Microbial Metabolomes in Alzheimer’s Disease: From Pathogenesis to Therapeutic Potential
Abstract
1. Introduction
2. Method
3. Alzheimer’s Disease and Gut Microbiome Dysbiosis
3.1. Alteration in Gut Microbiome Composition in Alzheimer’s Disease
3.2. Relationship Between the Gut Microbiome and Biomarkers in Alzheimer’s Disease
3.3. The Role of the Gut Microbiome in the Pathogenesis of Alzheimer’s Disease
4. Microbial Metabolomes
| Microbial Compounds | Bacterial Producers | Major Effects | References |
|---|---|---|---|
| Sphingolipids | Bacteroides, members of Pseudomonadota. | - Restore the intestinal mucosal barrier. - Regulate microglia. - Induce neuroinflammation | [102,103,104] |
| Phospholipids | Akkermansia, Desulfovibrio. |
| [105,106,107] |
| LPS | Bacteroides, Escherichia/Shigella. | - Microglial activation. - Release pro-inflammatory factors. - Regulate Aβ accumulation. | [93,108] |
| SCFAs | Akkermansia, Bacteroides, members of Bacillota. | - Inhibit tau protein phosphorylation. - Reduce oxidative stress. - Release pro-inflammatory factors. - Regulate mitochondrial homeostasis. | [109,110,111] |
| BAs | Bacteroides, Bifidobacterium. | - Regulate tau and Aβ accumulation. - Promote mitochondrial biogenesis. | [112,113,114,115] |
| Amino acids | Clostridium, Escherichia, Lactobacillus. | - Activate oxidative stress. - Regulate microglial polarization. - Reduce oxidative stress. | [116,117,118] |
| GABA | Bacteroides, Bifidobacterium, Escherichia, Lacticigenium. | - Promotes the spread of tau and Aβ pathologies. - Promotes neuronal differentiation. | [119,120,121] |
| Serotonin | Bifidobacterium, Lacticigenium, Roseburia. | - Increases vagus neuron activity. - Regulates astrocyte and microglia activities. - Reduces Aβ aggregation and tau phosphorylation. | [122,123,124] |
| ACh | Bacillus, Escherichia, Lactiplantibacillus, Staphylococcus. | - Promotes the deposition of Aβ plaques. - Induces hippocampal atrophy. | [125,126] |
| Dopamine | Bacillus, Bacteroides, Bifidobacterium, Brevilactibacter. | - Regulates the intensity of synapses in neurons. - Affects cognition and mood. | [127,128,129] |
| Norepinephrine | Bacillus, Escherichia, Proteus. | - Regulates synaptic plasticity. - Upregulates BDNF. - Reduces pro-inflammatory factors. - Increases amyloid clearance. | [130,131,132] |
4.1. Bacterial Components
4.2. Bioactive Microbial Metabolites
4.2.1. Short-Chain Fatty Acids
4.2.2. Bile Acids
4.3. Tryptophan Metabolites
4.4. Neurotransmitters
4.4.1. Gamma-Amino Butyric Acid
4.4.2. Serotonin
4.4.3. Acetylcholine
4.4.4. Dopamine
4.4.5. Norepinephrine
5. Therapeutic Approaches Targeting the Gut Microbiome
5.1. Probiotics
5.2. Prebiotics
5.3. Fecal Microbiota Transplantation
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| ACh | Acetylcholine |
| AD | Alzheimer’s disease |
| AhR | Aryl hydrocarbon receptor |
| APP | Amyloid precursor protein |
| BAs | Bile acids |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BFCNs | Basal forebrain cholinergic neurons |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| FMT | Fecal microbiota transplantation |
| GABA | γ-aminobutyric acid |
| GAD | Glutamate decarboxylase |
| GM | Gut microbiome |
| GPRs | G protein-coupled receptors |
| 3-HAA | Antioxidant 3-hydroxyanthranilic acid |
| HDACs | Histone deacetylases |
| 3-HK | 3-hydroxykynurenine |
| HPA | Hypothalamic–pituitary–adrenal |
| KP | Kynurenine pathway |
| KYN | Kynurenic acid |
| LC | Locus coeruleus |
| LPS | Lipopolysaccaharides |
| mAChR | Muscarinic acetylcholine receptors |
| MAO | Monoamine oxidase |
| MAPK | Mitogen-activated protein kinase |
| MCI | Mild cognitive impairment |
| MGB | Microbiota–gut–brain |
| MYD88 | Myeloid differentiation primary response 88 |
| nAChR | Nicotinic acetylcholine receptors |
| NF-κB | Nuclear factor-κB |
| NLRP3 | NOD-like receptor protein 3 |
| NOX2 | NADPH oxidase 2 |
| p-tau | Phosphorylated tau |
| QUIN | Quinolinic acid |
| SCFAs | Short-chain fatty acids |
| SSRIs | Selective serotonin reuptake inhibitors |
| TLR4 | Toll-like receptor 4 |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| Trp | Tryptophan |
| TRYCATs | Tryptophan catabolites |
| TUDCA | Tauroursodeoxycholic acid |
| WT | Wild-type |
References
- Borrego-Ruiz, A.; Borrego, J.J. Influence of human gut microbiome on the healthy and the neurodegenerative aging. Exp. Gerontol. 2024, 194, 112497. [Google Scholar] [CrossRef]
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 22 August 2025).
- Hao, M.; Chen, J. Trend analysis and future predictions of global burden of alzheimer’s disease and other dementias: A study based on the global burden of disease database from 1990 to 2021. BMC Med. 2025, 23, 378. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Chen, X.; Holtzman, D.M. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity 2022, 55, 2236–2254. [Google Scholar] [CrossRef]
- Sierksma, A.; Escott-Price, V.; De Strooper, B. Translating genetic risk of Alzheimer’s disease into mechanistic insight and drug targets. Science 2020, 370, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Kametani, F.; Hasegawa, M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, S.; Commins, C.; Lathuiliere, A.; Beerepoot, P.; Fernandes, A.R.; Kamath, T.V.; De Los Santos, M.B.; Klickstein, N.; Corjuc, D.L.; Corjuc, B.T.; et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 2020, 26, 1256–1263, Erratum in Nat. Med. 2021, 27, 356. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Bostanciklioğlu, M. The role of gut microbiota in pathogenesis of Alzheimer’s disease. J. Appl. Microbiol. 2019, 127, 954–967. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef]
- Haukedal, H.; Freude, K.K. Implications of Glycosylation in Alzheimer’s Disease. Front. Neurosci. 2021, 14, 625348. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Vidal, C.; Zhang, L. An Analysis of the Neurological and Molecular Alterations Underlying the Pathogenesis of Alzheimer’s Disease. Cells 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut–brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Varesi, A.; Pierella, E.; Romeo, M.; Piccini, G.B.; Alfano, C.; Bjørklund, G.; Oppong, A.; Ricevuti, G.; Esposito, C.; Chirumbolo, S.; et al. The Potential Role of Gut Microbiota in Alzheimer’s Disease: From Diagnosis to Treatment. Nutrients 2022, 14, 668. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Baltriukiene, D.; Bukelskiene, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, Q.; Guan, Y.; Sun, Z.; Li, W.; Guo, S.; Zhang, A. The communication mechanism of the gut-brain axis and its effect on central nervous system diseases: A systematic review. Biomed. Pharmacother. 2024, 178, 117207. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Hollywood, K.A.; Goodacre, R. Metabolomics for the masses: The future of metabolomics in a personalized world. New Horiz. Transl. Med. 2017, 3, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Horgusluoglu, E.; Neff, R.; Song, W.M.; Wang, M.; Wang, Q.; Arnold, M.; Krumsiek, J.; Galindo-Prieto, B.; Ming, C.; Nho, K.; et al. Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 1260–1278. [Google Scholar] [CrossRef]
- Xi, J.; Ding, D.; Zhu, H.; Wang, R.; Su, F.; Wu, W.; Xiao, Z.; Liang, X.; Zhao, Q.; Hong, Z.; et al. Disturbed microbial ecology in Alzheimer’s disease: Evidence from the gut microbiota and fecal metabolome. BMC Microbiol. 2021, 21, 226. [Google Scholar] [CrossRef]
- Seo, D.O.; Holtzman, D.M. Current understanding of the Alzheimer’s disease—Associated microbiome and therapeutic strategies. Exp. Mol. Med. 2024, 56, 86–94. [Google Scholar] [CrossRef]
- Cammann, D.; Lu, Y.; Cummings, M.J.; Zhang, M.L.; Cue, J.M.; Do, J.; Ebersole, J.; Chen, X.; Oh, E.C.; Cummings, J.L.; et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci. Rep. 2023, 13, 5258. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef]
- Heravi, F.S.; Naseri, K.; Hu, H. Gut Microbiota Composition in Patients with Neurodegenerative Disorders (Parkinson’s and Alzheimer’s) and Healthy Controls: A Systematic Review. Nutrients 2023, 15, 4365. [Google Scholar] [CrossRef]
