1. Introduction
Tumor drug resistance remains one of the most formidable obstacles in cancer therapy. It markedly reduces treatment efficacy and severely compromises patients’ long-term prognosis and quality of life [
1]. In recent years, the tumor immune microenvironment (TIME) has emerged as a major contributor to therapeutic resistance, with immune evasion and immunosuppressive conditions playing pivotal roles in tumor progression. In addition, a variety of biological processes (including epigenetic dysregulation, extracellular vesicle-mediated drug efflux, and malfunction of membrane transporters) have been identified as key drivers of resistance across multiple malignancies [
2,
3]. These interconnected mechanisms contribute to the significant heterogeneity observed in treatment responses and present major challenges for traditional predictive models.
Conventional biomarker-based approaches often rely on single-gene indicators, which are insufficient to capture the complex, multifactorial nature of drug resistance phenotypes. In contrast, the advent of high-throughput transcriptomic technologies, particularly bulk and single-cell RNA sequencing, coupled with artificial intelligence (AI) methodologies has enabled the development of models capable of capturing high-dimensional gene expression patterns to more comprehensively predict therapeutic resistance [
4].
Recent advances in foundation models, such as scFoundation and Geneformer, have demonstrated strong capabilities in extracting biologically meaningful transcriptional embeddings from single-cell data [
5]. Building upon these representations, deep learning architectures such as variational autoencoders (VAEs) [
6], as well as ensemble machine learning algorithms including Random Forest (RF) and eXtreme Gradient Boosting (XGB) [
7], have shown promise in integrating bulk RNA-seq data for drug sensitivity prediction.
However, most prior studies have primarily focused on transferring transcriptomic features between bulk and single-cell datasets without incorporating real-world clinical outcomes [
8]. In clinical practice, patient-level variables (such as first-line therapeutic regimens, treatment responses, and survival outcomes) are essential for model validation and translational relevance. These variables, however, remain underutilized in many current computational approaches [
9]. Moreover, few AI-based frameworks directly combine drug resistance prediction with prognosis modeling at the patient level.
To address these limitations, we developed three novel deep learning models based on scATD and benchmarked them against two widely used classical methods. The proposed models include the following: (i) the LLM-large VAE (VAE_LL), a variational autoencoder constructed using scFoundation-derived transcriptomic features; (ii) the LLM-small VAE VAE_LS), which leverages Geneformer embeddings; (iii) the LLM-distillation VAE (VAE_LD), a residual-structured autoencoder optimized through a knowledge distillation strategy. In addition, RF and XGB were used as baseline ensemble learning models. All five models were trained using the Genomics of Drug Sensitivity in Cancer (GDSC) dataset, which encompasses 72 chemotherapeutic agents and represents a diverse range of tumor types and resistance profiles. Based on cross-validated performance metrics, the four best-performing models (VAE_LL, VAE_LD, RF, and XGB) were selected for subsequent clinical validation [
10].
For external evaluation, these four models were applied to bulk RNA-seq and clinical survival data from five representative cancer types in The Cancer Genome Atlas (TCGA), namely lung adenocarcinoma (LUAD,
n = 589), glioblastoma (GBM,
n = 175), acute myeloid leukemia (LAML,
n = 151), melanoma (SKCM,
n = 473), and stomach adenocarcinoma (STAD,
n = 448), resulting in a total cohort of 1836 patients. For each cancer type, resistance to nine clinically relevant first-line drugs (Cediranib, Dabrafenib, Dinaciclib, Entinostat, Foretinib, Gefitinib, Temozolomide, Trametinib, and AZD2014) was modeled, yielding a total of 180 drug–cancer prediction tasks [
11]. These drugs span multiple therapeutic categories, including anti-angiogenic agents, cell cycle inhibitors, epigenetic regulators, DNA damage response modulators, and inhibitors of key oncogenic signaling pathways, thereby providing a comprehensive platform for biomarker discovery and resistance stratification.
Given the complexity of gene–gene interactions in drug response, we further incorporated explainable AI (XAI) methodologies, including Integrated Gradients, GradientSHAP, and TreeSHAP, to interrogate the molecular mechanisms underlying each model’s predictions [
12,
13]. The identification of key features was supported by gene importance ranking, interaction network construction, and pathway enrichment analysis, which together reinforced the biological plausibility of the identified biomarkers.
Across various tumor–drug contexts, the models demonstrated robust predictive accuracy and reproducible biomarker identification. In LUAD, for example, elevated expression of
SFTPC, a marker of the terminal respiratory unit (TRU) subtype, was associated with resistance to Cediranib [
14]. Although
SFTPC is generally considered a favorable prognostic indicator, our results suggest that its high expression may confer intrinsic resistance by promoting vascular normalization and reducing dependence on VEGF-A signaling, a key target of Cediranib, which primarily inhibits VEGFR-1, -2, and -3 [
15]. Dysregulated VEGF–VEGFR signaling is a canonical driver of therapeutic resistance at the interface of tumor vasculature and immunity. Excess VEGF fosters structurally and functionally abnormal vessels that aggravate hypoxia, hinder the intratumoral delivery of cytotoxic and targeted agents, and reinforce immune escape by limiting effector cell trafficking and antigen presentation. Conceptually, restoring vessel structure and function through vascular normalization can reopen a therapeutic window in which perfusion and oxygenation are improved, thereby potentiating the activity of chemotherapy, radiation therapy, and immune checkpoint blockade [
16]. At the same time, resistance to anti-angiogenic strategies frequently emerges and may be shaped by isoform-level heterogeneity within the VEGF family, exemplified by VEGF165b, an alternatively spliced, anti-angiogenic variant that competes for VEGFR binding and can attenuate pro-angiogenic signaling. These considerations underscore the need to model VEGF pathway dependence when predicting drug resistance and to interpret anti-angiogenic contexts alongside immune and stromal features [
17]. Prognostic biomarkers do not necessarily translate into predictive markers for treatment response, and therapeutic pathway dependencies must be accounted for when designing individualized treatment strategies.
In summary, the VAE_LD architecture proposed in this study demonstrated the best overall performance, achieving accurate prediction of multidrug resistance across both cell line and patient-derived datasets. Beyond predictive accuracy, this study provides a unified and interpretable pipeline that enables systematic identification of resistance-associated biomarkers and facilitates the elucidation of their underlying biological mechanisms.
3. Results
We first benchmark the five models on pharmacogenomic cell-line data to establish predictive performance and feature stability. We then extend the best performers to TCGA cohorts to explore cross-tumor applicability and to generate biomarker hypotheses in clinically relevant settings. Finally, we interrogate tumor- and drug-specific mechanisms through interpretability, network analyses, pathway enrichment, and prognostic stratification.
3.1. Drug Resistance Prediction and Key Gene Identification in Cell Lines
This subsection evaluates whether residual VAE variants and ensemble learners can accurately classify resistance in vitro and yield stable feature attributions. We use five-fold cross-validation on GDSC to compare discrimination, calibration, and robustness across drugs, and we examine concordance of gene-level importance between deep and classical models.
