Metabolome and Transcriptome Analyses of the Molecular Mechanism Underlying Light-Induced Anthocyanin Accumulation in Pepper (Capsicum annuum L.) Peel
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Metabolite Detection and Analysis
2.3. RNA Sequencing
2.4. Differentially Expressed Gene Screening and Enrichment Analysis
2.5. Association Analysis of Key Metabolites and Genes Involved in Anthocyanin Synthesis
2.6. Quantitative Reverse Transcription PCR (qRT-PCR)
3. Results
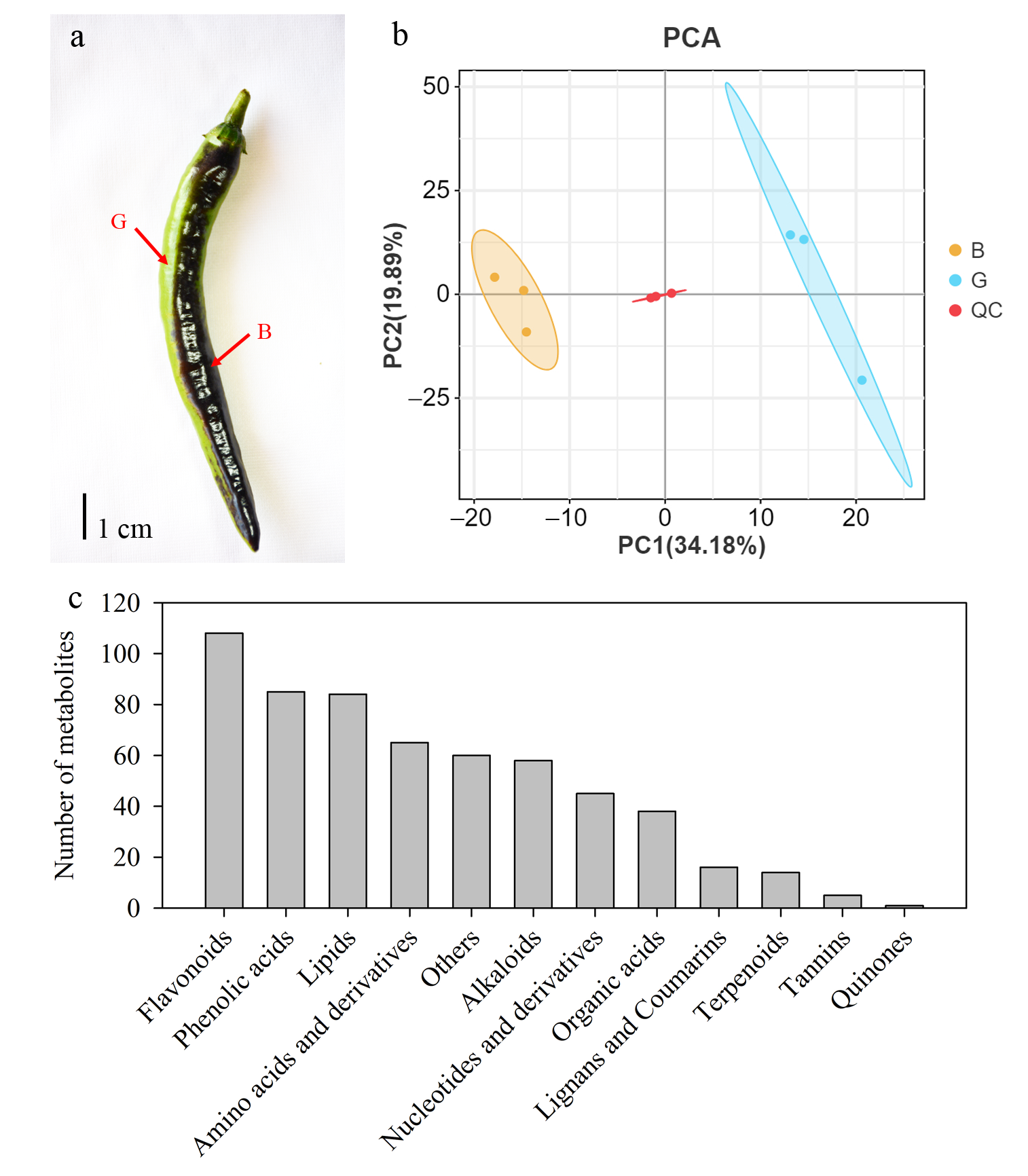
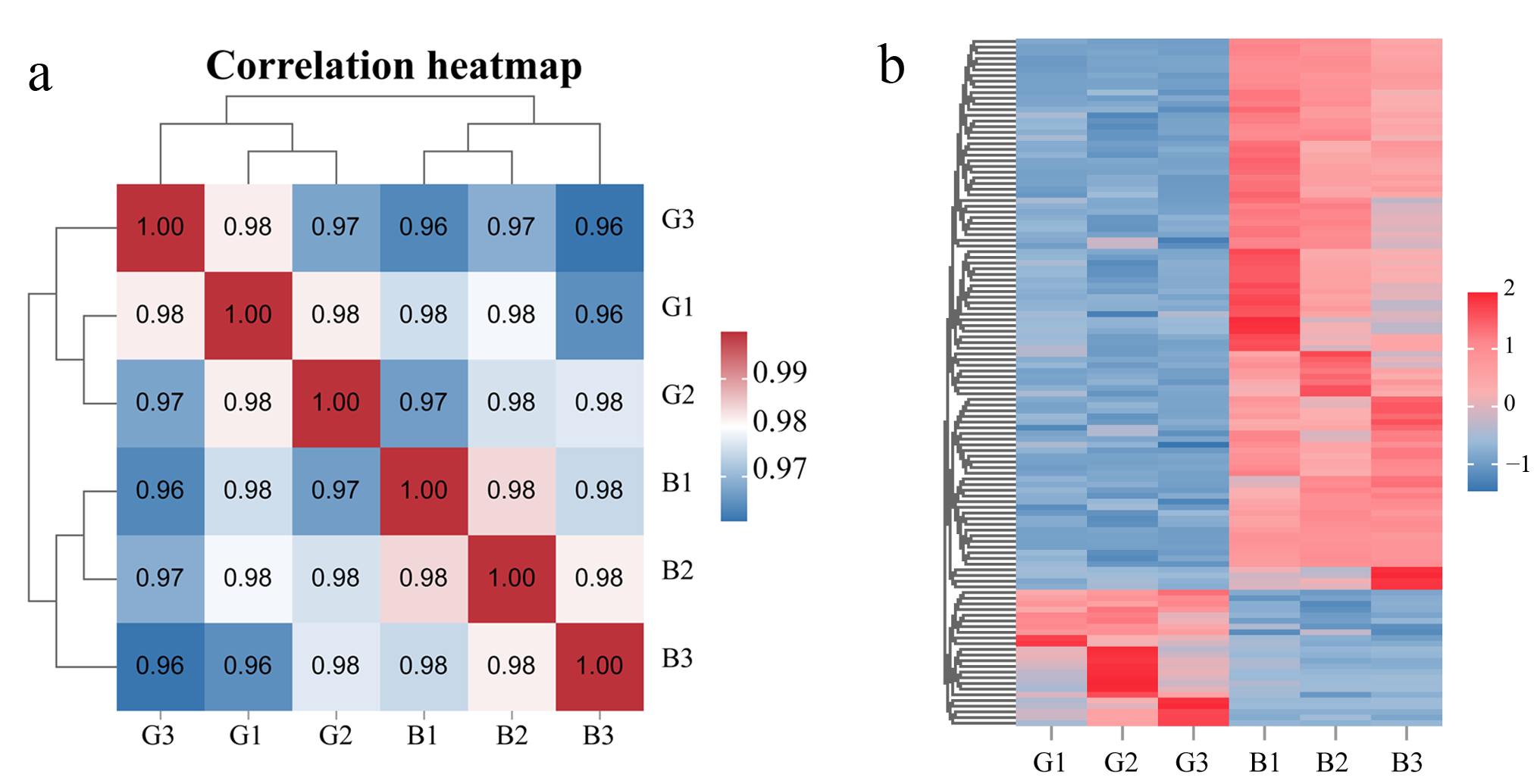
3.1. Global Metabolic Characteristics of Different-Colored Pepper Peels
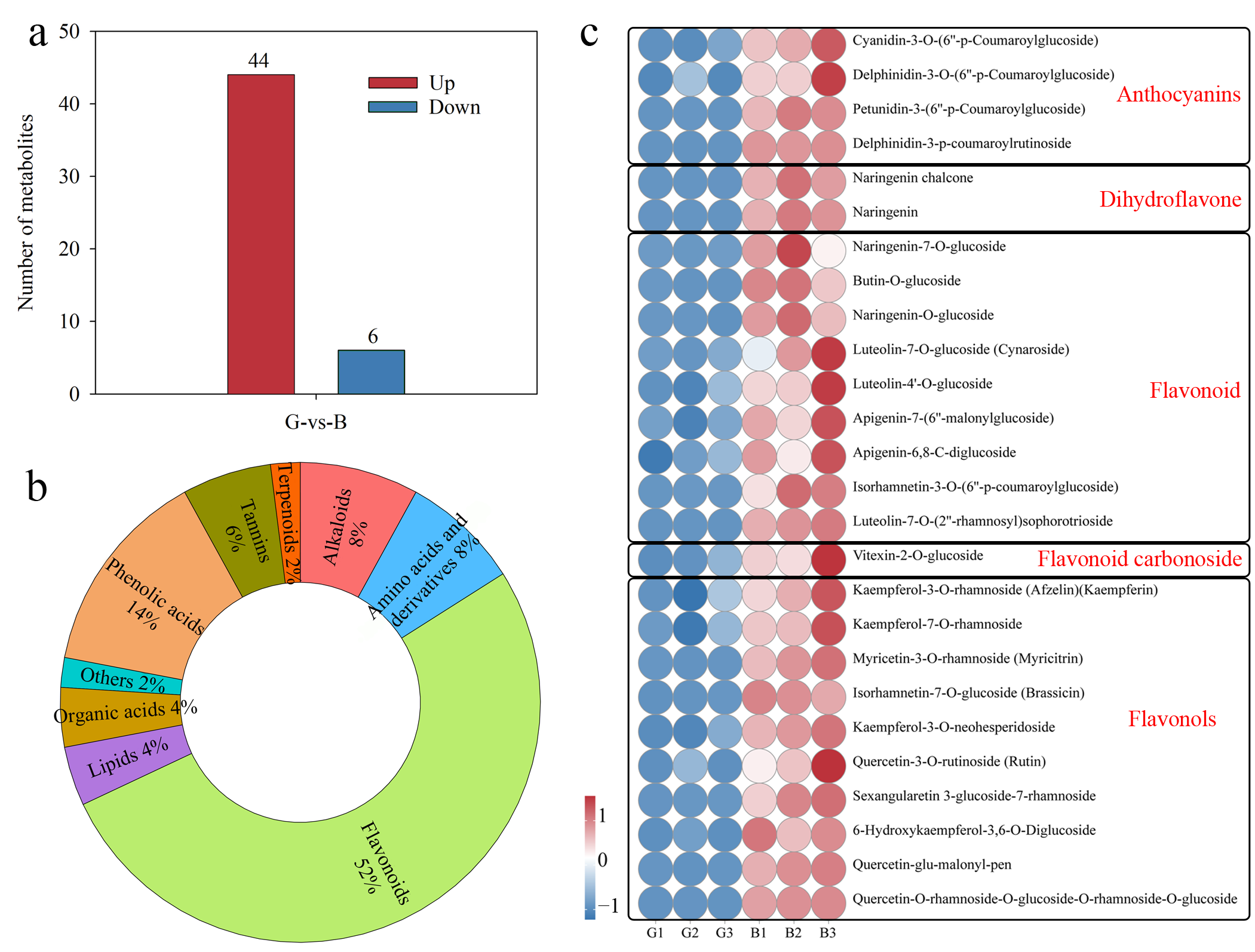
3.2. DAMs Between Two Different-Colored Pepper Peels
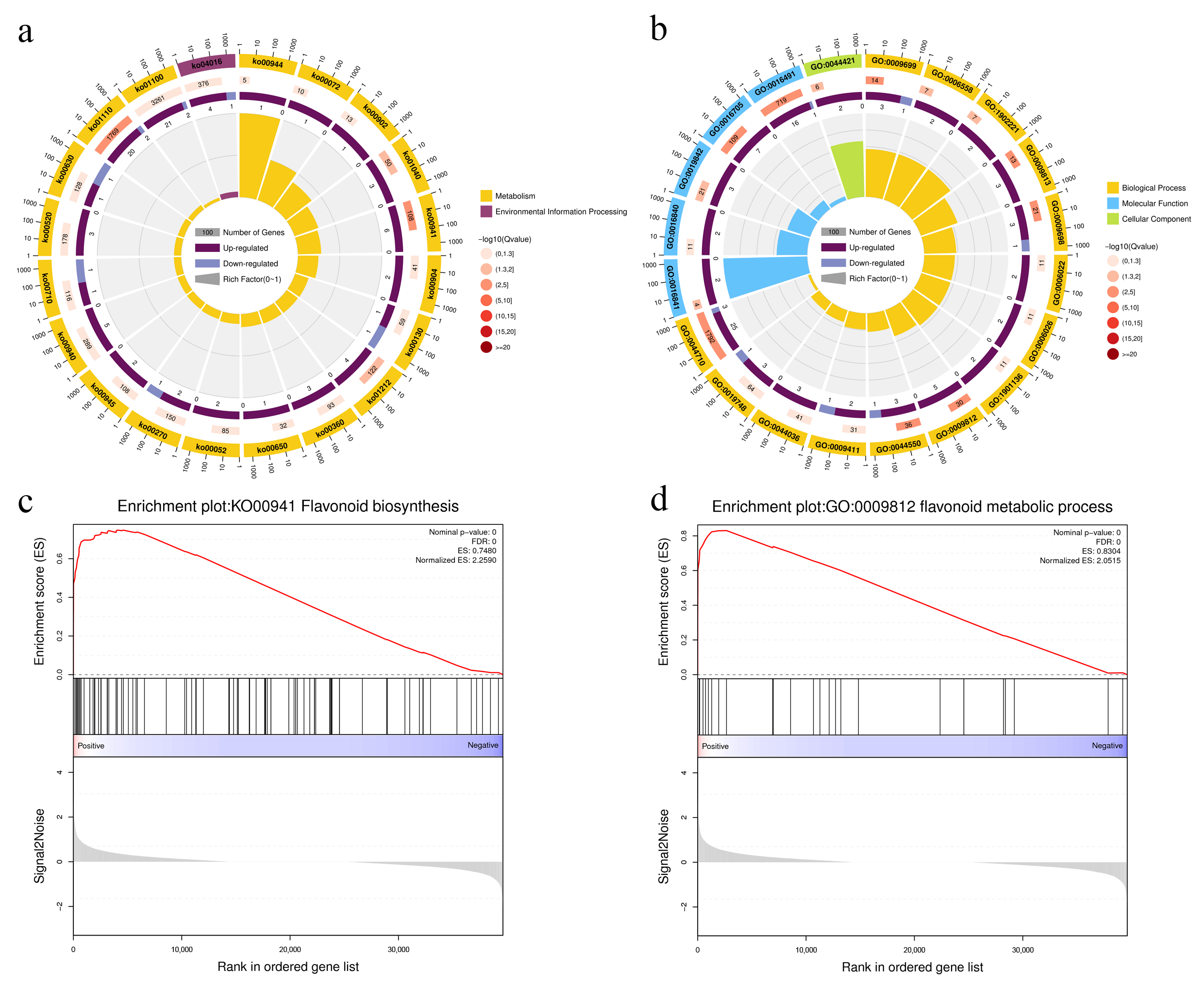
3.3. Analysis of Differentially Expressed Genes Between the Two Different-Colored Pepper Fruit Peels
3.4. Functional Analysis of DEGs of Two Different-Colored Peppers Fruit Peels
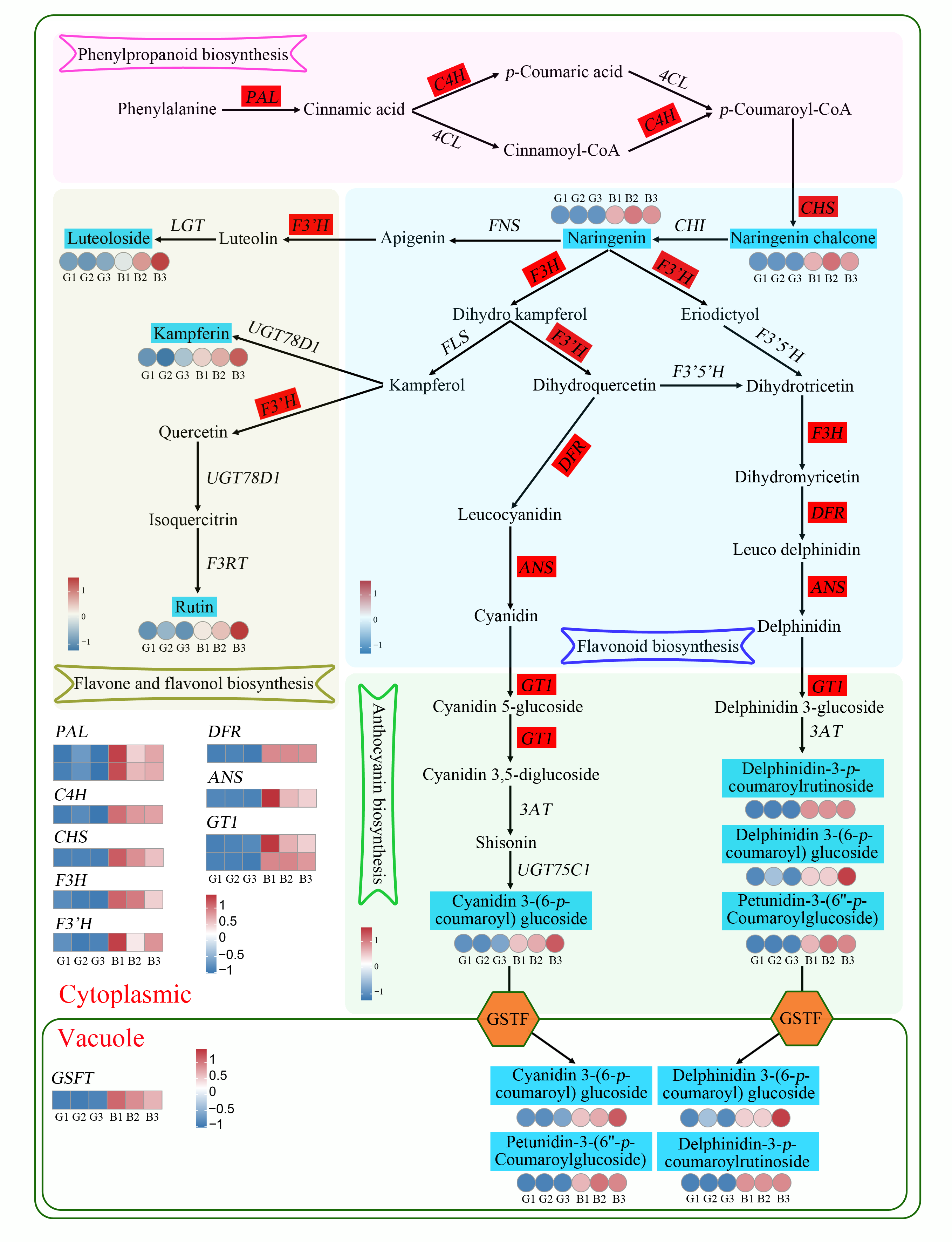
3.5. Transcriptome and Metabolome Correlation Analysis in Anthocyanin Biosynthesis Process
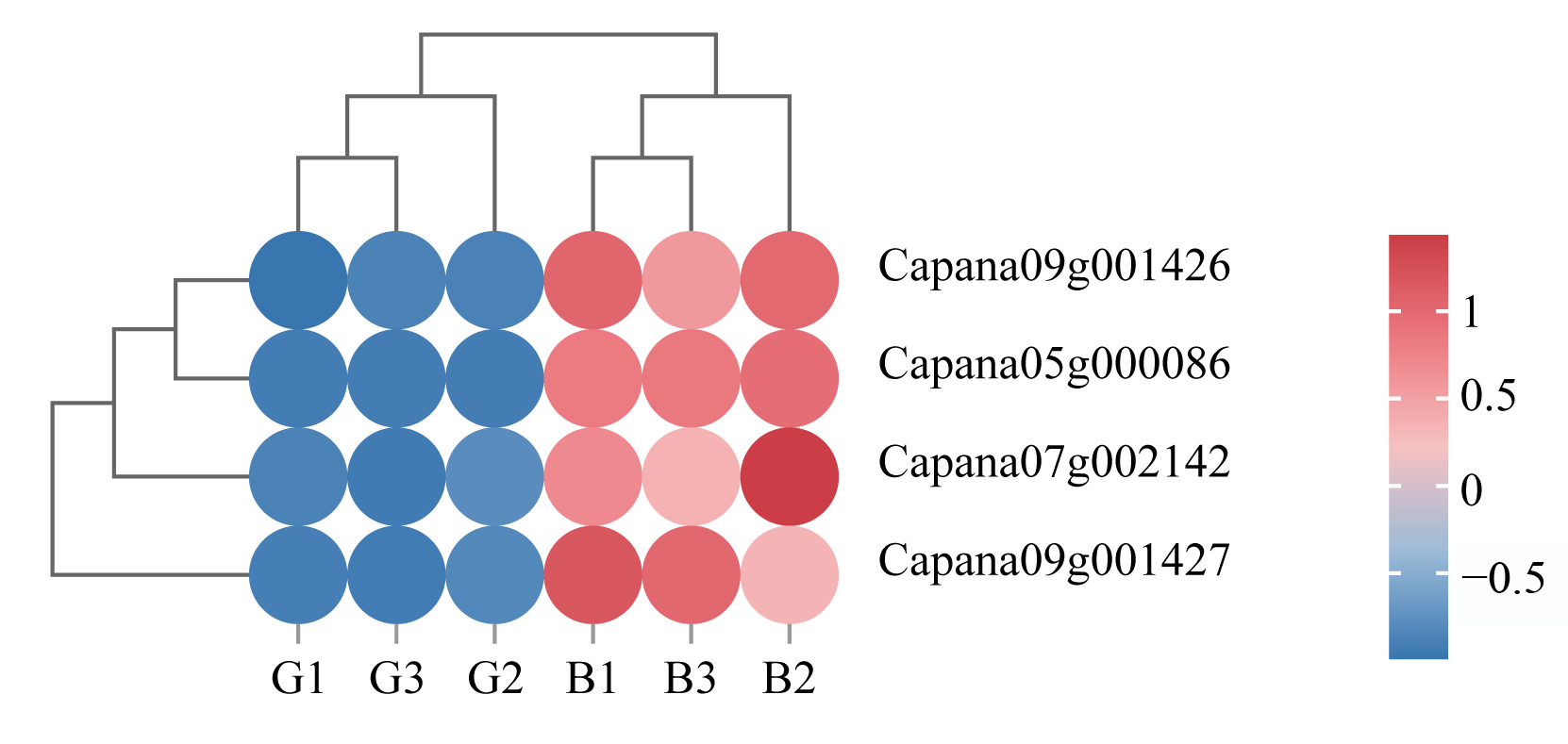
3.6. Correlation Analysis of Transcription Factors Related to Anthocyanin Synthesis with Differentially Accumulated Metabolites and Differentially Expressed Genes
3.7. qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VIP | variable importance in projection |
| QC | quality control |
| ROS | reactive oxygen species |
| PAL | Phenylalanine ammonia lyase |
| C4H | cinnamic acid hydroxylase |
| 4CL | coumarin CoA ligase |
| CHS | chalcone synthase |
| CHI | chalcone isomerase |
| F3H | flavonoid 3-hydroxylase, |
| F3′H | flavonoid 3′-hydroxylase |
| F3,5′H | flavonoid 3′-hydroxylase |
| DFR | dihydroflavonol 4-reductase |
| ANS | anthocyanin synthase |
| UGT (GT1) | UDP glucosyltransferase |
| GSTF | glutathione S-transferase |
| TIC | total ion chromatogram |
| GSEA | gene set enrichment analysis |
| OPLS-DA | Orthogonal partial least squares—discriminant analysis |
| PCA | PCA |
References
- Gu, L.B.; Pang, H.L.; Lu, K.K.; Liu, H.M.; Wang, X.D.; Qin, G.Y. Process optimization and characterization of fragrant oil from red pepper (Capsicum annuum L.) seed extracted by subcritical butane extraction. J. Sci. Food Agric. 2017, 97, 1894–1903. [Google Scholar] [CrossRef]
- Rahmani, F.; Kouki, H.; Hamdi, M.; Bouazizi, S.; Khammassi, M.A.; Zernadji, W.; Bulka, S.; Gryczka, U.; Hmaied, F. Implementation of low energy e-beam for dried vegetable treatment: Effect on microbial and physicochemical qualities of dried red chili pepper (Capsicum annuum L.). Innov. Food Sci. Emerg. 2025, 101, 103954. [Google Scholar] [CrossRef]
