Beyond Purification: Evolving Roles of Fusion Tags in Biotechnology
Abstract
1. Introduction
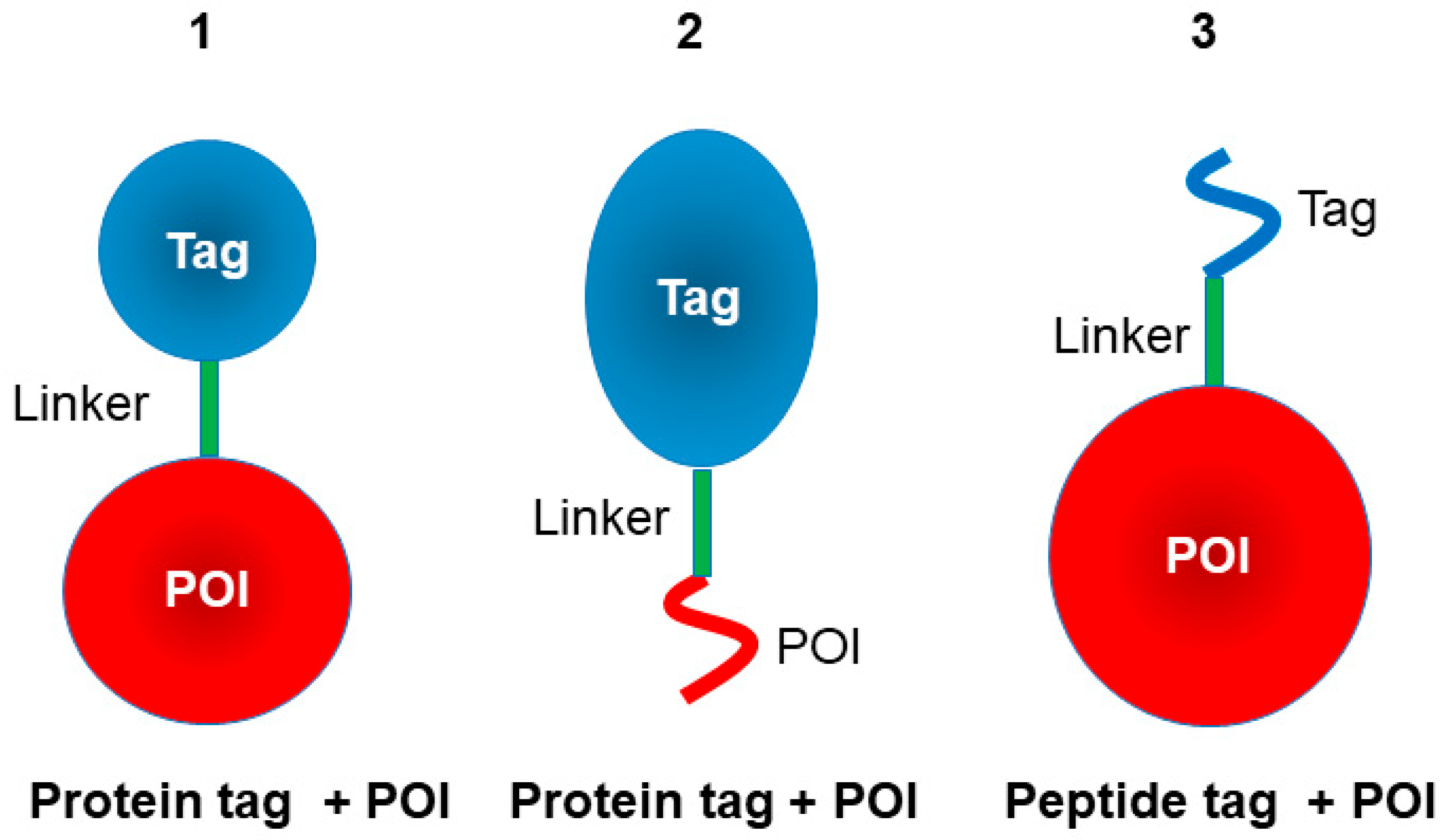
2. Protein Tag
2.1. Green Fluorescent Protein (GFP)
2.2. Thioredoxin (Trx)
| Protein Tag | Size | Main Uses | Oligomerization | Advantages (Pros) | Limitations (Cons) | Ref. |
|---|---|---|---|---|---|---|
| GFP | 27 kDa | Detection, solubility | N | Direct fluorescence monitoring; stabilizes fusion proteins | Moderate (~27 kDa); may affect folding/function | [21,22,23,24,25] |
| Trx | 12 kDa | Solubility, folding | N | Enhances folding in E. coli; improves solubility | Limited purification use; may require removal | [39,40,45] |
| MBP | 42.5 kDa | Solubility, purification | N | Strong solubility enhancer; affinity purification on amylose resin | Large (~42 kDa); may alter activity | [47,48,49,50,51,52,53,54,55] |
| NusA | 55 kDa | Solubility | N | Very strong solubility enhancer for insoluble proteins | Very large (~55 kDa); usually removed | [56,57,58,59] |
| HSA | 66 kDa | Stability, half-life extension | N | Extends serum half-life; enhances solubility; clinically validated | Large (~66 kDa); may interfere with activity | [60,61,62,63,64,65] |
| SUMO | 11 kDa | Solubility, cleavage | N | Enhances folding/solubility; precise cleavage by SUMO protease | Requires SUMO protease; adds extra step | [66,67,68,69,70,71,72] |
| BLA | 29 kDa | Solubility, stability | N | Highly stable under extreme/halophilic conditions | Not widely adopted; limited affinity purification | [73,74,75,76,77] |
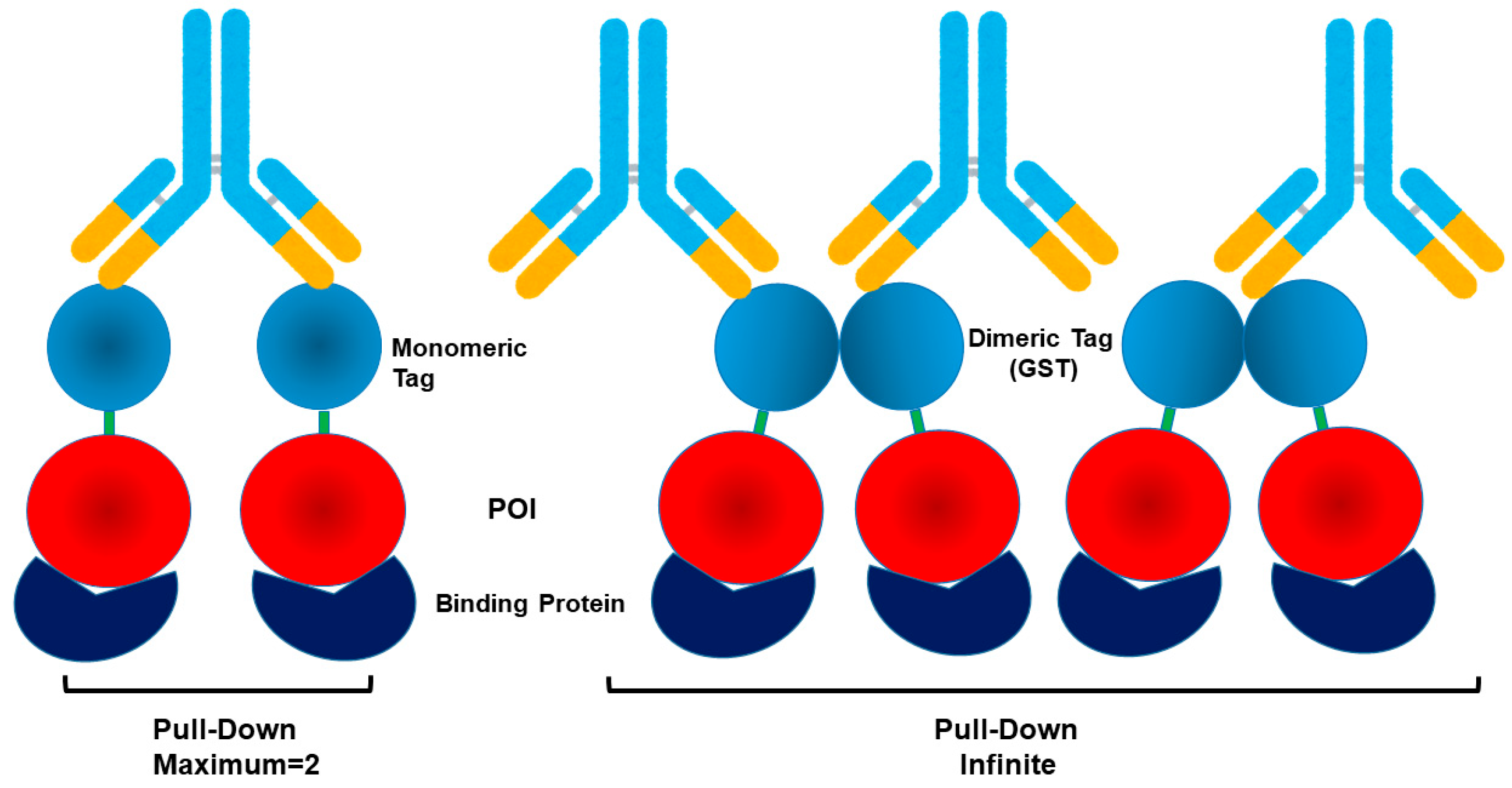
| GST | 26 kDa (mono) 52 kDa (dimer) | Purification, solubility, IP | Dimer | Affinity purification with glutathione resin; moderate solubility enhancer | Dimerization (26 kDa × 2); may alter activity and lead to false-positive in IP | [78,79,80,81,82,83,84,85,86,87,88] |
| Fc | 25 kDa (mono), 50 kDa (dimer) | Purification, stability | Dimer | Protein A/G affinity purification; increases stability and half-life, Fc receptor binding | Large (~25 kDa/chain); promotes dimerization | [89,90,91,92,93,94,95,96,97] |
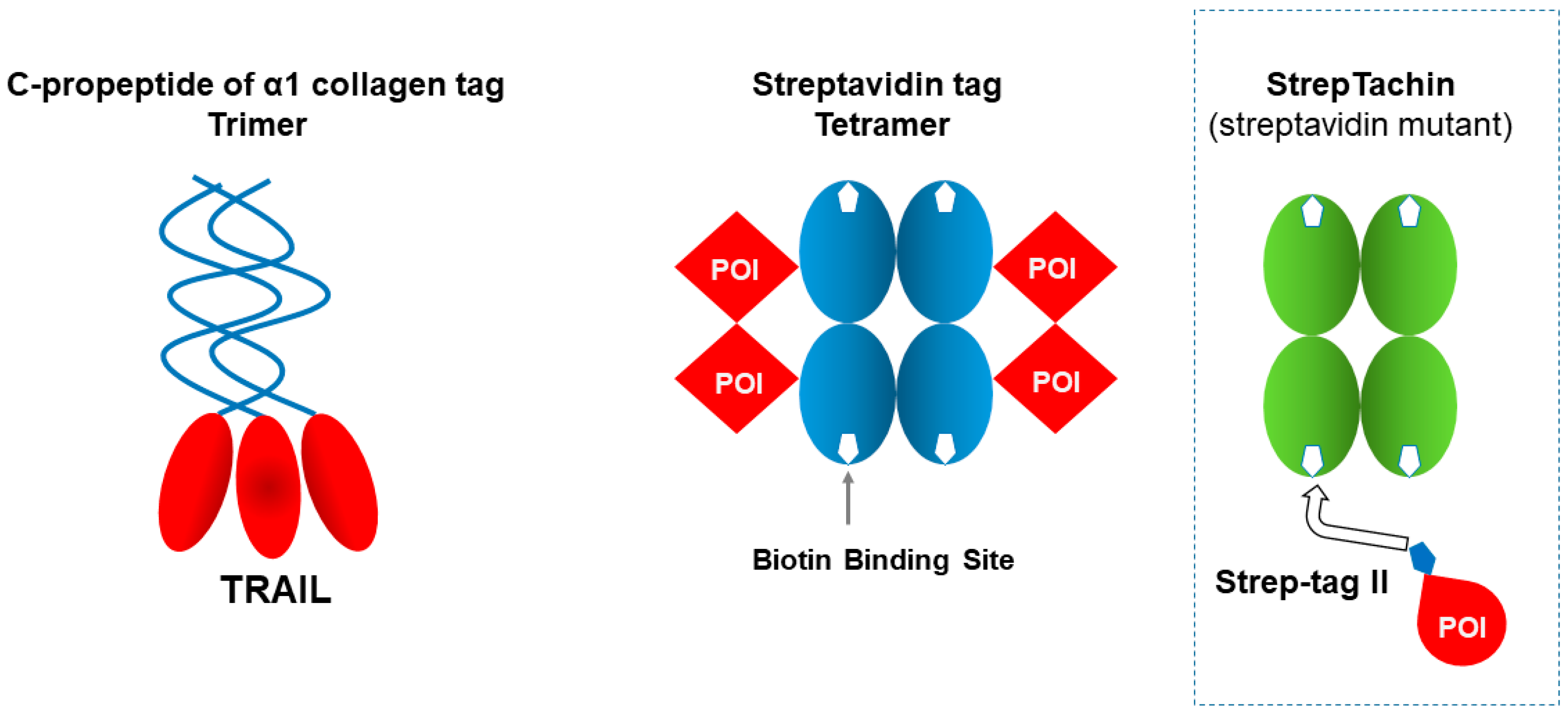
| C-prpeptide of α1 collagen | 34 kDa | Folding, stability | Trimer | Enhanced activity (e.g., TRAIL) | Large domain; mainly suited for limited target | [98,99] |
| Streptavidin | 13 kDa (mono), 52 kDa (tetramer) | Immobilization, purification | Tetramer | Extremely high biotin affinity; useful for fusion constructs (e.g., VHH-Streptavidin for column binding) | Tetramerization may affect fusion partner; very strong binding can hinder elution | [100,101,102] |
2.3. Maltose-Binding Protein (MBP)
2.4. NusA
2.5. Human Serum Albumin (HSA)
2.6. SUMO
2.7. Halophilic β-Lactamase (BLA)
2.8. Dimeric Glutathione-S-Transferase (GST)
2.9. Dimeric Fc
2.10. Trimeric Human C-Propeptide of α1 Collagen
2.11. Tetrameric Streptavidin
3. Peptide Tags
3.1. His
3.2. Flag
3.3. Myc
3.4. PA
3.5. HA
3.6. V5
| Protein Tag | Sequence | Size (aa) | Main Uses | Ligand/ Antibody | Advantages (Pros) | Limitations (Cons) | Ref. |
|---|---|---|---|---|---|---|---|
| His | HHHHHH | 6.8 | Affinity purification + detection | Ni2+/Co2+-NTA resin, anti-His antibody | Highly standardized purification Can bind in the presence of denaturant | Non-specific binding, requires metal ions, can co-purify contaminants | [82,103,104] |
| Flag | DYKDDDDK | 8 | Affinity purification + detection | Anti-FLAG antibody, FLAG-beads | Small size, efficient immunoprecipitation and elution | Acidic elution may affect proteins | [105,106,107,108] |
| PA | GVAMPGAEDDVV | 12 | Affinity purification + detection | NZ-1 antibody | High-affinity antibody binding, efficient IP and purification | Less commonly used than FLAG/HA/Myc | [112,113] |
| Strept tag II | WSHPQFEK | 8 | Affinity purification + detection | Strep-Tactin resin | Mild elution, biotin-based binding, good for native proteins | Lower affinity than Twin-Strep | [121,122,123,124] |
| Twin-Strept tag | WSHPQFEK × 2 (linked) | 16 | High-efficiency purification + detection | Strep-Tactin XT resin | Much higher affinity than single Strep-tag, works well in mammalian systems | Larger size than single tags | [121,122,123,124] |
| Myc | EQKLISEEDL | 10 | Detection (WB, IF, IP) | Anti-Myc antibody | Widely used, multiple antibodies available | Poor for purification | [109,110,111] |
| HA | YPYDVPDYA | 9 | Detection (WB, IF, IP) | Anti-HA antibody | Excellent for immunostaining and localization | Not efficient for purification | [114,115,116,117] |
| VA (V5) | GKPIPNPLLGLDST | 14 | Detection (WB, IF) | Anti-V5 antibody | Affinity purification with glutathione resin; moderate solubility enhancer | Dimerization (26 kDa × 2) may alter activity and lead to false-positive in IP | [118,119,120] |
| Dimerization | EFLIVIKS | 7 | Dimerization | Not available | Protein A/G affinity purification; increases stability and half-life, Fc receptor binding | Large (~25 kDa/chain); promotes dimerization | [125,126,127] |
| Insoluble | GIFQINSRY GILQINSRW | 9.10 | Purification by precipitation | Not available | Enhanced activity (e.g., TRAIL) | Large domain; mainly suited for limited targets Requires solubilization for application | [128,129,130,131,132,133,134,135,136,137] |
3.7. Strep-Tag II or Twin-Strep-Tag
3.8. Dimerization Tag
3.9. Insoluble (Ins)
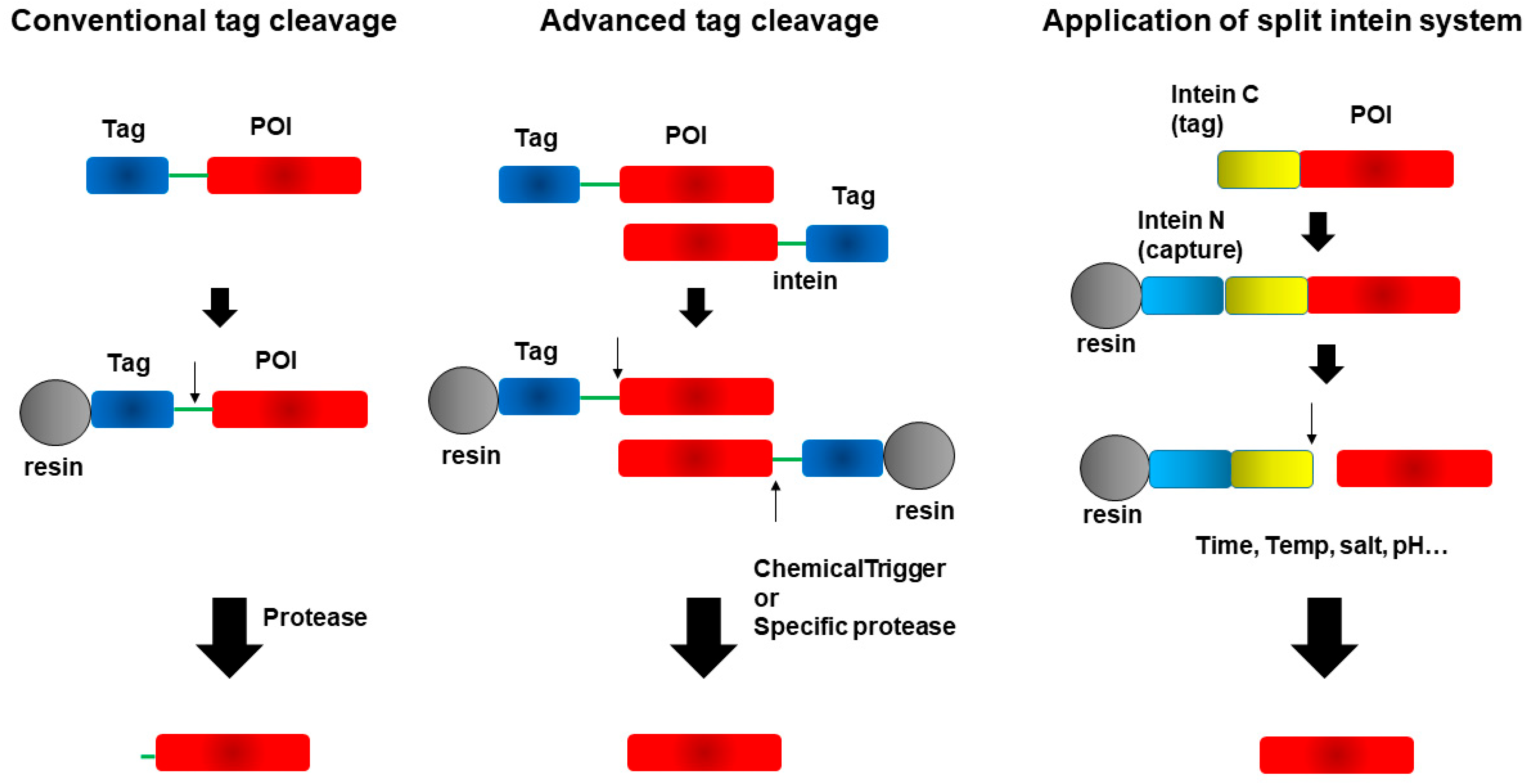
4. Cleavage of Fusion Tags from Target Proteins
4.1. Cleavage Enzymes Degrade Proteins Through Cleaving Peptide Bonds
| Cleavage Enzyme | Recognized Sequence | Ref. |
|---|---|---|
| Thrombin | Leu-Val-Pro-Arg↓Gly-Ser↓ | [141,142,143,144] |
| Factor Xa | Ile-(Glu or Asp)-Gly-Arg↓X | [141,145] |
| Enterokinase | Asp-Asp-Asp-Asp-Lys↓X | [146] |
| HRV 3C/PreScission Protease | Leu-Glu-Val-Leu-Phe-Gln↓Gly-Pro | [147] |
| TEV protease | Glu-Asn-Leu-Tyr-Phe-Gln↓Gly/Ser | [148] |
4.1.1. Thrombin
4.1.2. Factor Xa
4.1.3. Enterokinase
4.1.4. HRV 3C and PreScission Protease
4.1.5. Tobacco Etch Virus (TEV) Protease
4.2. Advances in Tagging Systems That Preserve Native Protein Sequences
4.2.1. eXact Tag and Profinity eXact Resin
4.2.2. SUMO Tag and Protease
4.2.3. CASPON Tag and cpCasp2 Protease
4.2.4. IMPACT
4.3. Novel Tagging and Purification Strategies Using the Splint Intein System
4.3.1. ProteinSelect Tag
4.3.2. iCap Tag
4.3.3. Gp41-1 Tag
4.3.4. Cfa DnaE Tag
4.3.5. Npu DnaE
4.3.6. Aes (Cysteine-Less Aes123 PolB1 Intein)
| System | Tag | Size | Main Uses (with Fusion/Supplier/Resin/Cleavage Info) | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Protease-based system | eXact-tag | 8 kDa | N-terminal fusion; Bio-Rad Laboratories; Profinity eXact resin; cleavage by NaF (requires halogen-free buffers) → Enhancing solubility and purification of expressed proteins | Stable expression in E. coli; self-cleavage leaves no extra residues | Requires specific cleavage conditions; commercial system dependent; binding and washing require halogen-free buffers (no Cl−, etc.) | [163,164] |
| SUMO-tag | 12 kDa | N-terminal fusion; Merck KGaA; SUMO-tag TRAP or His/GST resin (if fused); cleavage by SUMO protease (Ulp1) → Solubilization, increased expression, purification | Strong solubilization effect; SUMO protease provides precise cleavage without residual amino acids | Protease cost is high; may interfere with endogenous SUMO in eukaryotic systems | [167,168,169] | |
