Bioactive Component Screening and Mechanistic Study of the Anti-Diabetic Activity of Lophatherum gracile Brongn Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Method of LGB Preparation
2.3. Chromatographic Conditions
2.4. Determination of Antioxidant Activity In Vitro Using DPPH and ABTS Methods
2.5. Inhibition Activity of α-Glucosidase
2.6. Animal Experimental Design
2.7. Evaluation of the Pharmacodynamic Effects of LGB on T2DM Mice
2.8. Non-Targeted Metabolomics Analysis of Fecal Samples
2.9. 16S rRNA Sequencing Analysis
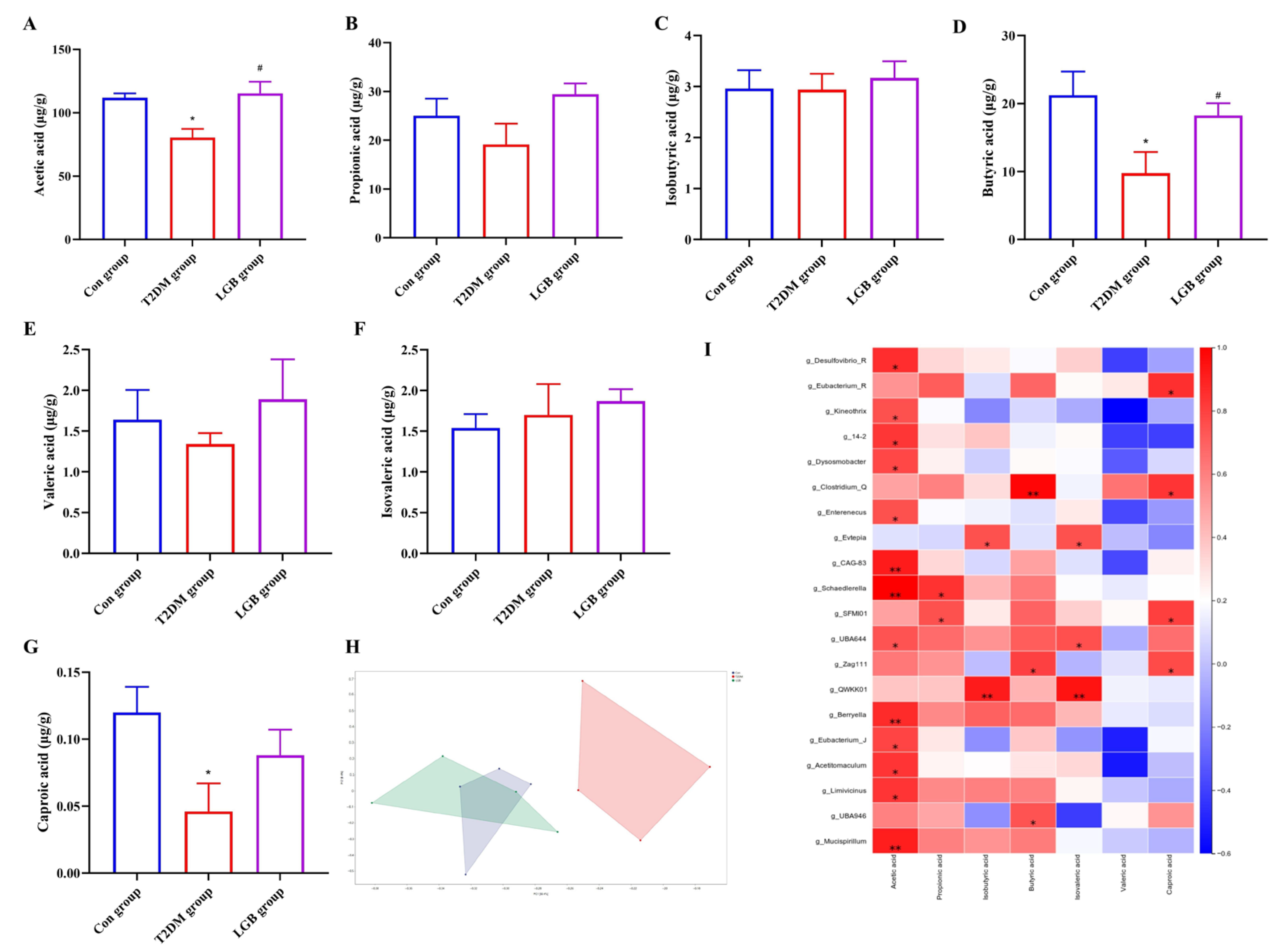
2.10. Determination of Short-Chain Fatty Acid Content
2.11. Western Blotting Analysis
2.12. Statistical Analysis
3. Results
3.1. HPLC Fingerprint of the LGB Samples
3.2. Qualitative and Quantitative Analysis of Common Peaks in LGB
3.3. Analysis of Pearson Correlations Between Chemical Fingerprints of LGB and Their Biological Activity Profiles In Vitro
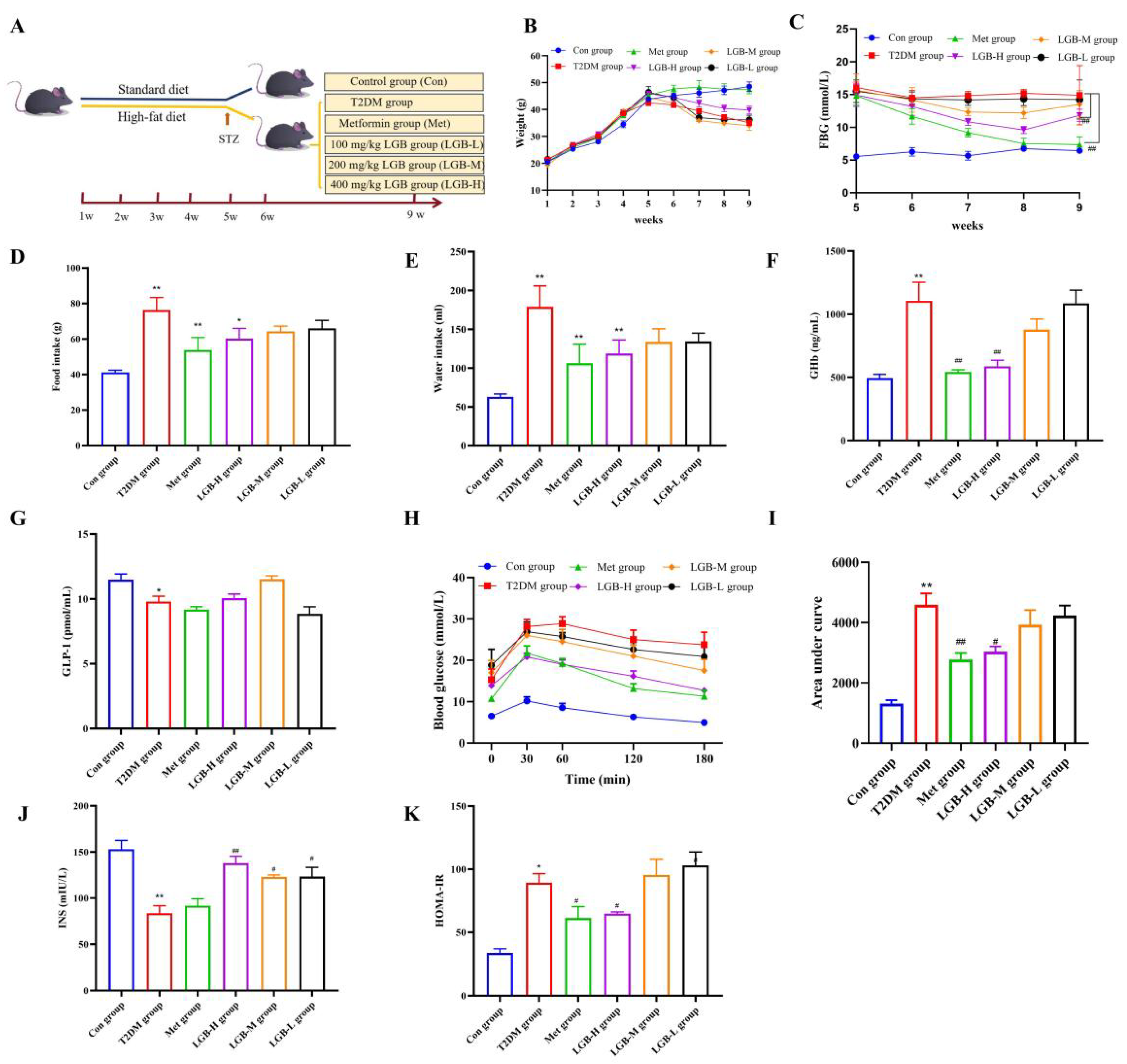
3.4. LGB Enhanced the Pathological State and Blood Glucose Homeostasis in Mice with Diabetes
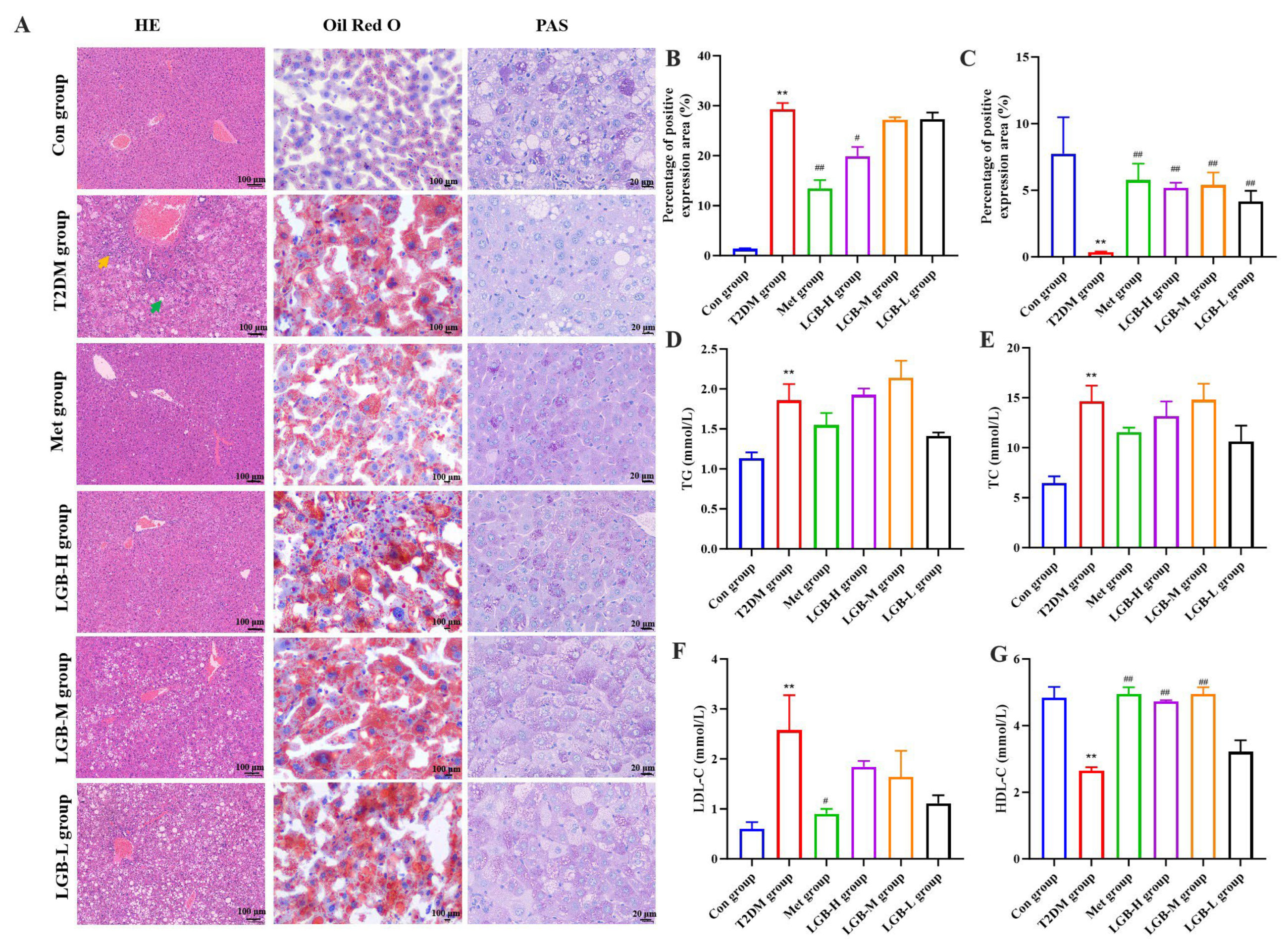
3.5. LGB Alleviated Lipid Profile Dysregulation and Mitigated Hepatic Injury in Diabetic Mice
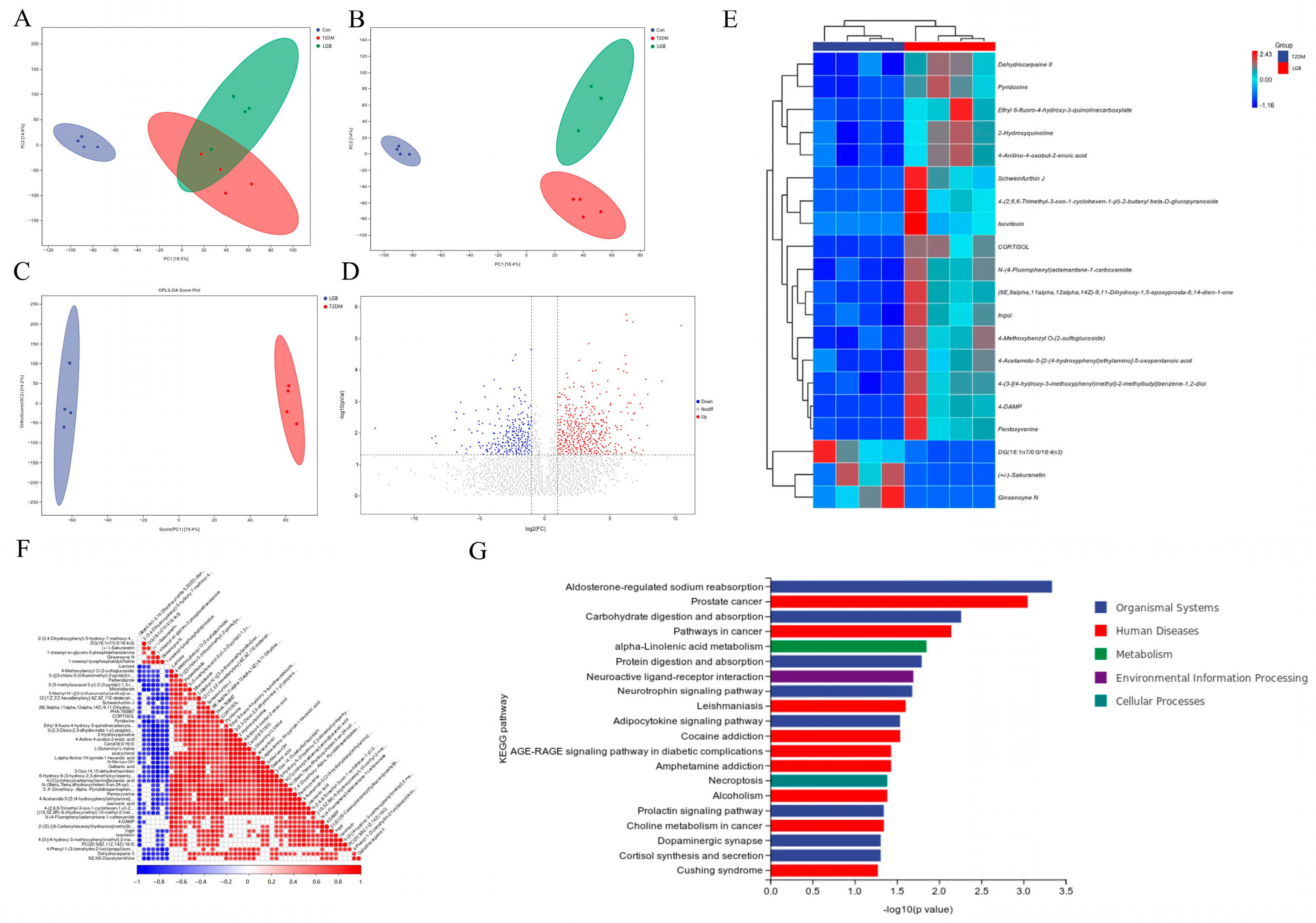
3.6. LGB Enhanced Fecal Metabolic Profile in Mice Suffering from T2DM
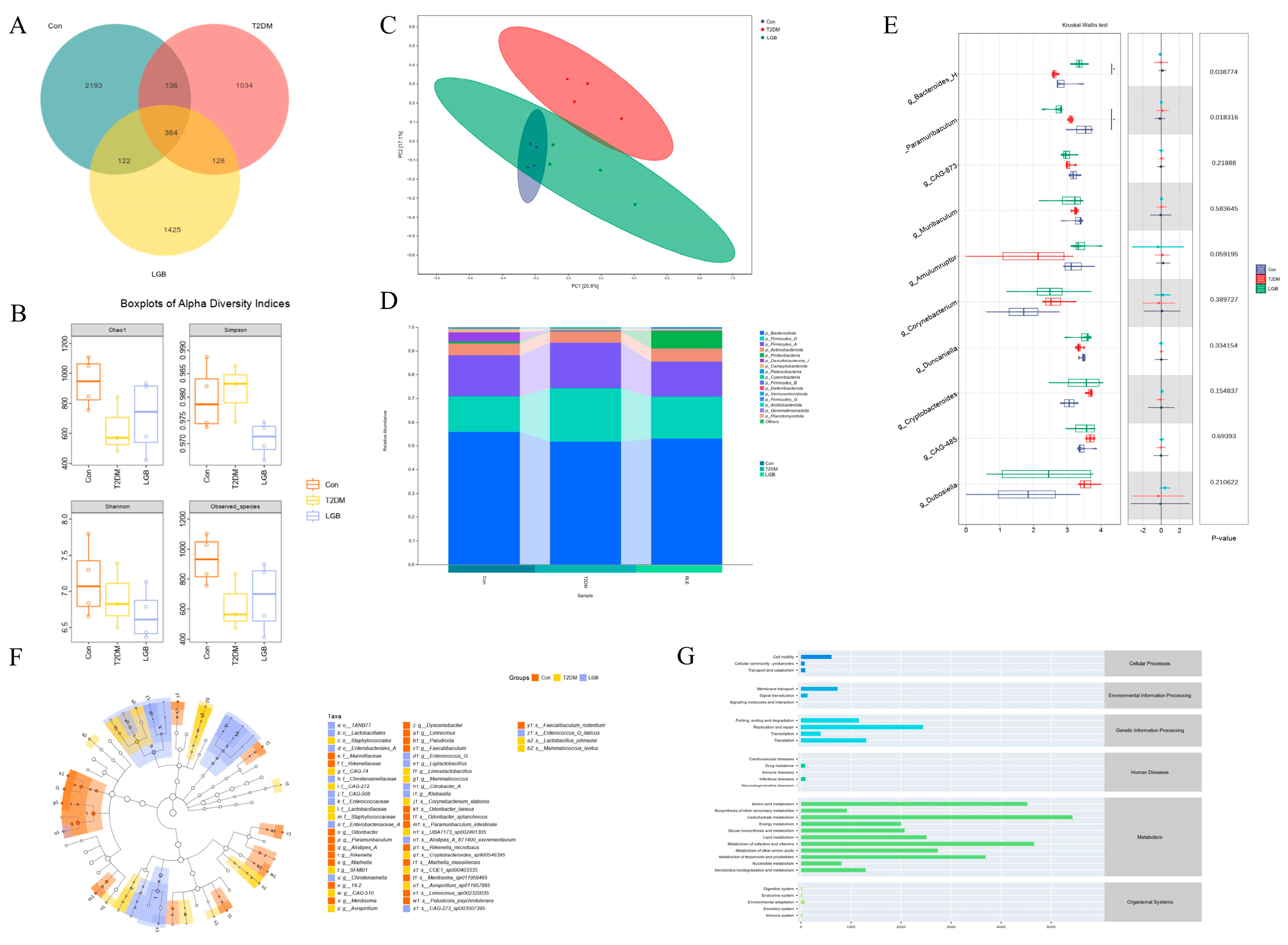
3.7. LGB Improved Gut Microbiota Dysbiosis in T2DM Mice
3.8. LGB Altered the Microbial Metabolites in the Feces of T2DM Mice
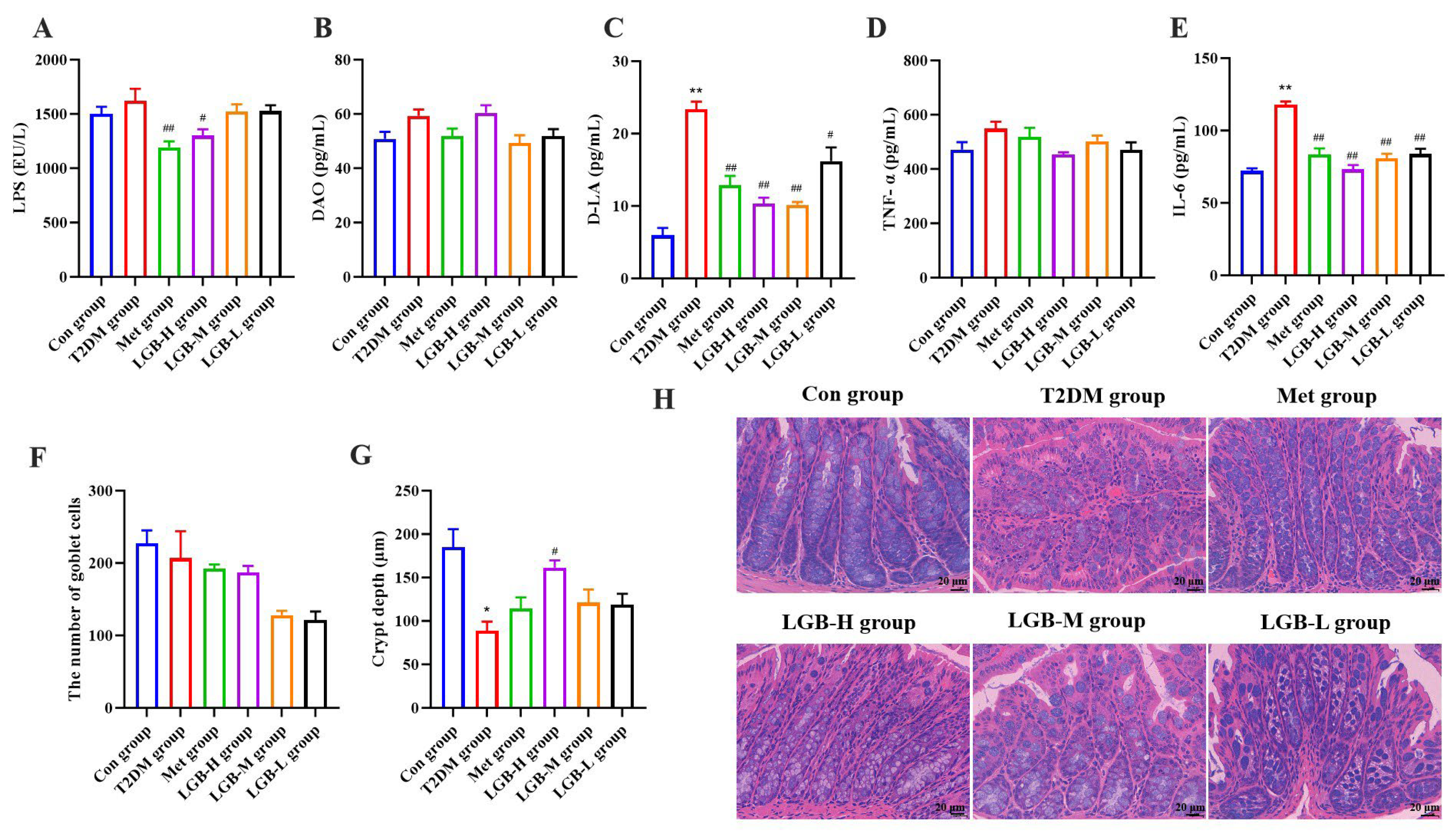
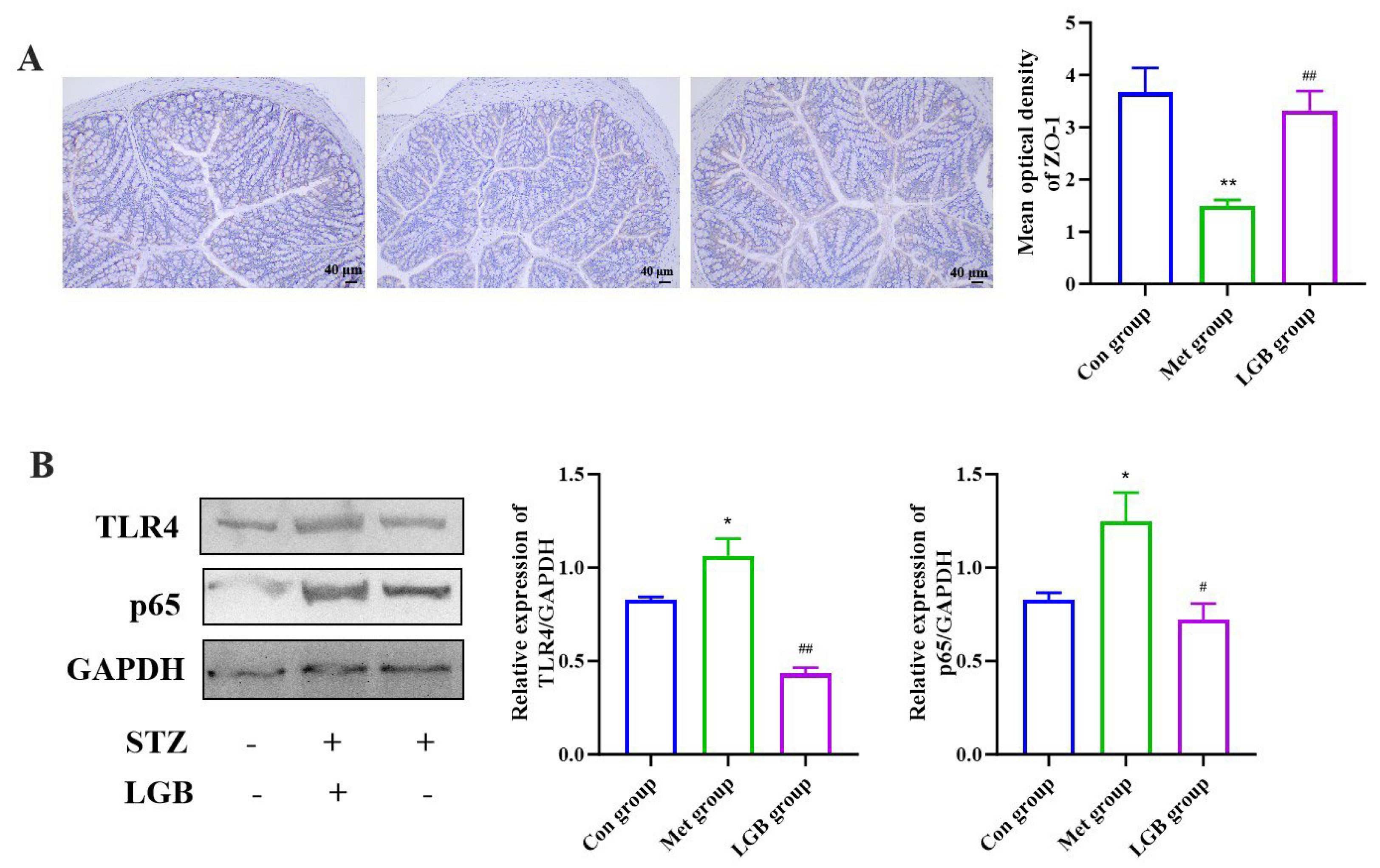
3.9. LGB Alleviated Inflammation and Improved Intestinal Barrier Damage in T2DM Mice by Inhibiting the LPS/TLR4/NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| LGB | Lophatherum gracile Brongn |
| GM | Gut microbiota |
| HE | Hematoxylin and eosin |
| PAS | Periodic acid–Schiff |
| STZ | Streptozotocin |
| INS | Insulin |
| FBG | Fasting blood glucose |
| OGTT | Oral glucose tolerance test |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| GHb | Glycosylated hemoglobin |