- Batiha, G.E.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Huei, C.S.; Azlan, A.; Ismail, A.; Shafie, N.H.; Sultana, S. Antioxidant and anti-obesity properties of local chilies varieties in Malaysia. J. Food Sci. Technol. 2020, 10, 3677–3687. [Google Scholar] [CrossRef]
- Wu, D.; Duan, R.; Tang, L.; Zhou, D.; Zeng, Z.; Wu, W.; Hu, J.; Sun, Q. In-vitro binding analysis and inhibitory effect of capsaicin on lipase. LWT 2022, 154, 112674. [Google Scholar] [CrossRef]
- Sanatombi, K. Antioxidant potential and factors influencing the content of antioxidant compounds of pepper: A review with current knowledge. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3011–3052. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, C.; Jiang, W.; Yang, W.; Qin, Q.; Tan, Q.; Lian, B.; Liang, Z.; Wei, C. Capsaicin inhibits proliferation and induces apoptosis in breast cancer by down-regulating FBI-1-mediated NF-κB pathway. Drug. Des. Dev. Ther. 2021, 15, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Que, T.; Ren, B.; Fan, Y.; Liu, T.; Hou, T.; Dan, W.; Liu, B.; Wei, Y.; Lei, Y.; Zeng, J.; et al. Capsaicin inhibits the migration, invasion and EMT of renal cancer cells by inducing AMPK/mTOR-mediated autophagy. Chem. Biol. Interact 2022, 366, 110043. [Google Scholar] [CrossRef]
- Brudzyńska, P.; Sionkowska, A.; Grisel, M. Plant-derived colorants for food, cosmetic and textile industries: A review. Materials 2021, 14, 3484. [Google Scholar] [CrossRef]
- Mate, D.; Ramlunalanaglu, K.; Pimple, B.; Vadge, S.; Kuchekar, M.; Nipate, S.; Tare, M.; Baheti, D. Comparative evaluation of lipsticks developed using isolated pigments of Capsicum annuum and Lycopersicon esculentum fruits and various natural bases. Int. J. Exp. Res. Rev. 2023, 30, 352–358. [Google Scholar] [CrossRef]
- Guo, Y.; Bai, J.; Duan, X.; Wang, J. Accumulation characteristics of carotenoids and adaptive fruit color variation in ornamental pepper. Sci. Hortic. 2021, 275, 109699. [Google Scholar] [CrossRef]
- Wang, J.; Duan, X.; An, Y.; He, J.; Li, J.; Xian, J.; Zhou, D. An Analysis of capsaicin, dihydrocapsaicin, Vitamin C and flavones in different tissues during the development of ornamental pepper. Plants 2024, 13, 2038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, Y.; Liu, J.; Ma, R.; Miao, Y.; Chen, W.; Zheng, J.; Pang, X.; Wan, H. Pigment biosynthesis and molecular genetics of fruit color in pepper. Plants 2023, 12, 2156. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, M.H.; Han, Y.S. Physicochemical properties and antioxidant activity of colored peppers (Capsicum annuum L.). Food Sci. Biotech. 2023, 32, 209–219. [Google Scholar] [CrossRef]