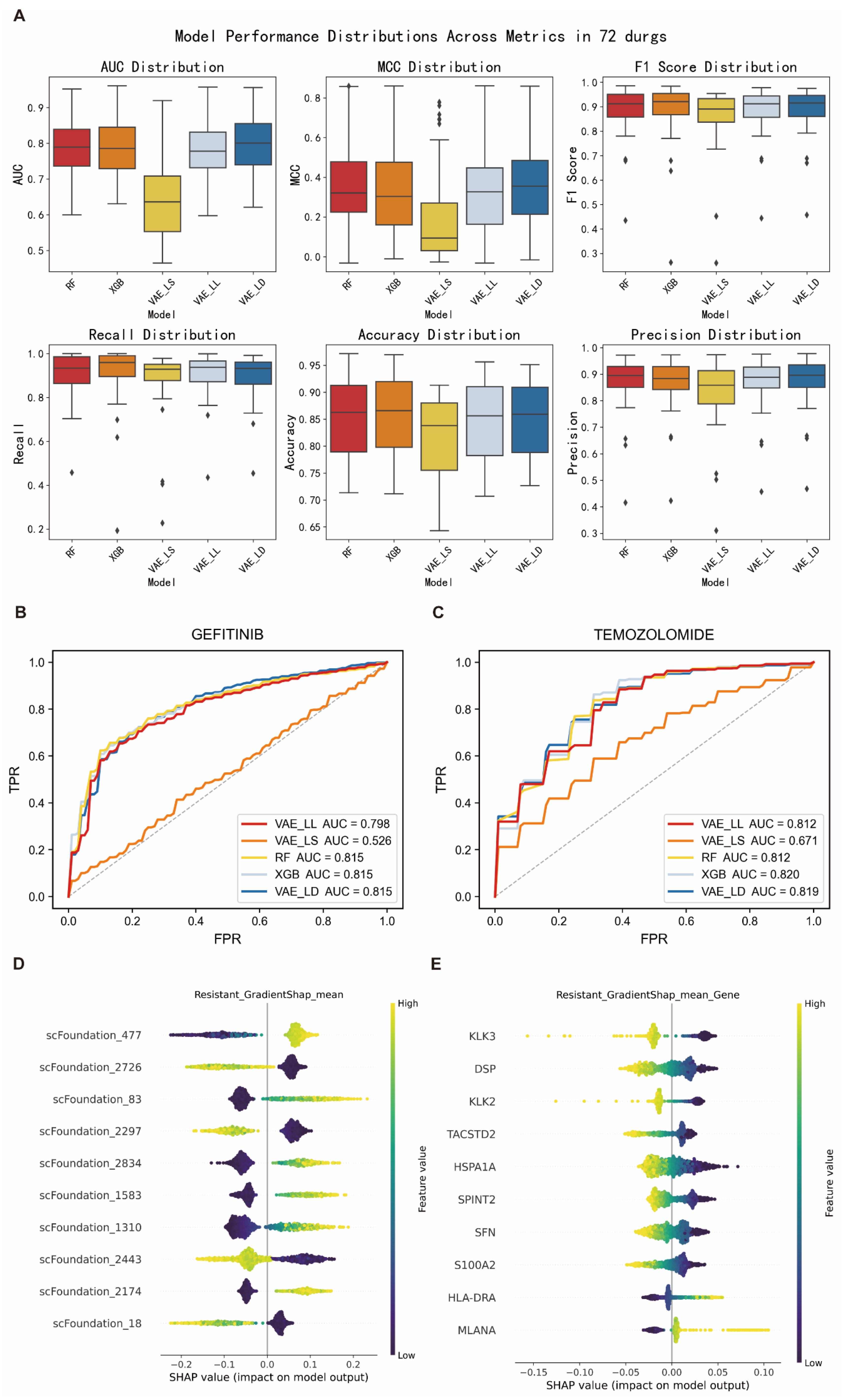
To systematically evaluate the performance of different machine learning frameworks in predicting tumor drug resistance, we employed five-fold cross-validation on the GDSC cell line drug sensitivity dataset. The dataset was split into training and validation sets, and the performance of five models (VAE_LD, VAE_LS, VAE_LL, RF, and XGB) was compared using six evaluation metrics: AUC, MCC, F1-score, recall, accuracy, and precision.
The results showed that VAE_LD outperformed all other models across all metrics, achieving an average AUC of 0.81, MCC of 0.37, F1-score of 0.89, recall of 0.91, accuracy of 0.86, and precision of 0.88 (
Figure 2A,
Table 1). It also demonstrated stable performance across nine commonly used anticancer drugs (
Table 2 and
Supplementary Figure S1). Furthermore, VAE_LD consistently achieved the highest average AUC across individual drugs (
Figure 2B,C). The two traditional machine learning models, RF and XGB, also exhibited comparable and robust performance. In contrast, the conventional deep learning model VAE_LS performed significantly worse across all evaluation metrics, with a median AUC of only 0.65, suggesting its limited ability to capture key features related to drug resistance. Consequently, VAE_LS was excluded from further analysis, and VAE_LD was selected as the primary model for subsequent interpretability studies.
Given that the VAE_LD model does not support direct feature attribution, we employed its backbone architecture, VAE_LL, to perform feature-level interpretability analysis using IG and GradientSHAP algorithms. In the context of gefitinib resistance prediction, the latent feature “scFoundation_477” was consistently identified as the most important positive predictor, while “scFoundation_2726” showed a strong negative correlation with resistance, it was markedly reduced in resistant samples and elevated in sensitive ones (
Figure 2D and
Supplementary Figure S2). This directional consistency supports the biological relevance of the model-learned features.
To further interpret these latent features, we mapped them back to specific genes and identified three key genes:
KLK3,
TACSTD2, and
PAGE4. Previous studies have shown that
KLK3 plays a critical role in inhibiting prostate cancer progression and restoring chemotherapy sensitivity [
19], while
PAGE4 has been reported to significantly enhance tumor cell responsiveness to gefitinib and other therapies [
20] (
Figure 2E and
Supplementary Figure S2). These three genes were consistently ranked as the most important across both IG and GradientSHAP analyses, reinforcing the robustness of the model’s interpretability.
To explore the biological context of these key genes, we conducted GO and KEGG pathway enrichment analyses. The results revealed that DSP, KRT19, and members of the S100 family were predominantly enriched in pathways related to epithelial–mesenchymal transition (EMT) [
21], cell adhesion, and migration, while HSPA1A and SFN were enriched in apoptosis-related pathways [
22]. Additional significantly enriched pathways included those related to angiogenesis, lymphangiogenesis, and Wnt signaling regulation, all of which are highly relevant to the development of drug resistance.
