Anti-TB Drugs for Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis: A Review
Abstract
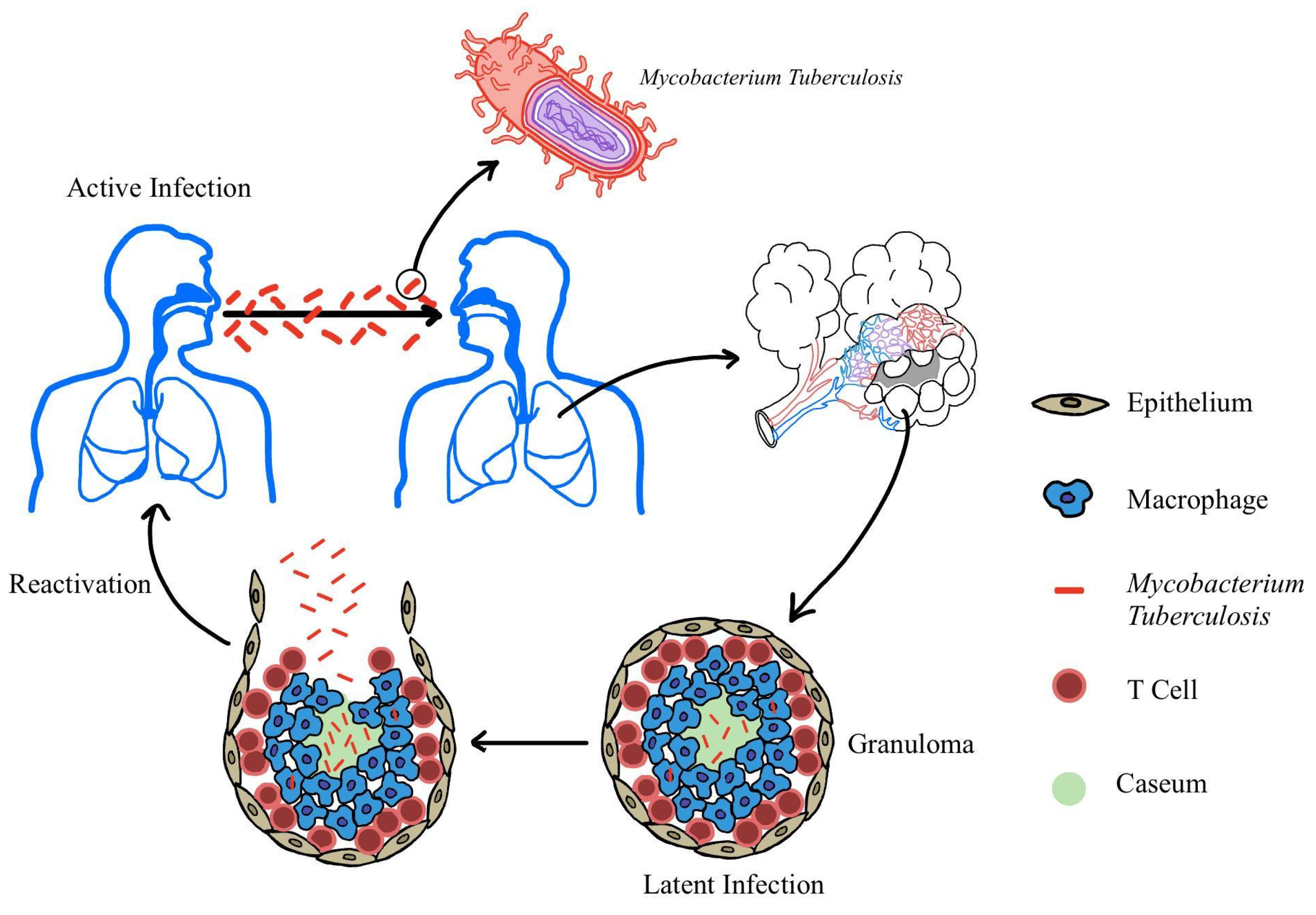
1. Introduction
2. Methods
3. Diagnosing TB
4. Vaccines
5. Anti-TB Drugs
5.1. Drug-Sensitive TB
| Regimen | Drugs Used | Treatment Duration | Key Findings | Study/Source |
|---|---|---|---|---|
| Standard Treatment (Reference) | Isoniazid, Rifampicin, Pyrazinamide, Ethambutol | 6 months | Baseline Comparison Regimen | [2] |
| 4-month Rifapentine–Moxifloxacin Regimen | Isoniazid, Rifapentine, Moxifloxacin, Pyrazinamide | 4 months | Non-inferior to 6-month standard regimen; moxifloxacin essential for efficacy | [14,36,37] |
| Higher Dose Pyrazinamide Regimen | Standard drugs with Pyrazinamide 35–45 mg/kg | 12 weeks | Higher culture conversion rates | [38] |
| Flat-Dosing Pyrazinamide Regimen | Standard drugs with Pyrazinamide 1000 mg | 4 months | 1000 mg flat dose achieves most consistent therapeutic levels | [39] |
| Faropenem Substitution Regimen | Isoniazid, Rifampicin, Pyrazinamide, Faropenem (instead of Ethambutol) | 6 months | Non-inferior to standard treatment; fewer side effects; avoids ocular toxicity | [40] |
| Pretomanid-Containing Regimen | Pretomanid, Rifamycin, Pyrazinamide | 12 weeks | Higher microbiological activity; increased risk of adverse events | [41] |
| Sitafloxacin Monotherapy Regimen | Sitafloxacin Monotherapy | 7 days | Similar EBA and compared to levofloxacin and isoniazid greater prolonged EBA | [42] |
5.2. Drug-Resistant TB
| Regimen | Drugs Used | Treatment Duration | Key Findings | Study/Source |
|---|---|---|---|---|
| BPaLM Standard Treatment (Reference) | Bedaquiline, Pretomanid, Linezolid, Moxifloxacin | 6 months | Baseline Comparison Regimen for Rifampicin-Resistant, Fluoroquinolone-Susceptible TB | [2] |
| BPaL Standard Treatment (Reference) | Bedaquiline, Pretomanid, Linezolid | 6 months | Baseline Comparison Regimen for Rifampicin- and Fluoroquinolone- Resistant TB | [36] |
| 8-Week Intensive Bedaquiline-Linezolid Regimen | Bedaquiline, Linezolid, Isoniazid, Pyrazinamide, Ethambutol | 8 weeks (Followed by Continuation Phase) | Non-inferior to standard 24-week treatment; Improved Adherence | [48,49] |
| Shortened Regimen with Levofloxacin or Bedaquiline Regimen | Linezolid, Cycloserine, Clofazimine, Pyrazinamide, Levofloxacin or Bedaquiline | 24 weeks | Less hepatotoxicity than injectable-based regimens | [50] |
| Lower Exposure to Linezolid Regimen | 600 mg Linezolid for 9–13 weeks + 300 mg Linezolid for remaining treatment (as part of BPaL) | 26 weeks | Maintains efficacy of standard treatment | [51] |
| Lower Dose Linezolid Regimen | 600 mg of Linezolid (as part of BPaL) | 26 weeks | Fewer incidents of peripheral neuropathy, myelosuppression, and bacteriologic failure | [52] |
| Higher Exposure Linezolid | 1200 mg Linezolid with multiple dosing regimens | N/A * | Increased bactericidal clearance | [53] |
