Metabolic Engineering of Terpenoid Biosynthesis in Medicinal Plants: From Genomic Insights to Biotechnological Applications
Abstract
1. Introduction
2. Comparative Analysis of Terpenoid Production Systems
3. Fundamentals of Terpene Biosynthesis in Medicinal Plants: Biosynthetic Pathways and Regulatory Mechanisms
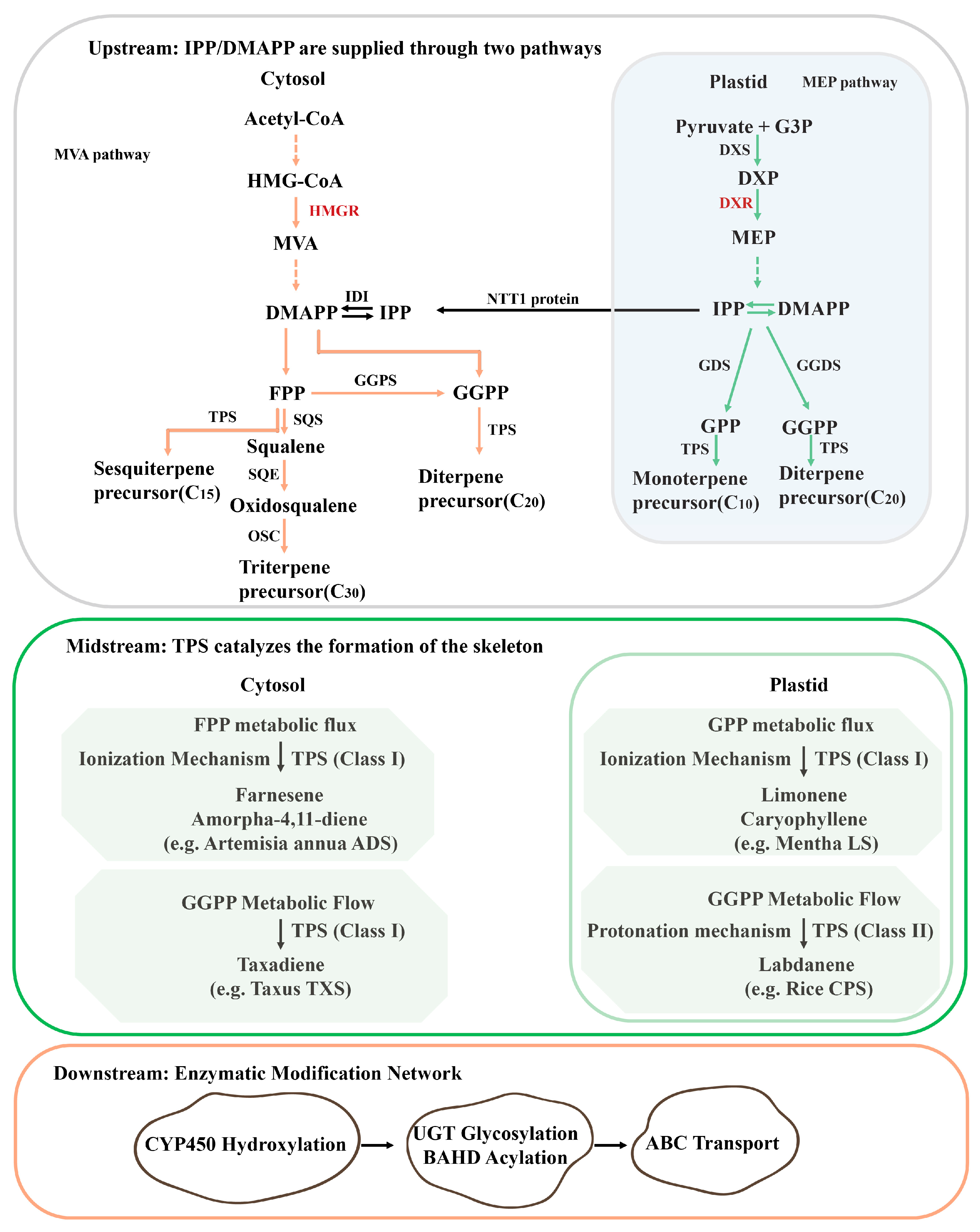
3.1. Core Pathways: From Precursor Biosynthesis to Structural Diversification
3.2. Medicinal Plant-Specific Regulatory Adaptations
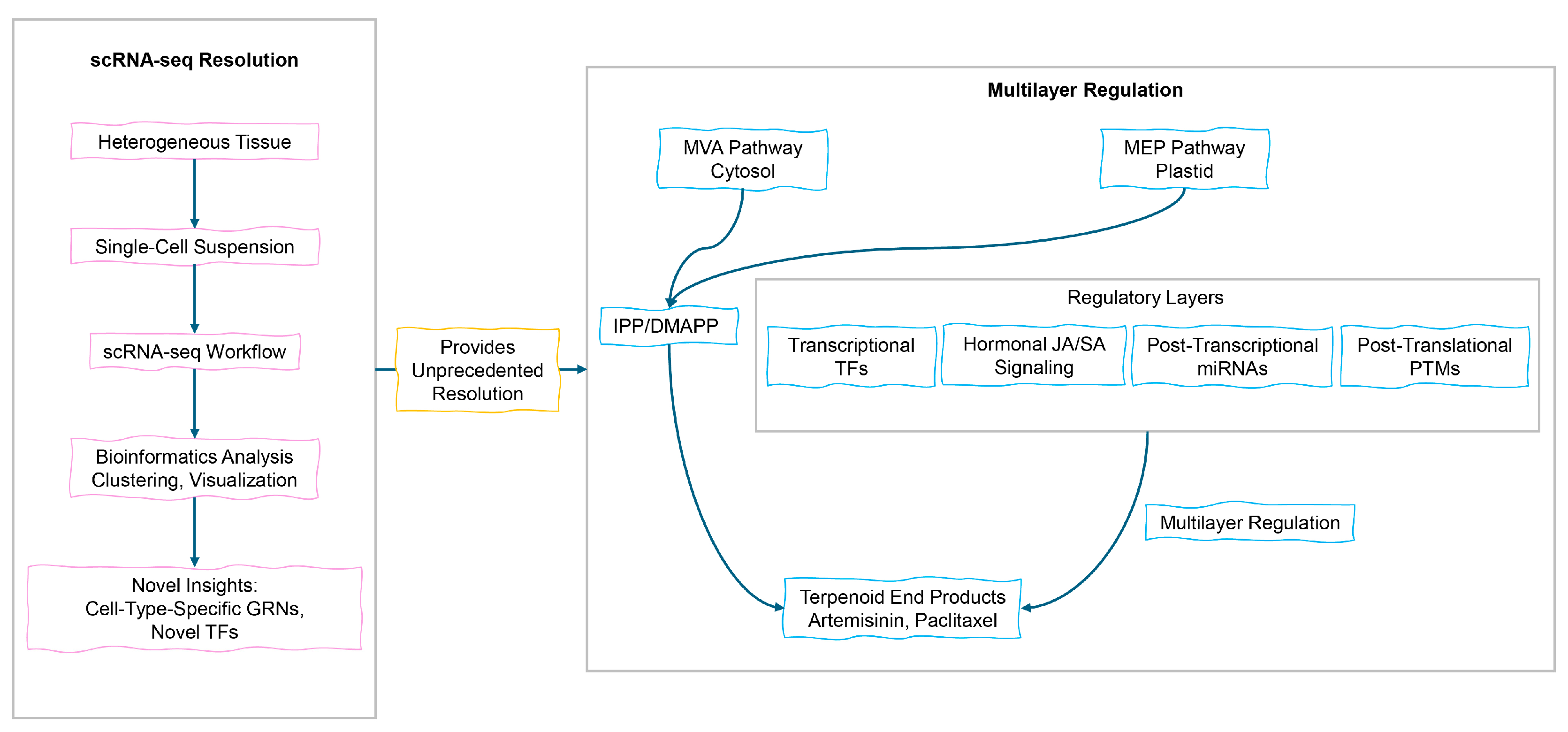
3.3. Multilayer Regulatory Networks in Plant Systems
3.4. Engineering Regulatory Networks to Enhance Terpenoid Production
3.5. Emerging Insights and Persistent Challenges
4. Metabolic Engineering Strategies Across Platforms
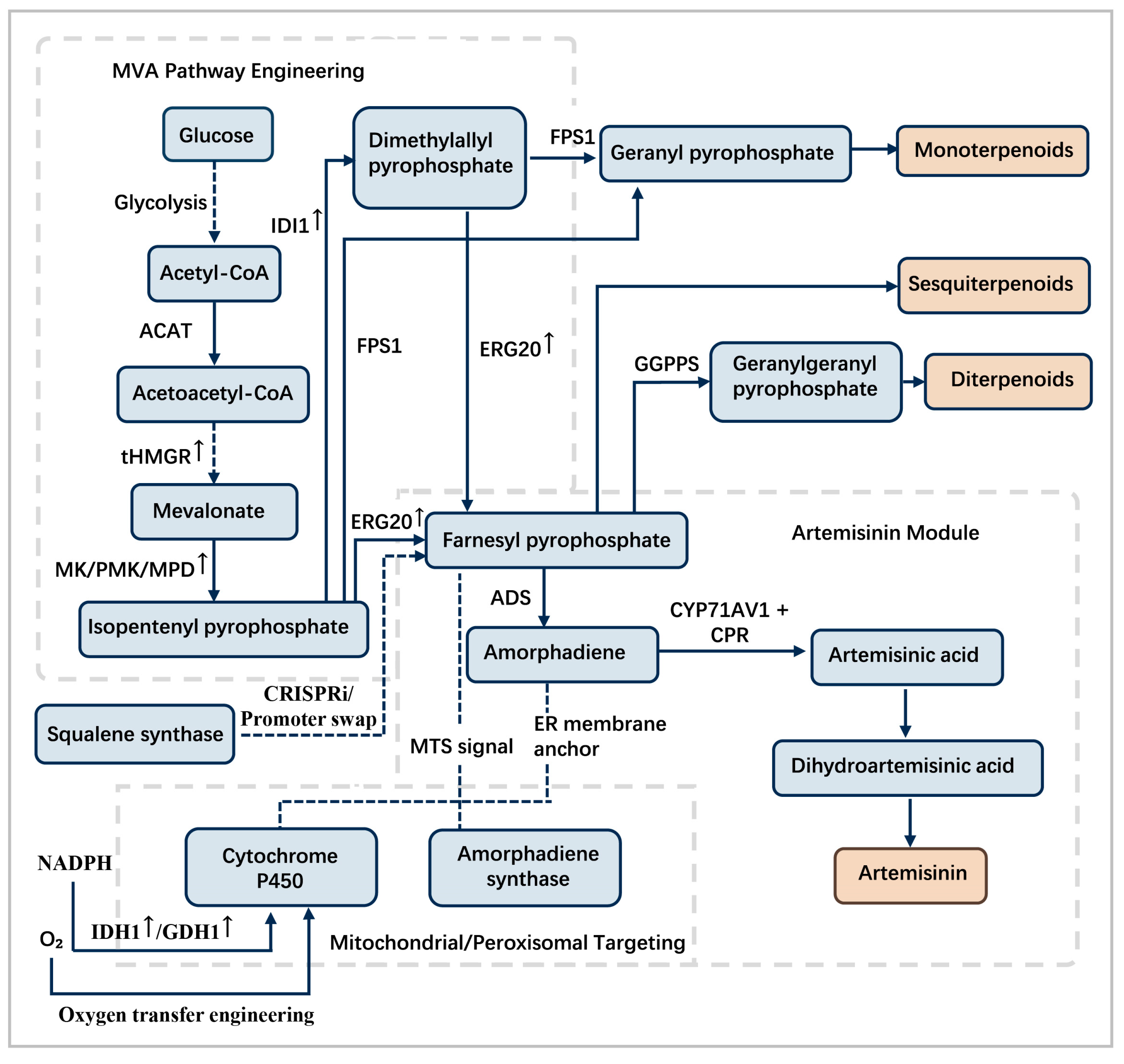
4.1. Overexpression of Rate-Limiting Enzymes and Enhancement of Metabolic Pathways
4.2. Precise Suppression of Competing Metabolic Pathways
4.3. Hierarchical Regulation of Transcription Factors (TFs)
4.4. Heterologous Pathway Reconstruction and Enzyme Engineering
4.5. Directed Subcellular Metabolic Channeling
4.6. Cofactor Balancing and Dynamic Regulatory Mechanisms
4.7. Advances in Synthetic Biology Tools
4.8. Critical Analysis of Metabolic Engineering Strategies: Mechanistic Insights into Success and Failure
4.8.1. The Paradigm Shift from ‘Rate-Limiting Steps’ to Distributed Metabolic Control
4.8.2. Precursor Availability as a Critical Metabolic Bottleneck: The Case of ADS in Artemisia Annua
4.8.3. Pathway Balancing Through Attenuation of Competing Metabolic Fluxes
4.8.4. Integrated Multi-Gene Engineering: The “Push, Pull, and Block” Strategy
4.8.5. Analysis of Persistent Challenges in Terpenoid Metabolic Engineering
5. Genomics and Multi-Omics: Elucidating the Blueprint and Potential Targets
5.1. Foundation: Genome Sequencing and Gene Identification
5.2. Decoding Dynamic Processes: Transcriptomic and Metabolomic Profiling
5.3. Beyond Abundance: Proteomic and Epigenomic Regulatory Mechanisms
5.4. Systems Integration: Constructing Predictive Models for Engineering Applications
6. Biotechnological Applications: From Laboratory Research to Prospective Industrialization
6.1. High-Yielding Cultivation of Medicinal Plants and Cell Lines
6.2. Production of Rare and Structurally Complex Terpenoids
6.3. Biosynthesis of Novel Terpenoid Derivatives
6.4. Enhanced Plant Stress Tolerance
6.5. Plant Cell and Tissue Culture: Challenges in Scale-Up and Industrial Application
6.6. Plant-Based Systems as Sustainable Cell Factories
7. Current Challenges and Limitations
7.1. Complex Metabolic Pathways and Incompletely Characterized Regulatory Mechanisms
7.2. Limitations in Genetic Transformation and Regeneration Efficiency
7.3. Metabolic Imbalance, Growth-Associated Penalties, and Cellular Toxicity
7.4. Compartmentalization and Transport Limitations
7.5. Suboptimal Enzymatic Characteristics
7.6. Absence of Universal Chassis Plant Systems
7.7. Scale-Up Challenges: Process Engineering Constraints and Economic Barriers
7.8. Regulatory and Societal Challenges
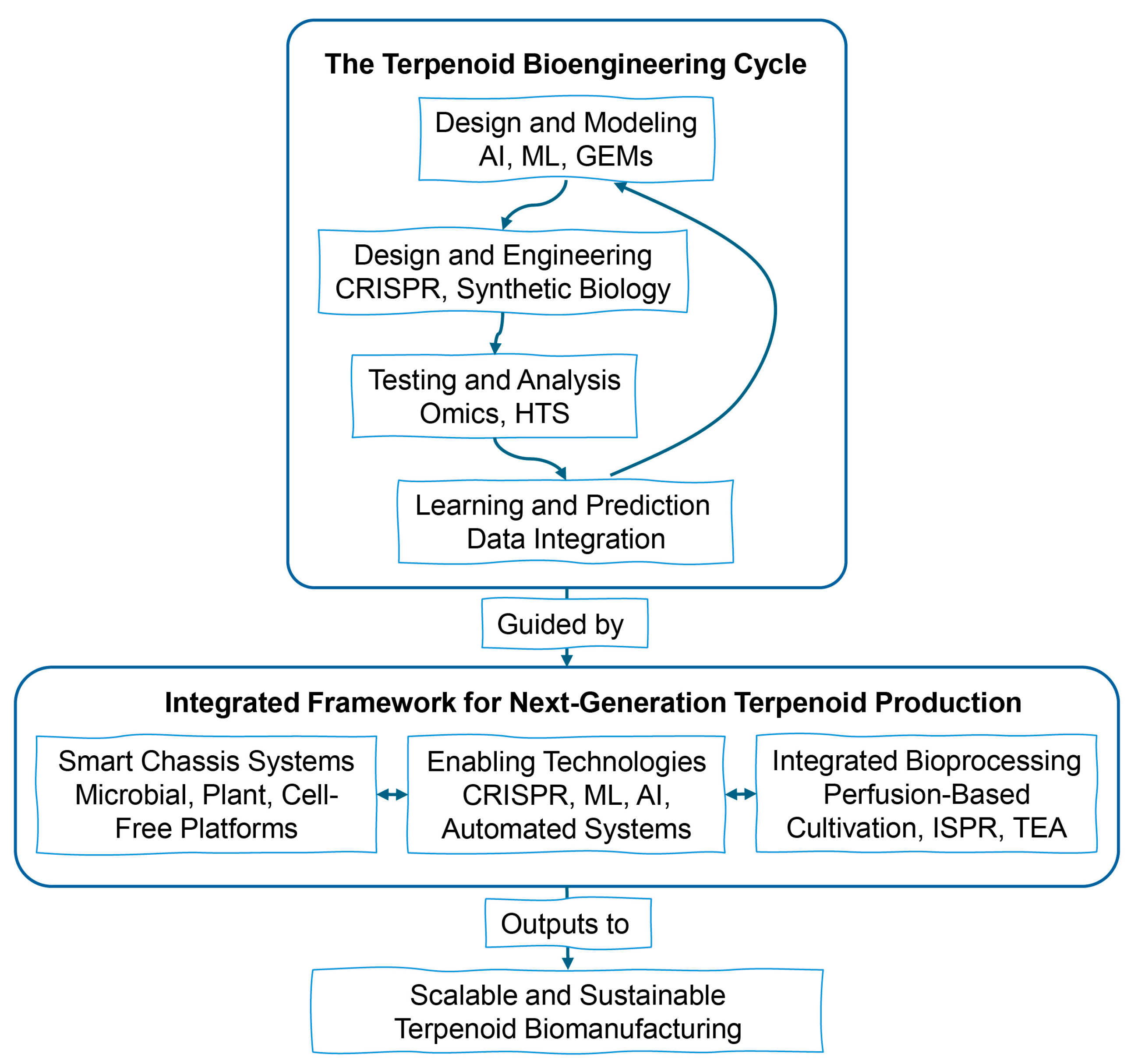
8. Prospects and Frontier Directions
8.1. Integrated Bioprocessing Strategies for Industrial Application and Scale-Up
8.2. Deep Integration of Multi-Omics and Systems Biology for Predictive Modeling
8.3. Advances in Gene Editing Technologies
8.4. Synthetic Biology and Modular Design Approaches
8.5. Enzyme Engineering and Directed Evolutionary Strategies
8.6. Subcellular Compartment Engineering
8.7. Development of an Efficient Universal Host System
8.8. Cell-Free Synthetic Biology Systems
8.9. Integration of Artificial Intelligence and Machine Learning
8.10. Focus on Non-Model Medicinal Plants
8.11. End-to-End Integration and Collaborative Innovation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-Binding Cassette |
| ADS | Amorpha-4,11-Diene Synthase |
| AI | Artificial Intelligence |
| ALDH1 | Aldehyde Dehydrogenase 1 |
| ATAC-seq | Assay for Transposase-Accessible Chromatin with high-throughput sequencing |
| ATP | Adenosine Triphosphate |
| BE | Base Editing |
| ChIP-seq | Chromatin Immunoprecipitation sequencing |
| COX-2 | Cyclooxygenase-2 |
| CPR | Cytochrome P450 Reductase |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CRISPR-Cas9 | CRISPR-associated protein 9 |
| CRISPRi | CRISPR interference |
| CYP | Cytochrome P450 |
| DMAPP | Dimethylallyl Diphosphate |
| DXS | 1-Deoxy-D-Xylulose-5-Phosphate Synthase |
| EFSA | European Food Safety Authority |
| ER | Endoplasmic Reticulum |
| FAD3 | Fatty Acid Desaturase 3 |
| FPP | Farnesyl Diphosphate |
| G6PDH | Glucose-6-Phosphate Dehydrogenase |
| GA3 | Gibberellin A3 |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| GEMs | Genome-scale Metabolic Models |
| GGPP | Geranylgeranyl Diphosphate |
| GMO | Genetically Modified Organism |
| GPP | Geranyl Diphosphate |
| GRNs | Gene Regulatory Networks |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl-CoA |
| HMGR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase |
| HRMS | High-Resolution Mass Spectrometry |
| IDI | Isopentenyl Diphosphate Isomerase |
| IMS | Ion Mobility Spectrometry |
| IPK | Isopentenyl Phosphate Kinase |
| IPP | Isopentenyl Diphosphate |
| iNOS | Inducible Nitric Oxide Synthase |
| IUP | Isopentenol Utilization Pathway |
| JA | Jasmonate |
| JAZ | Jasmonate ZIM-domain |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| MAFF | Ministry of Agriculture, Forestry and Fisheries (Japan) |
| MD | Molecular Dynamics |
| MeJA | Methyl Jasmonate |
| MEP | Methylerythritol Phosphate pathway |
| ML | Machine Learning |
| MVA | Mevalonate pathway |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate (reduced form) |
| NF-κB | Nuclear Factor kappa B |
| NMR | Nuclear Magnetic Resonance |
| OptoAMP | Optogenetic Amplification |
| oxPPP | Oxidative Pentose Phosphate Pathway |
| PAL | Phenylalanine Ammonia-Lyase |
| PDH | Pyruvate Dehydrogenase |
| PE | Prime Editing |
| PEPC | Phosphoenolpyruvate Carboxylase |
| POR | Protochlorophyllide Oxidoreductase |
| PSY | Phytoene Synthase |
| PTMs | Post-Translational Modifications |
| RNAi | RNA Interference |
| RNA-seq | RNA Sequencing |
| RNP | Ribonucleoprotein |
| ROS | Reactive Oxygen Species |
| SA | Salicylic Acid |
| scRNA-seq | Single-cell RNA Sequencing |
| SDN-1 | Site-Directed Nuclease 1 |
| SEM | Structural Equation Modeling |
| SQS | Squalene Synthase |
| SRM | Selected Reaction Monitoring |
| SWATH-MS | Sequential Window Acquisition of All Theoretical Mass Spectra |
| TF | Transcription Factor |
| TLA | Three Letter Acronym (included as per example) |
| TMT | Tandem Mass Tag |
| TPS | Terpene Synthase |
| UGT | UDP-Glycosyltransferase |
| USDA | United States Department of Agriculture |
| VIGS | Virus-Induced Gene Silencing |
| WGCNA | Weighted Gene Co-expression Network Analysis |