The VAE_LD model not only demonstrated superior predictive performance in drug resistance modeling but also enabled the identification of biologically meaningful features and key regulatory genes through interpretability analysis. These findings create a closed loop among molecular mechanisms, model attributions, and potential translational applications, providing a solid foundation for further exploration of tumor heterogeneity and personalized cancer treatment strategies.
3.2. Identification of Drug Sensitivity Biomarkers Across Multiple Cancers in a Clinical Prognostic Context
To examine clinical relevance, we transferred the best-performing models to TCGA cohorts spanning five tumor types. Here we assess whether gene signatures prioritized in cell lines retain signal in patient transcriptomes and whether they stratify prognosis, recognizing that these analyses are exploratory in the absence of direct treatment-response data.
In clinical applications, the identification of robust biomarkers is essential for elucidating cancer heterogeneity and evaluating the translational potential of predictive models. Our previous analyses were primarily conducted using the GDSC dataset, which provides drug sensitivity data derived from cancer cell lines. While this resource is valuable for model training and mechanistic exploration, it lacks critical components such as the tumor microenvironment, thereby limiting its ability to fully capture actual drug responses observed in patients.
To address this limitation, we incorporated data from The Cancer Genome Atlas (TCGA), which more accurately reflects clinical reality, particularly in terms of patient heterogeneity and its relevance to precision medicine. VAE_LD demonstrated strong predictive performance and interpretability on the GDSC training set. To further assess its predictive capability in clinically relevant settings, we applied the model to RNA-Seq data from five cancer types in the TCGA cohort: GBM (n = 175), LUAD (n = 589), LAML (n = 151), SKCM (n = 473), and STAD (n = 448).
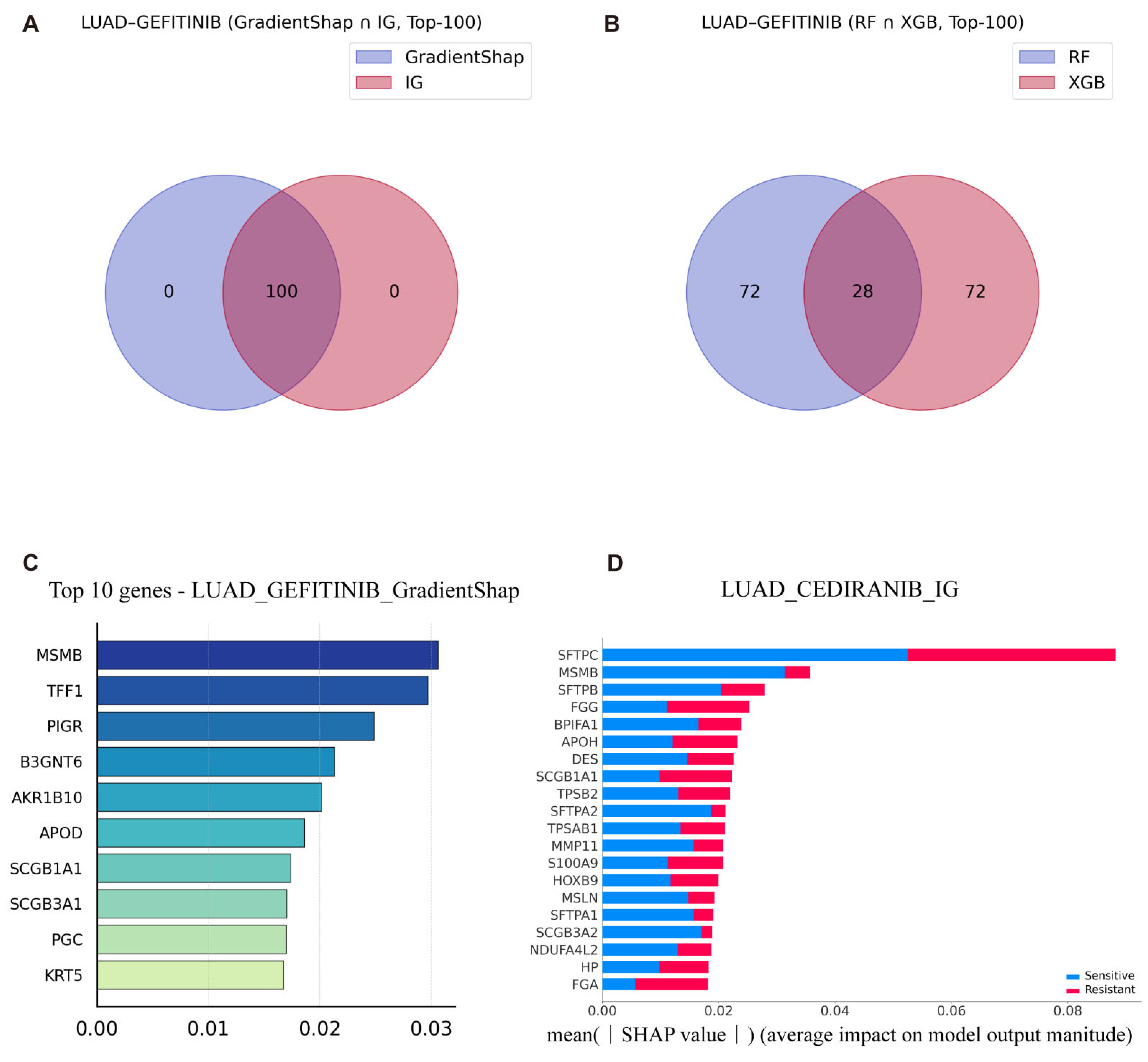
To maintain consistency with our prior predictive analyses, we focused in particular on LUAD patient data from TCGA to investigate the molecular mechanisms underlying gefitinib resistance. This approach enhances the clinical relevance of the identified biomarkers and supports their potential utility in guiding individualized therapeutic strategies. We first used the intersection of the T100 genes selected based on different interpretability methods to analyze gefitinib-related drug resistance in LUAD patients (
Figure 3A,B).
Gefitinib is most commonly used for the treatment of EGFR-mutant LUAD patients. By comparing the interpretability results of the VAE_LD model using IG and GradientSHAP with those of traditional machine learning models (RF with TreeSHAP, and XGB), we identified key genes implicated in drug resistance, including
TFF1 and
B3GNT6 (
Figure 3C and See
Supplementary Figures S3 and S4 for details).
Previous studies have reported that elevated expression of
TFF1 promotes cell proliferation and survival in LUAD and is significantly associated with shorter overall survival [
23]. Consistently, both IG and GradientSHAP interpretability analyses indicated that high expression of
TFF1 (highlighted in yellow for strong enrichment) positively contributes to gefitinib resistance. Similarly,
B3GNT6 was also identified by both interpretability methods as a strong positive regulator of resistance. Huang et al. previously demonstrated that elevated
B3GNT6 expression is closely linked to LUAD progression [
24]. In patients treated with EGFR-TKIs, it has been shown that TKIs modulate the MUC1 glycosylation axis, in which
B3GNT6 acts as a key regulator. MUC1 glycan isomerization has been implicated in altering EGFR recycling and promoting immune evasion, thereby contributing to the development of a drug-resistant tumor microenvironment. Notably, elevated expression of
B3GNT6 is a hallmark of this glycan isomerization process, directly supporting VAE_LD model’s prediction that
B3GNT6 promotes gefitinib resistance in LUAD patients.