| CASPON-tag | 4.1 kDa | N-terminal fusion; Boehringer Ingelheim (patent); IMAC capture; cleavage by Caspase → Purification and high-purity protein production | Residue-free cleavage; compatible with His-tag purification | Dependent on CASPON protease; requires proprietary technology | [171,172,173] | |
| Intein-based system | IMPACT (Intein-Mediated Purification with an Affinity Chitin-binding Tag) | 27 kDa (CBD-tag) | N- or C-terminal fusion; New England BioLabs; chitin resin; cleavage by reducing reagent (DTT) → Purification of insoluble proteins (intein + CBD system) | Intein-mediated self-cleavage allows tag removal; suitable for large-scale purification | Cleavage efficiency depends on optimized conditions; incomplete cleavage possible | [174,175] |
| Split intein-based system | Protein select (modified Ssp DnaE) | 4 kDa | N-terminal fusion; Cytiva; Protein Select resin; natural on-column self-cleavage → Affinity capture and traceless purification | Self-cleaving tag enabling one-step capture and traceless release (no protease required); simplifies workflows | Proprietary system requiring specialized resin; tag cleavage is not time-controllable (autocleavage occurs spontaneously upon folding/binding); limited to N-terminal fusion; efficiency can vary with target folding | [184,185,186,187] |
| iCap | 4 kDa | N-terminal fusion; Protein Capture Science (Ohio Univ. tech); NpuN immobilized resin; cleavage triggered by pH shift (8.5 → 6.2) → Precise cleavage for native protein recovery | Protease-mediated exact cleavage without residual residues | Limited applicability; strongly condition dependent | [188,189] | |
| Modified Npu DNaE | 17.2 kDa | N-terminal fusion; Merck KGaA; NpuN immobilized resin; cleavage triggered by pH shift → Split intein-based protein ligation/editing | High-efficiency splicing and self-cleavage; seamless ligation | Complex system design; requires optimization in vitro | [179,196] | |
| Gp41-1 | 24.2 kDa | N- or C-terminal fusion; immobilized Gp41-1N resin; cleavage triggered by pH shift (9 → 7) → Split intein-mediated protein ligation/editing | Highly efficient; functions under broad conditions | Increases protein size; requires careful design | [190,191,192,193] | |
| Cfa | 17.2 kDa | C-terminal fusion; IMAC or chitin resin; cleavage triggered by reassociation of N/C intein fragments → Split intein-mediated protein ligation | Efficient, residue-free editing | Strongly condition-dependent; complex recombination construction | [194,195] |
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| POI | Protein of Interest |
| GFP | Green Fluorescent Protein |
| GST | Glutathione S Transferase |
| Trx | Thioredoxin |
| MBP | Maltose-Binding Protein |
| NusA | N-Utilization Substance A |
| HSA | Human Serum Albumin |
| SUMO | Small Ubiquitin-like Modifier |
| BLA | β-lactamase |
| Fc | (antibody) Fragment Crystallizable |
| TNF | Tumor Necrosis Factor |
| TRAIL | TNF-related Apoptosis-Inducing Ligand |
| scFv | Single-Chain Fab Fragment |
| SLG | Sodium Lauroyl Glutamate |
| TPO | Thrombopoietin |
| IMAC | Immobilized Metal Affinity Chromatography |
| CASPON | Caspase-Based Fusion Process |
| IMPACT | Intein-Mediated Purification with an Affinity Chitin-binding Tag |
| DTT | Dithiothreitol |
| HRV | Human Rhinovirus |
| TEV | Tobacco Etch Virus |
References
- Chou, C.P. Engineering cell physiology to enhance recombinant protein production in Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 76, 521–532. [Google Scholar] [CrossRef]
- Sahdev, S.; Khattar, S.K.; Saini, K.S. Production of active eukaryotic proteins through bacterial expression systems: A review of the extisting biotechnology strategies. Mol. Cell. Biochem. 2008, 307, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Yang, M.; Feng, X. Comparative efficacy and safety of rhTPO, romiplostim, and eltrombopag in the treatment of pediatric primary immune thrombocytopenia: A systematic review and network meta-analysis. Front. Immunol. 2025, 16, 1595774. [Google Scholar] [CrossRef]
- Almatar, E.; Alsharidah, S.; Hashem, O.A. Outcomes of recombinant activated factor VIIa (NovoSeven) therapy in glanzmann thromnasthenia: Two case reports. Blood Coagul. Fibrinolysis 2025, 36, 293–295. [Google Scholar] [CrossRef]
- Haugh, A.; Frigault, M.J. Cytokines and immune effector cell therapy. Best Pract. Res. Clin. Haematol. 2025, 38, 101628. [Google Scholar] [CrossRef]
- Xue, R.; Gong, X.; Huang, Y.; Zhong, W.; Jin, H.; Chen, Z.; Chen, Y.; Yan, S.; Hu, H.; Yuan, C.; et al. One-year outcomes after intravenous recombinant tissue plasminogen activator for ischemic stroke: A real-world study. CNS Neurosci. Ther. 2025, 31, e70543. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Liu, S.; Luo, J.; Chen, J.; Luo, H.; Wu, W.; Chen, X. High-yield biosynthesis process for producing insulin Lispro. Protein J. 2025, 44, 363–376. [Google Scholar] [CrossRef]
- Kim, M.; Langley, R.J.; Perry, J.K.; Wang, Y. Recovery of mouse growth hormone from E. coli inclusion bodies using a mild solubilization and repeated freeze-thaw approach. Mol. Biol. Rep. 2025, 52, 627. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, J.; Zhang, N.; Xu, H.; Yang, J.; Tao, R.; Jiang, J. Efficient production of α-L-rhamnosidase podoRha through multi-strategy synergistic regulation involving molecular chaperones, osmolytes, and high-density fermentation management in Escherichia coli, and its application in isoquercitrin production via resting cell transformation. Int. J. Biol. Macromol. 2025, 320, 145755. [Google Scholar] [CrossRef]
- Mital, S.; Christie, G.; Alcasabas, A.; Mellor, R.; Dikicioglu, D. pH-modulated soluble expression of alcohol dehydrogenase in Escherichai coli using adaptive laboratory evolution. Trends Biotechnol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Rüdt, M.; Bussien, A.; Cortada-Garcia, J.; Morschett, H.; Holm, H.; Mazzucchelli, C.; Flicker, K. The missing link: Connecting cultivation conditions and refolding performance via inclusion body biophysical properties. Biotechnol. Bioeng. 2025. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Akuta, T.; Ejima, D.; Tsumoto, K. Solubilization and refolding of inclusion bodies by detergents. Protein Expr. Purif. 2025, 236, 106791. [Google Scholar] [CrossRef]
- Kulkarni, N.A.; Das, P.K.; P, A.; Veeranki, V.D. Molecular chaperones: A revolutionary approach for increases solubility of recombinant mAbs from bacterial and yeast systems. Protein Expr. Purif. 2025, 234, 106764. [Google Scholar] [CrossRef]
- Baranowski, C.; Martin, H.G.; Oyarzún, D.A.; Spinner, A.; Desai, B.; Petzold, C.J.; Nikolados, E.M.; Jaaks-Kraatz, S.; Scannell, D.; Sevey, R.; et al. Can protein expression be ‘solved’? Trends Biotechnol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Arnau, J.; Lauritzen, C.; Petersen, G.E.; Pedersen, J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif. 2006, 48, 1–13. [Google Scholar] [CrossRef]
- Esposito, D.; Chatterjee, D.K. Enhancement of soluble protein expression through the use of fusion tags. Curr. Opin. Biotechnol. 2006, 17, 353–358. [Google Scholar] [CrossRef]
- Waugh, D.S. Making the most of affinity tags. Trends Biotechnol. 2005, 23, 316–320. [Google Scholar] [CrossRef]