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Jain, D.; Bisht, S.; Parvez, A.; Singh, K.; Bhaskar, P.; Koubouris, G. Effective biotic elicitors for augmentation of secondary metabolite production in medicinal plants. Agriculture 2024, 14, 796. [Google Scholar] [CrossRef]
- Volk, M.J.; Tran, V.G.; Tan, S.-I.; Mishra, S.; Fatma, Z.; Boob, A.; Li, H.; Xue, P.; Martin, T.A.; Zhao, H. Metabolic engineering: Methodologies and applications. Chem. Rev. 2022, 123, 5521–5570. [Google Scholar] [CrossRef]
- Ro, D.-K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Kloti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef]
- Jennewein, S.; Wildung, M.R.; Chau, M.; Walker, K.; Croteau, R. Random sequencing of an induced Taxus cell cDNA library for identification of clones involved in Taxol biosynthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 9149–9154. [Google Scholar] [CrossRef]
- Ma, D.; Pu, G.; Lei, C.; Ma, L.; Wang, H.; Guo, Y.; Chen, J.; Du, Z.; Wang, H.; Li, G. Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4, 11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol. 2009, 50, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, Y.; Zhang, X.; Shi, M.; Wang, B.; Wang, D.; Huang, L.; Zhang, X. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab. Eng. 2013, 20, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Courdavault, V.; O’Connor, S.E.; Jensen, M.K.; Papon, N. Metabolic engineering for plant natural products biosynthesis: New procedures, concrete achievements and remaining limits. Nat. Prod. Rep. 2021, 38, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Lin, D.-W.; Eames, A.; Chandrasekaran, S. Next-generation genome-scale metabolic modeling through integration of regulatory mechanisms. Metabolites 2021, 11, 606. [Google Scholar] [CrossRef]
- Tharasirivat, V.; Jantaro, S. Increased biomass and polyhydroxybutyrate production by Synechocystis sp. PCC 6803 Overexpressing RuBisCO Genes. Int. J. Mol. Sci. 2023, 24, 6415. [Google Scholar] [CrossRef]
- Cao, K.; Cui, Y.; Sun, F.; Zhang, H.; Fan, J.; Ge, B.; Cao, Y.; Wang, X.; Zhu, X.; Wei, Z. Metabolic engineering and synthetic biology strategies for producing high-value natural pigments in Microalgae. Biotechnol. Adv. 2023, 68, 108236. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Porter, J.A.; Janick, J.; Ferreira, J.F.S.; Mutui, T.M. Selection and clonal propagation of high artemisinin genotypes of Artemisia annua. Front. Plant Sci. 2018, 9, 358. [Google Scholar] [CrossRef]
- Wheeler, N.C.; Jech, K.; Masters, S.; Brobst, S.W.; Alvarado, A.B.; Hoover, A.J.; Snader, K.M. Effects of genetic, epigenetic, and environmental factors on taxol content in Taxus brevifolia and related species. J. Nat. Prod. 1992, 55, 432–440. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Xiao, W.H.; Tyo, K.E.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Zhao, G.; Yan, X.; Zhou, Z. Systematic optimization of the yeast cell factory for sustainable and high efficiency production of bioactive ginsenoside compound K. Synth. Syst. Biotechnol. 2021, 6, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mutanda, I.; Wang, K.; Yang, L.; Wang, J.; Wang, Y. Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana benthamiana. Nat. Commun. 2019, 10, 4850. [Google Scholar] [CrossRef] [PubMed]
- Romsuk, J.; Yasumoto, S.; Fukushima, E.O.; Miura, K.; Muranaka, T.; Seki, H. High-yield bioactive triterpenoid production by heterologous expression in Nicotiana benthamiana using the Tsukuba system. Front. Plant Sci. 2022, 13, 991909. [Google Scholar] [CrossRef]
- Reed, J.; Osbourn, A. Engineering terpenoid production through transient expression in Nicotiana benthamiana. Plant Cell Rep. 2018, 37, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019, 10, 2142. [Google Scholar] [CrossRef]
- Mani, V.; Park, S.; Kim, J.A.; Lee, S.I.; Lee, K. Metabolic perturbation and synthetic biology strategies for plant terpenoid production-an updated overview. Plants 2021, 10, 2179. [Google Scholar] [CrossRef]
- Wang, C.; Liwei, M.; Park, J.B.; Jeong, S.H.; Wei, G.; Wang, Y.; Kim, S.W. Microbial platform for terpenoid production: Escherichia coli and Yeast. Front. Microbiol. 2018, 9, 2460. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, K. Production of terpenoids by synthetic biology approaches. Front. Bioeng. Biotechnol. 2020, 8, 347. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, L.; Tang, K.; Hao, X.; Kai, G. Advanced metabolic engineering strategies for increasing artemisinin yield in Artemisia annua L. Hortic. Res. 2024, 11, uhad292. [Google Scholar]
- Hong, G.-J.; Hu, W.-L.; Li, J.-X.; Chen, X.-Y.; Wang, L.-J. Increased accumulation of artemisinin and anthocyanins in Artemisia annua expressing the Arabidopsis blue light receptor CRY1. Plant Mol. Biol. Report. 2009, 27, 334–341. [Google Scholar] [CrossRef]
- Pick, T.R.; Weber, A.P.M. Unknown components of the plastidial permeome. Front. Plant Sci. 2014, 5, 410. [Google Scholar] [CrossRef] [PubMed]
- Sirirungruang, S.; Markel, K.; Shih, P.M. Plant-based engineering for production of high-valued natural products. Nat. Prod. Rep. 2022, 39, 1492–1509. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jiang, Z.; Kempinski, C.; Eric Nybo, S.; Husodo, S.; Williams, R.; Chappell, J. Engineering triterpene metabolism in tobacco. Planta 2012, 236, 867–877. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, Y.; Wu, Y.; Zhao, L.; Pei, J. Functional characterization and screening of promiscuous kinases and isopentenyl phosphate kinases for the synthesis of DMAPP via a one-pot enzymatic cascade. Int. J. Mol. Sci. 2022, 23, 12904. [Google Scholar] [CrossRef] [PubMed]
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl. Acad. Sci. USA 2020, 117, 31789–31799. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth. Biol. 2014, 3, 298–306. [Google Scholar] [CrossRef]
- Ouyang, X.; Cha, Y.; Li, W.; Zhu, C.; Zhu, M.; Li, S.; Zhuo, M.; Huang, S.; Li, J. Stepwise engineering of Saccharomyces cerevisiae to produce (+)-valencene and its related sesquiterpenes. RSC Adv. 2019, 9, 30171–30181. [Google Scholar] [CrossRef]
- Promdonkoy, P.; Sornlek, W.; Preechakul, T.; Tanapongpipat, S.; Runguphan, W. Metabolic engineering of Saccharomyces cerevisiae for production of fragrant terpenoids from agarwood and sandalwood. Fermentation 2022, 8, 429. [Google Scholar] [CrossRef]
- Boutanaev, A.M.; Moses, T.; Zi, J.; Nelson, D.R.; Mugford, S.T.; Peters, R.J.; Osbourn, A. Investigation of terpene diversification across multiple sequenced plant genomes. Proc. Natl. Acad. Sci. USA 2015, 112, E81–E88. [Google Scholar] [CrossRef]
- Trapp, S.C.; Croteau, R.B. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001, 158, 811–832. [Google Scholar] [CrossRef]
- Li, B.; Cui, G.; Shen, G.; Zhan, Z.; Huang, L.; Chen, J.; Qi, X. Targeted mutagenesis in the medicinal plant Salvia miltiorrhiza. Sci. Rep. 2017, 7, 43320. [Google Scholar] [CrossRef]
