Inflammatory Molecule Elaboration in Secondhand Smoke (SHS)-Induced or Conditional RAGE Transgenic Modeling of Chronic Rhinosinusitis (CRS)
Abstract
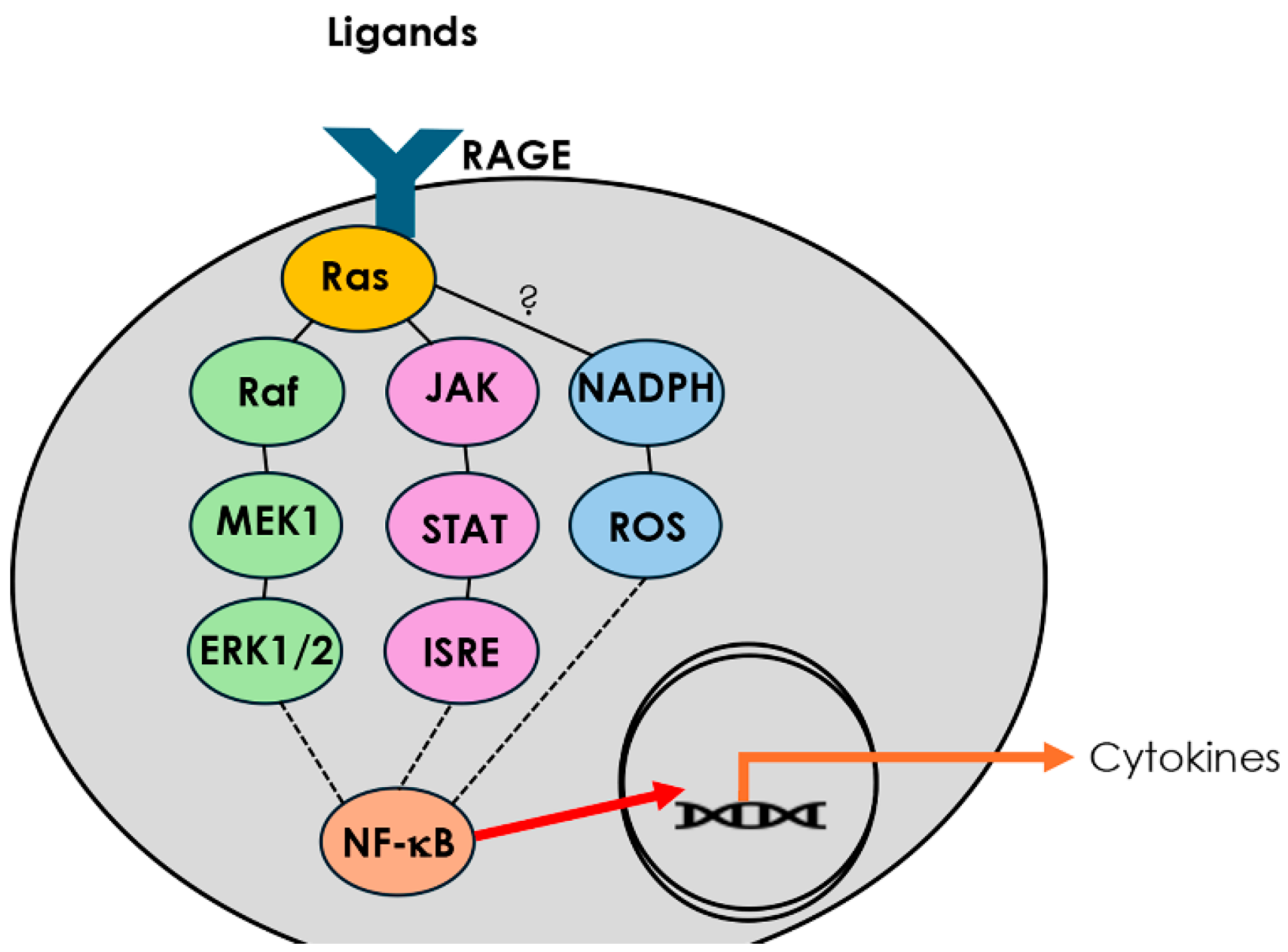
1. Introduction
2. Materials and Methods
2.1. Transgenic Mice and Experimental Exposures
2.2. Tissue Collection and Histological Processing
2.3. Cytokine Screening by Antibody-Based Dot Blot
2.4. Statistical Analysis
3. Results
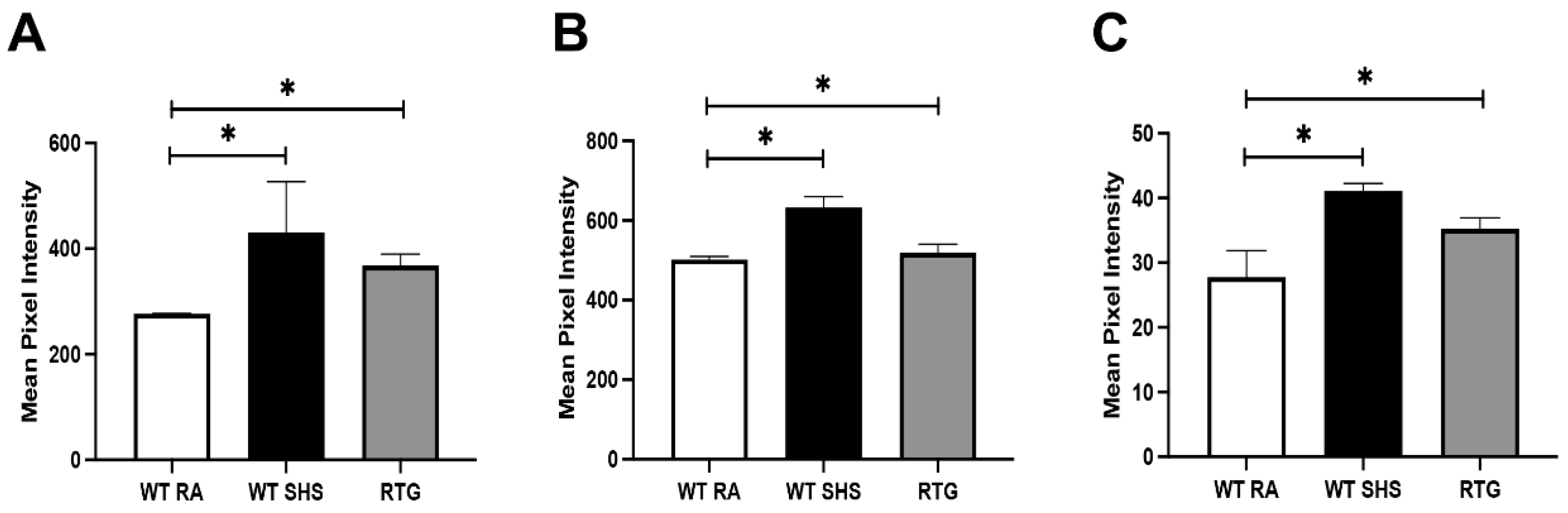
3.1. RAGE Overexpression and SHS Exposure Induce Sinonasal Epithelial Remodeling
3.2. SHS and RAGE Drive Upregulation of Th1 and Pro-Inflammatory Cytokines
3.3. SHS and RAGE Promote Type 2 and Type 17 Cytokine Responses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlBloushi, S.; Al-Ahmad, M. Exploring the immunopathology of type 2 inflammatory airway diseases. Front. Immunol. 2024, 15, 1285598. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of T(H) cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zeng, Y.; Huang, Z.; Tang, Y.; Zeng, Q.; Liu, W.; Shi, J. Immunopathologic characteristics of Chinese pediatric patients with chronic rhinosinusitis. World Allergy Organ. J. 2021, 14, 100616. [Google Scholar] [CrossRef] [PubMed]
- Ramezanpour, M.; Moraitis, S.; Smith, J.L.; Wormald, P.J.; Vreugde, S. Th17 Cytokines Disrupt the Airway Mucosal Barrier in Chronic Rhinosinusitis. Mediat. Inflamm. 2016, 2016, 9798206. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, N.; Zheng, M.; Li, Y.; Meng, L.; Ruan, Y.; Han, J.; Zhao, N.; Wang, X.; Zhang, L.; et al. Cross-talk between T(H)2 and T(H)17 pathways in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2019, 144, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- van den Bekerom, M.P.; Geervliet, P.C.; Somford, M.P.; van den Borne, M.P.; Boer, R. Total shoulder arthroplasty versus hemiarthroplasty for glenohumeral arthritis: A systematic review of the literature at long-term follow-up. Int. J. Shoulder Surg. 2013, 7, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Humpert, P.M.; Stern, D.M.; Arnold, B.; Nawroth, P.P. Advanced glycation end product receptor-mediated cellular dysfunction. Ann. N. Y. Acad. Sci. 2005, 1043, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Hirschi-Budge, K.M.; Tsai, K.Y.F.; Curtis, K.L.; Davis, G.S.; Theurer, B.K.; Kruyer, A.M.M.; Homer, K.W.; Chang, A.; Van Ry, P.M.; Arroyo, J.A.; et