- Hung, C.C.; Chang, C.C.; Huang, C.W.; Nouchi, R.; Cheng, C.H. Gut microbiota in patients with Alzheimer’s disease spectrum: A systematic review and meta-analysis. Aging 2022, 14, 477–496. [Google Scholar] [CrossRef]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimer’s Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients With Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 8, 634069. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Paley, E.L. Discovery of Gut Bacteria Specific to Alzheimer’s Associated Diseases is a Clue to Understanding Disease Etiology: Meta-Analysis of Population-Based Data on Human Gut Metagenomics and Metabolomics. J. Alzheimer’s Dis. 2019, 72, 319–355. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, X.; Song, Y.; Zhao, W.; Sun, Z.; Zhu, J.; Yu, Y. Multi-omics analyses identify gut microbiota-fecal metabolites-brain-cognition pathways in the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2025, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.Q.; Shen, L.L.; Li, W.W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host-Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef]
- Cirstea, M.S.; Kliger, D.; MacLellan, A.D.; Yu, A.C.; Langlois, J.; Fan, M.; Boroomand, S.; Kharazyan, F.; Hsiung, R.G.Y.; MacVicar, B.A.; et al. The Oral and Fecal Microbiota in a Canadian Cohort of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 87, 247–258. [Google Scholar] [CrossRef]
- Sheng, C.; Yang, K.; He, B.; Du, W.; Cai, Y.; Han, Y. Combination of gut microbiota and plasma amyloid-β as a potential index for identifying preclinical Alzheimer’s disease: A cross-sectional analysis from the SILCODE study. Alzheimer’s Res. Ther. 2022, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Jemimah, S.; Chabib, C.M.M.; Hadjileontiadis, L.; AlShehhi, A. Gut microbiome dysbiosis in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0285346. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Peng, J.; Huang, X.; Xiao, L.; Huang, F.; Zuo, Z. Gut Microbiome Features of Chinese Patients Newly Diagnosed with Alzheimer’s Disease or Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 80, 299–310. [Google Scholar] [CrossRef]
- Yıldırım, S.; Nalbantoğlu, Ö.U.; Bayraktar, A.; Ercan, F.B.; Gündoğdu, A.; Velioğlu, H.A.; Göl, M.F.; Soylu, A.E.; Koç, F.; Gülpınar, E.A.; et al. Stratification of the Gut Microbiota Composition Landscape across the Alzheimer’s Disease Continuum in a Turkish Cohort. mSystems 2022, 7, e0000422. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Quan, M.; Zhao, H.; Jia, J. Gut Microbiota Changes and Their Correlation with Cognitive and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 81, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Hendriksen, H.M.A.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 2022, 12, 794519. [Google Scholar] [CrossRef] [PubMed]
- Wanapaisan, P.; Chuansangeam, M.; Nopnipa, S.; Mathuranyanon, R.; Nonthabenjawan, N.; Ngamsombat, C.; Thientunyakit, T.; Muangpaisan, W. Association between Gut Microbiota with Mild Cognitive Impairment and Alzheimer’s Disease in a Thai Population. Neurodegener. Dis. 2022, 22, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, D.; Giannakopoulos, P.; Borgwardt, S.; Rodriguez, C.; Haller, S. Hippocampal and Amygdala Gray Matter Loss in Elderly Controls with Subtle Cognitive Decline. Front. Aging Neurosci. 2017, 9, 50. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Hampel, H.; Cummings, J.; Blennow, K.; Gao, P.; Jack, C.R.; Vergallo, A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 2021, 17, 580–589. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Rangasamy, S.B.; Jana, M.; Roy, A.; Corbett, G.T.; Kundu, M.; Chandra, S.; Mondal, S.; Dasarathi, S.; Mufson, E.J.; Mishra, R.K.; et al. Selective disruption of TLR2-MyD88 interaction inhibits inflammation and attenuates Alzheimer’s pathology. J. Clin. Investig. 2018, 128, 4297–4312. [Google Scholar] [CrossRef]
- Quan, W.; Luo, Q.; Hao, W.; Tomic, I.; Furihata, T.; Schulz-Schäffer, W.; Menger, M.D.; Fassbender, K.; Liu, Y. Haploinsufficiency of microglial MyD88 ameliorates Alzheimer’s pathology and vascular disorders in APP/PS1-transgenic mice. Glia 2021, 69, 1987–2005. [Google Scholar] [CrossRef]
- Jong Huat, T.; Onraet, T.; Camats-Perna, J.; Newcombe, E.A.; Ngo, K.C.; Sue, A.N.; Mirzaei, M.; LaFerla, F.M.; Medeiros, R. Deletion of MyD88 in astrocytes prevents β-amyloid-induced neuropathology in mice. Glia 2023, 71, 431–449. [Google Scholar] [CrossRef]
- Weitz, T.M.; Gate, D.; Rezai-Zadeh, K.; Town, T. MyD88 is dispensable for cerebral amyloidosis and neuroinflammation in APP/PS1 transgenic mice. Am. J. Pathol. 2014, 184, 2855–2861. [Google Scholar] [CrossRef][Green Version]
- Chang, C.W.; Shao, E.; Mucke, L. Tau: Enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies. Science 2021, 371, eabb8255. [Google Scholar] [CrossRef] [PubMed]
- Younas, N.; Saleem, T.; Younas, A.; Zerr, I. Nuclear face of Tau: An inside player in neurodegeneration. Acta Neuropathol. Commun. 2023, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Clayton, E.; Kaminski Schierle, G.S. Synaptic tau: A pathological or physiological phenomenon? Acta Neuropathol. Commun. 2021, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar] [CrossRef]
- Udeochu, J.C.; Amin, S.; Huang, Y.; Fan, L.; Torres, E.R.S.; Carling, G.K.; Liu, B.; McGurran, H.; Coronas-Samano, G.; Kauwe, G.; et al. Tau activation of microglial cGAS-IFN reduces MEF2C-mediated cognitive resilience. Nat. Neurosci. 2023, 26, 737–750. [Google Scholar] [CrossRef]
- Mattsson-Carlgren, N.; Collij, L.E.; Stomrud, E.; Pichet Binette, A.; Ossenkoppele, R.; Smith, R.; Karlsson, L.; Lantero-Rodriguez, J.; Snellman, A.; Strandberg, O.; et al. Plasma Biomarker Strategy for Selecting Patients With Alzheimer Disease for Antiamyloid Immunotherapies. JAMA Neurol. 2024, 81, 69–78. [Google Scholar] [CrossRef]
- Aoyagi, A.; Condello, C.; Stöhr, J.; Yue, W.; Rivera, B.M.; Lee, J.C.; Woerman, A.L.; Halliday, G.; van Duinen, S.; Ingelsson, M.; et al. Aβ and tau prion-like activities decline with longevity in the Alzheimer’s disease human brain. Sci. Transl. Med. 2019, 11, eaat8462. [Google Scholar] [CrossRef]
- Gomes, L.A.; Hipp, S.A.; Rijal Upadhaya, A.; Balakrishnan, K.; Ospitalieri, S.; Koper, M.J.; Largo-Barrientos, P.; Uytterhoeven, V.; Reichwald, J.; Rabe, S.; et al. Aβ-induced acceleration of Alzheimer-related τ-pathology spreading and its association with prion protein. Acta Neuropathol. 2019, 138, 913–941. [Google Scholar] [CrossRef]
- Self, W.K.; Holtzman, D.M. Emerging diagnostics and therapeutics for Alzheimer disease. Nat. Med. 2023, 29, 2187–2199. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Kharitonova, E.K.; Snyder, A.C.; Hou, S.S.; Sanchez-Mico, M.V.; Das, S.; Fan, Z.; Shirani, H.; Nilsson, K.P.R.; Serrano-Pozo, A.; et al. Real-time imaging of mitochondrial redox reveals increased mitochondrial oxidative stress associated with amyloid β aggregates in vivo in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 6. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, S.; Wang, R.; Bao, X.; Li, Y. Neural Stem Cells in the Treatment of Alzheimer’s Disease: Current Status, Challenges, and Future Prospects. J. Alzheimer’s Dis. 2023, 94, S173–S186. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.R.; Kemp, M.; Nguyen, T.T. The Microbiota-Gut-Brain Axis in Alzheimer’s Disease: A Review of Taxonomic Alterations and Potential Avenues for Interventions. Arch. Clin. Neuropsychol. 2022, 37, 595–607. [Google Scholar] [CrossRef]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Daglia, M.; Argüelles Castilla, S.; Sanadgol, N.; Fazel Nabavi, S.; Khan, H.; Belwal, T.; Jeandet, P.; Marchese, A.; Pistollato, F.; et al. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 2020, 151, 104582. [Google Scholar] [CrossRef] [PubMed]
- Elkins, M.; Jain, N.; Tükel, Ç. The menace within: Bacterial amyloids as a trigger for autoimmune and neurodegenerative diseases. Curr. Opin. Microbiol. 2024, 79, 102473. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Hufnagel, D.A.; Tükel, C.; Chapman, M.R. Disease to dirt: The biology of microbial amyloids. PLoS Pathog. 2013, 9, e1003740. [Google Scholar] [CrossRef]