- Lanier, E.R.; Andersen, T.B.; Hamberger, B. Plant terpene specialized metabolism: Complex networks or simple linear pathways? Plant J. 2023, 114, 1178–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, J.; Ma, L.; Tu, L.; Hu, T.; Wu, X.; Su, P.; Zhao, Y.; Liu, Y.; Li, D. Tandemly duplicated CYP82Ds catalyze 14-hydroxylation in triptolide biosynthesis and precursor production in Saccharomyces cerevisiae. Nat. Commun. 2023, 14, 875. [Google Scholar] [CrossRef]
- Tippmann, S.; Chen, Y.; Siewers, V.; Nielsen, J. From flavors and pharmaceuticals to advanced biofuels: Production of isoprenoids in Saccharomyces cerevisiae. Biotechnol. J. 2013, 8, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, W.H.; Han, B.-S.; Lee, J.H.; Doo, E.K.; Kim, J.-H. Molecular drug discovery of single ginsenoside compounds as a potent bruton’s tyrosine kinase inhibitor. Int. J. Mol. Sci. 2020, 21, 3065. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, M.; Dong, S.; Wu, X.; Zhang, G.; He, L.; Jiao, Y.; Chen, S.; Li, L.; Luo, H. A chromosome-scale genome assembly of Artemisia argyi reveals unbiased subgenome evolution and key contributions of gene duplication to volatile terpenoid diversity. Plant Commun. 2023, 4, 100516. [Google Scholar] [CrossRef]
- Guo, C.; Xu, S.; Guo, X. Genome-wide analysis of oxidosqualene cyclase genes in Artemisia annua: Evolution, expression, and potential roles in triterpenoid biosynthesis. Curr. Issues Mol. Biol. 2025, 47, 545. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cui, G.; Chen, T.; Ma, X.; Wang, R.; Jin, B.; Yang, J.; Kang, L.; Tang, J.; Lai, C.; et al. Expansion within the CYP71D subfamily drives the heterocyclization of tanshinones synthesis in Salvia miltiorrhiza. Nat. Commun. 2021, 12, 685. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Jiang, Y.; Jin, B.; Wang, L. Functions of representative terpenoids and their biosynthesis mechanisms in medicinal plants. Biomolecules 2023, 13, 1725. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, M.; Wen, W.; Yu, R. Biosynthesis and regulation of terpenoid indole alkaloids in Catharanthus roseus. Pharmacogn. Rev. 2015, 9, 24–28. [Google Scholar] [CrossRef]
- Hofberger, J.A.; Ramirez, A.M.; Bergh, E.; Zhu, X.; Bouwmeester, H.J.; Schuurink, R.C.; Schranz, M.E. Large-scale evolutionary analysis of genes and supergene clusters from terpenoid modular pathways provides insights into metabolic diversification in flowering plants. PLoS ONE 2015, 10, e0128808. [Google Scholar] [CrossRef]
- Köllner, T.G.; Degenhardt, J.; Gershenzon, J. The product specificities of maize terpene synthases TPS4 and TPS10 are determined both by active site amino acids and residues adjacent to the active site. Plants 2020, 9, 552. [Google Scholar] [CrossRef]
- Li, F.; Wu, C.; Dewer, Y.; Liang, Y.; Zhou, J.J.; Luo, C.; Wang, G. Tandem duplication of P450 genes is involved in homoterpene biosynthesis in lima bean (Phaseolus lunatus). Pest. Manag. Sci. 2025. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Zhi, J.; Huang, D.; Zhang, Y.; Zhang, L.; Duan, X.; Zhang, P.; Qiu, S.; Geng, J. The ABC transporter SmABCG1 mediates tanshinones export from the peridermic cells of Salvia miltiorrhiza root. J. Integr. Plant Biol. 2025, 67, 135–149. [Google Scholar] [CrossRef]
- Heinig, U.; Scholz, S.; Jennewein, S. Getting to the bottom of taxol biosynthesis by fungi. Fungal Divers. 2013, 60, 161–170. [Google Scholar] [CrossRef]
- Ishii, Y.; Takeda, S.; Yamada, H.; Oguri, K. Functional protein-protein interaction of drug metabolizing enzymes. Front. Biosci. 2005, 10, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.A.; Kolewe, M.E.; Normanly, J.; Walker, E.L.; Roberts, S.C. Contribution of taxane biosynthetic pathway gene expression to observed variability in paclitaxel accumulation in Taxus suspension cultures. Biotechnol. J. 2012, 7, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Croteau, R. Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 583–587. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Szala, K.; Pilarek, M.; Orzechowski, R.; Pietrosiuk, A. A cellulase-supported two-phase in situ system for enhanced biosynthesis of paclitaxel in Taxus× media hairy roots. Acta Physiol. Plant 2018, 40, 201. [Google Scholar] [CrossRef]
- Bellucci, M.; De Marchis, F.; Pompa, A. The endoplasmic reticulum is a hub to sort proteins toward unconventional traffic pathways and endosymbiotic organelles. J. Exp. Bot. 2018, 69, 7–20. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P. Acyl-lipid metabolism. Arab. Book. 2013, 11, e0161. [Google Scholar] [CrossRef]
- Czechowski, T.; Li, Y.; Gilday, A.D.; Harvey, D.; Swamidatta, S.H.; Lichman, B.R.; Ward, J.L.; Graham, I.A. Evolution of linear triterpenoid biosynthesis within the Euphorbia genus. Nat. Commun. 2025, 16, 5602. [Google Scholar] [CrossRef]
- Yi, X.; Wang, X.; Wu, L.; Wang, M.; Yang, L.; Liu, X.; Chen, S.; Shi, Y. Integrated analysis of basic helix loop helix transcription factor family and targeted terpenoids reveals candidate AarbHLH Genes involved in terpenoid biosynthesis in Artemisia argyi. Front. Plant Sci. 2022, 12, 811166. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, X.; Chen, Y.; Wei, M.; Liao, W.; Zhao, S.; Fu, C.; Yu, L. TcMYC2a, a basic helix-loop-helix transcription factor, transduces JA-signals and regulates taxol biosynthesis in Taxus chinensis. Front. Plant Sci. 2018, 9, 863. [Google Scholar] [CrossRef]
- Ma, Y.N.; Xu, D.B.; Yan, X.; Wu, Z.K.; Kayani, S.I.; Shen, Q.; Fu, X.Q.; Xie, L.H.; Hao, X.L.; Hassani, D. Jasmonate-and abscisic acid-activated AaGSW1-AaTCP15/AaORA transcriptional cascade promotes artemisinin biosynthesis in Artemisia annua. Plant Biotechnol. J. 2021, 19, 1412–1428. [Google Scholar] [CrossRef]
- Matías-Hernández, L.; Jiang, W.; Yang, K.; Tang, K.; Brodelius, P.E.; Pelaz, S. AaMYB1 and its orthologue AtMYB61 affect terpene metabolism and trichome development in Artemisia annua and Arabidopsis thaliana. Plant J. 2017, 90, 520–534. [Google Scholar] [CrossRef]
- Li, L.; Hao, X.; Liu, H.; Wang, W.; Fu, X.; Ma, Y.; Shen, Q.; Chen, M.; Tang, K. Jasmonic acid-responsive AabHLH1 positively regulates artemisinin biosynthesis in Artemisia annua. Biotechnol. Appl. Biochem. 2019, 66, 369–375. [Google Scholar] [CrossRef]
- Ma, Y.-N.; Xu, D.-B.; Li, L.; Zhang, F.; Fu, X.-Q.; Shen, Q.; Lyu, X.-Y.; Wu, Z.-K.; Pan, Q.-F.; Shi, P. Jasmonate promotes artemisinin biosynthesis by activating the TCP14-ORA complex in Artemisia annua. Sci. Adv. 2018, 4, eaas9357. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, X.; Fu, X.; Taheri, A.; Zhang, W.; Liu, P.; Liu, H.; Li, L.; Wang, Y.; Tang, K. Comprehensive analysis of SPL gene family and miR156a/SPLs in the regulation of terpenoid indole alkaloid biosynthesis in Catharanthus roseus L. BMC Plant Biol. 2025, 25, 817. [Google Scholar] [CrossRef]