- Kapust, R.B.; Waugh, D.S. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 1999, 8, 1668–1674. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Howin, J.; McCorkle, S.; Lawrence, P.; Springer, K.; Freimuth, P. Protein aggregation during overexpression limited by peptide extensions with large net negative charges. Protein Expr. Purif. 2004, 36, 207–216. [Google Scholar] [CrossRef]
- Gurrieri, E.; Carradori, G.; Roccuzzo, M.; Pancher, M.; Peroni, D.; Belli, R.; Trevisan, C.; Notarangelo, M.; Huang, W.Q.; Carreira, A.S.A.; et al. CD81-guided heterologous EVs present heterologous interactions with breast cancer cells. J. Biomed. Sci. 2024, 31, 92. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, D.M.; Kim, E.M.; Kim, K.K. Establishments of G3BP1-GFP stress granule monitoring system for real-time stress assessment in human neuroblastome cell. Chemosphere 2024, 361, 142485. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, R.; Iwai, K.; Albertson, A.; Dang, G.; Christopher, D.A. Protein disulfide isomerase-9 interacts with the lumenal region of the transmembrane endoplasmic reticulum stress sensor kinase, IRE1, to modulate the unfolded protein response Arabidopsis. Front. Plant Sci. 2024, 15, 1389658. [Google Scholar] [CrossRef]
- Visser, C.; Rivieccio, F.; Krüger, T.; Schmidt, F.; Cseresnyés, Z.; Rohde, M.; Figge, M.T.; Kniemeyer, O.; Blango, M.G.; Brakhage, A.A. Tracking the uptake of labelled host-derived extracellular vesicles by the human fungal pathogen Aspergillus fumigatus. Microlife 2024, 5, uqae022. [Google Scholar] [CrossRef]
- Cheng, S.; Qiu, Z.; Zhang, Z.; Li, Y.; Zhu, Y.; Zhou, Y.; Yang, Y.; Zhang, Y.; Yang, D.; Zhang, Y.; et al. USP39 phase separates into the nucleolus and drives lung adenocarcinoma progression by promoting GLI1 expression. Cell Commun. Signal. 2025, 23, 56. [Google Scholar] [CrossRef]
- Ward, W.W.; Bokman, S.H. Reversible denaturation of Aequorea Green-Fluorescent Protein: Physical Separation and Characterization of the Renatured protein. Biochemistry 1982, 21, 4335–4540. [Google Scholar] [CrossRef]
- Melnik, B.S.; Povarnitsyna, T.V.; Melnik, T.N. Can the fluorescence of green fluorescent protein chromophore be related directly to the natuve protein structure? Biochem. Biophys. Res. Commun. 2009, 390, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Alkaabi, K.M.; Yafea, A.; Ashraf, S.S. Effect of pH on thermal- and chemical-induced denaturation of GFP. Appl. Biochem. Biotechnol. 2005, 126, 149–156. [Google Scholar] [CrossRef]
- Eissazadeh, S.; Moeini, H.; Dezfouli, M.G.; Heidary, S.; Nelofer, R.; Abdullah, M.P. Production of recombinant human epidermal growth factor in Pichia pastoris. Braz. J. Microbiol. 2017, 48, 286–293. [Google Scholar] [CrossRef]
- Bai, M.; Liu, Y.; Liu, H.; Jia, Y.; Tian, X.; Sun, C. Production of recombinant human epidermal growth factor fused with HaloTag protein and characterization of its biological functions. PeerJ 2024, 12, e17806. [Google Scholar] [CrossRef]
- George-Nascimento, C.; Gyenes, A.; Halloran, S.M.; Merryweather, J.; Valenzuela, P.; Steimer, K.S.; Masiarz, F.R.; Randolph, A. Characterization of recombinant human epidermal growth factor produced in yeast. Biochemistry 1988, 27, 797–802. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, J.; Wei, Z.; Xing, S. Efficient expression of fusion human epidermal growth factor in tobacco chloroplasts. BMC Biotechnol. 2023, 23, 1. [Google Scholar] [CrossRef]
- Motevalli, F.; Khodaei, A.; Bohhassani, A. Generation of a fluorescent oncoprotein in soluble form and its delivery into mammalian cells. Bratisl. Lek. Listy 2019, 120, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Jiang, X.; Wang, Z. GFP fusion promotes the soluble and active expression of a pea actin isoform (PEAc1) in Escherichia coli. Prep. Biochem. Biotechnol. 2023, 53, 557–564. [Google Scholar] [CrossRef]
- Liu, A.X.; Zhang, S.B.; Xu, X.J.; Ren, D.T.; Liu, G.Q. Soluble expression and characterization of a GFP-fused actin isoform (PEAc1). Cell Res. 2004, 14, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sullivan, D.S.; Huffaker, T.C. Two yeast genes with similarity to TCP-1 are required for microtubule and actin function in vivo. Proc. Natl. Acad. Sci. USA 1994, 91, 9111–9115. [Google Scholar] [CrossRef]
- Vinh, D.B.N.; Drubin, D.G. A yeast TCP-1-like protein is required for actin function in vivo. Proc. Natl. Acad. Sci. USA 1994, 91, 9116–9120. [Google Scholar] [CrossRef]
- Song, H.; Wang, F.; Xie, W.; Chen, Z.; He, R.; Fang, Y.; He, Q.; Yang, S.; Ma, L. A dual-tag strategy with superfolder green fluorescent protein (sfGFP) and small ubiquitin-like modifier (SUMO) for soluble expression of SARS-CoV-2 RNA polymerase visibly in Escherichia coli. Int. J. Biol. Macromol. 2025, 320, 145882. [Google Scholar] [CrossRef]
- Razali, R.; Budiman, C.; Kamaruzaman, K.A.; Subbiah, V.K. Soluble expression and catalytic properties of codon-optimized recombinant bromelain from MD2 pineapple in Escherichi coli. Protein J. 2021, 40, 406–418. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Ren, Y.; Wei, D. The expression, purification, and functional evaluation of the novel tumor suppressor fusion IL-24-CN. Appl. Microbiol. Biotechnol. 2021, 105, 7889–7898. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Kanei-Ishii, C.; Maekawa, T.; Fujimoto, J.; Yamamoto, T.; Ishii, S. Increase of solubility of foreign proteins in Escherichia coli by coproduction of the bacterial thioredoxin. J. Biol. Chem. 1995, 270, 25328–25331. [Google Scholar] [CrossRef]
- Nishi, K.; Fukunaga, N.; Ono, T.; Akuta, T.; Yumita, N.; Watanabe, H.; Kadowaki, D.; Suenaga, A.; Maruyama, T.; Otagiri, M. Construction of an expression system for human alpha(1)-acid glycoprotein in E. coli: The roles of oligosaccharide moieties in structural and functional properties. Drug Metab. Pharmacokinet. 2010, 25, 200–207. [Google Scholar] [CrossRef]
- Imaizumi, K.; Nishikawa, S.; Tarui, H.; Akuta, T. High-level expression and efficient one-step chromatographic purification of a soluble human leukemia inhibitory factor (LIF) in Escherichia coli. Protein Expr. Purif. 2013, 90, 20–26. [Google Scholar] [CrossRef]
- Akuta, T.; Kikuchi-Ueda, T.; Imaizumi, K.; Oshikane, H.; Nakaki, T.; Okada, Y.; Sultana, S.; Kobayashi, K.; Kiyokawa, N.; Ono, Y. Expression of bioactive soluble human stem cell factor (SCF) from recombinant Escherichia coli by coproduction of thioredoxin and efficient purification using arginine in affinity chromatography. Protein Expr. Purif. 2015, 105, 1–7. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, G.D. An efficient strategy for producing RNA-free Nucleocapsid protein of SARS-CoV-2 for biochemical and structural investigations. FEBS Open Bio 2025. [Google Scholar] [CrossRef]
- Schenkel, M.; Treff, A.; Deber, C.M.; Krainer, G.; Schlierf, M. Heat treatment of thioredoxin fusions increases the purity of α-helical transmembrane protein constructs. Protein Sci. 2021, 30, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Tropea, J.E.; Waught, D.S. Enhancing the solubility of recombinant proteins in Escherichia coli by using hexahistidine-tagged maltose-binding protein as a fusion partner. Methods Mol. Biol. 2011, 705, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Tropea, J.E.; Cherry, S.; Waugh, D.S. Expression and purification of soluble His(6)-tagged TEV protease. Methods Mol. Biol. 2009, 498, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.M.; Milling, L.M.; Elliott, B.M.; Hawes, M.R.; Wickens, J.M.; Webber, D.E. Bacterial expression of a snake venom metalloproteinase inhibitory protein from the North American opossum (D. virginiana). Toxicon 2021, 30, 1–10. [Google Scholar] [CrossRef]