- Inan, S.; Wilson, R.P.; Tükel, Ç. IUPHAR review: From gut to brain: The role of gut dysbiosis, bacterial amyloids, and metabolic disease in Alzheimer’s disease. Pharmacol. Res. 2025, 215, 107693. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef]
- Golan, N.; Engelberg, Y.; Landau, M. Structural Mimicry in Microbial and Antimicrobial Amyloids. Annu. Rev. Biochem. 2022, 91, 403–422. [Google Scholar] [CrossRef]
- Schwartz, K.; Boles, B.R. Microbial amyloids—Functions and interactions within the host. Curr. Opin. Microbiol. 2013, 16, 93–99. [Google Scholar] [CrossRef]
- Scialò, C.; De Cecco, E.; Manganotti, P.; Legname, G. Prion and Prion-Like Protein Strains: Deciphering the Molecular Basis of Heterogeneity in Neurodegeneration. Viruses 2019, 11, 261. [Google Scholar] [CrossRef]
- Wu, Y.C.; Bogale, T.A.; Koistinaho, J.; Pizzi, M.; Rolova, T.; Bellucci, A. The contribution of β-amyloid, Tau and α-synuclein to blood-brain barrier damage in neurodegenerative disorders. Acta Neuropathol. 2024, 147, 39. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Choi, Y.J.; Chen, L.; Zhang, B.; Eum, S.Y.; Abreu, M.T.; Toborek, M. Lipopolysaccharide potentiates polychlorinated biphenyl-induced disruption of the blood-brain barrier via TLR4/IRF-3 signaling. Toxicology 2012, 302, 212–220. [Google Scholar] [CrossRef]
- McCarthy, G.M.; Bridges, C.R.; Blednov, Y.A.; Harris, R.A. CNS cell-type localization and LPS response of TLR signaling pathways. F1000Research 2017, 6, 1144. [Google Scholar] [CrossRef]
- Zhao, Y.; Cong, L.; Lukiw, W.J. Lipopolysaccharide (LPS) Accumulates in Neocortical Neurons of Alzheimer’s Disease (AD) Brain and Impairs Transcription in Human Neuronal-Glial Primary Co-cultures. Front. Aging Neurosci. 2017, 9, 407. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Heneka, M.T. The endotoxin hypothesis of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zou, G.; Zou, X.; Wang, K.; Chen, Z. Gut microbiota and its metabolites in Alzheimer’s disease: From pathogenesis to treatment. PeerJ 2024, 12, e17061. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Hur, J.Y.; Frost, G.R.; Wu, X.; Crump, C.; Pan, S.J.; Wong, E.; Barros, M.; Li, T.; Nie, P.; Zhai, Y.; et al. The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer’s disease. Nature 2020, 586, 735–740. [Google Scholar] [CrossRef]
- Yao, A.Y.; Yan, R. Activity of Alzheimer’s γ-secretase is linked to changes of interferon-induced transmembrane proteins (IFITM) in innate immunity. Mol. Neurodegener. 2020, 15, 69. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Heaver, S.L.; Le, H.H.; Tang, P.; Baslé, A.; Mirretta Barone, C.; Vu, D.L.; Waters, J.L.; Marles-Wright, J.; Johnson, E.L.; Campopiano, D.J.; et al. Characterization of inositol lipid metabolism in gut-associated Bacteroidetes. Nat. Microbiol. 2022, 7, 986–1000. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Waters, J.L.; Kim, B.I.; Bretin, A.; Goodman, A.L.; Gewirtz, A.T.; Worgall, T.S.; Ley, R.E. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 2020, 11, 2471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Sheng, Y.; He, B.; Liu, Z.; Li, W.; Yu, S.; Wang, J.; Zhang, Y.; Chen, J.; et al. The function of sphingolipids in different pathogenesis of Alzheimer’s disease: A comprehensive review. Biomed. Pharmacother. 2024, 171, 116071. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Luo, Y.X.; Yang, L.L.; Yao, X.Q. Gut microbiota-host lipid crosstalk in Alzheimer’s disease: Implications for disease progression and therapeutics. Mol. Neurodegener. 2024, 19, 35. [Google Scholar] [CrossRef]
- Andreadou, E.G.; Katsipis, G.; Tsolaki, M.; Pantazaki, A.A. Involvement and relationship of bacterial lipopolysaccharides and cyclooxygenases levels in Alzheimer’s Disease and Mild Cognitive Impairment patients. J. Neuroimmunol. 2021, 357, 577561. [Google Scholar] [CrossRef]
- Chen, H.; Meng, L.; Shen, L. Multiple roles of short-chain fatty acids in Alzheimer disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.F.; Miao, J.; Zheng, R.F.; Li, J.Y. Short-chain fatty acids: Important components of the gut-brain axis against AD. Biomed. Pharmacother. 2024, 175, 116601. [Google Scholar] [CrossRef]
- Qian, X.H.; Xie, R.Y.; Liu, X.L.; Chen, S.D.; Tang, H.D. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022, 13, 1252–1266. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.M.; Barnes, K.; Clemmens, H.; Al-Rafiah, A.R.; Al-Ofi, E.A.; Leech, V.; Bandmann, O.; Shaw, P.J.; Blackburn, D.J.; Ferraiuolo, L.; et al. Ursodeoxycholic Acid Improves Mitochondrial Function and Redistributes Drp1 in Fibroblasts from Patients with Either Sporadic or Familial Alzheimer’s Disease. J. Mol. Biol. 2018, 430, 3942–3953. [Google Scholar] [CrossRef]
- Mulak, A. Bile Acids as Key Modulators of the Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 84, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, J.; Wang, L.; Hu, X.; Li, J.; Zhu, L.; Pang, R.; Zhang, A. Tauroursodeoxycholic acid: A bile acid that may be used for the prevention and treatment of Alzheimer’s disease. Front. Neurosci. 2024, 18, 1348844. [Google Scholar] [CrossRef]
- Wu, M.; Cheng, Y.; Zhang, R.; Han, W.; Jiang, H.; Bi, C.; Zhang, Z.; Ye, M.; Lin, X.; Liu, Z. Molecular mechanism and therapeutic strategy of bile acids in Alzheimer’s disease from the emerging perspective of the microbiota-gut-brain axis. Biomed. Pharmacother. 2024, 178, 117228. [Google Scholar] [CrossRef] [PubMed]
- Bakker, L.; Köhler, S.; Eussen, S.J.P.M.; Choe, K.; van den Hove, D.L.A.; Kenis, G.; Rutten, B.P.F.; Ulvik, A.; Ueland, P.M.; Verhey, F.R.J.; et al. Correlations between kynurenines in plasma and CSF, and their relation to markers of Alzheimer’s disease pathology. Brain Behav. Immun. 2023, 111, 312–319. [Google Scholar] [CrossRef]
- Jacobs, K.R.; Lim, C.K.; Blennow, K.; Zetterberg, H.; Chatterjee, P.; Martins, R.N.; Brew, B.J.; Guillemin, G.J.; Lovejoy, D.B. Correlation between plasma and CSF concentrations of kynurenine pathway metabolites in Alzheimer’s disease and relationship to amyloid-β and tau. Neurobiol. Aging 2019, 80, 11–20. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, Y.; Ye, T.; Kong, Y.; Cui, X.; Yuan, S.; Liu, J.; Zhang, Y. A High-Tryptophan Diet Alleviated Cognitive Impairment and Neuroinflammation in APP/PS1 Mice through Activating Aryl Hydrocarbon Receptor via the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2024, 68, e2300601. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, C.H.; Lane, H.Y. D-glutamate and Gut Microbiota in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2676. [Google Scholar] [CrossRef] [PubMed]
- Conn, K.A.; Borsom, E.M.; Cope, E.K. Implications of microbe-derived ɣ-aminobutyric acid (GABA) in gut and brain barrier integrity and GABAergic signaling in Alzheimer’s disease. Gut Microbes 2024, 16, 2371950. [Google Scholar] [CrossRef]
- Osse, A.M.L.; Pandey, R.S.; Wirt, R.A.; Ortiz, A.A.; Salazar, A.; Kimmich, M.; Toledano Strom, E.N.; Oblak, A.; Lamb, B.; Hyman, J.M.; et al. Reduction in GABAB on glia induce Alzheimer’s disease related changes. Brain Behav. Immun. 2023, 110, 260–275. [Google Scholar] [CrossRef]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef]
- Sood, A.; Wilson, R.S.; Yu, L.; Wang, T.; Schneider, J.A.; Honer, W.G.; Bennett, D.A. Selective serotonin reuptake inhibitor use, age-related neuropathology and cognition in late-life. Psychiatry Res. 2023, 328, 115471. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Gut Microbiota and its Metabolites: Bridge of Dietary Nutrients and Alzheimer’s Disease. Adv. Nutr. 2023, 14, 819–839. [Google Scholar] [CrossRef]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef] [PubMed]
- Possemato, E.; La Barbera, L.; Nobili, A.; Krashia, P.; D’Amelio, M. The role of dopamine in NLRP3 inflammasome inhibition: Implications for neurodegenerative diseases. Ageing Res. Rev. 2023, 87, 101907. [Google Scholar] [CrossRef] [PubMed]