- Das, S.; Kwon, M.; Kim, J.-Y. Enhancement of specialized metabolites using CRISPR/Cas gene editing technology in medicinal plants. Front. Plant Sci. 2024, 15, 1279738. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Leivar, P.; Antolín-Llovera, M.; Ferrero, S.; Closa, M.; Arro, M.; Ferrer, A.; Boronat, A.; Campos, N. Multilevel control of Arabidopsis 3-hydroxy-3-methylglutaryl coenzyme: A reductase by protein phosphatase 2A. Plant Cell 2011, 23, 1494–1511. [Google Scholar] [CrossRef] [PubMed]
- Gruchattka, E.; Kayser, O. In vivo validation of in silico predicted metabolic engineering strategies in yeast: Disruption of α-ketoglutarate dehydrogenase and expression of ATP-citrate lyase for terpenoid production. PLoS ONE 2015, 10, e0144981. [Google Scholar] [CrossRef]
- Liu, C.-L.; Xue, K.; Yang, Y.; Liu, X.; Li, Y.; Lee, T.S.; Bai, Z.; Tan, T. Metabolic engineering strategies for sesquiterpene production in microorganism. Crit. Rev. Biotechnol. 2022, 42, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xiang, L.; Zhang, F.; Tang, K.; Liao, Z. Metabolic regulation and engineering of artemisinin biosynthesis in A. annua. Med. Plant Biol. 2023, 2, 4. [Google Scholar]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T. Biosynthetic pathways of triterpenoids and strategies to improve their biosynthetic efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef]
- Zhao, E.M.; Suek, N.; Wilson, M.Z.; Dine, E.; Pannucci, N.L.; Gitai, Z.; Avalos, J.L.; Toettcher, J.E. Light-based control of metabolic flux through assembly of synthetic organelles. Nat. Chem. Biol. 2019, 15, 589–597. [Google Scholar] [CrossRef]
- Ma, Y.; Zu, Y.; Huang, S.; Stephanopoulos, G. Engineering a universal and efficient platform for terpenoid synthesis in yeast. Proc. Natl. Acad. Sci. USA 2023, 120, e2207680120. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Wang, J.; Tang, F. Enhancement of linalool production in Saccharomyces cerevisiae by utilizing isopentenol utilization pathway. Microb. Cell Fact. 2022, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Liu, P.; Shi, Y.; Feng, Y.; Gao, J.; Chen, L.; Zhang, A.; Cheng, X.; Wei, H.; Zhang, T. Single-cell transcriptome and network analyses unveil key transcription factors regulating mesophyll cell development in maize. Genes 2022, 13, 374. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhong, Y.; Wu, Y.; Li, Z.; Xu, L.-A.; Xu, M. Transcriptome analysis and identification of genes related to terpenoid biosynthesis in Cinnamomum camphora. BMC Genom. 2018, 19, 550. [Google Scholar] [CrossRef] [PubMed]
- Flügge, U.I.; Gao, W. Transport of isoprenoid intermediates across chloroplast envelope membranes. Plant Biol. 2005, 7, 91–97. [Google Scholar] [CrossRef]
- Han, J.; Wu, Y.; Zhou, Y.; Li, S. Engineering Saccharomyces cerevisiae to produce plant benzylisoquinoline alkaloids. Abiotech 2021, 2, 264–275. [Google Scholar] [CrossRef]
- Wijma, H.J.; Floor, R.J.; Bjelic, S.; Marrink, S.J.; Baker, D.; Janssen, D.B. Enantioselective enzymes by computational design and in silico screening. Angew. Chem. Int. Ed. 2015, 54, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Masakapalli, S. Network Flux Analysis of Central Metabolism in Plants. Ph.D. Thesis, Oxford University, Oxford, UK, 2011. [Google Scholar]
- Masakapalli, S.K.; Bryant, F.M.; Kruger, N.J.; Ratcliffe, R.G. The metabolic flux phenotype of heterotrophic Arabidopsis cells reveals a flexible balance between the cytosolic and plastidic contributions to carbohydrate oxidation in response to phosphate limitation. Plant J. 2014, 78, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Bureau, J.A.; Oliva, M.E.; Dong, Y.; Ignea, C. Engineering yeast for the production of plant terpenoids using synthetic biology approaches. Nat. Prod. Rep. 2023, 40, 1822–1848. [Google Scholar] [CrossRef]
- Scossa, F.; Benina, M.; Alseekh, S.; Zhang, Y.; Fernie, A.R. The integration of metabolomics and next-generation sequencing data to elucidate the pathways of natural product metabolism in medicinal plants. Planta Med. 2018, 84, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Fu, X.; Liu, M.; Shen, Q.; Jiang, W.; Li, L.; Sun, X.; Tang, K. Promotion of artemisinin content in Artemisia annua by overexpression of multiple artemisinin biosynthetic pathway genes. Plant Cell Tissue Organ Cult. 2017, 129, 251–259. [Google Scholar] [CrossRef]
- Hassani, D.; Taheri, A.; Fu, X.; Qin, W.; Hang, L.; Ma, Y.; Tang, K. Elevation of artemisinin content by co-transformation of artemisinin biosynthetic pathway genes and trichome-specific transcription factors in Artemisia annua. Front. Plant Sci. 2023, 14, 1118082. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Ghorai, M.; Kant, N.; Kumar, M.; Radha; Jha, N.K.; Swamy, M.K.; Proćków, J.; de la Lastra, J.M.P. Genome editing technologies, mechanisms and improved production of therapeutic phytochemicals: Opportunities and prospects. Biotechnol. Bioeng. 2023, 120, 82–94. [Google Scholar] [CrossRef]
- Hao, G.; Ji, H.; Li, Y.; Shi, R.; Wang, J.; Feng, L.; Huang, L. Exogenous ABA and polyamines enhanced salvianolic acids contents in hairy root cultures of Salvia miltiorrhiza Bge. f. alba. Plant Omics 2012, 5, 446–452. [Google Scholar]
- Bhuyan, S.J.; Kumar, M.; Ramrao Devde, P.; Rai, A.C.; Mishra, A.K.; Singh, P.K.; Siddique, K.H.M. Progress in gene editing tools, implications and success in plants: A review. Front. Genome Ed. 2023, 5, 1272678. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.; Liao, Z.; Wang, S.; Yan, T.; Shi, P.U.; Liu, M.; Fu, X.; Pan, Q.; Wang, Y. The genome of Artemisia annua provides insight into the evolution of Asteraceae family and artemisinin biosynthesis. Mol. Plant 2018, 11, 776–788. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, H.; Zhao, Y.; Xie, L.; He, Q.; Zhong, Y.; Wang, Y.; Wang, Y.; Tang, K. The transcription factor Aabzip9 positively regulates the biosynthesis of artemisinin in Artemisia annua. Front. Plant Sci. 2019, 10, 1294. [Google Scholar] [CrossRef]
- Traverse, K.K.F.; Breselge, S.; Trautman, J.G.; Dee, A.; Wang, J.; Childs, K.L.; Lee-Parsons, C.W.T. Characterization of the ZCTs, a subgroup of Cys2-His2 zinc finger transcription factors regulating alkaloid biosynthesis in Catharanthus roseus. Plant Cell Rep. 2024, 43, 209. [Google Scholar] [CrossRef]
- Graham, M.W.; Craig, S.; Waterhouse, P.M. Expression patterns of vascular-specific promoters RolC and Sh in transgenic potatoes and their use in engineering PLRV-resistant plants. Plant Mol. Biol. 1997, 33, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, W.; Huang, W.; Wang, B.; Li, S.; Zhang, J.; Chang, L. Importation of taxadiene synthase into chloroplast improves taxadiene production in tobacco. Planta 2021, 253, 107. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.; Zhao, G.-R.; Lu, W. Alpha-terpineol production from an engineered Saccharomyces cerevisiae cell factory. Microb. Cell Fact. 2019, 18, 160. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Han, J.Y.; Choi, Y.E. Production of ginsenoside aglycone (protopanaxatriol) and male sterility of transgenic tobacco co-overexpressing three Panax ginseng genes: PgDDS, CYP716A47, and CYP716A53v2. J. Ginseng Res. 2019, 43, 261–271. [Google Scholar] [CrossRef]