Although machine learning models have demonstrated high predictive accuracy in previous studies, whether their interpretability matches that of deep learning models remains unclear. To address this, we performed an interpretability analysis using XGB and RF in combination with TreeSHAP. Interestingly, the results yielded contradictory conclusions compared to those from deep learning models, and this inconsistency was observed across multiple datasets (See
Supplementary Figures S3 and S4 for details). In the context of resistance prediction,
IRF6 emerged as a key gene. While previous studies have shown that
IRF6 suppression contributes to acquired drug resistance in tumors [
25], TreeSHAP attributed a role for
IRF6 that contradicted these findings. This discrepancy highlights the potential limitations of interpretability in machine learning models, when applied to complex biological datasets. These results underscore the need for caution when relying solely on traditional interpretability tools in high-dimensional, biologically heterogeneous contexts.
Additionally, to explore drug resistance beyond gefitinib in LUAD, we analyzed resistance mechanisms associated with cediranib. Both IG and GradientSHAP identified key genes involved in cediranib resistance, including
SFTPC,
FGG,
AZGP1, and
FGA (
Figure 3D and See
Supplementary Figures S3 and S4 for details). Previous studies have shown that low expression of
SFTPC promotes cell proliferation and epithelial–mesenchymal transition (EMT) in LUAD and is associated with shorter overall survival [
26]. However, both IG and GradientSHAP analyses indicated that high expression of
SFTPC (visualized as yellow for high enrichment) positively regulates cediranib resistance. This finding is particularly intriguing. Deeper mechanistic studies revealed that
SFTPC is a marker of alveolar type II (AT2) cell differentiation, and its high expression suggests that tumors retain features of the TRU subtype [
27]. TRU-type tumors typically exhibit high vascular maturity, low microvessel density, and reduced dependency on VEGF-A. Given that cediranib primarily targets VEGFR-1/2/3, tumors with these characteristics may develop intrinsic resistance. Thus, high
SFTPC expression may promote cediranib resistance in LUAD, even in patients with otherwise favorable prognostic features. This underscores that genes associated with good prognosis do not necessarily imply drug sensitivity and must be interpreted within the context of specific therapeutic mechanisms.
Similarly, both interpretability methods identified
FGA as a strong positive regulator of cediranib resistance. Shang et al. previously reported that high
FGA expression in EGFR-mutant LUAD is negatively correlated with chemotherapy response, directly supporting our findings that
FGA contributes to cediranib resistance [
28]. In the cediranib sensitivity analysis, both IG and GradientSHAP further confirmed that high
SFTPC expression negatively regulates drug sensitivity, providing additional validation of the model’s accuracy. Both interpretability approaches consistently ranked
SFTPC among the top 20 most important genes, with low expression favoring drug sensitivity and high expression promoting resistance.
3.3. Biomarker Analysis for Temozolomide Response in GBM
As a focused exemplar, we analyze temozolomide in GBM to link model predictions with gene-level mechanisms. We integrate attributions from deep and classical models to highlight convergent and divergent biomarkers and to position these signals within the GBM literature.
Biomarkers play a critical role in predicting tumor response to treatment, and the identification of effective biomarkers enables the accurate selection of appropriate treatment candidates in advance. In this study, the deep learning model VAE_LD was integrated with two interpretability algorithms, IG and GradientSHAP. These algorithms operate directly on gene expression data and provide joint visualization of predictive results and the contributing genes. In addition, the simultaneous presentation of two traditional machine learning models further enabled a comparative evaluation of their performance in predicting tumor drug resistance and in generating interpretable outputs. This integrative framework provides a robust approach for biomarker discovery and enhances the clinical applicability of predictive models in oncology.
Temozolomide (TMZ) is a first-line chemotherapeutic agent for GBM [
29]. Using this drug as an example, we conducted an in-depth analysis of predictive biomarkers in GBM patients treated with TMZ from the TCGA database, utilizing the aforementioned models and interpretability algorithms. Both VAE_LD and IG/GradientSHAP identified
OPALIN,
LTF, and
IL2RA as the most important genes (
Figure 4A). These genes ranked 3/19,264, 9/19,264, and 5/19,264, respectively, in the resistance prediction group, and 2/17,006, 8/17,006, and 1/17,006 in the sensitivity prediction group (
Figure 4B,C,
Table 3). Among these,
OPALIN was consistently ranked highest and showed a strong degree of symmetry across both resistance and sensitivity predictions.
Previous studies have shown that
OPALIN is highly enriched in adult brain tissue and is primarily involved in oligodendrocyte differentiation [
30]. However, its role in GBM remains poorly characterized. One study reported a significant association between
OPALIN expression and decreased Karnofsky Performance Status (KPS) scores in elderly GBM patients, while no such association was found in younger patients. This suggests that
OPALIN may not directly mediate drug resistance, but instead may reflect cell differentiation status in the brain.
In contrast,
LTF displayed a more asymmetric pattern, with stronger contributions to resistance predictions (
Figure 4D). Prior research has demonstrated a significant positive correlation between
LTF overexpression and poor prognosis in GBM patients, as well as a strong association with immune evasion, thus supporting the biological relevance and accuracy of the model’s predictions [
31].
Additionally,
SLC17A7 emerged as a top-ranked gene in the sensitivity group (
Figure 4E), consistent with previous findings.
SLC17A7 is considered a tumor suppressor, and its overexpression has been shown to inhibit GBM cell proliferation and invasion [
32].
Since TreeSHAP and XGB are based on binary classification frameworks, their interpretability outputs are generally symmetric. The RF model combined with TreeSHAP identified
TRPM7,
CHODL, and
SMAP2 as the most important genes (See
Supplementary Figures S3–S7 for details). In the context of drug resistance,
TRPM7 was suggested by TreeSHAP to be lowly expressed in GBM and associated with resistance alleviation (
Figure 4F). However, this finding is inconsistent with prior studies. Liu et al. reported that
TRPM7 is highly expressed in GBM and promotes both proliferation and resistance, primarily by upregulating tumor-associated stem cell markers [
33]. Consistent with this, the XGB model identified
TRPM7 as highly expressed and positively associated with drug resistance (See
Supplementary Figures S3–S7 for details). Comprehensive analyses of other cancer types and their corresponding drugs are included in the
Supplementary Materials and are not discussed in detail here. (See
Supplementary Figures S3–S7 for details).