- Spoleti, E.; La Barbera, L.; Cauzzi, E.; De Paolis, M.L.; Saba, L.; Marino, R.; Sciamanna, G.; Di Lazzaro, V.; Keller, F.; Nobili, A.; et al. Dopamine neuron degeneration in the Ventral Tegmental Area causes hippocampal hyperexcitability in experimental Alzheimer’s Disease. Mol. Psychiatry 2024, 29, 1265–1280. [Google Scholar] [CrossRef]
- Ojeda, J.; Ávila, A.; Vidal, P.M. Gut Microbiota Interaction with the Central Nervous System throughout Life. J. Clin. Med. 2021, 10, 1299. [Google Scholar] [CrossRef]
- Pourahmad, R.; Saleki, K.; Zare Gholinejad, M.; Aram, C.; Soltani Farsani, A.; Banazadeh, M.; Tafakhori, A. Exploring the effect of gut microbiome on Alzheimer’s disease. Biochem. Biophys. Rep. 2024, 39, 101776. [Google Scholar] [CrossRef]
- van Hooren, R.W.E.; Verhey, F.R.J.; Ramakers, I.H.G.B.; Jansen, W.J.; Jacobs, H.I.L. Elevated norepinephrine metabolism is linked to cortical thickness in the context of Alzheimer’s disease pathology. Neurobiol. Aging 2021, 102, 17–22. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. An Overview of the Epigenetic Modifications in the Brain Under Normal and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 3881. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Ubeda, C.; Vázquez-Carretero, M.D.; Luque-Tirado, A.; Ríos-Reina, R.; Rubio-Sánchez, R.; Franco-Macías, E.; García-Miranda, P.; Calonge, M.L.; Peral, M.J. Fecal Volatile Organic Compounds and Microbiota Associated with the Progression of Cognitive Impairment in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 707. [Google Scholar] [CrossRef] [PubMed]
- Alsegiani, A.S.; Shah, Z.A. The influence of gut microbiota alteration on age-related neuroinflammation and cognitive decline. Neural Regen. Res. 2022, 17, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Perea, J.R.; Bolós, M.; Avila, J. Microglia in Alzheimer’s Disease in the Context of Tau Pathology. Biomolecules 2020, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Białecka-Dębek, A.; Granda, D.; Szmidt, M.K.; Zielínska, D. Gut Microbiota, Probiotic Interventions, and Cognitive Function in the Elderly: A Review of Current Knowledge. Nutrients 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulba, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative stress and the microbiota-gut-brain axis. Oxid. Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef]
- Jęśko, H.; Stepien, A.; Lukiw, W.J.; Strosznajder, R.P. The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration. Mol. Neurobiol. 2019, 56, 3501–3521. [Google Scholar] [CrossRef]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef]
- Xu, J.; Bankov, G.; Kim, M.; Wretlind, A.; Lord, J.; Green, R.; Hodges, A.; Hye, A.; Aarsland, D.; Velayudhan, L.; et al. Integrated lipidomics and proteomics network analysis highlights lipid and immunity pathways associated with Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 36. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, R.; Garcia-Barrera, T.; Gomez-Ariza, J.L. Combination of metabolomic and phospholipid-profiling approaches for the study of Alzheimer’s disease. J. Proteom. 2014, 104, 37–47. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Slack, B.E. Accelerated breakdown of phosphatidylcholine and phosphatidylethanolamine is a predominant brain metabolic defect in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 93, 1285–1289. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Jin, L.W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cong, L.; Jaber, V.; Lukiw, W.J. Microbiome-Derived Lipopolysaccharide Enriched in the Perinuclear Region of Alzheimer’s Disease Brain. Front. Immunol. 2017, 8, 1064. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, S.; Shin, S.J.; Park, Y.H.; Nam, Y.; Kim, C.W.; Lee, K.W.; Kim, S.M.; Jung, I.D.; Yang, H.D.; et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: Pathologic roles and therapeutic implications. Transl. Neurodegener. 2021, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhu, M.; Che, X.; Wang, H.; Liang, X.J.; Wu, C.; Xue, X.; Yang, J. Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation. J. NeuroInflamm. 2020, 17, 18. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; García-Rodriguez, C.; Villalobos, C.; Núñez, L. Role of Toll Like Receptor 4 in Alzheimer’s Disease. Front. Immunol. 2020, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Epigenetic Mechanisms in Aging: Extrinsic Factors and Gut Microbiome. Genes 2024, 15, 1599. [Google Scholar] [CrossRef]
- Ge, X.; Zheng, M.; Hu, M.; Fang, X.; Geng, D.; Liu, S.; Wang, L.; Zhang, J.; Guan, L.; Zheng, P.; et al. Butyrate ameliorates quinolinic acid-induced cognitive decline in obesity models. J. Clin. Investig. 2023, 133, e154612. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef]
- Soliman, M.L.; Ohm, J.E.; Rosenberger, T.A. Acetate reduces PGE2 release and modulates phospholipase and cyclooxygenase levels in neuroglia stimulated with lipopolysaccharide. Lipids 2013, 48, 651–662. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z.; Liu, J. Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol. Nutr. Food Res. 2020, 64, e1900636. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Y.; Gong, Y.; Yang, R.; Chen, Z.; Hu, W.; Wu, Y.; Gao, M.; Xu, X.; Qin, Y.; et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol. Dis. 2018, 111, 12–25. [Google Scholar] [CrossRef]
- Soliman, M.L.; Combs, C.K.; Rosenberger, T.A. Modulation of inflammatory cytokines and mitogen-activated protein kinases by acetate in primary astrocytes. J. Neuroimmune Pharmacol. 2013, 8, 287–300. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, K.; Li, X.; Xu, L.; Yang, Z. Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem. Biol. Interact. 2021, 341, 109452. [Google Scholar] [CrossRef]
- Saw, G.; Krishna, K.; Gupta, N.; Soong, T.W.; Mallilankaraman, K.; Sajikumar, S.; Dheen, S.T. Epigenetic regulation of microglial phosphatidylinositol 3-kinase pathway involved in long-term potentiation and synaptic plasticity in rats. Glia 2020, 68, 656–669. [Google Scholar] [CrossRef]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimer’s Dement. 2019, 15, 76–92, Erratum in Alzheimer’s Dement. 2019, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Baloni, P.; Funk, C.C.; Yan, J.; Yurkovich, J.T.; Kueider-Paisley, A.; Nho, K.; Heinken, A.; Jia, W.; MahmoudianDehkordi, S.; Louie, G.; et al. Metabolic Network Analysis Reveals Altered Bile Acid Synthesis and Metabolism in Alzheimer’s Disease. Cell Rep. Med. 2020, 1, 100138. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Rajani, C.; Kaddurah-Daouk, R.; Li, H. Expert insights: The potential role of the gut microbiome-bile acid-brain axis in the development and progression of Alzheimer’s disease and hepatic encephalopathy. Med. Res. Rev. 2020, 40, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mejia, R.O.; Mucke, L. Phospholipase A2 and arachidonic acid in Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 784–790. [Google Scholar] [CrossRef]
- Yin, F. Lipid metabolism and Alzheimer’s disease: Clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2023, 290, 1420–1453. [Google Scholar] [CrossRef]
- Dixon, S.J.; Winter, G.E.; Musavi, L.S.; Lee, E.D.; Snijder, B.; Rebsamen, M.; Superti-Furga, G.; Stockwell, B.R. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 2015, 10, 1604–1609. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef]