| DAO | Diamine oxidase |
| D-LA | D-lactic acid |
| TC | Total cholesterol |
| TG | Total triglyceride |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharide |
| TLR4 | Toll-like receptor 4 |
| NF-κB | Nuclear factor kappa-B |
| PCoA | Principal coordinate analysis |
| PLS-DA | Partial least squares discriminant analysis |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PCA | Principal component analysis |
| ZO-1 | Zonula occludens-1 |
| SCFAs | Short-chain fatty acids |
References
- Ding, Y.; Xu, T.; Mao, G.; Chen, Y.; Qiu, X.; Yang, L.; Zhao, T.; Xu, X.; Feng, W.; Wu, X. Di-(2-ethylhexyl) phthalate-induced hepatotoxicity exacerbated type 2 diabetes mellitus (t2dm) in female pubertal t2dm mice. Food Chem. Toxicol. 2021, 149, 112003. [Google Scholar] [CrossRef]
- Nova, E.; Redondo-Useros, N.; Martinez-Garcia, R.M.; Gomez-Martinez, S.; Diaz-Prieto, L.E.; Marcos, A. Potential of moringa oleifera to improve glucose control for the prevention of diabetes and related metabolic alterations: A systematic review of animal and human studies. Nutrients 2020, 12, 2050. [Google Scholar] [CrossRef]
- Peng, X.; Yang, B.; Wei, X.; Wang, L.; Kan, J. Zanthoxylum alkylamides improves hepatic glucose metabolism by regulating gut microbiota in stz-induced t2dm rats. Fitoterapia 2025, 184, 106623. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, W.; Chen, J.; Wang, X.; Lv, Y.; Huang, Y.; Zhang, Z.; Xu, F. Pharmacometabolomic prediction of individual differences of gastrointestinal toxicity complicating myelosuppression in rats induced by irinotecan. Acta Pharm. Sin. B 2019, 9, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q. Bioinformatics analysis of the diversity of gut microbiota and different microbiota on insulin resistance in diabetes mellitus patients. Heliyon 2023, 9, e22117. [Google Scholar] [CrossRef]
- Mccreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. A new possiLGB mechanism by which punicalagin protects against liver injury induced by type 2 diabetes mellitus: Upregulation of autophagy via the akt/foxo3a signaling pathway. J. Agric. Food Chem. 2019, 67, 13948–13959. [Google Scholar] [CrossRef]