- Maddi, E.R.; Natesh, R. Optimization strategies for expression and purification of soluble N-terminal domain of human centriolar protein SAS-6 in Escherichia coli. Protein Expr. Purif. 2021, 183, 195856. [Google Scholar] [CrossRef]
- Nemergut, M.; Škrabana, R.; Berta, M.; Plückthun, A.; Sedlák, E. Purification of MBP fusion proteins using engineered DARP in affinity matrix. Int. J. Biol. Macromol. 2021, 187, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, M.; Yang, X.; Guo, Y.; Guo, R.; Wang, M.; Yang, G.; Guo, Y. Soluble overexpression and purification of infectious bursal disease virus capsid protein VP2 in Escherichia coli and its nanometer structure observation. Cell Cycle 2022, 21, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.A.; Jin, H.; Lee, Y.; Shin, H.B.; Son, Y.J. Purification and evaluation of the biological activity of recombinant mouse prolactin. J. Microbiol. Biotechnol. 2025, 35, e2503029. [Google Scholar] [CrossRef] [PubMed]
- Nugyen, M.T.; Prima, M.J.; Do, B.H.; Yoo, J.; Park, S.; Jang, J.; Lee, S.; Lee, E.; Novais, M.P.; Seo, M.L.; et al. Prokaryotic soluble overexpression and purification of oncostatin M using a fusion approach and genetically engineered E. coli strains. Sci. Rep. 2019, 9, 13706. [Google Scholar] [CrossRef]
- Shankar, K.; Lin, Y.; Carlson, L.A. Flotation assay with fluorescence readout to study membrane association of the enteroviral peripheral membrane protein 2C. Bio-Protocol 2025, 15, e5261. [Google Scholar] [CrossRef]
- Davis, G.D.; Elisee, C.; Newham, D.M.; Harrison, R.G. New fusion protein systems designed to give soluble expression in Escherichia coli. Biotechnol. Bioeng. 1999, 65, 382–388. [Google Scholar] [CrossRef]
- Hara, K.Y.; Yagi, S.; Hirono-Haya, Y.; Kikukawa, H. A method of solubilizing and concentrating astaxanthin and other cartenoids. Mar. Drugs 2021, 19, 462. [Google Scholar] [CrossRef]
- Zaxharchenko, T.; Barsukov, I.; Rigden, D.J.; Bennett, D.; Mayans, O. Biophysical analysis of the N-terminal domain from the human protein phosphatase subunit PNUTS suggests an extended transcription factor TFIIS-like fold. Protein J. 2016, 35, 340–345. [Google Scholar] [CrossRef]
- Smith, M.A.; Gonzalez, J.; Hussain, A.; Oldfield, R.N.; Johnston, K.A.; Lopez, K.M. Overexpression of soluble recombinant human lysyl oxidase by usning solubility tags: Effects on activity and solubility. Enzym. Res. 2016, 2016, 5098985. [Google Scholar] [CrossRef]
- Yeh, P.; Landais, D.; Lemaȋtre, M.; Maury, I.; Crenne, J.Y.; Becquart, J.; Murry-Brelier, A.; Boucher, F.; Montay, G.; Fleer, R.; et al. Design of yeast-secreted albumin derivatives for human therapy: Biological and antiviral properties of a serum albumin-CD4 genetic conjugate. Proc. Natl. Acad. Sci. USA 1992, 89, 1904–1908. [Google Scholar] [CrossRef]
- Tao, H.Y.; Wang, R.Q.; Sheng, W.J.; Zhen, Y.S. The development of human serum albumin-based drugs and relevatnt fusion proteins for cancer therapy. Int. J. Biol. Macromol. 2021, 30, 24–34. [Google Scholar] [CrossRef]
- Sung, C.; Nardelli, B.; LaFleur, D.W.; Blatter, E.; Corcoran, M.; Olsen, H.S.; Birse, C.E.; Pickeral, O.K.; Zhang, J.; Shah, D. An IFN-beta-albumin fusion protein that displays improved pharamcokinetic and pharmacodynamic properties in nonhuman primates. J. Interferon Cytokine Res. 2003, 23, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kubota, M.; Shimada, M.; Hayase, T.; Miyaguchi, M.; Kobayashi, N.; Ikeda, M.; Shima, Y.; Kawahara, M. Thioredoxin-albumin fusion protein prevents urban aerosol-induced lung injury via suppressing oxidative stress-related neutrophil extracellular trap formation. Environ. Pollut. 2021, 268, 115787. [Google Scholar] [CrossRef] [PubMed]
- Nopia, H.; Kurimoto, D.; Sato, A. Albumin fusion with human lactoferrin shows enhanced inhibition of cancer migration. Biometals 2023, 36, 629–638. [Google Scholar] [CrossRef]
- Nilse, J.; Aaen, K.H.; Benjakul, S.; Ruso-Julve, F.; Greiner, T.U.; Bejan, D.; Stensland, M.; Singh, S.; Schlothauer, T.; Sandlie, I.; et al. Enhanced plasma half-life and efficacy of engineered human serum albumin-fused GLP-1 despite enzymatic cleavage of its C-terminal end. Commun. Biol. 2025, 8, 810. [Google Scholar] [CrossRef]
- Lii, R.J.P.; Prcutt, S.J.; Strickler, J.S.; Butt, T.R. SUMO fusion technology for enhanced protein expression and purification in prokaryotes and eukaryotes. Methods Mol. Biol. 2011, 705, 15–30. [Google Scholar] [CrossRef]
- Falco, A.D.; Greene-Cramer, R.; Shurina, B.A.; Zakian, S.; Acton, T.B.; Ramelot, T.A.; Montelione, G.T. Protocol for production and characterization of SARS-CoV-1 PLPro in Escherichai coli. STAR Protoc. 2025, 6, 103952. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, B.; Chen, Z.; Su, Y.; Cheng, C.; He, B. Scale up of fermentation of recombinant Escherichia coli for efficient production of spider drag silk protein MaSp1s and its dimers. Microb. Cell Factories 2025, 24, 108. [Google Scholar] [CrossRef]
- Ferraz, K.F.; Caetano, L.H.D.L.; Orefice, D.P.; Calabria, P.A.L.; Della-Casa, M.S.; Freitas-de-Sousa, L.A.; Beraldo-Neto, E.; Sanabani, S.S.; Magalhães, G.S.; Clissa, P.B. Bicistronic vector expression of recombinant Jararhagin-C and its effects on endothelian cells. Toxins 2024, 16, 524. [Google Scholar] [CrossRef] [PubMed]
- Lakhshei, P.; Ahangarzadeh, S.; Yarian, F.; Koochaki, A.; Kazemi, B.; Kiamehr, Z.; Mohammadi, E.; Alibakhshi, A. Cytotoxic effects of a novel tagged apoptin in breast cancer cell lines. Adv. Biomed. Res. 2024, 29, 46. [Google Scholar] [CrossRef]
- Devi, N.; Adivitiya; Khasa, Y.P. A combinatorial approach of N-terminus blocking and codon optimization strategies to enhance the soluble expression of recombinant hIL-7 in E. coli fed-batch culture. Appl. Microbiol. Biotechnol. 2016, 100, 9979–9994. [Google Scholar] [CrossRef]
- Butt, T.R.; Edavettal, S.C.; Hall, J.P.; Mattern, M.R. SUMO fusion technology for difficult-to-express proteins. Protein Expr. Purif. 2005, 43, 1–9. [Google Scholar] [CrossRef]
- Mevarech, M.; Frolow, F.; Gloss, L.M. Halophilic enzymes: Proteins with a grain of salt. Biophys. Chem. 2000, 86, 155–164. [Google Scholar] [CrossRef]
- Tokunaga, H.; Ishibashi, M.; Arakawa, T.; Tokunaga, M. Highly efficient renaturation of beta-lactamase isolated from moderately halophilic bacteria. FEBS Lett. 2004, 558, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, H.; Arakawa, T.; Fukada, H.; Tokunaga, M. Opposing effects of NaCl on reversibility and thermal stability of halophilic beta-lactamase from a moderate halophile, Chromohalobacter sp. 560. Biophys. Chem. 2006, 119, 316–320. [Google Scholar] [CrossRef]
- Daumy, G.O.; Merenda, J.M.; McColl, A.S.; Andrews, G.C.; Franke, A.E.; Geoghegan, K.F.; Otterness, I.G. Isolation and characterization of biologically active murine interleukin-1 alpha derived from expression of a synthetic gene in Escherichia coli. Biochim. Biophys. Acta. 1989, 998, 32–42. [Google Scholar] [CrossRef]
- Tokunaga, H.; Saito, S.; Sakai, K.; Yamaguchi, R.; Katsuyama, I.; Arakawa, T.; Onozaki, K.; Arakawa, T.; Tokunaga, M. Halophilic beta-lactamase as a new solubility- and folding-enhancing tag protein: Production of native human interleukin 1alpha and human neutrophil alpha-defensin. Appl. Microbiol. Biotechnol. 2010, 86, 649–658. [Google Scholar] [CrossRef]