- Gómez-García, M.D.R.; Ochoa-Alejo, N. Biochemistry and molecular biology of carotenoid biosynthesis in Chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xu, Z.; Wu, C.T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, S98–S102. [Google Scholar] [CrossRef]
- Shifriss, C.; Pilovsky, M. Studies of the inheritance of mature fruit color in Capsicum annuum L. Euphytica 1992, 60, 123–126. Euphytica 1992, 60, 123–126. [Google Scholar] [CrossRef]
- Kayesh, E.; Shangguan, L.; Korir, N.K.; Sun, X.; Bilkish, N.; Zhang, Y.; Han, J.; Song, C.; Cheng, Z.; Fang, J. Fruit skin color and the role of anthocyanin. Acta Physiol. Plantar. 2013, 35, 2879–2890. [Google Scholar] [CrossRef]
- Passeri, V.; Koes, R.; Quattrocchio, F.M. New challenges for the design of high value plant products: Stabilization of anthocyanins in plant vacuoles. Front. Plant Sci. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Harborne, J.B. Correlations between anthocyanin type, pollinator and flower colour in the Labiatae. Phytochemistry 1992, 31, 3009–3015. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Arista, M.; Buide, M.L.; Ortiz, P.L. Major flower pigments originate different colour signals to pollinators. Front. Ecol. Evol. 2021, 9, 743850. [Google Scholar] [CrossRef]
- Nevo, O.; Valenta, K.; Razafimandimby, D.; Melin, A.D.; Ayasse, M.; Chapman, C.A. Frugivores and the evolution of fruit colour. Biol. Lett. 2018, 14, 20180377. [Google Scholar] [CrossRef]
- Martin-Eberhardt, S.A.; Weber, M.G.; Gilbert, K.J. Anthocyanin impacts multiple plant-insect interactions in a carnivorous plant. Amer. Natural. 2025, 205, 502–515. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins are key regulators of drought stress tolerance in tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, S.; Yang, X.; Wang, L.; Pan, R.; Zhang, W. Evolutionary analysis of anthocyanin biosynthetic genes: Insights into abiotic stress adaptation. Plant Mol. Biol. 2025, 115, 6. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, R.; Li, Y.; Dai, W.; Shang, J.; He, Y.; Xiang, F.; Yang, Y.; Wang, J.; Huang, Z.; et al. Anthocyanin biosynthesis and transport synergistically modulated by RcMYB75 and RcGSTFL11 play a pivotal role in the feedforward loop in response to drought stress. Plant J. 2025, 121, e17240. [Google Scholar] [CrossRef]