- Marksteiner, J.; Blasko, I.; Kemmler, G.; Koal, T.; Humpel, C. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer’s disease. Metabolomics 2018, 14, 1. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmüller, G.; et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimer’s Dement. 2019, 15, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Pariante, C.M.; Borsini, A. From dried bear bile to molecular investigation: A systematic review of the effect of bile acids on cell apoptosis, oxidative stress and inflammation in the brain, across pre-clinical models of neurological, neurodegenerative and neuropsychiatric disorders. Brain Behav. Immun. 2022, 99, 132–146. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Barreda-Manso, M.A.; Nieto-Sampedro, M.; Romero-Ramírez, L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J. Cell. Physiol. 2017, 232, 2231–2245. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Tornese, P.; Cocco, A.; Albanese, A. Tauroursodeoxycholic acid: A potential therapeutic tool in neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, J.; Cao, S.; Xu, X.; Zhou, J.; Zhang, R.; Huang, B.; Lu, P.; Peng, L.; Liu, M. The Role of Gut Microbiota-Derived Trimethylamine N-Oxide in the Pathogenesis and Treatment of Mild Cognitive Impairment. Int. J. Mol. Sci. 2025, 26, 1373. [Google Scholar] [CrossRef]
- Chen, X.; Gu, M.; Hong, Y.; Duan, R.; Zhou, J. Association of Trimethylamine N-Oxide with Normal Aging and Neurocognitive Disorders: A Narrative Review. Brain Sci. 2022, 12, 1203. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Giil, L.M.; Midttun, Ø.; Refsum, H.; Ulvik, A.; Advani, R.; Smith, A.D.; Ueland, P.M. Kynurenine Pathway Metabolites in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 495–504. [Google Scholar] [CrossRef]
- Garcez, M.L.; Jacobs, K.R.; Guillemin, G.J. Microbiota alterations in Alzheimer’s disease: Involvement of the kynurenine pathway and inflammation. Neurotox. Res. 2019, 36, 424–436. [Google Scholar] [CrossRef]
- van der Velpen, V.; Teav, T.; Gallart-Ayala, H.; Mehl, F.; Konz, I.; Clark, C.; Oikonomidi, A.; Peyratout, G.; Henry, H.; Delorenzi, M.; et al. Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 93. [Google Scholar] [CrossRef]
- Salminen, A. Activation of aryl hydrocarbon receptor (AhR) in Alzheimer’s disease: Role of tryptophan metabolites generated by gut host-microbiota. J. Mol. Med. 2023, 101, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Kono, M.; Lee, Y.T.; Byrnes, C.; Li, C.; Tuymetova, G.; Proia, R.L. A genome-wide CRISPR/Cas9 screen reveals that the aryl hydrocarbon receptor stimulates sphingolipid levels. J. Biol. Chem. 2020, 295, 4341–4349. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Perry, G.; Fang, X.; Zagorski, M.; Sambamurti, K.; Poeggeler, B. Indoles as essential mediators in the gut-brain axis. Their role in Alzheimer’s disease. Neurobiol. Dis. 2021, 156, 105403. [Google Scholar] [CrossRef] [PubMed]
- Zott, B.; Konnerth, A. Impairments of glutamatergic synaptic transmission in Alzheimer’s disease. Semin. Cell Dev. Biol. 2023, 139, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, Y.; Lee, G.H. The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch. Pharm. Res. 2019, 42, 1031–1039. [Google Scholar] [CrossRef]
- Govindpani, K.; Turner, C.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Impaired Expression of GABA Signaling Components in the Alzheimer’s Disease Middle Temporal Gyrus. Int. J. Mol. Sci. 2020, 21, 8704. [Google Scholar] [CrossRef]
- Carello-Collar, G.; Bellaver, B.; Ferreira, P.C.L.; Ferrari-Souza, J.P.; Ramos, V.G.; Therriault, J.; Tissot, C.; De Bastiani, M.A.; Soares, C.; Pascoal, T.A.; et al. The GABAergic system in Alzheimer’s disease: A systematic review with meta-analysis. Mol. Psychiatry 2023, 28, 5025–5036. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate Decarboxylase from Lactic Acid Bacteria-A Key Enzyme in GABA Synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.A.; Poluektova, E.U.; Dyachkova, M.S.; Klimina, K.M.; Kovtun, A.S.; Averina, O.V.; Orlova, V.S.; Danilenko, V.N. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef]
- Eicher, T.P.; Mohajeri, M.H. Overlapping mechanisms of action of brain-active bacteria and bacterial metabolites in the pathogenesis of common brain diseases. Nutrients 2020, 4, 2661. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, Z.; Xie, G.; Liu, M.; Yuan, B.; Chai, H.; Wang, W.; Cheng, P. Implications of Gut Microbiota in Neurodegenerative Diseases. Front. Immunol. 2022, 13, 785644. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Q.; Sun, X.; Chen, D.; Wei, C.; Yu, X.; Liu, C.; Li, Y.; Li, J. Activation of GABAA Receptors in Colon Epithelium Exacerbates Acute Colitis. Front. Immunol. 2018, 9, 987. [Google Scholar] [CrossRef]
- Glover, J.S.; Ticer, T.D.; Engevik, M.A. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci. Rep. 2022, 12, 8456. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Li, J.; Zhao, Y.; Jiang, H.; Luo, S.; He, G. Intestinal changes in permeability, tight junction and mucin synthesis in a mouse model of Alzheimer’s disease. Int. J. Mol. Med. 2023, 52, 113. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; Basterfield, L.; McCleave, P.M.; Carter, S.M.; Simmons, N.L. Gamma-Aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br. J. Pharmacol. 2000, 129, 457–464. [Google Scholar] [CrossRef]
- Fuke, N.; Yamashita, T.; Shimizu, S.; Matsumoto, M.; Sawada, K.; Jung, S.; Tokuda, I.; Misawa, M.; Suzuki, S.; Ushida, Y.; et al. Association of Plasma Lipopolysaccharide-Binding Protein Concentration with Dietary Factors, Gut Microbiota, and Health Status in the Japanese General Adult Population: A Cross-Sectional Study. Metabolites 2023, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.J.; Frauen, H.D.; Zhang, B.; Nordhues, B.A.; Bijan, S.; Lin, Y.C.; Zamudio, F.; Hernandez, L.D.; Sabbagh, J.J.; Selenica, M.L.; et al. Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy. Acta Neuropathol. Commun. 2015, 3, 8. [Google Scholar] [CrossRef]
- Tang, X.; Jaenisch, R.; Sur, M. The role of GABAergic signalling in neurodevelopmental disorders. Nat. Rev. Neurosci. 2021, 22, 290–307. [Google Scholar] [CrossRef]
- Andersen, J.V.; Christensen, S.K.; Westi, E.W.; Diaz-del Castillo, M.; Tanila, H.; Schousboe, A.; Aldana, B.I.; Waagepetersen, H.S. Deficient astrocyte metabolism impairs glutamine synthesis and neurotransmitter homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 148, 105198. [Google Scholar] [CrossRef]
- Bi, D.; Wen, L.; Wu, Z.; Shen, Y. GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, 1312–1329. [Google Scholar] [CrossRef]
- Jiménez-Balado, J.; Eich, T.S. GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer’s disease. Semin. Cell Dev. Biol. 2021, 116, 146–159. [Google Scholar] [CrossRef]
- Michely, J.; Eldar, E.; Martin, I.M.; Dolan, R.J. A mechanistic account of serotonin’s impact on mood. Nat. Commun. 2020, 11, 2335. [Google Scholar] [CrossRef]
- Reddy, A.P.; Sawant, N.; Morton, H.; Kshirsagar, S.; Bunquin, L.E.; Yin, X.; Reddy, P.H. Selective serotonin reuptake inhibitor citalopram ameliorates cognitive decline and protects against amyloid beta-induced mitochondrial dynamics, biogenesis, autophagy, mitophagy and synaptic toxicities in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2021, 30, 789–810. [Google Scholar] [CrossRef]
- Sawant, N.; Kshirsagar, S.; Reddy, P.H.; Reddy, A.P. Protective effects of SSRI, Citalopram in mutant APP and mutant Tau expressed dorsal raphe neurons in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166942. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, Y.; Zhang, X.; Wei, X.; Zhang, Y.; Wang, D.; Huang, J.; Zhu, K.; Peng, G.; Sun, B. Aberrant serotonergic signaling contributes to the hyperexcitability of CA1 pyramidal neurons in a mouse model of Alzheimer’s disease. Cell Rep. 2023, 42, 112152. [Google Scholar] [CrossRef] [PubMed]
- Ackmann, J.; Brüge, A.; Gotina, L.; Lim, S.; Jahreis, K.; Vollbrecht, A.L.; Kim, Y.K.; Pae, A.N.; Labus, J.; Ponimaskin, E. Structural determinants for activation of the Tau kinase CDK5 by the serotonin receptor 5-HT7R. Cell Commun. Signal. 2024, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R. The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1273. [Google Scholar] [CrossRef]
- Sarter, M.; Parikh, V. Choline transporters, cholinergic transmission and cognition. Nat. Rev. Neurosci. 2005, 6, 48–56. [Google Scholar] [CrossRef]
- Fahnestock, M.; Shekari, A. ProNGF and Neurodegeneration in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 129. [Google Scholar] [CrossRef]
- Berry, A.S.; Harrison, T.M. New perspectives on the basal forebrain cholinergic system in Alzheimer’s disease. Neurosci. Biobehav. Rev. 2023, 150, 105192. [Google Scholar] [CrossRef]