3.4. Functional Network and Pathway Analysis of Temozolomide-Associated Biomarkers
To contextualize candidate genes, we map them onto interaction networks and biological processes. We test whether pathways implicated by interpretability methods converge on coherent programs related to angiogenesis, immune trafficking, and neural–tumor interactions that may underlie resistance.
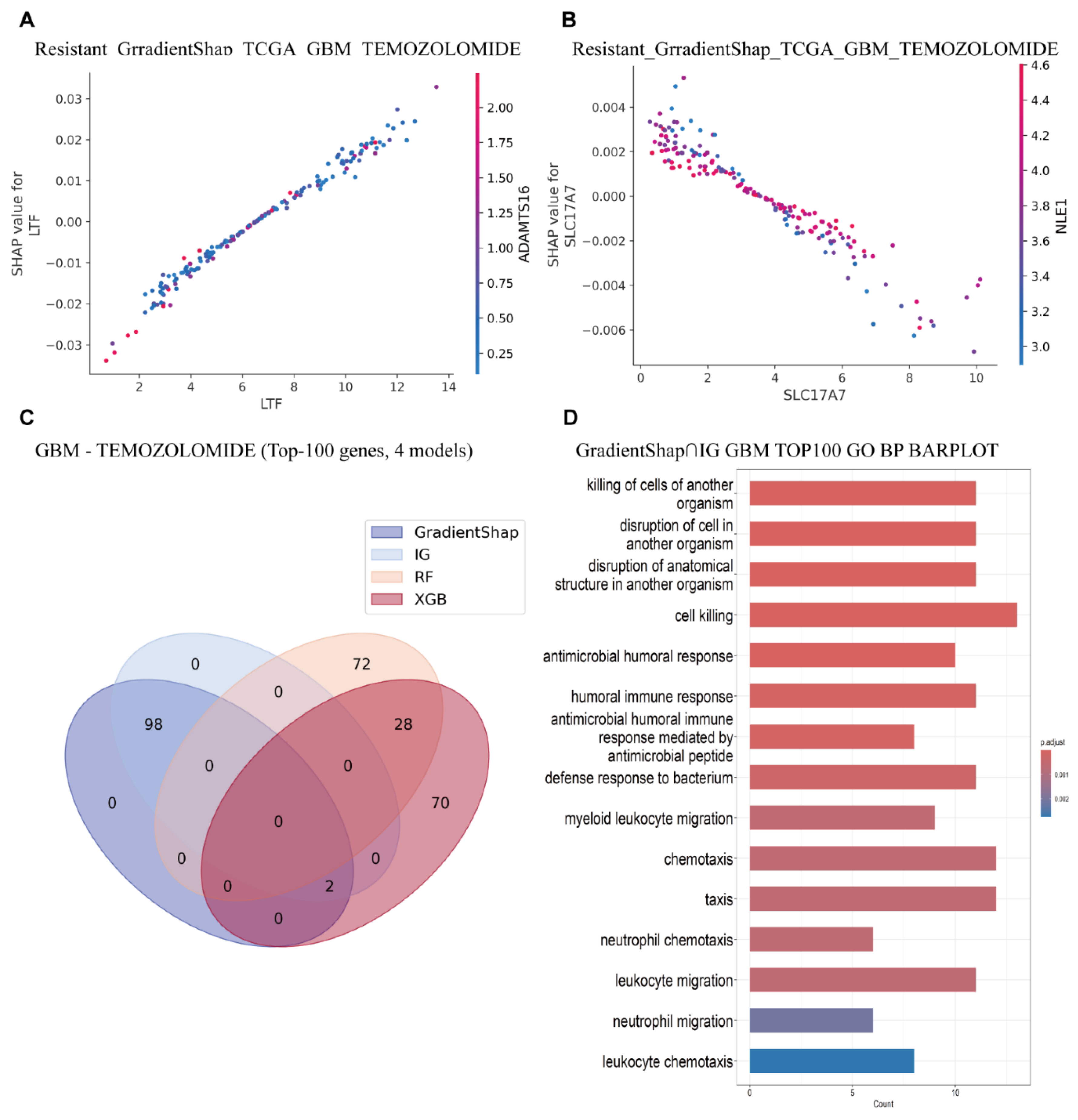
To further investigate the role of key genes in gene interaction networks and pathway enrichment, we employed the VAE_LD model in combination with IG/GradientSHAP and conventional machine learning algorithms. The four most important genes identified in the previous analysis were selected for gene interaction analysis. This revealed a particularly strong interaction between
LTF and
ADAMTS16, which appear to cooperatively contribute to GBM drug resistance (
Figure 5A and See
Supplementary Figures S8–S10 for details).
ADAMTS16 has been previously shown to drive epithelial–mesenchymal transition (EMT) and metastasis in various cancers, ultimately leading to the development of drug resistance [
34].
In addition, we observed an antagonistic relationship between
SLC17A7 and
NLE1, wherein high expression of
NLE1 appears to suppress
SLC17A7, thereby contributing to chemotherapy resistance (
Figure 5B and See
Supplementary Figures S8–S10 for details). Interestingly, previous studies have reported that
NLE1 is a critical regulator of brain tumor stem cell growth and survival in GBM. Targeting
NLE1 has been shown to inhibit stemness features and restore the sensitivity of GBM cells to radiotherapy [
35]. These findings suggest that elevated
NLE1 expression may downregulate
SLC17A7, thus promoting treatment resistance.
Based on the intersection of T100 genes selected by four interpretable methods, it was found that the T100 genes selected by the IG/GradientSHAP interpretable method were completely consistent. However, there were significant differences between the T100 genes selected by the two machine learning models (
Figure 5C).
Furthermore, to elucidate potential mechanisms of action, we selected the top 100 most important genes based on the VAE_LD model combined with IG and GradientSHAP, and conducted GO pathway enrichment analyses. GO enrichment results from both interpretability algorithms revealed consistent enrichment in pathways related to cell killing/antimicrobial defense, granulocyte/myeloid chemotaxis, synaptic vesicle endocytosis, and glial cell development. These findings suggest that necrosis- and inflammation-driven innate immune storms, in conjunction with neuro-tumor interactions, are prevalent in GBM, and are associated with poor prognosis, radio/chemotherapy resistance, and an immunosuppressive microenvironment (
Figure 5D). This insight supports several promising therapeutic strategies, such as CXCL8-CXCR2 axis inhibition, disruption of tumor–neuron synapses, and targeting of reactivated developmental pathways [
36].
Moreover, intersecting the most important genes identified by both interpretability algorithms yielded highly consistent enrichment patterns, particularly in cell killing and granulocyte/myeloid chemotaxis, further confirming their association with treatment resistance and adverse clinical outcomes.