- Ma, Y.L.; Wu, Z.F.; Li, Z.; Wang, Y.; Shang, Y.F.; Thakur, K.; Wei, Z.J. In vitro digestibility and hepato-protective potential of Lophatherum gracile Brongn. leave extract. Food Chem. 2024, 433, 137336. [Google Scholar] [CrossRef]
- Li, X.D.; Luo, Z.Q.; Hu, T.J.; Ou, D.Y.; Guo, R.R.; Li, C.J.; Yang, J.; Song, X.Q. Exploring the anti-inflammatory effects of total flavonoids from L. Gracile on LPS-induced inflammation: An integrated approach combining network pharmacology, molecular docking, and experimental validation. Int. Immunopharmacol. 2025, 161, 115027. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, J.J.; Qi, J.; Li, J.; Liu, Y.; Miao, Z.; Qiu, G.F.; Jia, W.K. Microwave-assisted extraction of flavonoids from Phyllostachys heterocycle leaves: Optimization, mechanism, and antioxidant activity in vitro. BioResources 2021, 4, 8060–8081. [Google Scholar] [CrossRef]
- Canas, S.; Rebollo-Hernanz, M.; Bermudez-Gomez, P.; Rodriguez-Rodriguez, P.; Braojos, C.; Gil-Ramirez, A.; Benitez, V.; Aguilera, Y.; Martin-Cabrejas, M.A. Radical scavenging and cellular antioxidant activity of the cocoa shell phenolic compounds after simulated digestion. Antioxidants 2023, 12, 1007. [Google Scholar] [CrossRef]
- Hong, D.F.; Hu, G.L.; Peng, X.R.; Wang, X.Y.; Wang, Y.B.; Al-Romaima, A.; Li, Z.R.; Qiu, M.H. Unusual ent-kaurane diterpenes from the Coffea cultivar s288 coffee beans and molecular docking to alpha-glucosidase. J. Agric. Food Chem. 2022, 70, 615–625. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, T.; Karrar, E.; Zheng, L.; Xie, D.; Jin, J.; Chang, M.; Wang, X.; Jin, Q. Insights into an alpha-glucosidase inhibitory profile of 4,4-dimethylsterols by multispectral techniques and molecular docking. J. Agric. Food Chem. 2021, 69, 15252–15260. [Google Scholar] [CrossRef]
