reticulum (ER) Stress and MAPK Signaling Activation
3.4. MG Inhibits CDDP-Induced Apoptosis and Ferroptosis
3.5. MG Restores CDDP-Suppressed Nrf2 Signaling Pathway in the Kidney
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Steinbach, E.J.; Zaher, A.; Riley, D.P.; Beardsley, R.A.; Keene, J.L.; Holmlund, J.T.; Anderson, C.M.; Zepeda-Orozco, D.; Buatti, J.M.; et al. Mitochondrial Superoxide Dismutase in Cisplatin-Induced Kidney Injury. Antioxidants 2021, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Domingo, I.K.; Latif, A.; Bhavsar, A.P. Pro-Inflammatory Signalling PRRopels Cisplatin-Induced Toxicity. Int. J. Mol. Sci. 2022, 23, 7227. [Google Scholar] [CrossRef] [PubMed]
- Volovat, S.; Apetrii, M.; Stefan, A.; Vlad, C.; Voroneanu, L.; Hogas, M.; Haisan, A.; Volovat, C.; Hogas, S. Cisplatin and AKI: An ongoing battle with new perspectives—A narrative review. Int. Urol. Nephrol. 2023, 55, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-Y.; Lou, D.-Y.; Zhou, L.-Q.; Wang, J.-C.; Yang, B.; He, Q.-J.; Wang, J.-J.; Weng, Q.-J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969, Erratum in Acta Pharmacol. Sin. 2023, 44, 488. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, X.; Wang, Y.; Wang, Y.; Teng, X.; Wang, S. Cardiovascular Modulating Effects of Magnolol and Honokiol, Two Polyphenolic Compounds from Traditional Chinese Medicine-Magnolia Officinalis. Curr. Drug Targets 2020, 21, 559–572. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, Toxicity, Bioavailability, and Formulation of Magnolol: An Update. Front. Pharmacol. 2021, 12, 632767. [Google Scholar] [CrossRef]
- Chen, H.; Fu, W.; Chen, H.; You, S.; Liu, X.; Yang, Y.; Wei, Y.; Huang, J.; Rui, W. Magnolol attenuates the inflammation and enhances phagocytosis through the activation of MAPK, NF-κB signal pathways in vitro and in vivo. Mol. Immunol. 2019, 105, 96–106. [Google Scholar] [CrossRef]
- Chen, J.H.; Kuo, H.C.; Lee, K.F.; Tsai, T.H. Magnolol protects neurons against ischemia injury via the downregulation of p38/MAPK, CHOP and nitrotyrosine. Toxicol. Appl. Pharmacol. 2014, 279, 294–302. [Google Scholar] [CrossRef]
- Lu, S.H.; Hsu, W.L.; Chen, T.H.; Chou, T.C. Activation of Nrf2/HO-1signaling pathway involves the anti-inflammatory activity of magnolol in Porphyromonas gingivalis lipopolysaccharide-stimulated mouse RAW 264.7 macrophages. Int. Immunopharmacol. 2015, 29, 770–778. [Google Scholar] [CrossRef]
- Lu, S.H.; Chen, T.H.; Chou, T.C. Magnolol Inhibits RANKL-induced osteoclast differentiation of raw 264.7 macrophages through heme oxygenase-1-dependent inhibition of NFATc1 expression. J. Nat. Prod. 2015, 78, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, M.; Sun, Q.; Guo, Z.; Lin, Y.; Li, S.; Huang, Y.; Li, Y.; Fu, Q. Magnolol attenuates macrophage pyroptosis triggered by Streptococcus equi subsp. zooepidemicus. Int. Immunopharmacol. 2024, 131, 111922. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Jin, L.; Yao, X.; Zhang, Y.; Zhang, G.; Wang, F.; Su, X.; Fang, Q.; Xiao, L.; Yang, Y.; et al. TRPM2 protects against cisplatin-induced acute kidney injury and mitochondrial dysfunction via modulating autophagy. Theranostics 2023, 13, 4356–4375. [Google Scholar] [CrossRef]