- Li, L.; Fu, J.; Liu, N. Advances in the structures, pharmacological activities, and biosynthesis of plant diterpenoids. J. Microbiol. Biotechnol. 2024, 34, 1563–1579. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Kampranis, S.C.; Makris, A.M. Developing a yeast cell factory for the production of terpenoids. Comput. Struct. Biotechnol. J. 2012, 3, e201210006. [Google Scholar] [CrossRef]
- Edgar, S.; Li, F.S.; Qiao, K.; Weng, J.K.; Stephanopoulos, G. Engineering of taxadiene synthase for improved selectivity and yield of a key taxol biosynthetic intermediate. ACS Synth. Biol. 2017, 6, 201–205. [Google Scholar] [CrossRef]
- Ndochinwa, O.G.; Wang, Q.Y.; Amadi, O.C.; Nwagu, T.N.; Nnamchi, C.I.; Okeke, E.S.; Moneke, A.N. Current status and emerging frontiers in enzyme engineering: An industrial perspective. Heliyon 2024, 10, e32673. [Google Scholar] [CrossRef]
- Paramasivan, K.; Mutturi, S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 2017, 37, 974–989. [Google Scholar] [CrossRef]
- Huttanus, H.M.; Senger, R.S. A synthetic biosensor to detect peroxisomal acetyl-CoA concentration for compartmentalized metabolic engineering. PeerJ 2020, 8, e9805. [Google Scholar] [CrossRef]
- Zhao, C.; Kim, Y.; Zeng, Y.; Li, M.; Wang, X.; Hu, C.; Gorman, C.; Dai, S.Y.; Ding, S.Y.; Yuan, J.S. Co-compartmentation of terpene biosynthesis and storage via synthetic droplet. ACS Synth. Biol. 2018, 7, 774–781. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, X.; Jiang, G.; Wu, J.; Zhang, J.L.; Lei, D.; Yuan, Y.J.; Qiao, J.; Zhao, G.R. Orthogonal engineering of biosynthetic pathway for efficient production of limonene in Saccharomyces cerevisiae. ACS Synth. Biol. 2019, 8, 968–975. [Google Scholar] [CrossRef]
- Wang, J.W.; Tian, H.; Yu, X.; Zheng, L.P. Glucose-6-phosphate dehydrogenase plays critical role in artemisinin production of Artemisia annua under salt stress. Biol. Plant 2017, 61, 529–539. [Google Scholar] [CrossRef]
- Hao, X.; Pu, Z.; Cao, G.; You, D.; Zhou, Y.; Deng, C.; Shi, M.; Nile, S.H.; Wang, Y.; Zhou, W. Tanshinone and salvianolic acid biosynthesis are regulated by SmMYB98 in Salvia miltiorrhiza hairy roots. J. Adv. Res. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Szymczyk, P.; Szymańska, G.; Kuźma, Ł.; Jeleń, A.; Balcerczak, E. Methyl jasmonate activates the 2C methyl-D-erithrytol 2, 4-cyclodiphosphate synthase gene and stimulates tanshinone accumulation in Salvia miltiorrhiza solid callus cultures. Molecules 2022, 27, 1772. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Kanagarajan, S.; Lundgren, A.; Brodelius, P.E. Trichome-specific expression of the amorpha-4,11-diene 12-hydroxylase (cyp71av1) gene, encoding a key enzyme of artemisinin biosynthesis in Artemisia annua, as reported by a promoter-GUS fusion. Plant Mol. Biol. 2013, 81, 119–138. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Ebrahimi, V.; Hashemi, A. CRISPR-based gene editing in plants: Focus on reagents and their delivery tools. BioImpacts BI 2025, 15, 30019. [Google Scholar] [CrossRef]
- Vu, T.V.; Sivankalyani, V.; Kim, E.J.; Doan, D.T.H.; Tran, M.T.; Kim, J.; Sung, Y.W.; Park, M.; Kang, Y.J.; Kim, J.Y. Highly efficient homology-directed repair using CRISPR/Cpf1-geminiviral replicon in tomato. Plant Biotechnol. J. 2020, 18, 2133–2143. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Jin, L.; Zhang, T.; Pu, X.; Qiu, B.; Li, G. A chromosome-level genome assembly of the Knoxia roxburghii (Rubiaceae). Sci. Data 2023, 10, 803. [Google Scholar] [CrossRef]
- Lee, J.E.; Neumann, M.; Duro, D.I.; Schmid, M. CRISPR-based tools for targeted transcriptional and epigenetic regulation in plants. PLoS ONE 2019, 14, e0222778. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhang, D.; Baltes, N.J.; Paul, J.W., 3rd; Tang, X.; Zheng, X.; Voytas, D.F.; Hsieh, T.F.; Zhang, Y.; Qi, Y. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiol. 2015, 169, 971–985. [Google Scholar] [CrossRef]
- Zhang, Y.; Iaffaldano, B.; Qi, Y. CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2021, 2, 100168. [Google Scholar] [CrossRef]
- Ding, X.; Yu, L.; Chen, L.; Li, Y.; Zhang, J.; Sheng, H.; Ren, Z.; Li, Y.; Yu, X.; Jin, S.; et al. Recent progress and future prospect of CRISPR/Cas-derived transcription activation (CRISPRa) system in plants. Cells 2022, 11, 3045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Du, H.; Wang, J.; Pu, Y.; Yang, C.; Yan, R.; Yang, H.; Cheng, H.; Yu, D. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 2020, 18, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L. Working with Randy: The diacylglycerol acyltransferase story. Lipids 2020, 55, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoven, E.J.; Lahtvee, P.J.; Nielsen, J. Applications of computational modeling in metabolic engineering of yeast. FEMS Yeast Res. 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Moreno-Sánchez, R.; Saavedra, E.; Rodríguez-Enríquez, S.; Olín-Sandoval, V. Metabolic control analysis: A tool for designing strategies to manipulate metabolic pathways. J. Biomed. Biotechnol. 2008, 2008, 597913. [Google Scholar] [CrossRef]
- Rinaldi, M.A.; Ferraz, C.A.; Scrutton, N.S. Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli. Nat. Prod. Rep. 2022, 39, 90–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, J.; Kanagarajan, S.; Lundgren, A.; Brodelius, P.E. Studies on the expression of sesquiterpene synthases using promoter-β-glucuronidase fusions in transgenic Artemisia annua L. PLoS ONE 2013, 8, e80643. [Google Scholar]
- Catania, T.M.; Branigan, C.A.; Stawniak, N.; Hodson, J.; Harvey, D.; Larson, T.R.; Czechowski, T.; Graham, I.A. Silencing amorpha-4,11-diene synthase Genes in Artemisia annua leads to FPP accumulation. Front. Plant Sci. 2018, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Abdin, M.Z. Over-expression of HMG-CoA reductase and amorpha-4,11-diene synthase genes in Artemisia annua L. and its influence on artemisinin content. Plant Cell Rep. 2011, 30, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jing, F.; Li, F.; Li, M.; Wang, Y.; Wang, G.; Sun, X.; Tang, K. Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA-mediated gene silencing. Biotechnol. Appl. Biochem. 2009, 52, 199–207. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Q.; Smith, N.A.; Liang, G.; Wang, M.B. RNA silencing in plants: Mechanisms, technologies and applications in horticultural crops. Curr. Genom. 2016, 17, 476–489. [Google Scholar] [CrossRef]