- Zhang, S.S.; Zhang, N.N.; Guo, S.; Liu, S.J.; Hou, Y.F.; Li, S.; Ho, C.T.; Bai, N.S. Glycosides and flavonoids from the extract of Pueraria thomsonii benth leaf alleviate type 2 diabetes in high-fat diet plus streptozotocin-induced mice by modulating the gut microbiota. Food Funct. 2022, 13, 3931–3945. [Google Scholar]
- Ying, C.; Mao, Y.; Chen, L.; Wang, S.; Ling, H.; Li, W.; Zhou, X. Bamboo leaf extract ameliorates diabetic nephropathy through activating the akt signaling pathway in rats. Int. J. Biol. Macromol. 2017, 105 Pt 3, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ge, X.; Song, L.; Wu, H.; Qian, X.; Jia, B.; Zhao, C.; Zhuang, Y. Zingiber striolatum phytochemicals ameliorated hyperglycemia symptoms by modulating gut microbial communities in mice with type 2 diabetes mellitus. Front. Nutr. 2025, 12, 1537932. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of schisandrachinensis bee pollen on nonalcoholic fatty liver disease and gut microbiota in highfat diet induced obese mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, K.; Liu, X.F.; Zhang, Y.Y.; Chen, Y.M.; Wang, N.N.; Yu, L.L.; Liu, S.J.; Hu, Y.Q.; Qin, B. The antidiabetic mechanisms of cinnamon extract: Insights from network pharmacology, gut microbiota, and metabolites. Curr. Issues Mol. Biol. 2025, 47, 543. [Google Scholar] [CrossRef]
- Cheng, Y.; Wan, S.; Yao, L.; Lin, D.; Wu, T.; Chen, Y.; Zhang, A.; Lu, C. Bamboo leaf: A review of traditional medicinal property, phytochemistry, pharmacology, and purification technology. J. Ethnopharmacol. 2023, 306, 116166. [Google Scholar] [CrossRef]
- Ikeda, M.; Shimazawa, R. Challenges to hemoglobin a1c as a therapeutic target for type 2 diabetes mellitus. J. Gen. Fam. Med. 2019, 20, 129–138. [Google Scholar] [CrossRef]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; GribLGB, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (glp-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, H.; Gu, Y.; Tang, Z.; Zhao, X.; Zhou, R.; Huang, P.; Zhang, R.; Wang, X. Association between early-stage diabetic nephropathy and the delayed monophasic glucose peak during oral glucose tolerance test in type 2 diabetes mellitus. J. Diabetes Investig. 