- Yan, M.; Shu, S.; Guo, C.; Tang, C.; Dong, Z. Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 2018, 50, 381–390. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Lai, K.; Chen, Z.; Lin, S.; Ye, K.; Yuan, Y.; Li, G.; Song, Y.; Ma, H.; Mak, T.W.; Xu, Y. The IDH1-R132H mutation aggravates cisplatin-induced acute kidney injury by promoting ferroptosis through disrupting NDUFA1 and FSP1 interaction. Cell Death Differ. 2025, 32, 242–255. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Zhao, W.; Wang, H.; Xue, X.; Chen, X.; Li, W.; Xu, P.; Wang, K.; Liu, P.; et al. VPA improves ferroptosis in tubular epithelial cells after cisplatin-induced acute kidney injury. Front. Pharmacol. 2023, 14, 1147772. [Google Scholar] [CrossRef]
- Dong, X.-Q.; Chu, L.-K.; Cao, X.; Xiong, Q.-W.; Mao, Y.-M.; Chen, C.-H.; Bi, Y.-L.; Liu, J.; Yan, X.-M. Glutathione metabolism rewiring protects renal tubule cells against cisplatin-induced apoptosis and ferroptosis. Redox Rep. 2023, 28, 2152607. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Y.; Luo, X.; Luo, Y.; Ji, J.; Li, J.; Lai, J.; Liu, Z.; Chen, Y.; Lin, Y.; et al. Dexmedetomidine inhibits ferroptosis and attenuates sepsis-induced acute kidney injury via activating the Nrf2/SLC7A11/FSP1/CoQ10 pathway. Redox Rep. 2024, 29, 2430929. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Pulliam, C.F.; Zepeda-Orozco, D.; Griffin, B.R.; Furqan, M.; Spitz, D.R.; Allen, B.G. Redox Regulation of Nrf2 in Cisplatin-Induced Kidney Injury. Antioxidants 2023, 12, 1728. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, K.; Mao, W.; Yu, B.; Liu, Z.; Huang, F.; Yang, Z. Morroniside alleviates cisplatin-induced renal injury and gut dysbiosis via the gut–kidney axis and ferroptosis. Int. Immunopharmacol. 2025, 153, 114430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yue, Z.; Wang, G.; Qin, J.; Ma, H.; Tang, D.; Yin, G. Smilax glabra roxb. alleviates cisplatin-induced acute kidney injury in mice by activating the Nrf2/HO-1 Signalling Pathway. Phytomedicine 2025, 139, 156550. [Google Scholar] [CrossRef]
- Yalinbas-Kaya, B.; Tureyen, A.; Cesur, S.; Zemheri-Navruz, F.; Demirel, H.H.; Ince, S. Iristectorin A Ameliorates Cisplatin-Induced Hepatorenal Injury in Mice Through Modulation of the Nrf2/HO-1 Signaling Pathway. J. Biochem. Mol. Toxicol. 2025, 39, e70136. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Wu, D.; Li, S.; Wang, C.; Han, Z.; Wang, J.; Wang, K.; Yang, Z.; Wei, Z. Magnolol Prevents Acute Alcoholic Liver Damage by Activating PI3K/Nrf2/PPARγ and Inhibiting NLRP3 Signaling Pathway. Front. Pharmacol. 2019, 10, 1459. [Google Scholar] [CrossRef]
- Kuo, N.C.; Huang, S.Y.; Yang, C.Y.; Shen, H.H.; Lee, Y.M. Involvement of HO-1 and Autophagy in the Protective Effect of Magnolol in Hepatic Steatosis-Induced NLRP3 Inflammasome Activation In Vivo and In Vitro. Antioxidants 2020, 9, 924, Correction in Antioxidants 2025, 14, 690. [Google Scholar] [CrossRef]
- Tao, W.; Hu, Y.; Chen, Z.; Dai, Y.; Hu, Y.; Qi, M. Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway. Phytomedicine 2021, 91, 153692. [Google Scholar] [CrossRef]
- Garg, A.D.; Kaczmarek, A.; Krysko, O.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. ER stress-induced inflammation: Does it aid or impede disease progression? Trends Mol. Med. 2012, 18, 589–598. [Google Scholar] [CrossRef]