- Pani, A.; Mahapatra, R.K.; Behera, N.; Naik, P.K. Computational identification of sweet wormwood (Artemisia annua) microRNA and their mRNA targets. Genom. Proteom. Bioinform. 2011, 9, 200–210. [Google Scholar] [CrossRef]
- Ma, T.; Gao, H.; Zhang, D.; Shi, Y.; Zhang, T.; Shen, X.; Wu, L.; Xiang, L.; Chen, S. Transcriptome analyses revealed the ultraviolet B irradiation and phytohormone gibberellins coordinately promoted the accumulation of artemisinin in Artemisia annua L. Chin. Med. 2020, 15, 67. [Google Scholar] [CrossRef]
- Wani, K.I.; Choudhary, S.; Zehra, A.; Naeem, M.; Weathers, P.; Aftab, T. Enhancing artemisinin content in and delivery from Artemisia annua: A review of alternative, classical, and transgenic approaches. Planta 2021, 254, 29. [Google Scholar] [CrossRef]
- Lyu, X.; Lyu, Y.; Yu, H.; Chen, W.; Ye, L.; Yang, R. Biotechnological advances for improving natural pigment production: A state-of-the-art review. Bioresour. Bioprocess. 2022, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Kamaluddin; Sharaf-Eldin, M.A.; Elkholy, S.F.; Abdin, M.Z. The effect of over-expression of rate limiting enzymes on the yield of artemisinin in Artemisia annua. Rend. Lincei 2015, 27, 311–319. [Google Scholar] [CrossRef]
- Zhao, M.L.; Cai, W.S.; Zheng, S.Q.; Zhao, J.L.; Zhang, J.L.; Huang, Y.; Hu, Z.L.; Jia, B. Metabolic engineering of the isopentenol utilization pathway enhanced the production of terpenoids in Chlamydomonas reinhardtii. Mar. Drugs 2022, 20, 577. [Google Scholar] [CrossRef]
- Pasoreck, E.K.; Su, J.; Silverman, I.M.; Gosai, S.J.; Gregory, B.D.; Yuan, J.S.; Daniell, H. Terpene metabolic engineering via nuclear or chloroplast genomes profoundly and globally impacts off-target pathways through metabolite signalling. Plant Biotechnol. J. 2016, 14, 1862–1875. [Google Scholar] [CrossRef]
- Vavitsas, K.; Fabris, M.; Vickers, C.E. Terpenoid metabolic engineering in photosynthetic microorganisms. Genes 2018, 9, 520. [Google Scholar] [CrossRef]
- Leonard, E.; Ajikumar, P.K.; Thayer, K.; Xiao, W.H.; Mo, J.D.; Tidor, B.; Stephanopoulos, G.; Prather, K.L. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc. Natl. Acad. Sci. USA 2010, 107, 13654–13659. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Xue, D.; Abdallah, I.I.; Dijkshoorn, L.; Setroikromo, R.; Lv, G.; Quax, W.J. Metabolic engineering of Bacillus subtilis for terpenoid production. Appl. Microbiol. Biotechnol. 2015, 99, 9395–9406. [Google Scholar] [CrossRef]
- Dong, Q.; Zou, Q.; Mao, L.; Tian, D.; Hu, W.; Cao, X.; Ding, H. The chromosome-scale assembly of the Curcuma alismatifolia genome provides insight into anthocyanin and terpenoid biosynthesis. Front. Plant Sci. 2022, 13, 899588. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Song, W.; Yin, Z.; Wu, S.; Liu, J.; Wang, N.; Jin, H.; Qiao, J.; Huo, Y.-X. Genomic analysis based on chromosome-level genome assembly reveals an expansion of terpene biosynthesis of Azadirachta indica. Front. Plant Sci. 2022, 13, 853861. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Q.Y.; Ge, Y.; Huang, Z.Y.; Hong, R.; Li, A.; Xu, J.H.; Yu, H.L. Hydroxylases involved in terpenoid biosynthesis: A review. Bioresour. Bioprocess. 2023, 10, 39. [Google Scholar] [CrossRef]
- Zhou, T.; Bai, G.; Hu, Y.; Ruhsam, M.; Yang, Y.; Zhao, Y. De novo genome assembly of the medicinal plant Gentiana macrophylla provides insights into the genomic evolution and biosynthesis of iridoids. DNA Res. 2022, 29, dsac034. [Google Scholar] [CrossRef]
- Liao, X.; Guo, S.; Liao, B.; Shen, X.; He, W.; Meng, Y.; Liang, C.; Pei, J.; Liu, J.; Zhang, Y. Chromatin architecture of two different strains of Artemisia annua reveals the alterations in interaction and gene expression. Planta 2023, 258, 74. [Google Scholar] [CrossRef]
- Kautsar, S.A.; Suarez Duran, H.G.; Blin, K.; Osbourn, A.; Medema, M.H. plantiSMASH: Automated identification, annotation and expression analysis of plant biosynthetic gene clusters. Nucleic Acids Res. 2017, 45, W55–W63. [Google Scholar] [CrossRef]
- Tai, Y.; Hou, X.; Liu, C.; Sun, J.; Guo, C.; Su, L.; Jiang, W.; Ling, C.; Wang, C.; Wang, H. Phytochemical and comparative transcriptome analyses reveal different regulatory mechanisms in the terpenoid biosynthesis pathways between Matricaria recutita L. and Chamaemelum Nobile L. BMC Genom. 2020, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, X.; Yang, L.; Peng, S.; Song, W.; Lin, Y.; Xiang, G.; Li, Y.; Ye, S.; Ma, C. Comparative genomics reveals the diversification of triterpenoid biosynthesis and origin of ocotillol-type triterpenes in Panax. Plant Commun. 2023, 4, 100591. [Google Scholar] [CrossRef] [PubMed]
- Zi, J.; Mafu, S.; Peters, R.J. To gibberellins and beyond! Surveying the evolution of (di) terpenoid metabolism. Annu. Rev. Plant Biol. 2014, 65, 259–286. [Google Scholar] [CrossRef]
- Liu, N.; Li, T.; Reid, W.R.; Yang, T.; Zhang, L. Multiple cytochrome P450 genes: Their constitutive overexpression and permethrin induction in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS ONE 2011, 6, e23403. [Google Scholar] [CrossRef]
- Spyropoulou, E.A.; Haring, M.A.; Schuurink, R.C. RNA sequencing on Solanum lycopersicum trichomes identifies transcription factors that activate terpene synthase promoters. BMC Genom. 2014, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wan, W.; Yin, D.; Deng, X.; Ma, Z.; Gao, T.; Cao, X. Genome-wide analysis of WRKY transcription factor genes in Toona sinensis: An insight into evolutionary characteristics and terpene synthesis. Front. Plant Sci. 2023, 13, 1063850. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, W.; Miao, Z. Integrative metabolomic and transcriptomic analyses reveals the accumulation patterns of key metabolites associated with flavonoids and terpenoids of Gynostemma pentaphyllum (Thunb.) Makino. Sci. Rep. 2024, 14, 8644. [Google Scholar] [CrossRef]
- Booth, J.K.; Yuen, M.M.S.; Jancsik, S.; Madilao, L.L.; Page, J.E.; Bohlmann, J. Terpene synthases and terpene variation in Cannabis sativa. Plant Physiol. 2020, 184, 130–147. [Google Scholar] [CrossRef]
- Chen, K.H.; Boettiger, A.N.; Moffitt, J.R.; Wang, S.; Zhuang, X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 2015, 348, aaa6090. [Google Scholar] [CrossRef]