2025, 16, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rellan, M.J.; Fondevila, M.F.; Fernandez, U.; Rodriguez, A.; Varela-Rey, M.; Veyrat-Durebex, C.; Seoane, S.; Bernardo, G.; Lopitz-Otsoa, F.; Fernandez-Ramos, D.; et al. O-glcnacylated p53 in the liver modulates hepatic glucose production. Nat. Commun. 2021, 12, 5068. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Xue, J.; Li, X.; Li, Y.; Ding, Y.; Feng, Y.; Zhang, X.; Su, J.; Chu, X. Optimization of liquid fermentation of Acanthopanax senticosus leaves and its non-targeted metabolomics analysis. Molecules 2024, 29, 4749. [Google Scholar] [CrossRef]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 2021, 117, 154712. [Google Scholar] [CrossRef]
- Wang, L.; Gou, X.; Ding, Y.; Liu, J.; Wang, Y.; Wang, Y.; Zhang, J.; Du, L.; Peng, W.; Fan, G. The interplay between herbal medicines and gut microbiota in metabolic diseases. Front. Pharmacol. 2023, 14, 1105405. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.A.; Zwickey, H.; Bradley, R. Associations between food-specific igg antibodies and intestinal permeability biomarkers. Front. Nutr. 2022, 9, 962093. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zeng, Y.; Zeng, R.; Gou, X.; Zhou, X.; Zhang, J.; Nhamdriel, T.; Fan, G. The total alkaloids of berberidis cortex alleviate type 2 diabetes mellitus by regulating gut microbiota, inflammation and liver gluconeogenesis. J. Ethnopharmacol. 2025, 337 Pt 3, 118957. [Google Scholar] [CrossRef]
- Yi, X.; Dong, M.; Guo, N.; Tian, J.; Lei, P.; Wang, S.; Yang, Y.; Shi, Y. Flavonoids improve type 2 diabetes mellitus and its complications: A review. Front. Nutr. 2023, 10, 1192131. [Google Scholar] [CrossRef]
- Karacaglar, N.; Bulat, T.; Boyaci, I.H.; Topcu, A. Raman spectroscopy coupled with chemometric methods for the discrimination of foreign fats and oils in cream and yogurt. J. Food Drug Anal. 2019, 27, 101–110. [Google Scholar] [CrossRef]