| Increased Isoniazid Dosing (in patients with inhA mutation) | Isoniazid 10 mg/kg (slow acetylators), 15 mg/kg (fast acetylators) | N/A * | Efficacy comparable to standard dose in drug-sensitive TB | [54] |
| BPaMZ Regimen | Bedaquiline, Pretomanid, Moxifloxacin, and Pyrazinamide | 4 months | Strong bactericidal activity; increased risk of hepatic adverse events | [55] |
| Clofazimine Regimen | Bedaquiline, Levofloxacin, Linezolid, Cycloserine, and 100 mg Clofazimine | 6 months | Improved cure rates | [56] |
| All-oral 9-month Regimens | Various combinations of Bedaquiline, Delamanid, Linezolid, Levofloxacin or Moxifloxacin, Clofazimine, and Pyrazinamide | 9 months | Comparable to standard treatment; increased hepatotoxic adverse events | [58] |
6. Adjunctive Therapies
6.1. Metformin
6.2. N-Acetylcysteine and Glutathione
6.3. Other Host Directed Therapies
| Therapy | Key Findings | Study/Source |
|---|---|---|
| Metformin | Decreased inflammation, increased immune balance, improved culture conversion, improved radiological outcomes | [64,65,66,67,68,69,70,71,72,73] |
| N-Acetylcysteine | Increased lung function (FEV1, FVC), reduced TNF, hepatoprotective effects, decreased oxidative stress | [74,75,76,77,78] |
| Low-Dose Aspirin | Improvement in clinical signs, increased rate of sputum conversion, reduced inflammatory markers and pulmonary cavitary lesions | [80] |
| CC-11050 | Improved FEV1 recovery | [81] |
| Everolimus | Improved FEV1 recovery | [81] |
| Atorvastatin | Faster sputum culture conversion, decreased mycobacterial burden, improved chest X-ray scores, reduced peripheral neuropathy | [82] |
| Azithromycin | Reduced lung inflammation and tissue turnover, decreased IP-10 and neutrophils | [83] |
7. Implementation Strategies
8. Limitations
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTS | Active Care and Treatment Strategy |
| BCG | Bacille Calmette-Guérin |
| BPaL | Bedaquiline, Pretomanid, and Linezolid |
| BPaLM | Bedaquiline, Pretomanid, Linezolid, and Moxifloxacin |
| CARP | Cas9/gRNA-assisted quantitative Real-Time PCR |
| CAMPER | Cas9 and Mutagenic Polymerase for Evolving Resistance |
| CRISPR-CAS | Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR Associated |
| DHEA | Dehydroepiandrosterone |
| DM | Diabetes Mellitus |
| DOT | Directly Observed Therapy |
| DST | Drug Susceptibility Test |
| EBA | Early Bactericidal Activity |
| EMB | Ethambutol |
| ESAT-6 | Early Secreted Antigenic Target 6 |
| HDT | Host-Directed Therapy |
| INH | Isoniazid |
| MDR-TB | Multi-Drug-Resistant Tuberculosis |
| NAC | N-Acetylcysteine |
| PZA | Pyrazinamide |
| RIF | Rifampicin |
| RRDR | RIF resistance-determining region |
| TB | Tuberculosis |
| TTP | Time-to-Positivity |
| VDOT | Video Directly Observed Therapy |
| WGS | Whole Genome Sequencing |
| WHO | World Health Organization |
References
- Alsayed, S.S.R.; Gunosewoyo, H. Tuberculosis: Pathogenesis, Current Treatment Regimens and New Drug Targets. Int. J. Mol. Sci. 2023, 24, 5202. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240101531 (accessed on 29 July 2025).