- Li, C.; Du, J.; Xu, H.; Feng, Z.; Chater, C.C.; Duan, Y.; Yang, Y.; Sun, X. UVR8-TCP4-LOX2 module regulates UV-B tolerance in Arabidopsis. J. Integ. Plant Biol. 2024, 66, 897–908. [Google Scholar] [CrossRef]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of anthocyanins on vascular health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Zhou, H.; Liu, Y.; Pang, W.; Ma, T.; Niu, C.; Yang, Z.; Chang, A.K.; Li, X.; et al. Sirtuin1 (SIRT1) is involved in the anticancer effect of black raspberry anthocyanins in colorectal cancer. Eur. J. Nutr. 2023, 62, 395–406. [Google Scholar] [CrossRef]
- Szot, I.; Łysiak, G.P.; Sosnowska, B.; Chojdak-Łukasiewicz, J. Health-promoting properties of anthocyanins from cornelian cherry (Cornus mas L.) fruits. Molecules 2024, 29, 449. [Google Scholar] [CrossRef]
- Abdelrahman, K.N.; Ghany, A.G.A.A.; Saber, R.A.; Osman, A.; Sitohy, B.; Sitohy, M. Anthocyanins from pomegranate peel (Punica granatum), chili pepper fruit (Capsicum annuum), and bougainvillea flowers (Bougainvillea spectabilis) with multiple biofunctions: Antibacterial, antioxidant, and anticancer. Heliyon 2024, 10. [Google Scholar] [CrossRef]
- Niu, B.; Gao, W.; Li, F.; Pei, Z.; Wang, H.; Tian, F.; Zhao, J.; Lu, W. Enhancing colonic health with encapsulated grape seed anthocyanins: Oral capsule for Colon-targeted delivery. Food Chem. 2025, 469, 142544. [Google Scholar] [CrossRef]
- Tang, B.; Li, L.; Hu, Z.; Chen, Y.; Tan, T.; Jia, Y.; Xie, Q.; Chen, G. Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in purple pepper. J. Agric. Food Chem. 2020, 68, 12152–12163. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, H.; Fan, Y.; Yan, L. Anthocyanins accumulation analysis of correlated genes by metabolome and transcriptome in green and purple peppers (Capsicum annuum). BMC Plant Biol. 2022, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Xu, R.R.; Wang, X.N.; Zhang, X.W.; You, C.X.; Han, Y. MdbHLH162 connects the gibberellin and jasmonic acid signals to regulate anthocyanin biosynthesis in apple. J. Integrat. Plant Biol. 2024, 66, 265–284. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, P.; Kumar, A.; Chawla, N.; Dhatt, A.S. Multifaceted regulation of anthocyanin biosynthesis in plants: A comprehensive review. J. Plant Growth Regul. 2024, 43, 3048–3062. [Google Scholar] [CrossRef]
- Shao, D.; Li, Y.; Zhu, Q.; Zhang, X.; Liu, F.; Xue, F.; Sun, J. GhGSTF12, a glutathione S-transferase gene, is essential for anthocyanin accumulation in cotton (Gossypium hirsutum L.). Plant Sci. 2021, 305, 110827. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Liu, T.; Zhao, Y.; Zhao, X.; Liu, J.; Zhang, J. Functional analysis of the anthocyanin-associated glutathione s-transferase gene StGST1 in potato. Potato Res. 2023, 66, 215–230. [Google Scholar] [CrossRef]