- Wang, X.; Ma, C.J.; Yang, P.M.; Wei, Y.L.; Jia, J.; Cao, G.S.; Li, J.; Xin, Y. Integrated HPLC fingerprinting and multivariate analysis differentiates between wild and cultivated Hedyotis diffusa Willd. Ind. Crops Prod. 2020, 148, 112223. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Zhu, M.; Yang, B.; Yang, Y.; Jia, X.; Feng, L. Moutan cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int. J. Biol. Macromol. 2022, 206, 849–860. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Hou, J.; Liu, H.; Zeng, R.; Li, X.; Han, M.; Li, Q.; Ji, L.; Pan, D.; et al. Plasma proteome profiling reveals the therapeutic effects of the ppar pan-agonist chiglitazar on insulin sensitivity, lipid metabolism, and inflammation in type 2 diabetes. Sci. Rep. 2024, 14, 638. [Google Scholar] [CrossRef]
- Xie, C.; Qi, C.; Zhang, J.; Wang, W.; Meng, X.; Aikepaer, A.; Lin, Y.; Su, C.; Liu, Y.; Feng, X.; et al. When short-chain fatty acids meet type 2 diabetes mellitus: Revealing mechanisms, envisioning therapies. Biochem. Pharmacol. 2025, 233, 116791. [Google Scholar] [CrossRef]
- Kochumon, S.; Malik, M.Z.; Sindhu, S.; Arefanian, H.; Jacob, T.; Bahman, F.; Nizam, R.; Hasan, A.; Thomas, R.; Al-Rashed, F.; et al. Gut dysbiosis shaped by cocoa butter-based sucrose-free HFD leads to steatohepatitis, and insulin resistance in mice. Nutrients 2024, 16, 1929. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ma, J.; Kang, M.; Tang, W.; Xia, S.; Yin, J.; Yin, Y. Flavonoids, gut microbiota, and host lipid metabolism. Eng. Life Sci. 2024, 24, 2300065. [Google Scholar] [CrossRef]
- Sepp, E.; Kolk, H.; Loivukene, K.; Mikelsaar, M. Higher blood glucose level associated with body mass index and gut microbiota in elderly people. Microb. Ecol. Health Dis. 2014, 25, 22857. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, X.; Wang, Y.; Zhang, J.; Sui, Y.; Yin, X.; Wu, N.; Yang, L.; Xu, J.; Zhou, H.; et al. Ruditapes philippinarum polysaccharide alleviates hyperglycemia by modulating gut microbiota in a mouse model of type 2 diabetes mellitus. Mol. Nutr. Food Res. 2025, 69, e202400996. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.F.; Yang, K.; Yu, L.L.; Liu, S.J.; Wang, N.N.; Chen, Y.M.; Hu, Y.Q.; Qin, B. Gypenosides alleviate hyperglycemia by regulating gut microbiota metabolites and intestinal permeability. Curr. Issues Mol. Biol. 2025, 47, 515. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shi, M.; Guo, G.; Xing, J.; Liu, Z.; Song, F.; Liu, S. In-depth investigation of the therapeutic effect of Tribulus terrestris l. On type 2 diabetes based on intestinal microbiota and feces metabolomics. J. Ethnopharmacol. 2024, 325, 117815. [Google Scholar] [CrossRef]
- Zhao, H.; Li, M.; Liu, L.; Li, D.; Zhao, L.; Wu, Z.; Zhou, M.; Jia, L.; Yang, F. Cordyceps militaris polysaccharide alleviates diabetic symptoms by regulating gut microbiota against tlr4/nf-kappab pathway. Int. J. Biol. Macromol. 2023, 230, 123241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, T.; Xu, W.; Huang, Y.; Ran, L.; Yan, Y.; Mi, J.; Lu, L.; Sun, Y.; Zeng, X.; et al. The polysaccharides from the fruits of Lycium barbarum l. Confer anti-diabetic effect by regulating gut microbiota and intestinal barrier. Carbohydr. Polym. 2022, 291, 119626. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, L.; Huang, B.; Lu, Q.; Liu, R. 3,4-dihydroxyphenylacetic acid ameliorates gut barrier dysfunction via regulation of MAPK-MLCK pathway in type 2 diabetes mice. Life Sci. 2022, 305, 120742. [Google Scholar] [CrossRef] [PubMed]

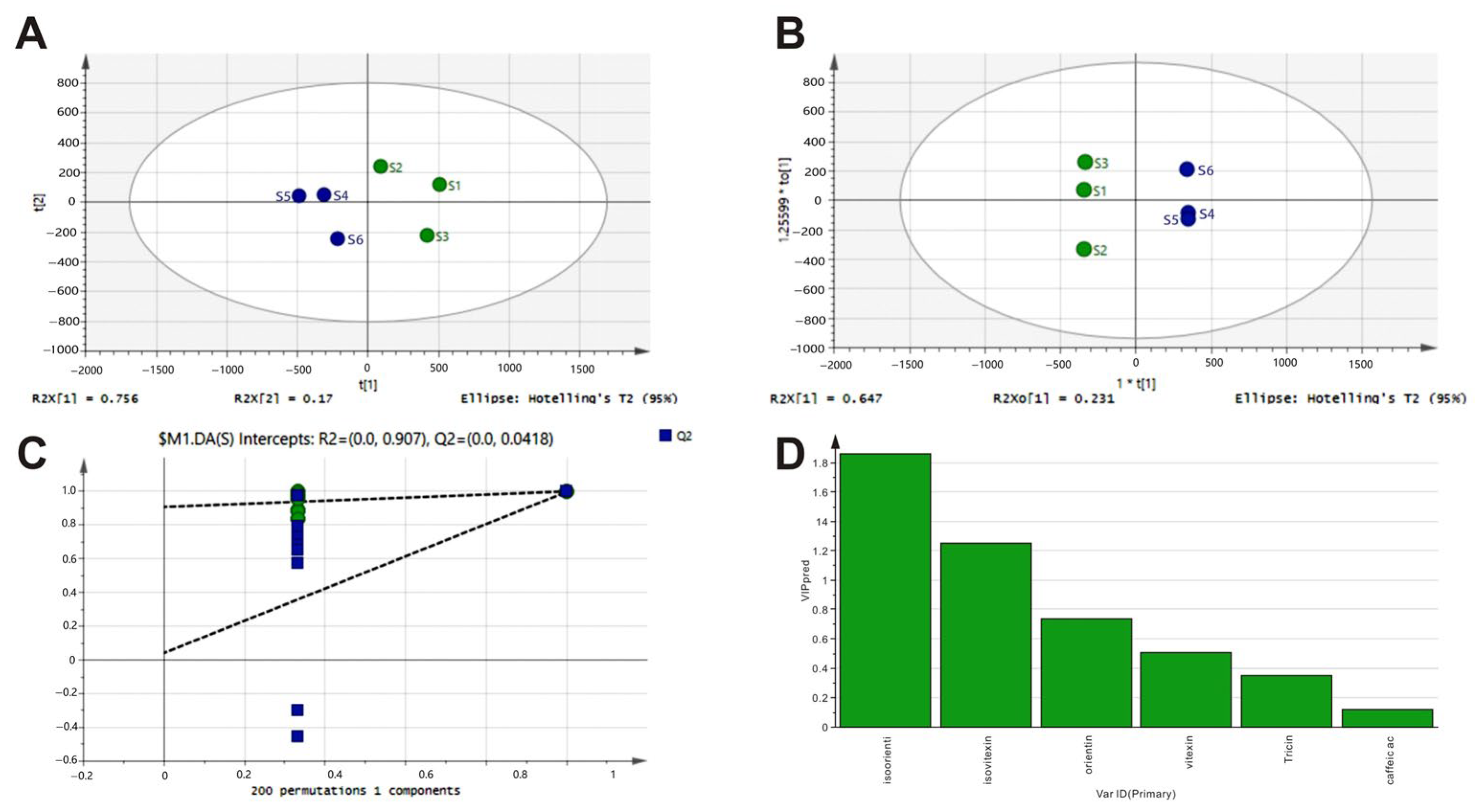
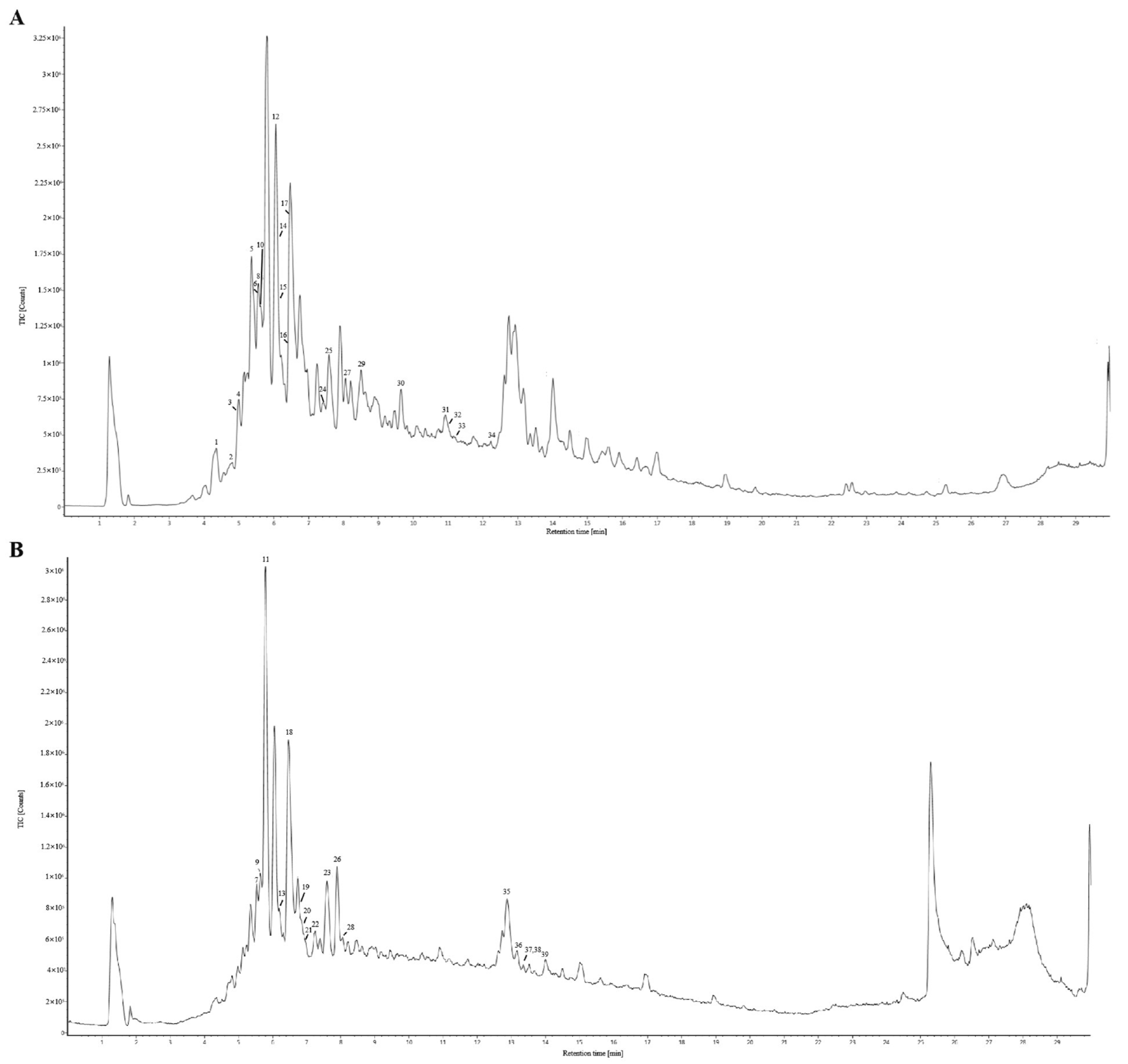
| Sample | Content of Components (mg/g) | |||||
|---|---|---|---|---|---|---|
| Isoorientin | Orientin | Vitexin | Isovitexin | Tricin | Caffeic Acid | |
| S1 | 117.86 ± 0.79 | 40.23 ± 0.25 | 9.40 ± 0.01 | 36.36 ± 0.21 | 5.43 ± 0.02 | 8.61 ± 0.17 |
| S2 | 97.97 ± 0.49 | 37.89 ± 0.02 | 8.94 ± 0.02 | 32.64 ± 0.05 | 4.41 ± 0.02 | 8.62 ± 0.13 |
| S3 | 112.94 ± 0.38 | 39.74 ± 0.38 | 8.76 ± 0.03 | 33.04 ± 0.17 | 6.22 ± 0.07 | 7.01 ± 0.22 |
| S4 | 117.66 ± 0.47 | 42.44 ± 0.07 | 10.09 ± 0.25 | 38.05 ± 0.19 | 4.49 ± 0.02 | 9.11 ± 0.06 |
| S5 | 128.60 ± 0.34 | 41.93 ± 0.10 | 9.59 ± 0.17 | 37.49 ± 0.14 | 6.71 ± 0.03 | 7.10 ± 0.08 |