In contrast, the two machine learning models produced differing enrichment profiles. The XGB algorithm primarily highlighted pathways associated with RNA/DNA editing, viral replication inhibition, vascular endothelial activation, neuronal dendritic self-avoidance, and immune enhancement. These features suggest that the corresponding patient subtype may be more responsive to interferon-based adjuvant therapies, oncolytic viruses, anti-angiogenic therapies, and immune checkpoint inhibitors, although they differ substantially from the pathways expected based on prior knowledge (See
Supplementary Figures S8–S10 for details). The TreeSHAP algorithm identified additional pathways, including Rac-GTPase signaling and cytoskeletal remodeling, postsynaptic structure assembly, and neuronal coupling, which are plausibly linked to drug resistance mechanisms (See
Supplementary Figures S8–S10 for details).
Overall, these findings suggest that the VAE_LD deep learning model, in combination with interpretable algorithms, provides superior accuracy in pathway-level enrichment analysis compared to traditional machine learning approaches. Analyses for additional tumor types and drug response patterns are provided in the
Supplementary Materials. (See
Supplementary Figures S8–S10 for details).
3.5. Prognostic Risk Analysis in GBM Patients Based on Gene Biomarkers
The next analysis evaluates whether gene sets derived from model attributions stratify overall survival in GBM. Compact and extended panels are compared to balance statistical power with clinical feasibility and to assess the portability of transcriptomic risk scores.
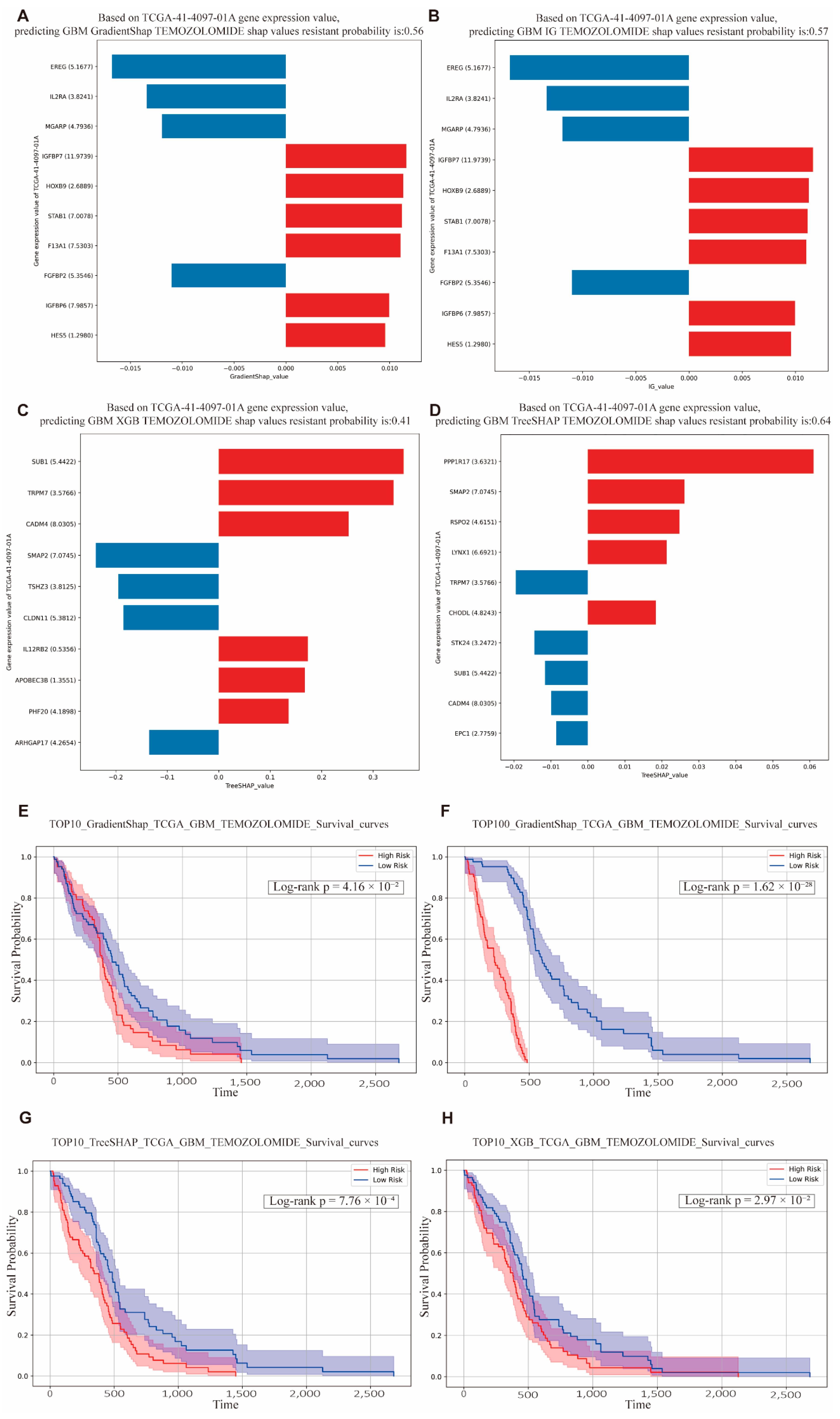
Based on the aforementioned model, we selected the five GBM patients with the longest survival times and the patient with the shortest survival time for detailed predictive drug response analysis. Among the patients with the shortest survival durations, the VAE_LD model combined with IG/GradientSHAP predicted drug resistance probabilities of 0.56 and 0.57, respectively, indicating a high degree of consistency (
Figure 6A,B). Importantly, the key contributing genes were also consistent across these cases, with
IGFBP7 emerging as the most influential gene in resistance prediction. Previous studies have demonstrated that
IGFBP7 is significantly associated with poor prognosis in GBM and promotes tumor angiogenesis, thereby contributing to drug resistance [
37,
38].
In comparison, machine learning models XGB and TreeSHAP predicted drug resistance probabilities of 0.41 and 0.64, respectively (
Figure 6C,D). Interestingly, although
SUB1 was not the most highly expressed gene in the XGB model, it was identified as a key contributor to resistance. Prior studies have shown that
SUB1 is upregulated in GBM and enhances tumor cell proliferation and migration. According to TreeSHAP, the gene
PPP1R17, despite its relatively low expression, accounted for a substantial proportion of resistance contribution. Research by Tokizane et al. found that neurons with high
Ppp1r17 expression are associated with aging phenotypes, and inhibition of
Ppp1r17 can alleviate neurological symptoms and extend lifespan in mouse models [
39]. This suggests a potential mechanism by which GBM may promote drug resistance through aging-related pathways involving
PPP1R17. Predicted drug resistance probabilities for other patients with short survival times also ranged between 0.5 and 0.9, reflecting substantial inter-individual variability.
Among the five patients with the longest survival durations, the VAE_LD and IG/GradientSHAP model predicted drug sensitivity probabilities of around 0.45 in some patients (
Supplementary Figure S11), while others showed predicted probabilities as high as 1.0 (
Supplementary Figure S11), again indicating pronounced individual variation. In patients with a predicted sensitivity probability of 1.0, the most important genes identified were
NOTCH3,
SOD3, and
NR2E1.