- Teipel, S.; Grothe, M.J.; Alzheimer’s Disease Neuroimaging Initiative. MRI-based basal forebrain atrophy and volumetric signatures associated with limbic TDP-43 compared to Alzheimer’s disease pathology. Neurobiol. Dis. 2023, 180, 106070. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Pacheco-Herrero, M.; Thyssen, D.; Murillo-Carretero, M.I.; Berrocoso, E.; Spires-Jones, T.L.; Bacskai, B.J.; Garcia-Alloza, M. Rapid β-amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J. Neuropathol. Exp. Neurol. 2013, 72, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, E.; Arulsamy, A.; Aslan, F.S.; Sarisözen, B.; Guney, B.; Hekimoglu, A.; Yilmaz, B.N.; Retinasamy, T.; Shaikh, M.F. An Expanded Narrative Review of Neurotransmitters on Alzheimer’s Disease: The Role of Therapeutic Interventions on Neurotransmission. Mol. Neurobiol. 2025, 62, 1631–1674. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Kobilka, B.K.; Gautam, D.; Sexton, P.M.; Christopoulos, A.; Wess, J. Muscarinic acetylcholine receptors: Novel opportunities for drug development. Nat. Rev. Drug Discov. 2014, 13, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar]
- Kutlu, M.G.; Gould, T.J. Nicotinic modulation of hippocampal cell signaling and associated effects on learning and memory. Physiol. Behav. 2016, 155, 162–171. [Google Scholar] [CrossRef]
- Inazu, M. Functional Expression of Choline Transporters in the Blood-Brain Barrier. Nutrients 2019, 11, 2265. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Li, A.; Yao, M.; Liu, G.; Chen, S.; Luo, Y.; Wang, Z.; Gong, H.; Li, X.; et al. Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer’s disease. Nat. Commun. 2022, 13, 998. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and cognitive control in prefrontal cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Lu, X.; Tao, F. Role of Descending Dopaminergic Pathways in Pain Modulation. Curr. Neuropharmacol. 2019, 17, 1176–1182. [Google Scholar] [CrossRef]
- Manzoor, S.; Hoda, N. A comprehensive review of monoamine oxidase inhibitors as Anti-Alzheimer’s disease agents: A review. Eur. J. Med. Chem. 2020, 206, 112787. [Google Scholar] [CrossRef]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wu, X.; Acheampong, K.; Liu, A. Dopamine and Dopamine Receptors in Alzheimer’s Disease: A Systematic Review and Network Meta-Analysis. Front. Aging Neurosci. 2019, 11, 175. [Google Scholar] [CrossRef]
- Gloria, Y.; Ceyzériat, K.; Tsartsalis, S.; Millet, P.; Tournier, B.B. Dopaminergic dysfunction in the 3xTg-AD mice model of Alzheimer’s disease. Sci. Rep. 2021, 11, 19412. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, L.; Nobili, A.; Cauzzi, E.; Paoletti, I.; Federici, M.; Saba, L.; Giacomet, C.; Marino, R.; Krashia, P.; Melone, M.; et al. Upregulation of Ca2+-binding proteins contributes to VTA dopamine neuron survival in the early phases of Alzheimer’s disease in Tg2576 mice. Mol. Neurodegener. 2022, 17, 76. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Kelly, L.; Seifi, M.; Ma, R.; Mitchell, S.J.; Rudolph, U.; Viola, K.L.; Klein, W.L.; Lambert, J.J.; Swinny, J.D. Identification of intraneuronal amyloid beta oligomers in locus coeruleus neurons of Alzheimer’s patients and their potential impact on inhibitory neurotransmitter receptors and neuronal excitability. Neuropathol. Appl. Neurobiol. 2021, 47, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Robbins, T.W.; Rowe, J.B. The role of noradrenaline in cognition and cognitive disorders. Brain 2021, 144, 2243–2256. [Google Scholar] [CrossRef]
- Fukabori, R.; Iguchi, Y.; Kato, S.; Takahashi, K.; Eifuku, S.; Tsuji, S.; Hazama, A.; Uchigashima, M.; Watanabe, M.; Mizuma, H.; et al. Enhanced Retrieval of Taste Associative Memory by Chemogenetic Activation of Locus Coeruleus Norepinephrine Neurons. J. Neurosci. 2020, 40, 8367–8385. [Google Scholar] [CrossRef] [PubMed]
- Giustino, T.F.; Ramanathan, K.R.; Totty, M.S.; Miles, O.W.; Maren, S. Locus coeruleus norepinephrine drives stress-induced increases in basolateral amygdala firing and impairs extinction learning. J. Neurosci. 2020, 40, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Portela-Moreira, I.; Henriques, T.; Vieira-Coelho, M.A.; Guimarães, J. Dysfunction of norepinephrine and its metabolites in Alzheimer’s dementia—A review with meta-analysis. Ageing Res. Rev. 2023, 83, 101784. [Google Scholar] [CrossRef]
- Cao, S.; Fisher, D.W.; Rodriguez, G.; Yu, T.; Dong, H. Comparisons of neuroinflammation, microglial activation, and degeneration of the locus coeruleusnorepinephrine system in APP/PS1 and aging mice. J. Neuroinflamm. 2021, 18, 10. [Google Scholar] [CrossRef]
- Malatt, C.; Tagliati, M. The role of the locus coeruleus/norepinephrine system in the pathogenesis of neurodegenerative disorders: An update. Curr. Opin. Neurol. 2022, 35, 220–229. [Google Scholar] [CrossRef]
- Chalermpalanupap, T.; Schroeder, J.P.; Rorabaugh, J.M.; Liles, L.C.; Lah, J.J.; Levey, A.I.; Weinshenker, D. Locus Coeruleus Ablation Exacerbates Cognitive Deficits, Neuropathology, and Lethality in P301S Tau Transgenic Mice. J. Neurosci. 2018, 38, 74–92. [Google Scholar] [CrossRef]
- Betts, M.J.; Kirilina, E.; Otaduy, M.C.G.; Ivanov, D.; Acosta-Cabronero, J.; Callaghan, M.F.; Lambert, C.; Cardenas-Blanco, A.; Pine, K.; Passamonti, L.; et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 2019, 142, 2558–2571. [Google Scholar] [CrossRef]
- Lin, C.P.; Frigerio, I.; Bol, J.G.J.M.; Bouwman, M.M.A.; Wesseling, A.J.; Dahl, M.J.; Rozemuller, A.J.M.; van der Werf, Y.D.; Pouwels, P.J.W.; van de Berg, W.D.J.; et al. Microstructural integrity of the locus coeruleus and its tracts reflect noradrenergic degeneration in Alzheimer’s disease and Parkinson’s disease. Transl. Neurodegener. 2024, 13, 9. [Google Scholar] [CrossRef]
- Flores-Aguilar, L.; Hall, H.; Orciani, C.; Foret, M.K.; Kovecses, O.; Ducatenzeiler, A.; Cuello, A.C. Early loss of locus coeruleus innervation promotes cognitive and neuropathological changes before amyloid plaque deposition in a transgenic rat model of Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2022, 48, e12835. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, Y.; Zhou, J.; Zou, H.; Ma, L.; Liu, C.; Xiao, Z.; Liu, X.; Tan, X.; Yu, T.; et al. Activation of locus coeruleus-spinal cord noradrenergic neurons alleviates neuropathic pain in mice via reducing neuroinflammation from astrocytes and microglia in spinal dorsal horn. J. Neuroinflamm. 2022, 19, 123. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Berardi, S.; Scarpona, S.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Gut microbiota manipulation through probiotics oral administration restores glucose homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2020, 87, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A.M. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cuccioloni, M.; Gong, C.; Cecarini, V.; Spina, M.; Zheng, Y.; Angeletti, M.; Eleuteri, A.M. Gut microbiota modulation in Alzheimer’s disease: Focus on lipid metabolism. Clin. Nutr. 2022, 41, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Z.; Zhao, Y.; Wang, Z.; Wang, C.; Yang, G.; Li, S. Lactobacillus plantarum DP189 prevents cognitive dysfunction in D-galactose/AlCl3 induced mouse model of Alzheimer’s disease via modulating gut microbiota and PI3K/Akt/GSK-3β signaling pathway. Nutr. Neurosci. 2022, 25, 2588–2600. [Google Scholar] [CrossRef]
- Wang, Q.J.; Shen, Y.E.; Wang, X.; Fu, S.; Zhang, X.; Zhang, Y.N.; Wang, R.T. Concomitant memantine and Lactobacillus plantarum treatment attenuates cognitive impairments in APP/PS1 mice. Aging 2020, 12, 628–649. [Google Scholar] [CrossRef]