- Borah, P.; Deb, P.K.; Venugopala, K.N.; Al-Shar’i, N.A.; Singh, V.; Deka, S.; Srivastava, A.; Tiwari, V.; Mailavaram, R.P. Tuberculosis: An Update on Pathophysiology, Molecular Mechanisms of Drug Resistance, Newer Anti-TB Drugs, Treatment Regimens and Host- Directed Therapies. Curr. Top. Med. Chem. 2021, 21, 547–570. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnabi, N.; Shamseddin, J.; Emadi, M.; Bodaghi, A.B.; Varseh, M.; Shariati, A.; Rezaei, M.; Dastranj, M.; Farahani, A. Mycobacterium tuberculosis: The Mechanism of Pathogenicity, Immune Responses, and Diagnostic Challenges. J. Clin. Lab. Anal. 2024, 38, e25122. [Google Scholar] [CrossRef]
- Russell, D.G.; Cardona, P.J.; Kim, M.J.; Allain, S.; Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009, 10, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Rahlwes, K.C.; Dias, B.R.S.; Campos, P.C.; Alvarez-Arguedas, S.; Shiloh, M.U. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence 2023, 14, 2150449. [Google Scholar] [CrossRef]
- Abrahem, R.; Cao, R.; Robinson, B.; Munjal, S.; Cho, T.; To, K.; Ashley, D.; Hernandez, J.; Nguyen, T.; Teskey, G.; et al. Elucidating the Efficacy of the Bacille Calmette-Guérin Vaccination in Conjunction with First Line Antibiotics and Liposomal Glutathione. J. Clin. Med. 2019, 8, 1556. [Google Scholar] [CrossRef]
- Datta, D.; Jamwal, S.; Jyoti, N.; Patnaik, S.; Kumar, D. Actionable mechanisms of drug tolerance and resistance in Mycobacterium tuberculosis. FEBS J. 2024, 291, 4433–4452. [Google Scholar] [CrossRef]
- Kwon, B.E.; Ahn, J.H.; Min, S.; Kim, H.; Seo, J.; Yeo, S.G.; Ko, H.J. Development of New Preventive and Therapeutic Vaccines for Tuberculosis. Immune Netw. 2018, 18, e17. [Google Scholar] [CrossRef]
- Lerner, T.R.; Borel, S.; Greenwood, D.J.; Repnik, U.; Russell, M.R.; Herbst, S.; Gutierrez, M.G. Mycobacterium tuberculosis replicates within necrotic human macrophages. J. Cell Biol. 2017, 216, 583–594. [Google Scholar] [CrossRef]
- Sarathy, J.P.; Dartois, V.; Lee, E.J. The role of transport mechanisms in mycobacterium tuberculosis drug resistance and tolerance. Pharmaceuticals 2012, 5, 1210–1235. [Google Scholar] [CrossRef]
- Wulandari, D.A.; Hartati, Y.W.; Ibrahim, A.U.; Pitaloka, D.A.E.; Irkham. Multidrug-resistant tuberculosis. Clin. Chim. Acta Int. J. Clin. Chem. 2024, 559, 119701. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Park, H.A.; Hyun, I.G.; Kim, J.H.; Hwang, Y.I.; Jang, S.H.; Sim, Y.S.; Shin, T.R.; Ko, Y.; Ban, G.Y.; et al. Incidence and outcomes of adverse drug reactions to first-line anti-tuberculosis drugs and their effects on the quality of life: A multicenter prospective cohort study. Pharmacoepidemiol. Drug Saf. 2022, 31, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Dorman, S.E.; Nahid, P.; Kurbatova, E.V.; Phillips, P.P.J.; Bryant, K.; Dooley, K.E.; Engle, M.; Goldberg, S.V.; Phan, H.T.T.; Hakim, J.; et al. Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis. N. Engl. J. Med. 2021, 384, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, E.H.; Abdelwahab, M.T.; Hendricks, B.; Gupte, N.; Narunsky, K.; Wiesner, L.; Barnes, G.; Dawson, R.; Dooley, K.E.; Denti, P. Pretomanid Pharmacokinetics in the Presence of Rifamycins: Interim Results from a Randomized Trial among Patients with Tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e01196-20. [Google Scholar] [CrossRef]
- Nasiri, M.J.; Venketaraman, V. Advances in Host-Pathogen Interactions in Tuberculosis: Emerging Strategies for Therapeutic Intervention. Int. J. Mol. Sci. 2025, 26, 1621. [Google Scholar] [CrossRef]
- Xiong, X.S.; Zhang, X.D.; Yan, J.W.; Huang, T.T.; Liu, Z.Z.; Li, Z.K.; Wang, L.; Li, F. Identification of Mycobacterium tuberculosis Resistance to Common Antibiotics: An Overview of Current Methods and Techniques. Infect. Drug Resist. 2024, 17, 1491–1506. [Google Scholar] [CrossRef]
- Tiberi, S.; Utjesanovic, N.; Galvin, J.; Centis, R.; D’Ambrosio, L.; van den Boom, M.; Zumla, A.; Migliori, G.B. Drug resistant TB-latest developments in epidemiology, diagnostics and management. Int. J. Infect. Dis. 2022, 124 (Suppl. S1), S20–S25. [Google Scholar] [CrossRef]
- Davies, P.D.; Pai, M. The diagnosis and misdiagnosis of tuberculosis. Int. J. Tuberc. Lung Dis. 2008, 12, 1226–1234. [Google Scholar]
- World Health Organization. Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis; World Health Organization: Geneva, Switzerland, 2018; Available online: https://iris.who.int/handle/10665/275469 (accessed on 6 September 2025).