- Aza-González, C.; Herrera-Isidrón, L.; Núñez-Palenius, H.G.; Martínez De La Vega, O.; Ochoa-Alejo, N. Anthocyanin accumulation and expression analysis of biosynthesis-related genes during chili pepper fruit development. Biol. Plant. 2013, 57, 49–55. [Google Scholar] [CrossRef]
- Zhou, Y.; Mumtaz, M.A.; Zhang, Y.; Shu, H.; Hao, Y.; Lu, X.; Cheng, S.; Zhu, G.; Wang, Z. Response of anthocyanin accumulation in pepper (Capsicum annuum) fruit to light days. Int. J. Mol. Sci. 2022, 23, 8357. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Wang, H.; Zhang, Y.; Li, W.; Liu, J.; Cheng, Q.; Sun, L.; Shen, H. Identification of the regulatory genes of UV-B-induced anthocyanin biosynthesis in pepper fruit. Int. J. Mol. Sci. 2022, 23, 1960. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Sun, Y.; Shen, Y.; Mao, L.; Dai, Y.; Yang, B.; Liu, Z. Integrated transcriptome and metabolome analysis reveals anthocyanin biosynthesis mechanisms in pepper (Capsicum annuum L.) leaves under continuous blue light irradiation. BMC Plant Biol. 2024, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Mu, H.; Chen, J.; Huang, W.; Huang, G.; Deng, M.; Hong, S.; Ai, P.; Gao, C.; Zhou, H. OmicShare tools: A zero-code interactive online platform for biological data analysis and visualization. Imeta 2024, 3, e228. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, L.; Tan, H.; Liu, J.; Qiu, Q.; Sun, Q.; Ouyang, L.; Han, H.; He, Q. Transcriptome and Physiological Analyses of Resistant and Susceptible Pepper (Capsicum annuum) to Verticillium dahliae Inoculum. Horticulturae 2024, 10, 1160. [Google Scholar] [CrossRef]
- Sunil, L.; Shetty, N.P. Biosynthesis and regulation of anthocyanin pathway genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, H.; Xu, J.; Zheng, P.; Liu, S.; Sun, B. CsHY5 Regulates Light-Induced Anthocyanin Accumulation in Camellia sinensis. Int. J. Mol. Sci. 2025, 26, 3253. [Google Scholar] [CrossRef]
- LaFountain, A.M.; Yuan, Y.W. Repressors of anthocyanin biosynthesis. New Phytol. 2021, 231, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, H.; Shi, J.; Duan, Y.; Wu, W.; Lyu, L.; Li, W. Effects of different light wavelengths on fruit quality and gene expression of anthocyanin biosynthesis in blueberry (Vaccinium corymbosm). Cells 2023, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; An, H.; Zhang, X.; Zhou, B. Integrated transcriptome and metabolome analysis reveals the anthocyanin biosynthesis mechanisms in blueberry (Vaccinium corymbosum L.) leaves under different light qualities. Front. Plant Sci. 2022, 13, 1073332. [Google Scholar] [CrossRef]