- Kaur, H.; Nagamoto-Combs, K.; Golovko, S.; Golovko, M.Y.; Klug, M.G.; Combs, C.K. Probiotics ameliorate intestinal pathophysiology in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2020, 92, 114–134. [Google Scholar] [CrossRef]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lv, S.; Li, Y.; Wei, D.; Zhou, X.; Niu, X.; Yang, Z.; Song, W.; Zhang, Z.; Peng, D. Prebiotics modulate the microbiota-gut-brain axis and ameliorate cognitive impairment in APP/PS1 mice. Eur. J. Nutr. 2023, 62, 2991–3007. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lai, D.M.; Huang, H.J.; Lee-Chen, G.J.; Chang, C.H.; Hsieh-Li, H.M.; Lee, G.C. Prebiotic Lactulose Ameliorates the Cognitive Deficit in Alzheimer’s Disease Mouse Model through Macroautophagy and Chaperone-Mediated Autophagy Pathways. J. Agric. Food Chem. 2021, 69, 2422–2437. [Google Scholar] [CrossRef]
- Sun, J.; Liu, S.; Ling, Z.; Wang, F.; Ling, Y.; Gong, T.; Fang, N.; Ye, S.; Si, J.; Liu, J. Fructooligosaccharides Ameliorating Cognitive Deficits and Neurodegeneration in APP/PS1 Transgenic Mice through Modulating Gut Microbiota. J. Agric. Food Chem. 2019, 67, 3006–3017. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Han, D.; Li, Z.; Liu, T.; Yang, N.; Li, Y.; He, J.; Qian, M.; Kuang, Z.; Zhang, W.; Ni, C.; et al. Prebiotics Regulation of Intestinal Microbiota Attenuates Cognitive Dysfunction Induced by Surgery Stimulation in APP/PS1 Mice. Aging Dis. 2020, 11, 1029–1045. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Fecal microbiota transplantation as a tool for therapeutic modulation of neurological and mental disorders. SciBase Neurol. 2024, 2, 1018. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef]
- Qian, X.; Hai, W.; Chen, S.; Zhang, M.; Jiang, X.; Tang, H. Multi-omics data reveals aberrant gut microbiota-host glycerophospholipid metabolism in association with neuroinflammation in APP/PS1 mice. Gut Microbes 2023, 15, 2282790. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jeon, S.H.; Ju, I.G.; Gee, M.S.; Do, J.; Oh, M.S.; Lee, J.K. Transplantation of gut microbiota derived from Alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav. Immun. 2021, 98, 357–365. [Google Scholar] [CrossRef]
- Jin, J.; Xu, Z.; Zhang, L.; Zhang, C.; Zhao, X.; Mao, Y.; Zhang, H.; Liang, X.; Wu, J.; Yang, Y.; et al. Gut-derived β-amyloid: Likely a centerpiece of the gut-brain axis contributing to Alzheimer’s pathogenesis. Gut Microbes 2023, 15, 2167172. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Xiang, H.; Wang, J.; Jiang, Y.; Pan, C.; Ji, B.; Zhang, A. Fecal microbiota transplantation: A novel strategy for treating Alzheimer’s disease. Front. Microbiol. 2023, 14, 1281233. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, J.H.; Shin, J.; Kim, J.S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive function improvement after fecal microbiota transplantation in Alzheimer’s dementia patient: A case report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef]
- Cao, J.; Amakye, W.K.; Qi, C.; Liu, X.; Ma, J.; Ren, J. Bifidobacterium lactis Probio-M8 regulates gut microbiota to alleviate Alzheimer’s disease in the APP/PS1 mouse model. Eur. J. Nutr. 2021, 60, 3757–3769. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Huang, Y.Y.; Tsai, S.Y.; Kuo, Y.W.; Lin, J.H.; Ho, H.H.; Chen, J.F.; Hsia, K.C.; Sun, Y. Efficacy of Probiotic Supplements on Brain-Derived Neurotrophic Factor, Inflammatory Biomarkers, Oxidative Stress and Cognitive Function in Patients with Alzheimer’s Dementia: A 12-Week Randomized, Double-Blind Active-Controlled Study. Nutrients 2023, 16, 16. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, K.E.; Kim, J.K.; Kim, D.H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019, 9, 11814. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Niu, X.; Li, P.; Tong, T.; Wang, Q.; Zhang, M.; Li, Y.; Liu, J.; Li, Z. Lactobacillaceae improve cognitive dysfunction via regulating gut microbiota and suppressing Aβ deposits and neuroinflammation in APP/PS1 mice. Arch. Microbiol. 2023, 205, 118. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, D.; Yang, J.; Liu, T.; Hu, G.; Liang, H.; Tang, X.; Lai, G.; Shuai, O.; Zheng, C.; et al. Effects of Oligosaccharides From Morinda officinalis on Gut Microbiota and Metabolome of APP/PS1 Transgenic Mice. Front. Neurol. 2018, 9, 412. [Google Scholar] [CrossRef]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Elangovan, S.; Borody, T.J.; Holsinger, R.M.D. Fecal Microbiota Transplantation Reduces Pathology and Improves Cognition in a Mouse Model of Alzheimer’s Disease. Cells 2022, 12, 119. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, H.; Lee, J.H.; Shin, J.; Cha, B.; Kwon, K.S.; Shin, Y.W.; Kim, Y.; Kim, Y.; Bae, J.S.; et al. Effect of altered gene expression in lipid metabolism on cognitive improvement in patients with Alzheimer’s dementia following fecal microbiota transplantation: A preliminary study. Ther. Adv. Neurol. Disord. 2024, 17, 17562864231218181. [Google Scholar] [CrossRef]
- Ratsika, A.; Cruz Pereira, J.S.; Lynch, C.M.K.; Clarke, G.; Cryan, J.F. Microbiota-immune-brain interactions: A lifespan perspective. Curr. Opin. Neurobiol. 2023, 78, 102652. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, R.F.; Golde, T.E.; Heneka, M.T.; Readhead, B. Do infections have a role in the pathogenesis of Alzheimer disease? Nat. Rev. Neurol. 2020, 16, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E.; DeKosky, S.T.; Galasko, D. Alzheimer’s disease: The right drug, the right time. Science 2018, 362, 1250–1251. [Google Scholar] [CrossRef]
- Malan-Müller, S.; Martín-Hernández, D.; Caso, J.R.; Matthijnssens, J.; Rodríguez-Urrutia, A.; Lowry, C.A.; Leza, J.C. Metagenomic symphony of the intestinal ecosystem: How the composition affects the mind. Brain Behav. Immun. 2025, 123, 510–523. [Google Scholar] [CrossRef]
- Ances, B.; Ferreiro, A.L.; Choi, J.H.; Ryou, J.; Newcomer, E.; Stark, S.L.; Benzinger, T.L.S.; Holtzman, D.M.; Fagan, A.M.; Schindler, S.E.; et al. The gut microbiome as an early biomarker of preclinical Alzheimer disease. Alzheimer’s Dement. 2023, 19, e078811. [Google Scholar] [CrossRef]
- Ferreiro, A.L.; Choi, J.; Ryou, J.; Newcomer, E.P.; Thompson, R.; Bollinger, R.M.; Hall-Moore, C.; Ndao, I.M.; Sax, L.; Benzinger, T.L.S.; et al. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl. Med. 2023, 15, eabo2984. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. The role of the gut microbiome in Alzheimer’s disease pathophysiology. Curr. Opin. Neurol. 2025, 38, 157–162. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Bustillos-López, A. Intervención social dirigida al envejecimiento saludable: Revisión de estudios recientes. Análisis Modif. Conducta 2024, 50, 21–38. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. Una revisión crítica sobre la aplicación de estimulación cognitiva en el contexto gerontológico. Escr. Psicol. 2024, 17, 31–43. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Una revisión actual sobre enfoques terapéuticos microbianos destinados a mejorar las funciones cognitivas en adultos mayores. Gerokomos 2024, 35, 235–243. [Google Scholar]
- Borrego-Ruiz, A.; Borrego, J.J. Microbial therapeutic tools for human brain disorders: A current overview. Brain Disord. 2025, 19, 100262. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. El envejecimiento tras la Covid-19. Paraninfo Digit. 2024, 38, e3815c. [Google Scholar]
- Arora, S.; Santiago, J.A.; Bernstein, M.; Potashkin, J.A. Diet and lifestyle impact the development and progression of Alzheimer’s dementia. Front. Nutr. 2023, 10, 1213223. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. Vegetarian and ketogenic diets: Their relationship with gut microbiome and mental health, and their clinical applications. Food Nutr. Chem. 2025, 3, 278. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Smith, P.J.; Mabe, S.; Hinderliter, A.; Lin, P.H.; Liao, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Kraus, W.E.; Doraiswamy, P.M.; et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology 2019, 92, e212–e223. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Osse, A.M.L.; Kinney, J.W.; Cammann, D.; Chen, J. Alzheimer’s Disease: Combination Therapies and Clinical Trials for Combination Therapy Development. CNS Drugs 2024, 38, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. Maximizing the benefit and managing the risk of anti-amyloid monoclonal antibody therapy for Alzheimer’s disease: Strategies and research directions. Neurotherapeutics 2025, 22, e00570. [Google Scholar] [CrossRef] [PubMed]