- Valli, A.; Achilonu, I. Comparative structural analysis of the human and Schistosoma glutathione transferase dimer interface using selective binding of bromosulfophthalei. Proteins 2022, 90, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Petiot, N.; Schwartz, M.; Delarue, P.; Senet, P.; Neiers, F.; Nicolaï, A. Structural analysis of the Drosophila melanogaster GSTome. Biomolecules 2024, 14, 759. [Google Scholar] [CrossRef]
- Davis, A.H.; Jowett, J.B.; Jones, I.M. Recombinant baculovirus vectors expressing glutathione-S-transferase fusion proteins. Biotechnology 1993, 11, 933–936. [Google Scholar] [CrossRef]
- Schầfer, F.; Seip, N.; Maertens, B.; Block, H.; Kubicek, J. Purification of GST-tagged proteins. Methods Enzymol. 2015, 559, 127–139. [Google Scholar] [CrossRef]
- Zweng, S.; Mendoza-Rojas, G.; Lepak, A.; Altegoer, F. Simplifying recombinant protein production: Combining golden gate cloning with a standerized protein purification scheme. Methods Mol. Biol. 2025, 2850, 229–249. [Google Scholar] [CrossRef]
- Lone, I.N.; Sekerogu, E.O.; Kafaz, G.I.; Haija, A.A.H.A.A.; Turker, E.; Batur, T.; Diril, M.K. Cost-effective production of biologically active leukemia inhibitory factor for mouse embryonic stem cell culture. Mol. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Watson, G.M.; Gunzburg, M.J.; Wilce, J.A. Using surface plasmon resonance to study SH2 domain-peptide interactions. Methods Mol. Biol. 2023, 2705, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hakoshima, T. GST pull-down assay to measure complex formations. Methods Mol. Biol. 2019, 1893, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kawamura-Tsuzuku, J.; Ohsugi, M.; Yoshida, M.; Emi, M.; Onda, M.; Yoshida, Y.; Nishiyama, A.; Yamamoto, T. Tob, a novel protein that interacts with p185erbB2, is associated with anti-proliferative activity. Oncogene 1996, 12, 705–713. [Google Scholar] [PubMed]
- Shinjo, H.; Nagano, G.; Ishii, S.; Himeno, N.; Yamamoto, Y.; Sagawa, J.; Baba, R.; Egusa, G.; Hattori, N.; Ohno, H. Immunoprecipitation with an anti-epitope tag affinity gel to study protein-protein interactions. J. Vis. Exp. 2024, 203, e66085. [Google Scholar] [CrossRef]
- Soeda, S.; Montrose, M.; Takahashi, A.; Ishida, R.; Burriel, S.; Ohmine, N.; Natsume, T.; Adachi, S.; Kim, M.; Yamamoto, T. The E3 ubuquitin ligase, RNF219, suppresses CNOT6L expression to exhibit antiproliferative activity. FEBS Open Bio 2025. [Google Scholar] [CrossRef]
- Sutherland, S. Peptibodies: The new cool technology. Durg Discov. Today 2004, 9, 683. [Google Scholar] [CrossRef]
- Mondéjar-Parreño, G.; Sánchez-Pérez, P.; Cruz, F.M.; Jalife, J. Promising tools for future drug discovery and development in antiarrhythmic therapy. Pharmacol. Rev. 2025, 77, 100013. [Google Scholar] [CrossRef]
- Kherzi, H.; Mostafavi, M.; Dabirmanesh, B.; Khajeh, K. Peptibodies: Bridging the gap between peptides and antibodies. Int. J. Biol. Macromol. 2024, 278, 134718. [Google Scholar] [CrossRef]
- Nichol, J.L. AMG 531: An investigational thrombopoiesis-stimulating peptibody. Pedriatr. Blood Cancer. 2006, 47, 723–725. [Google Scholar] [CrossRef]
- Chen, F.; McDonal, V.; Newland, A. Experts’ review: The emerging roles of romiplostim in immune thromcytopenia (ITP). Expert Opin. Biol. Ther. 2021, 21, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Ghoshoon, M.B.; Jamshidi, M.; Moradbeygi, F.; Hashemzehi, A. rEview on romiplostim mechanism of action and the expressive approach in E. coli. Recent Pat. Biotechnol. 2024, 2, 95–109. [Google Scholar] [CrossRef]
- Jendryczko, K.; Rzeszotko, J.; Krzyschik, M.A.; Szymczyk, J.; Otlewski, J.; Szachcic, A. Peptibody based on FGFR1-binding peptide from the FGF4 sequence as a cancer targeting agent. Front. Pharmacol. 2021, 12, 748936. [Google Scholar] [CrossRef] [PubMed]
- Hallaji, M.; Allahyri, M.; Teimoori-Toolabi, L.; Yasami-Khiabani, S.; Golkar, M.; Fard-Esfahani, P. Targeted cancer treatment using a novel EGFR-specific Fc-fusion peptide based on GE11 peptide. Sci. Rep. 2025, 15, 5107. [Google Scholar] [CrossRef] [PubMed]
- Chidpi, B.; Chang, M.; Cui, M.; Abou-Assali, O.; Resiser, M.; Pshenychnyi, S.; Logothetis, D.E.; Teng, M.N.; Noujaim, S.F. Bioengineered peptibodies as blockers of ion channels. Proc. Natl. Acad. Sci. USA 2022, 119, e2212564119. [Google Scholar] [CrossRef]
- Liu, H.; Su, D.; Zhang, J.; Ge, S.; Li, Y.; Wang, F.; Gravel, M.; Roulston, A.; Song, Q.; Xu, W.; et al. Improvement of pharmacokinetic profile of TRAIL via trimer-tag enhances its antitumor activity in vivo. Sci. Rep. 2017, 7, 8953, Correction in Sci. Rep. 2018, 8, 5266. [Google Scholar] [CrossRef]
- Montinaro, A.; Walczak, H. Harnessing TRAIL-induced cell death for cancer therapy: A long walk with thrilling discoveries. Cell Death Differ. 2023, 30, 237–249. [Google Scholar] [CrossRef]
- Lim, K.H.; Huang, H.; Pralle, A.; Park, S. Stable, high-affinity streptavidin monomer for protein labeling and monovalent biotin detection. Biotechnol. Bioeng. 2013, 110, 57–67. [Google Scholar] [CrossRef]
- Phelps, A.; Pazos-Castro, D.; Urselli, F.; Grydziuszko, E.; Mann-Delany, O.; Fang, A.; Walker, T.D.; Guruge, R.T.; Tome-Amat, J.; Diaz-Perales, A.; et al. Production and use of antigen tetramers to study antigen-specific B cells. Nat. Protoc. 2024, 19, 727–751, Correction in Nat. Protoc. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ejima, D.; Sato, H.; Sato, H.; Tsumoto, K.; Date, M. Method for Preparing Polymeric Protein Composed of Monomeric Protein Produced by Fusing Protein Having Immunoglobulin Fold Structure to Protein Capable of Serving as Subunit Structure. Patent WO-A1-2012/176919, 2012. Available online: https://patents.google.com/patent/US10308969 (accessed on 12 December 2013).
- Brown, B.L. Heterologous expression and purification of eukaryotic ALA synthase from E. coli. Methods Mol. Biol. 2024, 2939, 233–241. [Google Scholar] [CrossRef]
- Fonza, F.; Verardo, R.; Schneider, C. A streamlines tandem affinity purification of His-MBP-SpyCas9, without buffer exchange, suitable for in vitro cleavage applications. MethodsX 2025, 14, 103368. [Google Scholar] [CrossRef] [PubMed]
- Beugelink, J.W.; Sweep, E.; Janssen, B.J.C.; Snijder, J.; Pronker, M.F. Structural basis for recognition of the FLAG-tag by anti-FLAG M2. J. Mol. Biol. 2024, 436, 168649. [Google Scholar] [CrossRef] [PubMed]
- Koskela, M.; Skotnicová, P.; Kiss, Ė.; Sobotka, R. Purification of Protein-complexes from the Cyanobacterium Synechocystis sp. PCC 6803 Using FLAG-affinity Chromatography. Bio-Protocol 2020, 10, e3616. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, D.; Ono, F.; Sugino, M.; Suzuki, H.; Endo, N.; Shimada, A.; Ebihara, A. Production of recombinant His-tagged triple-FLAG peptide in Brevibacillus chroshinensis and its utilization as an easy-to-remove affinity peptide. Biosci. Biotechnol. Biochem. 2023, 87, 1029–1035. [Google Scholar] [CrossRef]
- Park, S.H.; Cheong, C.; Idoyaga, J.; Kim, J.Y.; Choi, J.H.; Do, Y.; Lee, H.; Jo, J.H.; Oh, Y.S.; Im, W.; et al. Generation and application of new rat monoclonal antibodies against synthetic FLAG and OLLAS tags for improved immunodetection. J. Immunol. Methods 2008, 331, 27–38. [Google Scholar] [CrossRef]