NOTCH3 is mainly involved in angiogenesis and is expressed in brain tissues. While it functions as an oncogene in many cancer types, some studies have reported a tumor-suppressive role. In the context of GBM,
NOTCH3 expression has been detected in some drug-resistant strains, while absent in others. These findings suggest that high
NOTCH3 expression may sensitize GBM cells to chemotherapy, although the role remains context-dependent [
40,
41].
SOD3 and
NR2E1 are known tumor suppressor genes, and both have been previously implicated in GBM, supporting their role in promoting treatment sensitivity.
The interpretable algorithms of the two machine learning models predicted sensitivity probabilities of 0.61 (TreeSHAP) and 0.69 (XGB) (
Supplementary Figure S11), respectively. The associated gene contributions also varied significantly. In XGB,
CIAPIN1 was found to be highly expressed and identified as a major regulator of apoptosis. Its elevated expression correlated positively with treatment sensitivity. Conversely, the TreeSHAP model considered
CIAPIN1 to be a non-contributory gene and instead highlighted
APOBEC3D, which is known to drive tumor resistance [
42]. This discrepancy persisted across repeated evaluations and may reflect model-specific differences in feature attribution. Additional analyses of drug sensitivity and resistance across other tumor types are provided in the
Supplementary Materials. (See
Supplementary Figure S11 for details).
In the TCGA-based survival analysis, the top 10 and top 100 genes identified by the models were used to construct gene sets, where high expression levels indicated high risk, and low expression levels indicated low risk. The results showed that, regardless of the algorithm applied, patients in the high-risk group exhibited significantly lower overall survival compared to those in the low-risk group, with
p values well below 0.05 (
Figure 6E). Moreover, the top 100 gene (T100) sets yielded even more statistically significant survival differences (
Figure 6F).
These findings suggest that the gene sets identified by the VAE_LD-based model and its associated interpretability algorithms (IG/GradientSHAP) can serve as robust prognostic biomarkers for predicting poor clinical outcomes.
Similarly, the top 10 and top 100 gene sets derived from the TreeSHAP and XGBoost (XGB) algorithms also showed significant differences in survival between risk groups (
p < 0.05) (
Figure 6G,H). While gene sets containing 100 genes provide increased statistical power due to the larger number of included features, their clinical utility is limited by the practical challenges of obtaining and analyzing large gene expression panels in routine clinical settings. In contrast, the top 10 gene sets offer a more feasible and clinically applicable solution, while still maintaining strong statistical significance (
Table 4). These smaller, high-impact gene panels hold promise for implementation in precision oncology workflows. Prognostic analyses for additional cancer types are provided in the
Supplementary Materials. (See
Supplementary Figure S12 for details).
4. Discussion
This study integrates large scale transcriptomic modeling with interpretable artificial intelligence to tackle the heterogeneity of tumor drug resistance in both preclinical and clinical settings. Five distinct models (VAE_LL, VAE_LS, RF, XGB, and VAE_LD) were retrained on the GDSC cell line dataset containing 72 chemotherapeutic agents, enabling the capture of broad resistance patterns across diverse tumor contexts. The four best performing models, selected according to predictive metrics, were then validated in five TCGA cancer cohorts with a total of 1836 patients. For each cancer type, response to nine clinically relevant first line drugs was modeled, resulting in 180 prediction tasks that span all drug and cancer combinations. This multi-level experimental design provides a pragmatic bridge from in vitro drug sensitivity profiling to clinically grounded biomarker discovery and risk stratification, with resulting clinical inferences considered hypothesis-generating rather than confirmatory.
VAE_LD, which applies a knowledge distillation strategy, achieved the highest accuracy, F1 score, and AUC on the GDSC training set (average AUC 0.81 and F1 score 0.92). These results suggest potential generalizability within preclinical settings, with clinical generalizability contingent on validation in treatment-annotated cohorts. By adapting the Deep learning transferred framework to fit bulk RNA sequencing, we enabled efficient transfer of models trained on cell lines to patient cohorts. This methodological adaptation underscores the flexibility of the framework and its potential translational relevance; however, real-world clinical utility remains to be established.
To ensure biological interpretability, we applied Integrated Gradients, GradientSHAP, and TreeSHAP to interrogate feature importance and reveal underlying mechanisms. In glioblastoma treated with Temozolomide, the models prioritized
OPALIN,
LTF,
IL2RA, and
SLC17A7 as candidate resistance related genes. Although these genes have known roles in tumor biology, their specific contributions to Temozolomide resistance are not well defined, suggesting the need for further experimental verification [
43] and indicating that these signals should be interpreted as hypothesis-generating. Gene interaction network analysis provided another layer of support. For instance, interactions between
LTF and
ADAMTS16 in glioblastoma indicate a possible role in epithelial to mesenchymal transition. Functional enrichment pointed to granulocyte recruitment, angiogenesis, and cancer stemness, reinforcing the biological plausibility of the predicted biomarkers.
Beyond correlation, several features support a causal role for the highlighted markers in context. In LUAD, TFF1 and B3GNT6 converge on epithelial differentiation and mucin-linked receptor trafficking, a biology that can modulate EGFR-TKI dependence and thereby rationalize resistance to Gefitinib. In GBM, LTF and IL2RA align with immune-evasive and angiogenic programs that are mechanistically plausible mediators of Temozolomide response, whereas SLC17A7 tracks with neuronal differentiation and has been reported to oppose proliferative signaling, consistent with a sensitivity-associated role. OPALIN behaves as a lineage marker, suggesting that lineage state rather than direct effector function may underlie its association. These convergences across pathway level, lineage context, and established drivers argue for biological plausibility. However, patient-level signals remain hypothesis-generating and require orthogonal validation in treatment-annotated cohorts and experimental perturbations to establish causality.
VAE_LS consistently underperformed relative to VAE_LL, VAE_LD, RF, and XGB. Possible reasons include limited sample size, uneven RNA sequencing quality, and mismatch between model complexity and dataset scale. Validation in larger, higher quality datasets will be necessary to refine architecture choices and confirm these observations [
44,
45]. Classical machine learning models such as RF and XGB delivered stable performance and, when coupled with TreeSHAP, successfully identified biologically meaningful genes including
TRPM7,
CHODL, and
SMAP2. These genes were enriched in pathways related to angiogenesis, epithelial to mesenchymal transition, and immune regulation. This finding underscores the value of ensemble methods for mechanistic discovery, even if their predictive metrics are slightly lower than those of VAE_LD [
46]. However, such models may struggle in complex clinical contexts because they rely on one dimensional gene features and do not fully capture inter patient heterogeneity. In contrast, deep learning frameworks, especially VAE based architectures, excel at learning non-linear representations and integrating modular biological signals, which makes them better suited for multiomics integration and interpretation.