- Samkumar, A.; Jones, D.; Karppinen, K.; Dare, A.P.; Sipari, N.; Espley, R.V.; Martinussen, I.; Jaakola, L. Red and blue light treatments of ripening bilberry fruits reveal differences in signalling through abscisic acid-regulated anthocyanin biosynthesis. Plant Cell Environ. 2021, 44, 3227–3245. [Google Scholar] [CrossRef] [PubMed]
- Satitmunnaithum, J.; Abe, I.; Mitsuzuka, R.; Onozawa, T.; Ikeda, T. Night blue-light radiation enhances anthocyanin production in purple paprika ‘Tequila’ by increasing its structural-gene expression during fruit growth. J. Am. Soc. Hortic. Sci 2024, 149, 294–301. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Qiu, H.; Chen, R.; Zhang, Z.; Xiong, A.; Xu, Z.; Chen, R.; Zhang, X.; Liu, X.; et al. Metabolomics and transcriptomics analyses provide insights into the dynamic of metabolites and the molecular mechanisms of natural pigment formation of different color-transition types of red pepper (Capsicum). Sci. Hortic. 2025, 342, 114062. [Google Scholar] [CrossRef]
- Gao, J.; Dou, Y.; Wang, X.; Zhang, D.; Wei, M.; Li, Y. Transcriptome analysis reveals the mechanism for blue-light–induced biosynthesis of delphinidin derivatives in harvested purple pepper fruit. Front. Plant Sci. 2023, 14, 1289120. [Google Scholar] [CrossRef]
- Wang, X.; Luo, S.; Li, Q.; Song, L.; Zhang, W.; Yu, P.; Xuan, S.; Wang, Y.; Zhao, J.; Chen, X.; et al. Delphinidins and naringenin chalcone underlying the fruit color changes during maturity stages in eggplant. Agronomy 2022, 12, 1036. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in develoment strawberry fruit. J. Am. Soc. Hortic. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Saengnil, K.; Lueangprasert, K.; Uthaibutra, J. Sunlight-stimulated phenylalanine ammonia-lyase (PAL) activity and anthocyanin accumulation in exocarp of ‘Mahajanaka’ mango. Maejo Int. J. Sci. Technol. 2011, 5, 365. [Google Scholar]
- Chen, X.; Wang, P.; Gu, M.; Hou, B.; Zhang, C.; Zheng, Y.; Sun, Y.; Jin, S.; Ye, N. Identification of PAL genes related to anthocyanin synthesis in tea plants and its correlation with anthocyanin content. Hortic. Plant J. 2022, 8, 381–394. [Google Scholar] [CrossRef]
- Shan, B.; Mo, J.; Yang, J.; Qin, X.; Yu, H. Cloning and functional characterization of a cinnamate 4-hydroxylase gene from the hornwort Anthoceros angustus. Plant Sci. 2024, 341, 111989. [Google Scholar] [CrossRef]
- Kuang, L.; Chen, J.; Bao, X.; Zhang, D.; Liu, J.; Wang, W.; Wei, Y.; Zong, C. Environmental and phytohormonal factors regulating anthocyanin biosynthesis in fruits. Horticulturae 2025, 11, 681. [Google Scholar] [CrossRef]