- Breitinger, U.; Zakaria, Z.I.S.; Mahgoub, H.A.; Wiessler, A.L.; Tuerker, E.; Villmann, C.; Breitinger, H.G. Activity and cellular distribution of ORF3a mutants of SARS-CoV-2 variants of concern. J. Gen. Virol. 2025, 106, 002135. [Google Scholar] [CrossRef]
- Lima, A.J.F.; Hajdu, K.L.; Abdo, L.; Batista-Silva, L.R.; Andrade, C.O.; Correia, E.M.; Aragão, E.A.A.; Bonamino, M.H.; Lourenzoni, M.R. In silico and in vivo analysis reveal impact of c-Myc tag in FMC63 scFv-CD19 protein interface and CAR-T cell efficacy. Comput. Struct. Biotechnol. J. 2024, 23, 2375–2387. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Hou, C.Y.; Jung, S.T.; Kang, T.J. Detection and purification of backbone-cyclized proteins using a bacterially expressed anti-myc-tag single chain amtibody. Anal. Biochem. 2017, 532, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; Nogi, T.; Kato, Y.; Takagi, J. PA tag: A versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr. Purif. 2014, 95, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, M.; Kato, Y.; Takagi, J. “PA tag”, a novel affinity tag system that enables protein purification and detection. PSSJ Arch. 2014, 7, e075. [Google Scholar]
- Gordaliza-Alagueto, I.; Sánchez-Fernández-de-Landa, P.; Radivojevikj, D.; Villarreal, L.; Arauz-Vicente, M.; Seco, J.; Martin-Malpartida, P.; Vilaseca, M.; Macías, M.; Palacin, M.; et al. Endogenous interactomes of MFN1 and MFN2 provide noveal insights into interorganelle communication and autophagy. Autophagy 2025, 21, 957–978. [Google Scholar] [CrossRef] [PubMed]
- Low, T.Y.; Lee, P.Y. Tandem affinity purification (TAP) of inetarcting prey proteins with FLAG- and HA-tagged bait proteins. Methods Mol. Biol. 2023, 2690, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.N.; Cai, S.T.; Liang, Y.F.; Weng, Z.J.; Song, T.Q.; Li, X.; Sun, Y.S.; Peng, Y.Z.; Huang, Z.; Gao, Q.; et al. Subcellular localization of viral proteins after porcine epidemic diarrhea virus infection and their roles in the viral life cycle. Int. J. Biol. Macomol. 2024, 274, 133401. [Google Scholar] [CrossRef]
- Kajimura, Y.; Dong, S.; Tessari, A.; Oriacchio, A.; Thoms, A.; Cufaro, M.C.; Di Marco, F.; Amari, F.; Chen, M.; Soliman, S.H.A.; et al. An in vivo “turning model” reveals new RanBP9 interactions in lung macrophage. Cell Death Discov. 2025, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Ishino, T.; Harrington, A.; Zaks-Zilberman, M.; Scibek, J.J.; Chaiken, I. Slow-dissociation effect of common siganling subunit beta c on IL5 and GM-CSF receptor assembly. Cytokine 2008, 42, 179–190. [Google Scholar] [CrossRef][Green Version]
- Ma, H.C.; Fang, C.P.; Hsieh, Y.C.; Chen, S.C.; Li, H.C.; Lo, S.Y. Expression and membrane inegration of SARS-CoV M protein. J. Biomed. Sci. 2008, 15, 301–310. [Google Scholar] [CrossRef]
- Zeghal, M.; Matte, K.; Venes, A.; Patel, S.; Laroche, G.; Sarvan, S.; Joshi, M.; Rain, J.C.; Couture, J.F.; Giguère, P.M. Development of a V5-tag-directed nanobody and its implementation as an intracellular biosensor of GPCR signaling. J. Biol. Chem. 2023, 299, 105107. [Google Scholar] [CrossRef]
- Schmidt, T.G.; Skerra, A. One-step affinity purification of bacterially produced proteins by means of the “Strep tag” and immobilized recombinant core streptavidin. J. Chromatogr. A 1994, 676, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, D.; Takahashi, N.; Inoue, T.; Nomura, S.M.; Matsubayashi, H.T. A unified purification method for actin-binding proteins using a TEV-cleavable His-Strep-tag. MethodsX 2024, 13, 102884. [Google Scholar] [CrossRef]
- Scarfe, J.; Kosmützky, D.; Nisbet, R.E.R. A game of tag: A review of protein tags for the successful detection, purification and fluorescence labelling of proteins expressed in microalgae. Plant J. 2025, 122, e70272. [Google Scholar] [CrossRef]
- Dong, X.; Lu, Q.; Zhang, Y.; Yang, L. Microfluidic magnetic solid-phase extraction of acetamiprid using dynamically manipulated apta-magnetic sorbents for chromatographic analysis. J. Chromatogr. A 2025, 1760, 466273. [Google Scholar] [CrossRef]
- Gururaja, T.L.; Narasimhamurthy, S.; Payan, D.G.; Anderson, D.C. A novel artificial loop scaffold for the noncovalent constraint of peptides. Chem. Biol. 2000, 7, 515–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Leo, C.; Jang, Y.; Chan, E.; Padilla, D.; Huang, B.C.; Lin, T.; Gururaja, T.; Hitoshi, Y.; Lorens, J.B.; et al. Dominant effector genetics in mammalian cells. Nat. Genet. 2001, 27, 23–29. [Google Scholar] [CrossRef]
- Terashita, K.; Hashimoto, Y.; Niikura, T.; Tajima, H.; Yamagishi, Y.; Ishizaka, M.; Kawasumi, M.; Chiba, T.; Kanekura, K.; Yamada, M.; et al. Two serine residues distinctly regulate the rescue function of Humanin, an inhibiting factore of Alzheimer’s disease-related neurotoxicity: Functional potentiation by isomerization and dimerization. J. Neurochem. 2003, 85, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Arakawa, T.; Tokunaga, Y.; Sugimoto, Y. Insoluble expression of highly soluble halophilic metal binding protein for metal ion biosorption: Application of aggregation-prone peptide from egg white lysozyme. Protein Expr. Purif. 2019, 156, 50–57. [Google Scholar] [CrossRef]
- Song, L.; Chen, Y.; Liu, H.; Zhang, X. Preparation, biological activities, and potential applications of hen egg-derived peptides: A review. Foods 2024, 13, 885. [Google Scholar] [CrossRef]
- Peternel, S.; Komel, R. Active protein aggregates produced in Escherichia coli. Int. J. Mol. Sci. 2011, 12, 8275–8287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xing, L.; Wu, W.; Zhang, X.E.; Lin, Z. Small surfactant-like peptides can drive soluble proteins into active aggregates. Microb. Cell Factories 2012, 11, 10. [Google Scholar] [CrossRef]
- Jong, W.S.; Vikström, D.; Houben, D.; van den Berg van Saparoes, H.B.; de Gier, J.W.; Luirink, J. Application of an E. coli signal sequence as a versatile inclusion body tag. Microb. Cell Factories 2017, 16, 50. [Google Scholar] [CrossRef]
- Yang, X.; Pisolozzi, M.; Lin, Z. New trends in aggregating tags for therapeutic protein purification. Biotechnol. Lett. 2018, 40, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Kamada, Y.; Tokunaga, Y.; Shinohara, H.; Matsumoto, M.; Kusakabe, T.; Ohkuri, T.; Ueda, T. Aggregates with lysozyme and ovalbumin show denatures of amyloid-like fibrils. Biochem. Cell Biol. 2011, 89, 533–544. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Matsumoto, M.; Sugimoto, Y. Amyloid fibril formation from a 9 amino acid peptide, 55th-63rd residues of human lysozyme. Int. J. Biol. Macromol. 2015, 80, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, Y.; Sakakibara, Y.; Kamada, Y.; Watanabe, K.; Sugimoto, Y. Analysis of core region from egg white lysozyme forming amyloid fibrils. Int. J. Biol. Sci. 2013, 9, 219–227. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Inoue, Y.; Tokunaga, H.; Ishibashi, M.; Arakawa, T. Halophilic characterization of starch-binding domain from Kucuria varient α-amylase. Int. J. Biol. Macromol. 2012, 50, 95–102. [Google Scholar] [CrossRef]
- Young, C.L.; Britton, Z.T.; Robinson, A.S. Recombinant protein expression and purification: A comprehensive review of affinity tags and microbial applications. Biotechnol. J. 2012, 7, 620–634. [Google Scholar] [CrossRef]
- Waugh, D.S. An overview of enzymatic reagents for the removal of affinity tags. Protein Expr. Purif. 2011, 80, 283–293. [Google Scholar] [CrossRef]