al. RAGE signaling during tobacco smoke-induced lung inflammation and potential therapeutic utility of SAGEs. BMC Pulm. Med. 2022, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.B.; Mejia, C.; Jordan, C.; Monson, T.D.; Bodine, J.S.; Dunaway, T.M.; Egbert, K.M.; Lewis, A.L.; Wright, T.J.; Ogden, K.C.; et al. Inhibition of the receptor for advanced glycation end-products (RAGE) protects from secondhand smoke (SHS)-induced intrauterine growth restriction IUGR in mice. Cell Tissue Res. 2017, 370, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Robin, H.; Trudeau, C.; Robbins, A.; Chung, E.; Rahman, E.; Gangmark-Strickland, O.; Licari, F.W.; Winden, D.R.; Orr, D.L.; Arroyo, J.A.; et al. A Potential Role for the Receptor for Advanced Glycation End-Products (RAGE) in the Development of Secondhand Smoke-Induced Chronic Sinusitis. Curr. Issues Mol. Biol. 2024, 46, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, C.; Wang, Y.; Yang, G.; Xu, Y.; Li, G.; Liao, F.; Tan, S. Interleukin-33 Promotes Th2/Th17 Response in Eosinophilic and Non-Eosinophilic Nasal Polyps. ORL J. Otorhinolaryngol. Relat. Spec. 2020, 82, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Song, J.; Xiong, P.; Cao, P.P.; Liao, B.; Ma, J.; Zhang, Y.N.; Zeng, M.; Liu, Y.; Wang, H.; et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2014, 190, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Jiang, J.; Zhang, Y.; Xiong, G. Role and Function of Regulatory T Cell in Chronic Rhinosinusitis with Nasal Polyposis. J. Immunol. Res. 2022, 2022, 1144563. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Schleimer, R.P.; Bleier, B.S. Mechanisms and pathogenesis of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, M.; Liu, Z. Th17 response and its regulation in inflammatory upper airway diseases. Clin. Exp. Allergy 2015, 45, 602–612. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Klemens, C.; Haack, M.; Nicoló, M.S.; Becker, S.; Kramer, M.F.; Gröger, M. Cytokine patterns in nasal secretion of non-atopic patients distinguish between chronic rhinosinusitis with or without nasal polys. Allergy Asthma Clin. Immunol. 