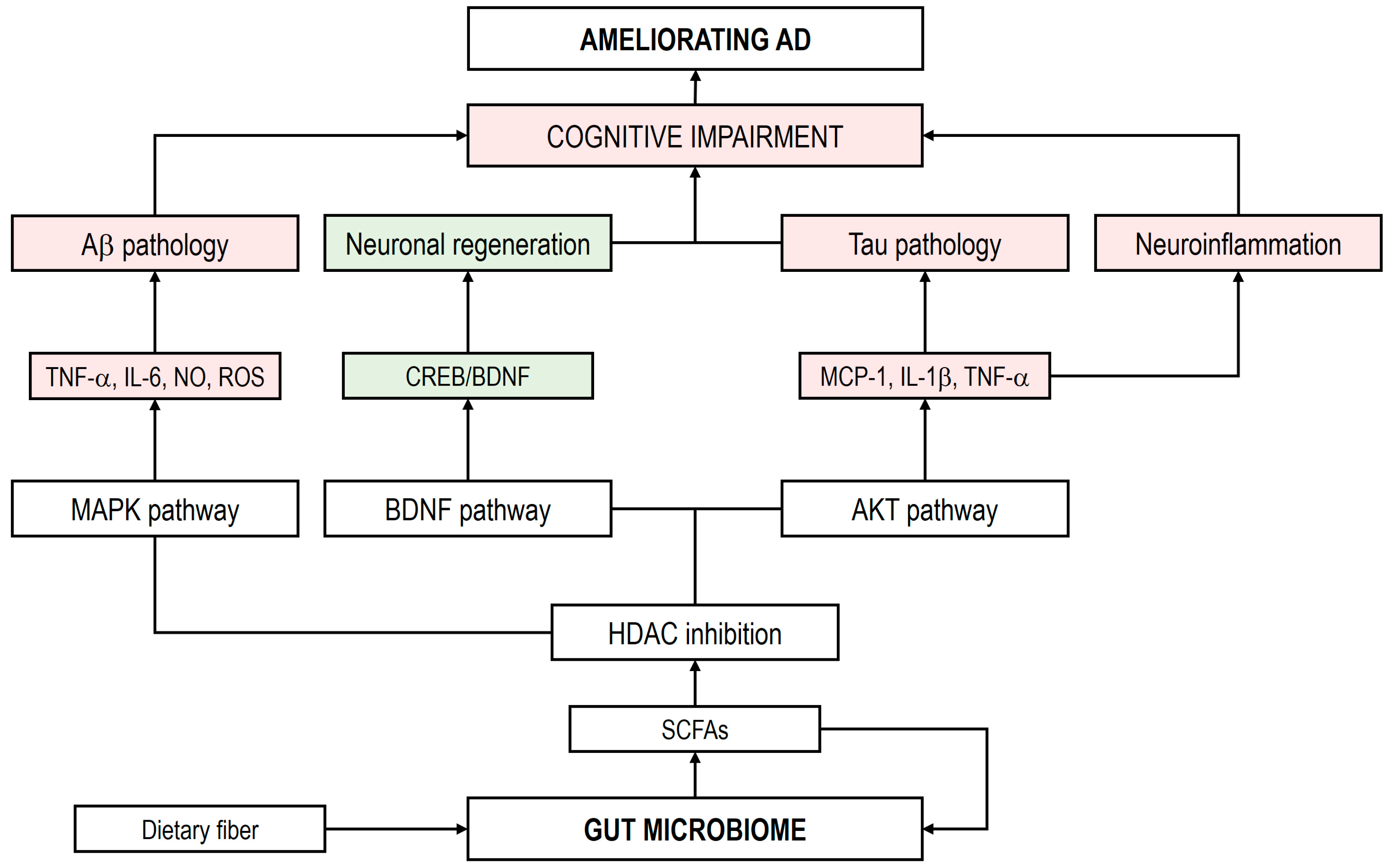
| Bacterial Taxa | Increased Abundance | Decreased Abundance | Characteristics of The Study | References |
|---|---|---|---|---|
| Phylum Acidobacteriota | X | Systematic review | [37] | |
| Phylum Actinomycetota | X | [37] | ||
| X | China/16S rRNA | [45] | ||
| Genus Adlercrutzia | X | USA/16S rRNA | [43] | |
| Genus Atopobium | X | China/16S rRNA | [32] | |
| Genus Bifidobacterium | X | Systematic review | [38] | |
| X | China/16S rRNA | [39] | ||
| X | China/16S rRNA | [40] | ||
| X | [43] | |||
| Phylum Bacillota | X | [37,38,45] | ||
| X | China/16S rRNA | [41] | ||
| Family Acidaminococcaceae | X | [37] | ||
| Family Clostridiaceae | X | [38,43] | ||
| Family Enterococcaceae | X | [45] | ||
| Family Gemellaceae | X | [43] | ||
| Family Lachnospiraceae | X | [37,41,43,45] | ||
| Family Lactobacillaceae | X | [45] | ||
| Family Mogibacteriaceae | X | [43] | ||
| Family Peptostreptococcaceae | X | [43] | ||
| Family Ruminococcaceae | X | [37,45] | ||
| X | [41,43] | |||
| Family Turicibacteraceae | X | [43] | ||
| Family Veillonellaceae | X | [45] | ||
| Genus Agathobacter | X | [32] | ||
| Genus Anaerostipes | X | China/16S rRNA | [44] | |
| Genus Bacillus | X | [44] | ||
| Genus Blautia | X | [39,43] | ||
| X | [41] | |||
| Genus Butyricicoccus | X | [40] | ||
| Genus Clostridium | X | [40,43] | ||
| Genus Coprococcus | X | [40] | ||
| X | [32] | |||
| Genus Dialister | X | [40,43] | ||
| Genus Dorea | X | [39] | ||
| Genus Erysipelatoclostridium | X | [32] | ||
| Genus Eubacterium | X | MCI/Italy/qPCR | [35] | |
| X | [32] | |||
| Genus Faecalibacterium | X | [40] | ||
| X | [32] | |||
| Genus Gemella | X | [43] | ||
| Genus Lachnoclostridium | X | USA/NextSeq500 | [36] | |
| Genus Lactobacillus | X | [39] | ||
| Genus Limosilactobacillus | X | [44] | ||
| Genus Parvimonas | X | [32] | ||
| Genus Phascolarctobacterium | X | [38,43] | ||
| Genus Romboutsia | X | [40] | ||
| Genus Roseburia | X | [40] | ||
| Genus Ruminiclostridium | X | [37] | ||
| Genus Ruminococcus | X | [45] | ||
| X | [41] | |||
| Genus Solobacterium | X | [32] | ||
| Genus Staphylococcus | X | [44] | ||
| Genus Streptococcus | X | [39] | ||
| Genus Subdoligranulum | X | [45] | ||
| Genus Turicibacter | X | [43] | ||
| Genus Tyzzerella | X | [32] | ||
| Phylum Bacteroidota | X | [43,45] | ||
| Family Bacteroidaceae | X | [43] | ||
| X | [45] | |||
| Family Rikenellaceae | X | [38] | ||
| X | [43] | |||
| Genus Alistipes | X | [36,43] | ||
| X | [39] | |||
| Genus Alloprevotella | X | [39] | ||
| X | [32] | |||
| Genus Bacteroides | X | [36,37,43,45] | ||
| Genus Barnesiella | X | [36] | ||
| X | [39] | |||
| Genus Butyricimonas | X | [39] | ||
| Genus Odoribacter | X | [36] | ||
| Genus Parabacteroides | X | [39] | ||
| Genus Paraprevotella | X | [39] | ||
| Genus Prevotella | X | [39] | ||
| X | Meta-analysis | [42] | ||
| Phylum Pseudomonadota | X | [38,41] | ||
| Family Enterobacteriaceae | X | [41] | ||
| Genus Acinetobacter | X | [39] | ||
| Genus Bosea | X | [44] | ||
| Genus Dyella | X | [44] | ||
| Genus Escherichia/Shigella | X | [35] | ||
| Genus Gemmiger | X | [40] | ||
| Genus Haemophilus | X | [39,42] | ||
| Genus Massilia | X | [44] | ||
| Genus Pseudomonas | X | [32] | ||
| Genus Sphingomonas | X | [44] | ||
| Genus Stenotrophomonas | X | [44] | ||
| Genus Succinivibrio | X | [39] | ||
| Genus Sutterella | X | [39,42] | ||
| Genus Variovorax | X | [44] | ||
| Phylum Synergistota | ||||
| Genus Cloacibacillus | X | [32] | ||
| Phylum Thermodesulfobacteriota | ||||
| Genus Bilophila | X | [43] | ||
| Phylum Verrucomicrobiota | X | [45] | ||
| Genus Akkermansia | X | [39,40] |
| Intervention | Alterations in the GM | Major Effects | Reference |
|---|---|---|---|
| Probiotics | |||
| Clostridium butyricum strain CGMCC 9831 (1 × 109 CFU/mL; oral; 4 weeks) APP/PS1 mice | Increase Alloprevotella and butyrate. Decrease Deferribacterota, Helicobacteraceae, Helicobacter. |
| [161] |
| SLAB51 (2 × 1011 bacteria/kg/d); oral; 4–12 months) 3xTg-AD mice | Increase Bifidobacterium and SCFAs. Decrease Campylobacterales. |
| [251,252,253,254] |
| Lactiplantibacillus plantarum strain DP189 (1 × 109 CFU/g; oral; 10 weeks) APP/PS1 mice | Decrease in TMA and TMAO. Restore GM dysbiosis. |
| [255,256] |
| VSL#3 (3.2 × 108 CFU/25 g; oral; 8 weeks) APP NL-GF mice | Increase Actinomycetota and Verrucomicrobiota. |
| [257] |
| Bifidobacterium animalis subsp. lactis strain Probio-M8 (1 × 109 CFU/g; oral; 45 days) APP/PS1 mice | Increase Desulfovibrionaceae, Coprococcus, Oscillospira, Clostridiales. Decrease Adlercreutzia, Lactobacillus, Streptococcus. |
| [272] |
| B. longum subsp. infantis strain BLI-02, B. breve strain Bv-889, B. animalis subsp. lactis strain CP-9, B. bifidum strain VDD088, and L. plantarum strain PL-02 (1 × 1010 CFU/capsule; oral; 12 weeks) Human AD patients | Increase Akkermansia, Bifidobacterium, Clostridium, Lactobacillus and Ruminococcus. Decrease Megamonas. |
| [273] |
| Bifidobacterium breve strain A1 (5 × 109 CFU/mL; oral; 11 days) Aβ injection mice. | Increase Actinomycetota, Bifidobacteriaceae, and acetate. Decrease Lachnospiraceae and Odoribacteraceae. |
| [274] |
| Bifidobacterium longum strain NK46 (1 × 109 CFU/mouse/day; oral; 2 months) 5xFAD-Tg mice | Increase Prevotellaceae. Decrease Lachnospiraceae, Helicobacteraceae, Pseudomonadaceae, Ruminococcaceae, and LPS. |
| [275] |
| Limosilactobacillus fermentum strain CGMCC 18206, L. fermentum strain CGMCC 18207, Lactiplantibacillus plantarum strain CGMCC 18208, and L. plantarum CGMCC 18209 (1 × 109 CFU/mL; oral; 12 weeks) APP/PS1 mice | Increase Bacteriodota, Staphylococcus, Acinetobacter, Butyricicoccus, Sphingobacterium, Weissella. Decrease Pseudomonadota, Desulfobacterota, Patescibacteria, Eisenbergiella. |
| [276] |
| Prebiotics | |||
| Mannanoligosaccharides (0.12%, w/v in the drinking water; oral; 8 weeks) 5xFAD-Tg mice | Increase Lactobacillus, Oscillospira, Prevotella and butyrate. Decrease Helicobacter and LPS. |
| [262] |
| Xylooligosaccharides (10%, w/v in PBS; oral; 5 weeks) APP/PS1 mice | Increase Bifidobacterium, Lactobacillus and Muribacterium. |
| [263] |
| Morinda officinalis oligosaccharides (50–100 mg/kg/d; 6 months) APP/PS1 mice | Increase Akkermansia, Allobaculum, Arthrobacter, Bifidobacterium, Brevilactibacter. |
| [277] |
| FMT preclinical trials | |||
| Donor: WT mice; Recipient: APP/PS1 mice | Increase Bacteroidota and butyrate. Decrease Pseudomonadota and Verrucomicrobiota. |
| [266] |
| Donor: C57BL/6 mice; Recipient: C57BL/6 mice treated with antibiotics | Reduction in SCFA-producers bacteria |
| [278] |
| Donor: B6S52 mice; Recipient: 5xFAD-Tg mice | Increase GM composition and SCFAs. |
| [279] |
| FMT clinical trials | |||
| Donor: 27-year-old healthy man; Recipient: 90-year-old woman with AD | Increase α-diversity and SCFAs |
| [271] |
| Donor: healthy man; Recipient: patients diagnosed with dementia with relapsed Clostridioides difficile infection | Increase the enrichment of Pseudomonadota and Bacteroidota |
| [280] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrego-Ruiz, A.; Borrego, J.J. Microbial Metabolomes in Alzheimer’s Disease: From Pathogenesis to Therapeutic Potential. Curr. Issues Mol. Biol. 2025, 47, 724. https://doi.org/10.3390/cimb47090724
Borrego-Ruiz A, Borrego JJ. Microbial Metabolomes in Alzheimer’s Disease: From Pathogenesis to Therapeutic Potential. Current Issues in Molecular Biology. 2025; 47(9):724. https://doi.org/10.3390/cimb47090724
Chicago/Turabian StyleBorrego-Ruiz, Alejandro, and Juan J. Borrego. 2025. "Microbial Metabolomes in Alzheimer’s Disease: From Pathogenesis to Therapeutic Potential" Current Issues in Molecular Biology 47, no. 9: 724. https://doi.org/10.3390/cimb47090724
APA StyleBorrego-Ruiz, A., & Borrego, J. J. (2025). Microbial Metabolomes in Alzheimer’s Disease: From Pathogenesis to Therapeutic Potential. Current Issues in Molecular Biology, 47(9), 724. https://doi.org/10.3390/cimb47090724