- Fu, D.; Qi, J.; Su, L.; Wang, X.; Wang, M.; Chen, B.; Yu, X.; Zhao, X.; Gao, W.; Guo, X.; et al. Chalcone synthase 2 (BpCHS2), a structural gene, was activated by low temperature to promote anthocyanin synthesis in Broussonetia papyrifera to improve its cold tolerance. Plant Physiol. Biochem. 2025, 222, 109656. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lai, G.; Lin, J.; Guo, A.; Yang, F.; Pan, R.; Che, J.; Lai, C. VdCHS2 Overexpression Enhances Anthocyanin Biosynthesis, Modulates the Composition Ratio, and Increases Antioxidant Activity in Vitis davidii Cells. Antioxidants 2024, 13, 1472. [Google Scholar] [CrossRef]
- Li, R.; Guo, S.; Hao, S.; Du, S.; Zhang, J.; Liu, R.; Li, J.; Zhang, Y.; Cheng, C. Characterization of the anthocyanin-related flavanone 3-hydroxylase (F3H) genes and functional analysis of VcF3H2 in blueberry. Fruit Res. 2025, 5, e022. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Yang, K.; Wu, H.; Chen, H.; Wu, Q.; Zhao, H. Tartary buckwheat FtF3′H1 as a metabolic branch switch to increase anthocyanin content in transgenic plant. Front. Plant Sci. 2022, 13, 959698. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, H.J.; Kim, J.H.; Kim, D.H.; Kim, H.K.; Cho, Y.G.; Bae, S.; Kang, K.K. CRISPR/Cas9-targeted mutagenesis of F3′ H, DFR and LDOX, genes related to anthocyanin biosynthesis in black rice (Oryza sativa L.). Plant Biotechnol. Rep. 2019, 13, 521–531. [Google Scholar] [CrossRef]
- Karamat, U.; Guo, J.; Jiang, S.; Khan, I.; Lu, M.; Li, G.; Fu, M. Integrated omics and functional insights into BjMYB90-mediated regulation of BjGSTF12 for enhanced anthocyanin biosynthesis in mustard (Brassica juncea). Plant Cell Rep. 2025, 44, 166. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef]
- Luo, H.; Dai, C.; Li, Y.; Feng, J.; Liu, Z.; Kang, C. Reduced Anthocyanins in petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry. J. Exp. Bot. 2018, 69, 2595–2608. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cao, H.; Pan, L.; Niu, L.; Wei, B.; Cui, G.; Wang, L.; Yao, J.; Zeng, W.; Wang, Z. Two loss-of-function alleles of the glutathione S-transferase (GST) gene cause anthocyanin deficiency in flower and fruit skin of peach (Prunus persica). Plant J. 2021, 107, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.W.; Wang, C.K.; Huang, X.Y.; Hu, D.G. Genome-wide analysis of the glutathione S-transferase (GST) genes and functional identification of MdGSTU12 reveals the involvement in the regulation of anthocyanin accumulation in apple. Genes 2021, 12, 1733. [Google Scholar] [CrossRef]