- Amini, H.; Naghavi, M.R.; Shen, T.; Wang, Y.; Nasiri, J.; Khan, I.A.; Fiehn, O.; Zerbe, P.; Maloof, J.N. Tissue-specific transcriptome analysis reveals candidate genes for terpenoid and phenylpropanoid metabolism in the medicinal plant Ferula assafoetida. Genes Genomes Genet. 2019, 9, 807–816. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Wang, F.; Chen, H.; Chen, L.; Hu, Y.; Liu, Y. Integrated HS-GC-IMS and UPLC-Q-Orbitrap HRMS-based metabolomics revealed the characteristics and differential volatile and nonvolatile metabolites of different citrus peels. Curr. Res. Food Sci. 2024, 8, 100755. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.-M.; Ofosu, F.K.; Chelliah, R.; Kim, J.-H.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Untargeted metabolomics of fermented rice using UHPLC Q-TOF MS/MS reveals an abundance of potential antihypertensive compounds. Foods 2020, 9, 1007. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-W.; Imran, M.; Kim, E.-H.; Park, S.-Y.; Lee, S.-G.; Park, H.-M.; Jung, J.-W.; Ryu, T.-H. Approach strategies and application of metabolomics to biotechnology in plants. Front. Plant Sci. 2023, 14, 1192235. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Huang, Q.; Li, C.; Yu, H.; Tan, G.; Wei, S.; El-Sappah, A.H.; Sooranna, S.; Zhang, K.; Pan, L. Integrated metabolome and transcriptome analysis identifies candidate genes involved in triterpenoid saponin biosynthesis in leaves of Centella asiatica (L.) Urban. Front. Plant Sci. 2024, 14, 1295186. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell Proteom. 2012, 11, O111–O016717. [Google Scholar] [CrossRef]
- Peterson, A.C.; Russell, J.D.; Bailey, D.J.; Westphall, M.S.; Coon, J.J. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell Proteom. 2012, 11, 1475–1488. [Google Scholar] [CrossRef]
- Domon, B.; Aebersold, R. Options and considerations when selecting a quantitative proteomics strategy. Nat. Biotechnol. 2010, 28, 710–721. [Google Scholar] [CrossRef]
- Lange, V.; Picotti, P.; Domon, B.; Aebersold, R. Selected reaction monitoring for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2008, 4, 222. [Google Scholar] [CrossRef]
- Reiter, L.; Rinner, O.; Picotti, P.; Hüttenhain, R.; Beck, M.; Brusniak, M.-Y.; Hengartner, M.O.; Aebersold, R. mProphet: Automated data processing and statistical validation for large-scale SRM experiments. Nat. Methods 2011, 8, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Faktor, J.; Michalová, E.; Bouchal, P. p-SRM, SWATH a HRM–cílené proteomické přístupy na hmotnostním spektrometru TripleTOF 5600+ a jejich aplikace v onkologickém výzkumu. Klin. Onkol. 2014, 27, 110–115. [Google Scholar] [CrossRef]
- Tee, M.K.; Miller, W.L. Phosphorylation of human cytochrome P450c17 by p38α selectively increases 17, 20 lyase activity and androgen biosynthesis. J. Biol. Chem. 2013, 288, 23903–23913. [Google Scholar] [CrossRef]
- Ma, R.; Su, P.; Ma, Q.; Guo, J.; Chen, S.; Jin, B.; Zhang, H.; Tang, J.; Zhou, T.; Xiao, C. Identification of (-)-bornyl diphosphate synthase from Blumea balsamifera and its application for (-)-borneol biosynthesis in Saccharomyces cerevisiae. Synth. Syst. Biotechnol. 2022, 7, 490–497. [Google Scholar] [CrossRef]
- Bai, W.; Li, C.; Li, W.; Wang, H.; Han, X.; Wang, P.; Wang, L. Machine learning assists prediction of genes responsible for plant specialized metabolite biosynthesis by integrating multi-omics data. BMC Genom. 2024, 25, 418. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, L.; Gordon, M.; Inzé, D.; Williams, C.; Sozzani, R. Gene regulatory network inference: Connecting plant biology and mathematical modeling. Front. Genet. 2020, 11, 457. [Google Scholar] [CrossRef]
- Dahal, S.; Yurkovich, J.T.; Xu, H.; Palsson, B.O.; Yang, L. Synthesizing systems biology knowledge from omics using genome-scale models. Proteomics 2020, 20, 1900282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wen, W.; Cheng, Y.; Alseekh, S.; Fernie, A.R. Integrating multiomics data accelerates elucidation of plant primary and secondary metabolic pathways. Abiotech 2023, 4, 47–56. [Google Scholar] [CrossRef]
- Espinel-Ríos, S.; Morabito, B.; Pohlodek, J.; Bettenbrock, K.; Klamt, S.; Findeisen, R. Toward a modeling, optimization, and predictive control framework for fed-batch metabolic cybergenetics. Biotechnol. Bioeng. 2024, 121, 366–379. [Google Scholar] [CrossRef]
- Lalwani, M.A.; Zhao, E.M.; Avalos, J.L. Current and future modalities of dynamic control in metabolic engineering. Curr. Opin. Biotechnol. 2018, 52, 56–65. [Google Scholar] [CrossRef]
- Lawson, C.E.; Martí, J.M.; Radivojevic, T.; Jonnalagadda, S.V.R.; Gentz, R.; Hillson, N.J.; Peisert, S.; Kim, J.; Simmons, B.A.; Petzold, C.J.; et al. Machine learning for metabolic engineering: A review. Metab. Eng. 2021, 63, 34–60. [Google Scholar] [CrossRef]
- Zhang, J.; Petersen, S.D.; Radivojevic, T.; Ramirez, A.; Pérez-Manríquez, A.; Abeliuk, E.; Sánchez, B.J.; Costello, Z.; Chen, Y.; Fero, M.J.; et al. Combining mechanistic and machine learning models for predictive engineering and optimization of tryptophan metabolism. Nat. Commun. 2020, 11, 4880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.M.; Lalwani, M.A.; Chen, J.M.; Orillac, P.; Toettcher, J.E.; Avalos, J.L. Optogenetic amplification circuits for light-induced metabolic control. ACS Synth. Biol. 2021, 10, 1143–1154. [Google Scholar] [CrossRef]
- Lu, X.; Tang, K.; Li, P. Plant metabolic engineering strategies for the production of pharmaceutical terpenoids. Front. Plant Sci. 2016, 7, 1647. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Wieczfinska, J.; Skała, E.; Śliwiński, T.; Sitarek, P. Transgenesis as a tool for the efficient production of selected secondary metabolites from plant in vitro cultures. Plants 2020, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, X.; Xu, Z.; Wang, Y.; Shen, X.; Zhang, L.; Zhou, Z.; Wang, P. Pathway elucidation of bioactive rhamnosylated ginsenosides in Panax ginseng and their de novo high-level production by engineered Saccharomyces cerevisiae. Commun. Biol. 2022, 5, 775. [Google Scholar] [CrossRef]
- Dueber, J.E.; Wu, G.C.; Malmirchegini, G.R.; Moon, T.S.; Petzold, C.J.; Ullal, A.V.; Prather, K.L.; Keasling, J.D. Synthetic protein scaffolds provide modular control over metabolic flux. Nat. Biotechnol. 2009, 27, 753–759. [Google Scholar] [CrossRef]
- Moon, T.S.; Dueber, J.E.; Shiue, E.; Prather, K.L. Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli. Metab. Eng. 2010, 12, 298–305. [Google Scholar] [CrossRef]
- Choi, K.R.; Shin, J.H.; Cho, J.S.; Yang, D.; Lee, S.Y. Systems metabolic engineering of Escherichia coli. EcoSal Plus 2017, 7, 10-1128. [Google Scholar] [CrossRef]
- Morisseau, C.; Hammock, B.D. Epoxide hydrolases: Mechanisms, inhibitor designs, and biological roles. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-C.; Kim, W.; Park, S.C.; Jeong, J.; Park, M.K.; Lim, S.; Lee, Y.; Im, W.-T.; Lee, J.H.; Choi, G. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014, 55, 2177–2188. [Google Scholar] [CrossRef]
- Bathe, U.; Frolov, A.; Porzel, A.; Tissier, A. CYP76 oxidation network of abietane diterpenes in Lamiaceae reconstituted in yeast. J. Agric. Food Chem. 2019, 67, 13437–13450. [Google Scholar] [CrossRef]