- He, G.; Zheng, Q.; Shi, J.; Wu, L.; Huang, B.; Yang, Y. Evaluation of WHO catalog of mutations and five WGS analysis tools for drug resistance prediction of Mycobacterium tuberculosis isolates from China. Microbiol. Spectr. 2024, 12, e03341-23. [Google Scholar] [CrossRef]
- Feng, S.; Liang, L.; Shen, C.; Lin, D.; Li, J.; Lyu, L.; Liang, W.; Zhong, L.L.; Cook, G.M.; Doi, Y.; et al. A CRISPR-guided mutagenic DNA polymerase strategy for the detection of antibiotic-resistant mutations in M. tuberculosis. Mol. Ther. Nucleic Acids 2022, 29, 354–367. [Google Scholar] [CrossRef]
- World Health Organization. Catalogue of Mutations in Mycobacterium tuberculosis Complex and Their Association with Drug Resistance, 2nd ed.; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240082410 (accessed on 26 July 2025).
- Pei, S.; Song, Z.; Yang, W.; He, W.; Ou, X.; Zhao, B.; He, P.; Zhou, Y.; Xia, H.; Wang, S.; et al. The catalogue of Mycobacterium tuberculosis mutations associated with drug resistance to 12 drugs in China from a nationwide survey: A genomic analysis. Lancet Microbe 2024, 5, 100899. [Google Scholar] [CrossRef]
- Augustin, L.; Agarwal, N. Designing a Cas9/gRNA-assisted quantitative Real-Time PCR (CARP) assay for identification of point mutations leading to rifampicin resistance in the human pathogen Mycobacterium tuberculosis. Gene 2023, 857, 147173. [Google Scholar] [CrossRef]
- Sodani, M.; Misra, C.S.; Rath, D.; Kulkarni, S. Harnessing CRISPR-Cas9 as an anti-mycobacterial system. Microbiol. Res. 2023, 270, 127319. [Google Scholar] [CrossRef]
- Maeda, T.; Kawada, M.; Sakata, N.; Kotani, H.; Furusawa, C. Laboratory evolution of Mycobacterium on agar plates for analysis of resistance acquisition and drug sensitivity profiles. Sci. Rep. 2021, 11, 15136. [Google Scholar] [CrossRef]
- Srivastava, S.; Dey, S.; Mukhopadhyay, S. Vaccines against Tuberculosis: Where Are We Now? Vaccines 2023, 11, 1013. [Google Scholar] [CrossRef]
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.C.; Croda, J.; Hill, P.C.; et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet Glob. Health 2022, 10, e1307–e1316. [Google Scholar] [CrossRef]
- An, Y.; Ni, R.; Zhuang, L.; Yang, L.; Ye, Z.; Li, L.; Parkkila, S.; Aspatwar, A.; Gong, W. Tuberculosis vaccines and therapeutic drug: Challenges and future directions. Mol. Biomed. 2025, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Sefat, K.M.S.R.; Kumar, M.; Kehl, S.; Kulkarni, R.; Leekha, A.; Paniagua, M.M.; Ackart, D.F.; Jones, N.; Spencer, C.; Podell, B.K.; et al. An intranasal nanoparticle vaccine elicits protective immunity against Mycobacterium tuberculosis. Vaccine 2024, 42, 125909. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, C.; Knudsen, N.P.H.; Sohn, I.; Izzo, A.A.; Kim, H.; Kristiansen, E.H.; Lindenstrøm, T.; Agger, E.M.; Rasmussen, M.; Shin, S.J.; et al. Immunization with Mycobacterium tuberculosis-Specific Antigens Bypasses T Cell Differentiation from Prior Bacillus Calmette-Guérin Vaccination and Improves Protection in Mice. J. Immunol. 2020, 205, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, J.S.; Clemmensen, H.S.; Battey, H.; Dijkman, K.; Lindenstrøm, T.; Laureano, R.S.; Taplitz, R.; Morgan, J.; Aagaard, C.; Rosenkrands, I.; et al. A Mycobacterium tuberculosis-specific subunit vaccine that provides synergistic immunity upon co-administration with Bacillus Calmette-Guérin. Nat. Commun. 2021, 12, 6658. [Google Scholar] [CrossRef]
- Fang, D.; Wang, R.; Fan, X.; Li, M.; Qian, C.; Cao, B.; Yu, J.; Liu, H.; Lou, Y.; Wan, K. Recombinant BCG vaccine expressing multistage antigens of Mycobacterium tuberculosis provides long-term immunity against tuberculosis in BALB/c mice. Hum. Vaccines Immunother. 2024, 20, 2299607. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Aguilo, N.; Marinova, D.; Gonzalo-Asensio, J. Update on TB Vaccine Pipeline. Appl. Sci. 2020, 10, 2632. [Google Scholar] [CrossRef]