- Li, S.; Zuo, D.; Cheng, H.; Ali, M.; Wu, C.; Ashraf, J.; Zhang, Y.; Feng, X.; Lin, Z.; Wang, Q.; et al. Glutathione S-transferases GhGSTF1 and GhGSTF2 involved in the anthocyanin accumulation in Gossypium hirsutum L. Int. J. Biol. Macromol. 2020, 165, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, H.; Sun, Y.; Zhang, P.; Li, L.; Luo, D.; Wu, Z. Identification of the GST gene family and functional analysis of RcGSTF2 related to anthocyanin in Rosa chinensis ‘Old Blush’. Plants 2025, 14, 932. [Google Scholar] [CrossRef]
- Yang, S.; Liu, M.; Zhao, C.; Wang, R.; Xue, L.; Lei, J. A novel bHLH transcription factor, FabHLH110, is involved in regulation of anthocyanin synthesis in petals of pink-flowered strawberry. Plant Physiol. Biochem. 2025, 222, 109713. [Google Scholar] [CrossRef]
- Meng, J.; Yin, J.; Wang, H.; Li, H. A TCP transcription factor in Malus halliana, MhTCP4, positively regulates Anthocyanins biosynthesis. Int. J. Mol. Sci. 2022, 23, 9051. [Google Scholar] [CrossRef]
- Tao, R.; Trivedi, I.; Trimborn, L.; Ponnu, J.; Tóth, B.V.; Hoecker, U. TCP3 is a substrate of the COP1/SPA ubiquitin ligase to regulate anthocyanin accumulation and flowering time in Arabidopsis. Proc. Natl. Acad. Sci. USA 2025, 122, e2426423122. [Google Scholar] [CrossRef]
- Bian, X.-H.; Li, W.; Niu, C.-F.; Wei, W.; Hu, Y.; Han, J.-Q.; Lu, X.; Tao, J.-J.; Jin, M.; Qin, H.; et al. A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol. 2020, 225, 268–283. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; He, L.; Feng, Z.; Xiao, Y.; Qiu, Q.; Liu, J.; Han, H.; Huang, X. Metabolome and Transcriptome Analyses of the Molecular Mechanism Underlying Light-Induced Anthocyanin Accumulation in Pepper (Capsicum annuum L.) Peel. Curr. Issues Mol. Biol. 2025, 47, 774. https://doi.org/10.3390/cimb47090774
He Q, He L, Feng Z, Xiao Y, Qiu Q, Liu J, Han H, Huang X. Metabolome and Transcriptome Analyses of the Molecular Mechanism Underlying Light-Induced Anthocyanin Accumulation in Pepper (Capsicum annuum L.) Peel. Current Issues in Molecular Biology. 2025; 47(9):774. https://doi.org/10.3390/cimb47090774
Chicago/Turabian StyleHe, Qinqin, Liming He, Zongqin Feng, Yunyi Xiao, Qiucheng Qiu, Jiefeng Liu, Hanbing Han, and Xinmin Huang. 2025. "Metabolome and Transcriptome Analyses of the Molecular Mechanism Underlying Light-Induced Anthocyanin Accumulation in Pepper (Capsicum annuum L.) Peel" Current Issues in Molecular Biology 47, no. 9: 774. https://doi.org/10.3390/cimb47090774
APA StyleHe, Q., He, L., Feng, Z., Xiao, Y., Qiu, Q., Liu, J., Han, H., & Huang, X. (2025). Metabolome and Transcriptome Analyses of the Molecular Mechanism Underlying Light-Induced Anthocyanin Accumulation in Pepper (Capsicum annuum L.) Peel. Current Issues in Molecular Biology, 47(9), 774. https://doi.org/10.3390/cimb47090774