Moreover, there are several shared limitations that warrant further clarification. First, the study depends on GDSC derived cell line data that mainly includes traditional chemotherapeutic agents and does not incorporate modern immunotherapies. Second, TCGA cohorts lack comprehensive treatment exposure and response annotations, and often exhibit variable sequencing quality, which means our external analyses are correlative and should be viewed as hypothesis-generating rather than confirmatory [
47]. GDSC offers high-throughput pharmacogenomic measurements in immortalized cell lines under controlled conditions, which facilitates comparative modeling but does not recapitulate stromal interactions, immune contexture, or pharmacokinetics in patients; plate conditions, assay protocols, and release-to-release differences can introduce technical heterogeneity that may affect generalizability. TCGA provides large, multi-center bulk transcriptomes with survival follow-up but lacks standardized, patient-level treatment exposure and response endpoints, and several clinical fields are incomplete or inconsistently annotated across disease programs. Variation in sequencing centers and preprocessing, together with differences in tumor purity and stromal admixture, can further modulate transcriptomic signals and complicate cross-tumor comparisons. These factors mean that the patient-level associations reported here are exploratory and hypothesis-generating rather than confirmatory. Where possible, harmonized preprocessing and prespecified correction procedures were applied to reduce technical variation, and claims have been limited to what is supported by the available data. Prospective evaluation in independent, treatment-annotated cohorts, together with functional validation in patient-derived systems, will be essential to determine whether the prioritized biomarkers and pathways add value beyond established clinical factors.
Future work should prioritize multicenter clinical datasets with detailed treatment metadata, develop AI frameworks that extract robust biological signals from small, high-quality real-world cohorts, and integrate additional molecular layers, including epigenomic, proteomic, and mutational profiles, to enhance robustness and clinical applicability [
48]. Beyond expression profiles, a multi-omics view is likely to strengthen both discrimination and mechanistic plausibility. Somatic drivers and copy number states provide complementary constraints on pathway dependence and can be incorporated as inputs to shared latent representations; for example, modeling interactions between
EGFR and
ALK alterations in LUAD, or conditioning on
IDH1 status and
MGMT promoter methylation in GBM when evaluating temozolomide response. Epigenetic context, including DNA methylation programs that modulate DNA-repair capacity and immune trafficking, can help distinguish lineage or microenvironmental influences from causal resistance mechanisms. Proteomic and phosphoproteomic measurements capture pathway activity not always apparent at the mRNA level and are particularly relevant for signaling nodes such as the VEGF–VEGFR axis, where protein abundance and phosphorylation states may mediate anti-angiogenic response more directly than VEGFA transcription. Methodologically, joint modeling can be framed with shared and private latent factors that fuse modalities while preserving modality-specific signal, with explicit handling of missing blocks and harmonized normalization to mitigate platform heterogeneity. Because multi-omics integration often reduces the number of complete cases, careful attention to imputation, sensitivity analyses, and external validation in treatment-annotated cohorts will be essential. Within this framework, the transcriptomic biomarkers highlighted here should be viewed as hypothesis-generating anchors that motivate multi-omics follow-up rather than standalone determinants of resistance.
The combination of VAE_LD and SHAP based interpretation provides a transparent analytic platform for hypothesis generation in modeling drug resistance mechanisms. This framework supports the prioritization of candidate biomarkers, reconstruction of resistance related signaling networks, and integration with survival modeling, while its translational utility will require confirmation in independent, treatment-annotated cohorts. Although multiple novel targets were identified across cancer types, experimental validation and evaluation in clinical cohorts with documented regimens and response endpoints is still required to establish their causal involvement in resistance.
Moving forward, the field should gradually transition from reliance on public resources like GDSC and TCGA to multi center, clinically annotated datasets that include documented therapies and on-treatment response measures. A systematic comparison of machine learning and deep learning models will be essential to establish standardized pipelines that meet the demands of precision medicine [
49]. In conclusion, the VAE_LD centered framework, trained on 72 drugs and validated through 180 drug and cancer prediction tasks, provides hypothesis-generating estimates of resistance risk using bulk RNA sequencing data and reveals putative molecular mechanisms and prognostic associations. With continued optimization in real world clinical cohorts and expansion to multi-omics integration, this approach holds strong promise for biomarker discovery, patient stratification, and clinical decision support in individualized cancer therapy.
5. Conclusions
In conclusion, an interpretable transfer-learning framework centered on a residual variational autoencoder was trained on GDSC (72 agents) and explored across five TCGA cancer types (n = 1836), yielding strong in vitro discrimination and consistent cross-tumor resistance signals across 180 tumor–drug tasks. The approach prioritizes biologically plausible candidate biomarkers and pathways and provides a transparent link from model attributions to mechanisms. As exemplars rather than confirmatory markers, TFF1 and B3GNT6 were repeatedly associated with Gefitinib resistance in LUAD, while OPALIN, LTF, IL2RA, and SLC17A7 were implicated in Temozolomide response in GBM, aligning with processes such as epithelial differentiation and angiogenesis. Because TCGA lacks treatment-response labels, all patient-level inferences are exploratory and hypothesis-generating. Practical translation will require validation in independent, treatment-annotated cohorts with standardized response endpoints and time-to-event measures, orthogonal confirmation in patient-derived models and targeted perturbation assays, and assay/reporting standardization for compact panels together with decision analytic evaluation of net clinical benefit. To facilitate reproduction and extension, all code, containers, configuration files, exact train–validation splits, and the end-to-end workflow (BPMN plus pseudocode) are publicly released under a permanent identifier. Subject to these validations and with expanded multi-omics integration, the framework has clear potential to support biomarker discovery and patient stratification in precision oncology.
Future work guidelines: Translation to practice requires validation in multi-institutional, treatment annotated cohorts with harmonized covariates and standardized endpoints, including objective response and time to event outcomes. Performance should be reported with discrimination and calibration together with clinical utility quantified by decision curve analysis under prespecified, locked thresholds. Robustness must be demonstrated across centers, platforms, sampling procedures, and patient subgroups, with sensitivity analyses for distribution shift, missingness, tumor purity, and other confounders, and with explicit procedures for detecting samples outside the training distribution. Interpretability should be reproducible and decision relevant by fixing attribution pipelines a priori, testing stability across resamples, and showing pathway level coherence rather than isolated features. Biological credibility should be examined through gain or loss of function perturbations in isogenic lines and patient derived organoids, dose–response assays, rescue experiments, and single cell or spatial profiling before and after drug exposure. Multi-omics integration that combines transcriptomics with copy number, methylation, chromatin accessibility, proteomics, and pathology should be assessed for incremental clinical utility under identical validation plans. Prospective evaluation should include a registry-based implementation study followed by a pragmatic, biomarker informed trial comparing a prespecified strategy with usual care. Reproducibility and governance should be ensured with versioned containers, model cards, data dictionaries, audit trails, fairness analyses, and documented privacy safeguards.