- Saukkonen, J.J.; Duarte, R.; Munsiff, S.S.; Winston, C.A.; Mammen, M.J.; Abubakar, I.; Acuña-Villaorduña, C.; Barry, P.M.; Bastos, M.L.; Carr, W.; et al. Updates on the treatment of drug-susceptible and drug-resistant tuberculosis: An official ATS/CDC/ERS/IDSA clinical practice guideline. Am. J. Respir. Crit. Care Med. 2025, 211, 15–33. [Google Scholar] [CrossRef]
- Chang, V.K.; Imperial, M.Z.; Phillips, P.P.J.; Velásquez, G.E.; Nahid, P.; Vernon, A.; Kurbatova, E.V.; Swindells, S.; Chaisson, R.E.; Dorman, S.E.; et al. Risk-stratified treatment for drug-susceptible pulmonary tuberculosis. Nat. Commun. 2024, 15, 9400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Savic, R.M.; Boeree, M.J.; Peloquin, C.A.; Weiner, M.; Heinrich, N.; Bliven-Sizemore, E.; Phillips, P.P.J.; Hoelscher, M.; Whitworth, W.; et al. Optimising pyrazinamide for the treatment of tuberculosis. Eur. Respir. J. 2021, 58, 2002013. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.Y.; Velásquez, G.E.; Zhang, N.; Chang, V.K.; Phillips, P.P.J.; Nahid, P.; Dorman, S.E.; Kurbatova, E.V.; Whitworth, W.C.; Sizemore, E.; et al. Pyrazinamide Safety, Efficacy, and Dosing for Treating Drug-Susceptible Pulmonary Tuberculosis: A Phase 3, Randomized Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2024, 210, 1358–1369. [Google Scholar] [CrossRef]
- Shangguan, Y.; Guo, W.; Feng, X.; Shi, Y.; Li, X.; Pan, Z.; Hu, M.; Shi, J.; Ding, C.; Xia, J.; et al. Randomized control study of the use of faropenem for treating patients with pulmonary tuberculosis. Int. J. Infect. Dis. 2023, 132, 99–107. [Google Scholar] [CrossRef]
- Dooley, K.E.; Hendricks, B.; Gupte, N.; Barnes, G.; Narunsky, K.; Whitelaw, C.; Smit, T.; Ignatius, E.H.; Friedman, A.; Dorman, S.E.; et al. Assessing Pretomanid for Tuberculosis (APT), a Randomized Phase 2 Trial of Pretomanid-Containing Regimens for Drug-Sensitive Tuberculosis: 12-Week Results. Am. J. Respir. Crit. Care Med. 2023, 207, 929–935. [Google Scholar] [CrossRef]
- Nie, L.; Tong, J.; Wu, G.; Du, J.; Shang, Y.; Wang, Y.; Wu, Z.; Xu, Y.; Ren, Y.; Rao, Y.; et al. Early bactericidal activity of sitafloxacin against pulmonary tuberculosis. Microbiol. Spectr. 2025, 13, e01645-24. [Google Scholar] [CrossRef]
- Farhat, M.; Cox, H.; Ghanem, M.; Denkinger, C.M.; Rodrigues, C.; Abd El Aziz, M.S.; Enkh-Amgalan, H.; Vambe, D.; Ugarte-Gil, C.; Furin, J.; et al. Drug-resistant tuberculosis: A persistent global health concern. Nat. Rev. Microbiol. 2024, 22, 617–635. [Google Scholar] [CrossRef]
- Li, M.C.; Lu, J.; Lu, Y.; Xiao, T.Y.; Liu, H.C.; Lin, S.Q.; Xu, D.; Li, G.L.; Zhao, X.Q.; Liu, Z.G.; et al. rpoB Mutations and Effects on Rifampin Resistance in Mycobacterium tuberculosis. Infect. Drug Resist. 2021, 14, 4119–4128. [Google Scholar] [CrossRef]
- Bastolla, U.; Rotkevich, M.; Arenas, M.; Arrayás, M.; Dogonadze, M.; Lavrova, A.; Molina-Sejas, J.; Tadesse, M.; Xulvi-Brunet, R.; Cox, J.A.G.; et al. Fitness Effect of the Isoniazid Resistance Mutation S315T of the Catalase-Peroxidase Enzyme KatG of Mycobacterium tuberculosis. Genome Biol. Evol. 2025, 17, evaf120. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, J.; Gong, Y.; Liu, W.; Xiao, G.; Liang, J.; Wang, X.; Bi, J.; Zhang, G. Application of engineered CRISPR/Cas12a variants with altered protospacer adjacent motif specificities for the detection of isoniazid resistance mutations in Mycobacterium tuberculosis. Microbiol. Spectr. 2025, e0016525. [Google Scholar] [CrossRef] [PubMed]
- Gausi, K.; Ignatius, E.H.; De Jager, V.; Upton, C.; Kim, S.; McKhann, A.; Moran, L.; Wiesner, L.; von Groote-Bidlingmaier, F.; Marzinek, P.; et al. High-Dose Isoniazid Lacks EARLY Bactericidal Activity against Isoniazid-resistant Tuberculosis Mediated by katG Mutations: A Randomized Phase II Clinical Trial. Am. J. Respir. Crit. Care Med. 2024, 210, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Nyang’wa, B.T.; Berry, C.; Kazounis, E.; Motta, I.; Parpieva, N.; Tigay, Z.; Moodliar, R.; Dodd, M.; Solodovnikova, V.; Liverko, I.; et al. Short oral regimens for pulmonary rifampicin-resistant tuberculosis (TB-PRACTECAL): An open-label, randomised, controlled, phase 2B-3, multi-arm, multicentre, non-inferiority trial. Lancet Respir. Med. 2024, 12, 117–128. [Google Scholar] [CrossRef]