- Kim, S.; Joe, Y.; Kim, H.J.; Kim, Y.-S.; Jeong, S.O.; Pae, H.-O.; Ryter, S.W.; Surh, Y.-J.; Chung, H.T. Endoplasmic reticulum stress–induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J. Immunol. 2015, 194, 4498–4506. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-Y.; Lai, C.-C.; Huang, P.-H.; Yang, A.-H.; Chiang, S.-C.; Huang, P.C.; Tseng, K.-W.; Huang, C.-H. Magnolol Reduces Renal Ischemia and Reperfusion Injury via Inhibition of Apoptosis. Am. J. Chin. Med. 2017, 45, 1421–1439. [Google Scholar] [CrossRef]
- Noh, M.R.; Kim, J.I.; Han, S.J.; Lee, T.J.; Park, K.M. C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim. Biophys. Acta 2015, 1852, 1895–1901. [Google Scholar] [CrossRef]
- Francescato, H.D.; Costa, R.S.; da Silva, C.G.; Coimbra, T.M. Treatment with a p38 MAPK inhibitor attenuates cisplatin nephrotoxicity starting after the beginning of renal damage. Life Sci. 2009, 84, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guo, X.; Lin, B.; Huang, R.; Li, H.; Wang, Q.; Zeng, Y.; Shang, Y.; Wu, Y. Magnolol against enterovirus 71 by targeting Nrf2-SLC7A11-GSH pathway. Biomed. Pharmacother. 2024, 176, 116866. [Google Scholar] [CrossRef] [PubMed]
- Zan, H.; Liu, J.; Yang, M.; Zhao, H.; Gao, C.; Dai, Y.; Wang, Z.; Liu, H.; Zhang, Y. Melittin alleviates sepsis-induced acute kidney injury by promoting GPX4 expression to inhibit ferroptosis. Redox Rep. 2024, 29, 2290864. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhang, Y.; Ci, X. Amentoflavone protects against cisplatin-induced acute kidney injury by modulating Nrf2-mediated oxidative stress and ferroptosis and partially by activating Nrf2-dependent PANoptosis. Front. Pharmacol. 2025, 16, 1508047. [Google Scholar] [CrossRef]
- Pan, M.; Wang, Z.; Wang, Y.; Jiang, X.; Fan, Y.; Gong, F.; Sun, Y.; Wang, D. Celastrol alleviated acute kidney injury by inhibition of ferroptosis through Nrf2/GPX4 pathway. Biomed. Pharmacother. 2023, 166, 115333. [Google Scholar] [CrossRef]
- Hu, J.; Gu, W.; Ma, N.; Fan, X.; Ci, X. Leonurine alleviates ferroptosis in cisplatin-induced acute kidney injury by activating the Nrf2 signalling pathway. Br. J. Pharmacol. 2022, 179, 3991–4009. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi-Jazi, F.; Nematbakhsh, M. Sex Difference in Cisplatin-Induced Nephrotoxicity: Laboratory and Clinical Findings. J. Toxicol. 2022, 2022, 3507721. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Gwon, M.-G.; Park, M.H.; Leem, J. Magnolol Ameliorates Cisplatin-Induced Acute Kidney Injury with Activation of Nrf2-Associated Antioxidant Responses. Curr. Issues Mol. Biol. 2026, 48, 96. https://doi.org/10.3390/cimb48010096
Gwon M-G, Park MH, Leem J. Magnolol Ameliorates Cisplatin-Induced Acute Kidney Injury with Activation of Nrf2-Associated Antioxidant Responses. Current Issues in Molecular Biology. 2026; 48(1):96. https://doi.org/10.3390/cimb48010096
Chicago/Turabian StyleGwon, Mi-Gyeong, Min Hui Park, and Jaechan Leem. 2026. "Magnolol Ameliorates Cisplatin-Induced Acute Kidney Injury with Activation of Nrf2-Associated Antioxidant Responses" Current Issues in Molecular Biology 48, no. 1: 96. https://doi.org/10.3390/cimb48010096
APA StyleGwon, M.-G., Park, M. H., & Leem, J. (2026). Magnolol Ameliorates Cisplatin-Induced Acute Kidney Injury with Activation of Nrf2-Associated Antioxidant Responses. Current Issues in Molecular Biology, 48(1), 96. https://doi.org/10.3390/cimb48010096