| S6 | 128.92 ± 0.72 | 41.73 ± 0.48 | 10.23 ± 0.07 | 39.31 ± 0.18 | 7.79 ± 0.03 | 9.16 ± 0.08 |
| Sample | IC50 (mg/L) | ||
|---|---|---|---|
| DPPH | ABTS | α-Glucosidase | |
| S1 | 22.97 ± 1.54 | 9.50 ± 0.42 | 0.18 ± 0.03 |
| S2 | 12.02 ± 1.15 | 7.42 ± 0.24 | 0.34 ± 0.04 |
| S3 | 20.03 ± 1.76 | 3.52 ± 0.16 | 0.24 ± 0.02 |
| S4 | 24.18 ± 2.62 | 12.40 ± 1.01 | 0.14 ± 0.07 |
| S5 | 15.85 ± 1.34 | 11.63 ± 0.93 | 0.13 ± 0.03 |
| S6 | 12.02 ± 1.92 | 3.67 ± 0.13 | 0.11 ± 0.01 |
| Indicator | IC50 (mg/L) | ||
|---|---|---|---|
| DPPH | ABTS | α-Glucosidase | |
| caffeic acid | 0.28 | 0.96 ** | −0.037 |
| isoorientin | 0.063 | 0.12 | −0.96 ** |
| orientin | 0.30 | 0.34 | −0.96 ** |
| vitexin | 0.19 | 0.73 | −0.78 |
| isovitexin | 0.17 | 0.58 | −0.91 * |
| tricin | −0.29 | −0.16 | −0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Liu, X.; Yang, K.; Liu, S.; Yu, L.; Chen, Y.; Wang, N.; Hu, Y.; Qin, B. Bioactive Component Screening and Mechanistic Study of the Anti-Diabetic Activity of Lophatherum gracile Brongn Extract. Curr. Issues Mol. Biol. 2025, 47, 779. https://doi.org/10.3390/cimb47090779
Wang R, Liu X, Yang K, Liu S, Yu L, Chen Y, Wang N, Hu Y, Qin B. Bioactive Component Screening and Mechanistic Study of the Anti-Diabetic Activity of Lophatherum gracile Brongn Extract. Current Issues in Molecular Biology. 2025; 47(9):779. https://doi.org/10.3390/cimb47090779
Chicago/Turabian StyleWang, Rong, Xuefeng Liu, Kuan Yang, Shaojing Liu, Lili Yu, Yunmei Chen, Nana Wang, Yaqi Hu, and Bei Qin. 2025. "Bioactive Component Screening and Mechanistic Study of the Anti-Diabetic Activity of Lophatherum gracile Brongn Extract" Current Issues in Molecular Biology 47, no. 9: 779. https://doi.org/10.3390/cimb47090779
APA StyleWang, R., Liu, X., Yang, K., Liu, S., Yu, L., Chen, Y., Wang, N., Hu, Y., & Qin, B. (2025). Bioactive Component Screening and Mechanistic Study of the Anti-Diabetic Activity of Lophatherum gracile Brongn Extract. Current Issues in Molecular Biology, 47(9), 779. https://doi.org/10.3390/cimb47090779