- Paton, N.I.; Cousins, C.; Suresh, C.; Burhan, E.; Chew, K.L.; Dalay, V.B.; Lu, Q.; Kusmiati, T.; Balanag, V.M.; Lee, S.L.; et al. Treatment Strategy for Rifampin-Susceptible Tuberculosis. N. Engl. J. Med. 2023, 388, 873–887. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Y.; Sun, F.; Lan, Y.; Tong, J.; Ge, S.; Feng, Z.; Li, R.; Yu, H.; Li, Y.; et al. Assessing hepatotoxicity in novel and standard short regimens for rifampicin-resistant tuberculosis: Insights from the TB-TRUST and TB-TRUST-plus trials. Int. J. Infect. Dis. 2024, 148, 107230. [Google Scholar] [CrossRef]
- Padmapriyadarsini, C.; Oswal, V.S.; Jain, C.D.; Mariappan, M.V.; Singla, N.; Kumar, S.; Daniel, B.D.; Dave, J.D.; Vadgama, P.; Ramraj, B.; et al. Effectiveness and Safety of Varying Doses of Linezolid With Bedaquiline and Pretomanid in Treatment of Drug-Resistant Pulmonary Tuberculosis: Open-Label, Randomized Clinical Trial. Clin. Infect. Dis. 2024, 79, 1375–1385. [Google Scholar] [CrossRef]
- Conradie, F.; Bagdasaryan, T.R.; Borisov, S.; Howell, P.; Mikiashvili, L.; Ngubane, N.; Samoilova, A.; Skornykova, S.; Tudor, E.; Variava, E.; et al. Bedaquiline-Pretomanid-Linezolid Regimens for Drug-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 810–823. [Google Scholar] [CrossRef]
- Simeon, S.; Garcia-Cremades, M.; Savic, R.; Solans, B.P. Pharmacokinetic-pharmacodynamic modeling of tuberculosis time to positivity and colony-forming unit to assess the response to dose-ranging linezolid. Antimicrob. Agents Chemother. 2024, 68, e0019024. [Google Scholar] [CrossRef]
- Gausi, K.; Ignatius, E.H.; Sun, X.; Kim, S.; Moran, L.; Wiesner, L.; von Groote-Bidlingmaier, F.; Hafner, R.; Donahue, K.; Vanker, N.; et al. A Semimechanistic Model of the Bactericidal Activity of High-Dose Isoniazid against Multidrug-Resistant Tuberculosis: Results from a Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 1327–1335. [Google Scholar] [CrossRef]
- Cevik, M.; Thompson, L.C.; Upton, C.; Rolla, V.C.; Malahleha, M.; Mmbaga, B.; Ngubane, N.; Abu Bakar, Z.; Rassool, M.; Variava, E.; et al. Bedaquiline-pretomanid-moxifloxacin-pyrazinamide for drug-sensitive and drug-resistant pulmonary tuberculosis treatment: A phase 2c, open-label, multicentre, partially randomised controlled trial. Lancet Infect. Dis. 2024, 24, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Zhu, M.; Nie, Q.; Chen, N.; Tu, S.; Zhou, Y.; Xiao, F.; Liu, Y.; Li, X.; Chen, H. Improved outcomes following addition of bedaquiline and clofazimine to a treatment regimen for multidrug-resistant tuberculosis. J. Int. Med. Res. 2023, 51, 3000605221148416. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.T.; Court, R.; Everitt, D.; Diacon, A.H.; Dawson, R.; Svensson, E.M.; Maartens, G.; Denti, P. Effect of Clofazimine Concentration on QT Prolongation in Patients Treated for Tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e0268720. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, L.; Khan, U.; Velásquez, G.E.; Gouillou, M.; Abubakirov, A.; Baudin, E.; Berikova, E.; Berry, C.; Bonnet, M.; Cellamare, M.; et al. Oral Regimens for Rifampin-Resistant, Fluoroquinolone-Susceptible Tuberculosis. N. Engl. J. Med. 2025, 392, 468–482. [Google Scholar] [CrossRef]
- Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. Int. J. Mol. Sci. 2025, 26, 1094. [Google Scholar] [CrossRef]
- Ye, Z.; Li, L.; Yang, L.; Zhuang, L.; Aspatwar, A.; Wang, L.; Gong, W. Impact of diabetes mellitus on tuberculosis prevention, diagnosis, and treatment from an immunologic perspective. Exploration 2024, 4, 20230138. [Google Scholar] [CrossRef]
- Boadu, A.A.; Yeboah-Manu, M.; Osei-Wusu, S.; Yeboah-Manu, D. Tuberculosis and diabetes mellitus: The complexity of the comorbid interactions. Int. J. Infect. Dis. 2024, 146, 107140. [Google Scholar] [CrossRef]