- Xie, J.; Fan, H.; Fu, Q.B. Strategies for Tag Design and Removal in the Expression and Purification of Recombinant Proteins. Health Metab. 2025, 2, 4. [Google Scholar] [CrossRef]
- Smith, D.B.; Johnson, K.S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 1988, 67, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.L.; Dixon, J.E. Eukaryotic proteins expressed in Escherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 1991, 192, 262–267. [Google Scholar] [CrossRef]
- Sticha, K.R.; Sieg, C.A.; Bergstrom, C.P.; Hanna, P.E.; Wagner, C.R. Overexpression and large-scale purification of recombinant hamster polymorphic arylamine N-acetyltransferase as a dihydrofolate reductase fusion protein. Protein Expr. Purif. 1997, 10, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Vothknecht, U.C.; Kannangara, C.G.; von Wettstein, D. Expression of catalytically active barley glutamyl tRNAGlu reductase in Escherichia coli as a fusion protein with glutathione S-transferase. Proc. Natl. Acad. Sci. USA 1996, 93, 9287–9291. [Google Scholar] [CrossRef]
- Osiro, K.O.; Duque, H.M.; Sampaio de Oliveira, K.B.; Melo, N.T.M.; Lima, L.F.; Paes, H.C.; Franco, O.L. Cleaving the way for heterologous peptide production: An overview of cleavage strategies. Methods 2025, 234, 36–44. [Google Scholar] [CrossRef]
- Hopp, T.P.; Prickett, K.S.; Price, V.L.; Libby, R.T.; March, C.J.; Cerretti, D.P.; Urdal, D.L.; Conlon, P.J. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio/Technology 1988, 6, 1204–1210. [Google Scholar] [CrossRef]
- Cordingley, M.G.; Callahan, P.L.; Sardana, V.V.; Garsky, V.M.; Colonno, R.J. Substrate requirements of human rhinovirus 3C protease for peptide cleavage in vitro. J. Biol. Chem. 1990, 265, 9062–9065. [Google Scholar] [CrossRef]
- Parks, T.D.; Leuther, K.K.; Howard, E.D.; Johnston, S.A.; Dougherty, W.G. Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Anal. Biochem. 1994, 216, 413–417. [Google Scholar] [CrossRef]
- Hefti, M.H.; Van Vugt-Van der Toorn, C.J.; Dixon, R.; Vervoort, J. A novel purification method for histidine-tagged proteins containing a thrombin cleavage site. Anal. Biochem. 2001, 295, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Jenny, R.J.; Mann, K.G.; Lundblad, R.L. A critical review of the methods for cleavage of fusion proteins with thrombin and factor Xa. Protein Expr. Purif. 2003, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rauniyar, K.; Akhondzadeh, S.; Gąciarz, A.; Künnapuu, J.; Jeltsch, M. Bioactive VEGF-C from E. coli. Sci. Rep. 2022, 12, 18157. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, J.H.; Lee, J.; Ka, S.Y.; Chae, H.D.; Jung, I.; Jung, S.T.; Na, J.H. Functional Expression of the Recombinant Spike Receptor Binding Domain of SARS-CoV-2 Omicron in the Periplasm of Escherichia coli. Bioengineering 2022, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhu, T.; Huang, X. Enhanced Soluble Expression of Linoleic Acid Isomerase by Coordinated Regulation of Promoter and Fusion Tag in Escherichia coli. Foods 2022, 11, 1515. [Google Scholar] [CrossRef]
- Srisaisap, M.; Suwankhajit, T.; Boonserm, P. A fusion protein designed for soluble expression, rapid purification, and enhanced stability of parasporin-2 with potential therapeutic applications. Biotechnol. Rep. 2024, 43, e00851. [Google Scholar] [CrossRef]
- Yuan, L.D.; Hua, Z.C. Expression, purification, and characterization of a biologically active bovine enterokinase catalytic subunit in Escherichia coli. Protein Expr. Purif. 2002, 25, 300–304. [Google Scholar] [CrossRef]
- Ko, H.; Kang, M.; Kim, M.J.; Yi, J.; Kang, J.; Bae, J.H.; Sohn, J.H.; Sung, B.H. A novel protein fusion partner, carbohydrate-binding module family 66, to enhance heterologous protein expression in Escherichia coli. Microb. Cell Factories 2021, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Do, V.G.; Yang, M.S. Production of Mature Recombinant Human Activin A in Transgenic Rice Cell Suspension Culture. Curr. Issues Mol. Biol. 2024, 46, 1164–1176. [Google Scholar] [CrossRef]
- Abdelkader, E.H.; Otting, G. NT*-HRV3CP: An optimized construct of human rhinovirus 14 3C protease for high-yield expression and fast affinity-tag cleavage. J. Biotechnol. 2021, 325, 145–151. [Google Scholar] [CrossRef]
- Amé, J.C.; Dantzer, F. Purification of Recombinant Human PARG and Activity Assays. Methods Mol. Biol. 2023, 2609, 399–418. [Google Scholar] [CrossRef]
- Carrington, J.C.; Dougherty, W.G. A viral cleavage site cassette: Identification of amino acid sequences required for tobacco etch virus polyprotein processing. Proc. Natl. Acad. Sci. USA 1988, 85, 3391–3395. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.Q.; Kim, D.H.; Shim, H.J.; Ta, H.K.K.; Vu, T.L.; Nguyen, T.K.O.; Lim, J.C.; Choe, H. Novel Anti-Mesothelin Nanobodies and Recombinant Immunotoxins with Pseudomonas Exotoxin Catalytic Domain for Cancer Therapeutics. Mol. Cells 2023, 46, 764–777. [Google Scholar] [CrossRef]
- Waddell, G.L.; Drew, E.E.; Rupp, H.P.; Hansen, S.D. Mechanisms controlling membrane recruitment and activation of the autoinhibited SHIP1 inositol 5-phosphatase. J. Biol. Chem. 2023, 299, 105022. [Google Scholar] [CrossRef]
- Ruan, B.; Fisher, K.E.; Alexander, P.A.; Doroshko, V.; Bryan, P.N. Engineering subtilisin into fluoride-triggered processing protease useful for one-step protein purification. Biochemistry 2004, 43, 14539–14546. [Google Scholar] [CrossRef]
- Peleg, Y.; Prabahar, V.; Bednarczyk, D.; Unger, T. Harnessing the Profinity eXact™ System for Expression and Purification of Heterologous Proteins in E. coli. Methods Mol. Biol. 2017, 1586, 33–43. [Google Scholar] [CrossRef]
- Nakagawa, M.; Tomioka, Y.; Akuta, T. Efficient expression and purification of tag-free recombinant human procalcitonin (hPCT) with precise sequence in E. coli. Protein Expr. Purif. 2024, 214, 106374. [Google Scholar] [CrossRef]
- Sato, R.; Tomioka, Y.; Sakuma, C.; Nakagawa, M.; Kurosawa, Y.; Shiba, K.; Arakawa, T.; Akuta, T. Detection of concentration-dependent conformational changes in SARS-CoV-2 nucleoprotein by agarose native gel electrophoresis. Anal. Biochem. 2023, 662, 114995. [Google Scholar] [CrossRef]
- Malakhov, M.P.; Mattern, M.R.; Malakhova, O.A.; Drinker, M.; Weeks, S.D.; Butt, T.R. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genom. 2004, 5, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ahler, E.; Simon, J.J.; Fang, L.; Potter, Z.E.; Sitko, K.A.; Stephany, J.J.; Guttman, M.; Fowler, D.M.; Maly, D.J. Profiling of drug resistance in Src kinase at scale uncovers a regulatory network coupling autoinhibition and catalytic domain dynamics. Cell Chem Biol. 2024, 31, 207–220.e11. [Google Scholar] [CrossRef]
- McKenna, S.; Giblin, S.P.; Bunn, R.A.; Xu, Y.; Matthews, S.J.; Pease, J.E. A highly efficient method for the production and purification of recombinant human CXCL8. PLoS ONE 2021, 16, e0258270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zheng, H.; Xian, Y.; Song, H.; Wang, S.; Yun, Y.; Yi, L.; Zhang, G. Profiling Substrate Specificity of the SUMO Protease Ulp1 by the YESS–PSSC System to Advance the Conserved Mechanism for Substrate Cleavage. Int. J. Mol. Sci. 2022, 23, 12188. [Google Scholar] [CrossRef] [PubMed]