2016, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Scheckenbach, K.; Wagenmann, M. Cytokine Patterns and Endotypes in Acute and Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Chinchilla, P.; Poveda, I.; Marco, F.M.; López-Fernández, J.A.; Peiro, G.; Illán, F.; Guijarro, J. Vulvar lymphedema and refractory VIN-III heralding GATA2 deficiency syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Das, S.; Ansari, M.A.; Singh, P.K.; Gupta, N.; Sharma, S.; Akhter, N.; Ramachandran, V.G.; Haque, S.; Dar, S.A. Phenotypic and functional profile of Th17 and Treg cells in allergic fungal sinusitis. Int. Immunopharmacol. 2018, 57, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Shaghayegh, G.; Cooksley, C.; Bouras, G.; Nepal, R.; Houtak, G.; Panchatcharam, B.S.; Fenix, K.A.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Staphylococcus aureus biofilm properties and chronic rhinosinusitis severity scores correlate positively with total CD4+ T-cell frequencies and inversely with its Th1, Th17 and regulatory cell frequencies. Immunology 2023, 170, 120–133. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponder, L.; Kinney, R.; Chatterjee, A.; Vu, K.; Sidhu, H.; Patel, N.; Desai, T.; Orr, D.L., II; Arroyo, J.A.; Reynolds, P.R. Inflammatory Molecule Elaboration in Secondhand Smoke (SHS)-Induced or Conditional RAGE Transgenic Modeling of Chronic Rhinosinusitis (CRS). Curr. Issues Mol. Biol. 2025, 47, 740. https://doi.org/10.3390/cimb47090740
Ponder L, Kinney R, Chatterjee A, Vu K, Sidhu H, Patel N, Desai T, Orr DL II, Arroyo JA, Reynolds PR. Inflammatory Molecule Elaboration in Secondhand Smoke (SHS)-Induced or Conditional RAGE Transgenic Modeling of Chronic Rhinosinusitis (CRS). Current Issues in Molecular Biology. 2025; 47(9):740. https://doi.org/10.3390/cimb47090740
Chicago/Turabian StylePonder, Logan, Ryan Kinney, Ankita Chatterjee, Kristina Vu, Harishma Sidhu, Neha Patel, Tejus Desai, Daniel L. Orr, II, Juan A. Arroyo, and Paul R. Reynolds. 2025. "Inflammatory Molecule Elaboration in Secondhand Smoke (SHS)-Induced or Conditional RAGE Transgenic Modeling of Chronic Rhinosinusitis (CRS)" Current Issues in Molecular Biology 47, no. 9: 740. https://doi.org/10.3390/cimb47090740
APA StylePonder, L., Kinney, R., Chatterjee, A., Vu, K., Sidhu, H., Patel, N., Desai, T., Orr, D. L., II, Arroyo, J. A., & Reynolds, P. R. (2025). Inflammatory Molecule Elaboration in Secondhand Smoke (SHS)-Induced or Conditional RAGE Transgenic Modeling of Chronic Rhinosinusitis (CRS). Current Issues in Molecular Biology, 47(9), 740. https://doi.org/10.3390/cimb47090740