- Cornejo-Báez, A.A.; Zenteno-Cuevas, R.; Luna-Herrera, J. Association Between Diabetes Mellitus-Tuberculosis and the Generation of Drug Resistance. Microorganisms 2024, 12, 2649. [Google Scholar] [CrossRef]
- Tetteh, P.; Danso, E.K.; Osei-Wusu, S.; Yeboah-Manu, D.; Asare, P. The role of metformin in tuberculosis control among TB and diabetes mellitus comorbid individuals. Front. Microbiol. 2025, 16, 1549572. [Google Scholar] [CrossRef]
- Padmapriydarsini, C.; Mamulwar, M.; Mohan, A.; Shanmugam, P.; Gomathy, N.S.; Mane, A.; Singh, U.B.; Pavankumar, N.; Kadam, A.; Kumar, H.; et al. Randomized Trial of Metformin with Anti-Tuberculosis Drugs for Early Sputum Conversion in Adults with Pulmonary Tuberculosis. Clin. Infect. Dis. 2022, 75, 425–434. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- McCreight, L.J.; Stage, T.B.; Connelly, P.; Lonergan, M.; Nielsen, F.; Prehn, C.; Adamski, J.; Brøsen, K.; Pearson, E.R. Pharmacokinetics of metformin in patients with gastrointestinal intolerance. Diabetes Obes. Metab. 2018, 20, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Nabrdalik, K.; Skonieczna-Żydecka, K.; Irlik, K.; Hendel, M.; Kwiendacz, H.; Łoniewski, I.; Januszkiewicz, K.; Gumprecht, J.; Lip, G.Y.H. Gastrointestinal adverse events of metformin treatment in patients with type 2 diabetes mellitus: A systematic review, meta-analysis and meta-regression of randomized controlled trials. Front. Endocrinol. 2022, 13, 975912. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.H.; Kadam, A.; Karunaianantham, R.; Tamizhselvan, M.; Padmapriyadarsini, C.; Mohan, A.; Jeyadeepa, B.; Radhakrishnan, A.; Singh, U.B.; Bapat, S.; et al. Effect of Metformin on Plasma Exposure of Rifampicin, Isoniazid, and Pyrazinamide in Patients on Treatment for Pulmonary Tuberculosis. Ther. Drug Monit. 2024, 46, 370–375. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Xia, L.; Feng, X.; Chen, F.; Cao, S.; Wei, X. Impact of metformin on the risk and treatment outcomes of tuberculosis in diabetics: A systematic review. BMC Infect. Dis. 2019, 19, 859. [Google Scholar] [CrossRef]
- Gonzalez-Muñiz, O.E.; Rodriguez-Carlos, A.; Santos-Mena, A.; Jacobo-Delgado, Y.M.; Gonzalez-Curiel, I.; Rivas-Santiago, C.; Navarro-Tovar, G.; Rivas-Santiago, B. Metformin modulates corticosteroids hormones in adrenals cells promoting Mycobacterium tuberculosis elimination in human macrophages. Tuberculosis 2024, 148, 102548. [Google Scholar] [CrossRef]
- Pavan Kumar, N.; Padmapriyadarsini, C.; Nancy, A.; Tamizhselvan, M.; Mohan, A.; Reddy, D.; Ganga Devi, N.P.; Rathinam, P.; Jeyadeepa, B.; Shandil, R.K.; et al. Effect of Metformin on systemic chemokine responses during anti-tuberculosis chemotherapy. Tuberculosis 2024, 148, 102523. [Google Scholar] [CrossRef]
- Lachmandas, E.; Eckold, C.; Böhme, J.; Koeken, V.A.C.M.; Marzuki, M.B.; Blok, B.; Arts, R.J.W.; Chen, J.; Teng, K.W.W.; Ratter, J.; et al. Metformin Alters Human Host Responses to Mycobacterium tuberculosis in Healthy Subjects. J. Infect. Dis. 2019, 220, 139–150. [Google Scholar] [CrossRef]
- Tukvadze, N.; Mashatole, S.; Jacobs, A.; Ngwanto, T.; Heinrich, N.; Van Rie, A.; Crudu, V.; Tudor, E.; Goginashvili, L.; Ibraim, E.; et al. DRTB-HDT: A randomized controlled trial of two adjunctive host-directed therapies in rifampin-resistant tuberculosis. BMC Infect. Dis. 2025, 25, 768. [Google Scholar] [CrossRef]
- Singh, M.; Vaughn, C.; Sasaninia, K.; Yeh, C.; Mehta, D.; Khieran, I.; Venketaraman, V. Understanding the Relationship between Glutathione, TGF-β, and Vitamin D in Combating Mycobacterium tuberculosis Infections. J. Clin. Med. 2020, 9, 2757. [Google Scholar] [CrossRef]
- Abnousian, A.; Vasquez, J.; Sasaninia, K.; Kelley, M.; Venketaraman, V. Glutathione Modulates Efficacious Changes in the Immune Response against Tuberculosis. Biomedicines 2023, 11, 1340. [Google Scholar] [CrossRef]
- Wallis, R.S.; Sabi, I.; Lalashowi, J.; Bakuli, A.; Mapamba, D.; Olomi, W.; Siyame, E.; Ngaraguza, B.; Chimbe, O.; Charalambous, S.; et al. Adjunctive N-Acetylcysteine and Lung Function in Pulmonary Tuberculosis. NEJM Evid. 2024, 3, EVIDoa2300332. [Google Scholar] [CrossRef] [PubMed]