- De Vos, J.; Pereira Aguilar, P.; Köppl, C.; Fischer, A.; Grünwald-Gruber, C.; Dürkop, M.; Klausberger, M.; Mairhofer, J.; Striedner, G.; Cserjan-Puschmann, M.; et al. Production of full-length SARS-CoV-2 nucleocapsid protein from Escherichia coli optimized by native hydrophobic interaction chromatography hyphenated to multi-angle light scattering detection. Talanta 2021, 235, 122691. [Google Scholar] [CrossRef]
- Müller, M.; Gibisch, M.; Brocard, C.; Cserjan-Puschmann, M.; Striedner, G.; Hahn, R. Purification of recombinantly produced somatostatin-28 comparing hydrochloric acid and polyethyleneimine as E. coli extraction aids. Protein Expr. Purif. 2024, 222, 106537. [Google Scholar] [CrossRef]
- Gibisch, M.; Müller, M.; Tauer, C.; Albrecht, B.; Hahn, R.; Cserjan-Puschmann, M.; Striedner, G. A production platform for disulfide-bonded peptides in the periplasm of Escherichia coli. Microb. Cell Factories 2024, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Mersha, F.B.; Comb, D.G.; Scott, M.E.; Landry, D.; Vence, L.M.; Perler, F.B.; Benner, J.; Kucera, R.B.; Hirvonen, C.A.; et al. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene 1997, 192, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.F.; Lorsch, J.R. Protein Affinity Purification using Intein/Chitin Binding Protein Tags. Methods Enzymol. 2015, 559, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pomorski, A.; Wu, S.; Peris-Díaz, M.D.; Czepczyńska-Krężel, H.; Krężel, A. The connection of α- and β-domains in mammalian metallothionein-2 differentiates Zn(II) binding affinities, affects folding, and determines zinc buffering properties. Metallomics 2023, 15, mfad029. [Google Scholar] [CrossRef]
- Petrišič, N.; Adamek, M.; Kežar, A.; Hočevar, S.B.; Žagar, E.; Anderluh, G.; Podobnik, M. Structural basis for the unique molecular properties of broad-range phospholipase C from Listeria monocytogenes. Nat. Commun. 2023, 14, 6474. [Google Scholar] [CrossRef]
- Volkmann, G.; Mootz, H.D. Recent progress in intein research: From mechanism to directed evolution and applications. Cell Mol. Life Sci. 2013, 70, 1185–1206. [Google Scholar] [CrossRef]
- Wood, D.W.; Belfort, M.; Lennon, C.W. Inteins-mechanism of protein splicing, emerging regulatory roles, and applications in protein engineering. Front. Microbiol. 2023, 14, 1305848. [Google Scholar] [CrossRef]
- Cui, C.; Zhao, W.; Chen, J.; Wang, J.; Li, Q. Elimination of in vivo cleavage between target protein and intein in the intein-mediated protein purification systems. Protein Expr. Purif. 2006, 50, 74–81. [Google Scholar] [CrossRef]
- Bleichner, L.; Eriksson, D.; Magnell, A.; Petersson, J. Self-Cleaving Tag Systems: The Future of Protein Purification. Bachelor’s Thesis, Uppsala University, Disciplinary Domain of Science and Technology, Biology, Biology Education Centre, Uppsala, Sweden, 2024. Available online: https://www.diva-portal.org/smash/get/diva2:1863493/FULLTEXT01.pdf (accessed on 31 May 2024).
- Anastassov, S.; Filo, M.; Khammash, M. Inteins: A Swiss army knife for synthetic biology. Biotechnol. Adv. 2024, 73, 108349. [Google Scholar] [CrossRef]
- Shepherd, C.; Lawson-Williams, M.; Holland, A.; Bello, A.J.; Sexton, D.W.; Olorunniji, F.J. Conditional Split Inteins: Adaptable Tools for Programming Protein Functions. Int. J. Mol. Sci. 2025, 26, 586. [Google Scholar] [CrossRef]
- Clifford, R.; Lindman, S.; Zhu, J.; Luo, E.; Delmar, J.; Tao, Y.; Ren, K.; Lara, A.; Cayatte, C.; McTamney, P.; et al. Production of native recombinant proteins using a novel split intein affinity technology. J. Chromatogr. A 2024, 1724, 464908. [Google Scholar] [CrossRef]
- Sevinsky, C.J.; Lundback, P.; Ohman, J.; Grossmann, G.; Dinn, S.R. Protein Purification Using a Split Intein System. Patent No. WO2021/099607 A1, 27 May 2021. Available online: https://patentimages.storage.googleapis.com/81/94/09/d1edc0d349710d/WO2021099607A1.pdf (accessed on 27 May 2021).
- Cytiva. CytivaTM Protein SelectTM Resin. WWW Document. 2024. Available online: https://www.cytivalifesciences.com/en/us/shop/chromatography/resins/affinity-tagged-protein/cytiva-protein-select-resin-p-43016?p (accessed on 28 August 2024).
- Cytiva. FAQs About CytivaTM Protein SelectTM Technology. WWW Document. 2024. Available online: https://www.cytivalifesciences.com/en/us/solutions/bioprocessing/knowledge-center/faqs-cytiva-protein-select-technology (accessed on 2 May 2024).
- Prabhala, S.V.; Mayone, S.A.; Moody, N.M.; Kanu, C.B.; Wood, D.W. A Convenient Self-Removing Affinity Tag Method for the Simple Purification of Tagless Recombinant Proteins. Curr. Protoc. 2023, 3, e901. [Google Scholar] [CrossRef]
- Fan, Y.; Miozzi, J.; Stimple, S.; Han, T.-C.; Wood, D. Column-Free Purification Methods for Recombinant Proteins Using Self-Cleaving Aggregating Tags. Polymers 2018, 10, 468. [Google Scholar] [CrossRef]
- Dassa, B.; London, N.; Stoddard, B.L.; Schueler-Furman, O.; Pietrokovski, S. Fractured genes: A novel genomic arrangement involving new split inteins and a new homing endonuclease family. Nucleic Acids Res. 2009, 37, 2560–2573. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Vallejos, P.; Pallisse, R.; Mootz, H.D.; Schmidt, S.R. Unprecedented rates and efficiencies revealed for new natural split inteins from metagenomic sources. J. Biol. Chem. 2012, 287, 28686–28696. [Google Scholar] [CrossRef]
- Knapp, M.; Kohl, V.; Best, T.; Rammo, O.; Ebert, S. Chromatographic single-step purification of tagless proteins using gp41-1 split inteins. Front. Bioeng. Biotechnol. 2024, 11, 1319916. [Google Scholar] [CrossRef]
- Beyer, J.N.; Serebrenik, Y.V.; Toy, K.; Najar, M.A.; Feierman, E.; Raniszewski, N.R.; Korb, E.; Shalem, O.; Burslem, G.M. Intracellular protein editing enables incorporation of noncanonical residues in endogenous proteins. Science 2025, 388, eadr5499. [Google Scholar] [CrossRef]
- Iwai, H.; Zuger, S.; Jin, J.; Tam, P.H. Highly efficient protein trans-splicing by a naturally split DnaE intein from Nostoc punctiforme. FEBS Lett. 2006, 580, 1853–1858. [Google Scholar] [CrossRef]
- Zettler, J.; Schütz, V.; Mootz, H.D. The naturally split Npu DnaE intein exhibits an extraordinarily high rate in the protein trans-splicing reaction. FEBS Lett. 2009, 583, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Valdes, N.; Guan, D.; Chen, Z. Engineering split intein DnaE from Nostoc punctiforme for rapid protein purification. Protein Eng. Des. Sel. 2013, 26, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Humberg, C.; Yilmaz, Z.; Fitzian, K.; Dörner, W.; Kümmel, D.; Mootz, H.D. A cysteine-less and ultra-fast split intein rationally engineered from being aggregation-prone to highly efficient in protein trans-splicing. Nat. Commun. 2025, 16, 2723. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arakawa, T.; Akuta, T. Beyond Purification: Evolving Roles of Fusion Tags in Biotechnology. Curr. Issues Mol. Biol. 2025, 47, 768. https://doi.org/10.3390/cimb47090768
Arakawa T, Akuta T. Beyond Purification: Evolving Roles of Fusion Tags in Biotechnology. Current Issues in Molecular Biology. 2025; 47(9):768. https://doi.org/10.3390/cimb47090768
Chicago/Turabian StyleArakawa, Tsutomu, and Teruo Akuta. 2025. "Beyond Purification: Evolving Roles of Fusion Tags in Biotechnology" Current Issues in Molecular Biology 47, no. 9: 768. https://doi.org/10.3390/cimb47090768
APA StyleArakawa, T., & Akuta, T. (2025). Beyond Purification: Evolving Roles of Fusion Tags in Biotechnology. Current Issues in Molecular Biology, 47(9), 768. https://doi.org/10.3390/cimb47090768