- Mapamba, D.A.; Sabi, I.; Lalashowi, J.; Sauli, E.; Buza, J.; Olomi, W.; Mtafya, B.; Kibona, M.; Bakuli, A.; Rachow, A.; et al. N-acetylcysteine modulates markers of oxidation, inflammation and infection in tuberculosis. J. Infect. 2025, 90, 106379. [Google Scholar] [CrossRef]
- Sukumaran, D.; Usharani, P.; Paramjyothi, G.K.; Subbalaxmi, M.V.S.; Sireesha, K.; Abid Ali, M. A study to evaluate the hepatoprotective effect of N-acetylcysteine on anti tuberculosis drug induced hepatotoxicity and quality of life. Indian J. Tuberc. 2023, 70, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.J.; Khoshdel, N.; Venketaraman, V. Glutathione and N-acetylcysteine in TB management. Int. J. Tuberc. Lung Dis. 2025, 29, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, Z.; Ni, M.; Wang, Z.; Liang, M.; Sheng, H.; Zhang, A.; Yang, J. Aspirin enhances the clinical efficacy of anti-tuberculosis therapy in pulmonary tuberculosis in patients with type 2 diabetes mellitus. Infect. Dis. 2020, 52, 721–729. [Google Scholar] [CrossRef]
- Wallis, R.S.; Ginindza, S.; Beattie, T.; Arjun, N.; Likoti, M.; Edward, V.A.; Rassool, M.; Ahmed, K.; Fielding, K.; Ahidjo, B.A.; et al. Adjunctive host-directed therapies for pulmonary tuberculosis: A prospective, open-label, phase 2, randomised controlled trial. Lancet Respir. Med. 2021, 9, 897–908, Erratum in Lancet Respir. Med. 2021, 9, e55. [Google Scholar] [CrossRef]
- Adewole, O.O.; Omotoso, B.A.; Ogunsina, M.; Aminu, A.; Ayoola, O.; Adedeji, T.; Awopeju, O.F.; Sogaolu, O.M.; Adewole, T.O.; Odeyemi, A.O.; et al. Atorvastatin improves sputum conversion and chest X-ray severity score. Int. J. Tuberc. Lung Dis. 2023, 27, 912–917. [Google Scholar] [CrossRef]
- Dekkers, B.G.J.; Kerstjens, H.A.M.; Breisnes, H.W.; Leeming, D.J.; Anthony, R.M.; Frijlink, H.W.; van der Werf, T.S.; Kosterink, J.G.W.; Alffenaar, J.C.; Akkerman, O.W. Azithromycin as Host-Directed Therapy for Pulmonary Tuberculosis: A Randomized Pilot Trial. J. Infect. Dis. 2025, 231, e891–e900. [Google Scholar] [CrossRef]
- Guo, P.; Qiao, W.; Sun, Y.; Liu, F.; Wang, C. Telemedicine Technologies and Tuberculosis Management: A Randomized Controlled Trial. Telemed. J. e-Health 2020, 26, 1150–1156. [Google Scholar] [CrossRef]
- Burzynski, J.; Mangan, J.M.; Lam, C.K.; Macaraig, M.; Salerno, M.M.; deCastro, B.R.; Goswami, N.D.; Lin, C.Y.; Schluger, N.W.; Vernon, A.; et al. In-Person vs. Electronic Directly Observed Therapy for Tuberculosis Treatment Adherence: A Randomized Noninferiority Trial. JAMA Netw. Open 2022, 5, e2144210. [Google Scholar] [CrossRef]
- Turyahabwe, S.; Ramachandra, S.S.; Quraishi, S.; Fasih, I.; Quraishi, H.; Peddapalegani, P.; Ahmad, A. A community based, bottom-up, multi-pronged, technology integrated approach to enhance tuberculosis related awareness and treatment adherence in Uganda: The ACTS model. PLoS ONE 2025, 20, e0318174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukas, K.; Dang, M.T.; Necas, C.; Venketaraman, V. Anti-TB Drugs for Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis: A Review. Curr. Issues Mol. Biol. 2025, 47, 776. https://doi.org/10.3390/cimb47090776
Lukas K, Dang MT, Necas C, Venketaraman V. Anti-TB Drugs for Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis: A Review. Current Issues in Molecular Biology. 2025; 47(9):776. https://doi.org/10.3390/cimb47090776
Chicago/Turabian StyleLukas, Kara, Madeleine T. Dang, Clare Necas, and Vishwanath Venketaraman. 2025. "Anti-TB Drugs for Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis: A Review" Current Issues in Molecular Biology 47, no. 9: 776. https://doi.org/10.3390/cimb47090776
APA StyleLukas, K., Dang, M. T., Necas, C., & Venketaraman, V. (2025). Anti-TB Drugs for Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis: A Review. Current Issues in Molecular Biology, 47(9), 776. https://doi.org/10.3390/cimb47090776