- Moses, T.; Pollier, J.; Almagro, L.; Buyst, D.; Van Montagu, M.; Pedreño, M.A.; Martins, J.C.; Thevelein, J.M.; Goossens, A. Combinatorial biosynthesis of sapogenins and saponins in Saccharomyces cerevisiae using a C-16α hydroxylase from Bupleurum falcatum. Proc. Natl. Acad. Sci. USA 2014, 111, 1634–1639. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Ma, Y.; Yuan, L.; Wu, B.; Li, X.; Chen, S.; Lu, S. Genome-wide identification and characterization of novel genes involved in terpenoid biosynthesis in Salvia miltiorrhiza. J. Exp. Bot. 2012, 63, 2809–2823. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Tao, W.-Y.; Cheng, L. Paclitaxel production using co-culture of Taxus suspension cells and paclitaxel-producing endophytic fungi in a co-bioreactor. Appl. Microbiol. Biotechnol. 2009, 83, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Motolinía-Alcántara, E.A.; Castillo-Araiza, C.O.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Cruz-Sosa, F. Engineering considerations to produce bioactive compounds from plant cell suspension culture in bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W.-Y.; Zhang, J.; Zuo, B.-M.; Zhang, L.-M.; Huang, L.-Q. Production of ginsenoside and polysaccharide by two-stage cultivation of Panax quinquefolium L. cells. Vitr. Cell Dev. Biol. Plant 2012, 48, 107–112. [Google Scholar] [CrossRef]
- Kochan, E.; Caban, S.; Szymańska, G.; Szymczyk, P.; Lipert, A.; Kwiatkowski, P.; Sienkiewicz, M. Ginsenoside content in suspension cultures of Panax quinquefolium L. cultivated in shake flasks and stirred-tank bioreactor. Ann. UMCS, Biol. 2018, 72, 15–26. [Google Scholar]
- Dejong, J.M.; Liu, Y.; Bollon, A.P.; Long, R.M.; Jennewein, S.; Williams, D.; Croteau, R.B. Genetic engineering of taxol biosynthetic genes in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2006, 93, 212–224. [Google Scholar] [CrossRef]
- Walls, L.E.; Martinez, J.L.; Del Rio Chanona, E.A.; Rios-Solis, L. Definitive screening accelerates Taxol biosynthetic pathway optimization and scale up in Saccharomyces cerevisiae cell factories. Biotechnol. J. 2022, 17, e2100414. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Li, J.-F.; Norville, J.E.; Aach, J.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 2013, 31, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, Z.; Hua, K.; Gao, X.; Mao, Y.; Botella, J.R.; Zhu, J.-K. A highly efficient cell division-specific CRISPR/Cas9 system generates homozygous mutants for multiple genes in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 3925. [Google Scholar] [CrossRef]
- Meng, J.; Qiu, Y.; Shi, S. CRISPR/Cas9 systems for the development of Saccharomyces cerevisiae cell factories. Front. Bioeng. Biotechnol. 2020, 8, 594347. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Y. Class 2 CRISPR/Cas: An expanding biotechnology toolbox for and beyond genome editing. Cell Biosci. 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, D.; Miller, A.R.; Horken, K.M.; Weeks, D.P.; Spalding, M.H. Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3- transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2009, 106, 5990–5995. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, H.; Miura, K.; Ishizaki, K.; Kucho, K.I.; Saito, T.; Kohinata, T.; Ohyama, K. Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc. Natl. Acad. Sci. USA 2001, 98, 5347–5352. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Y.; Fei, X.; Wright, D.A.; Spalding, M.H. Expression activation and functional analysis of HLA3, a putative inorganic carbon transporter in Chlamydomonas reinhardtii. Plant J. Cell Mol. Biol. 2015, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Sato, E.; Iguchi, H.; Fukuda, Y.; Fukuzawa, H. Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2015, 112, 7315–7320. [Google Scholar] [CrossRef]
- Rizvi, N.F.; Weaver, J.D.; Cram, E.J.; Lee-Parsons, C.W.T. Silencing the transcriptional repressor, ZCT1, illustrates the tight regulation of terpenoid indole alkaloid biosynthesis in Catharanthus roseus hairy roots. PLoS ONE 2016, 11, e0159712. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Yan, H.; Yang, J.; Xu, W.; Su, Z. croFGD: Catharanthus roseus functional genomics database. Front. Genet. 2019, 10, 238. [Google Scholar] [CrossRef]
- Mao, J.; Huang, L.; Hao, J.; Liu, T.; Huang, S. The evolutionary rate variation among genes of MVA and MEP pathways in plant terpenoid biosynthesis. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef]
- Wang, H.-m.; Zu, Y.-g. Agrobacterium-mediated genetic transformation of Camptotheca acuminata. J. For. Res. 2007, 18, 316–318. [Google Scholar] [CrossRef]
- Silva, T.N.; Thomas, J.B.; Dahlberg, J.; Rhee, S.Y.; Mortimer, J.C. Progress and challenges in sorghum biotechnology, a multipurpose feedstock for the bioeconomy. J. Exp. Bot. 2022, 73, 646–664. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.-J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef]
- Aharoni, A.; Giri, A.P.; Deuerlein, S.; Griepink, F.; de Kogel, W.-J.; Verstappen, F.W.A.; Verhoeven, H.A.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 2003, 15, 2866–2884. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; D’Auria, J.C.; Farooq, A.; Pichersky, E.; Gershenzon, J. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Aharoni, A.; Jongsma, M.A.; Kim, T.-Y.; Ri, M.-B.; Giri, A.P.; Verstappen, F.W.A.; Schwab, W.; Bouwmeester, H.J. Metabolic engineering of terpenoid biosynthesis in plants. Phytochem. Rev. 2006, 5, 49–58. [Google Scholar] [CrossRef]
- Payne, R.M.E.; Xu, D.; Foureau, E.; Teto Carqueijeiro, M.I.S.; Oudin, A.; de Bernonville, T.D.; Novak, V.; Burow, M.; Olsen, C.-E.; Jones, D.M. An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole. Nat. Plants 2017, 3, 16208. [Google Scholar] [CrossRef]
- Yu, F.; De Luca, V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc. Natl. Acad. Sci. USA 2013, 110, 15830–15835. [Google Scholar] [CrossRef] [PubMed]
- Hilvert, D. Spiers memorial lecture: Engineering biocatalysts. Faraday Discuss. 2024, 252, 9–28. [Google Scholar] [CrossRef] [PubMed]
- d’Oelsnitz, S.; Diaz, D.J.; Kim, W.; Acosta, D.J.; Dangerfield, T.L.; Schechter, M.W.; Minus, M.B.; Howard, J.R.; Do, H.; Loy, J.M.; et al. Biosensor and machine learning-aided engineering of an amaryllidaceae enzyme. Nat. Commun. 2024, 15, 2084. [Google Scholar] [CrossRef]
- Wei, T.; Gao, Y.; Deng, K.; Zhang, L.; Yang, M.; Liu, X.; Qi, C.; Wang, C.; Song, W.; Zhang, Y.; et al. Enhancement of tanshinone production in Salvia miltiorrhiza hairy root cultures by metabolic engineering. Plant Methods 2019, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sudheer, W.N.; Lakshmaiah, V.V.; Mukherjee, E.; Nizam, A.; Thiruvengadam, M.; Nagella, P.; Alessa, F.M.; Al-Mssallem, M.Q.; Rezk, A.A. Biotechnological approaches for production of artemisinin, an anti-malarial drug from Artemisia annua L. Molecules 2022, 27, 3040. [Google Scholar] [CrossRef]
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation strategies for production of pharmaceutical terpenoids in engineered yeast. Pharmaceuticals 2021, 14, 295. [Google Scholar] [CrossRef]
- Schmid, J.; Schwarz, S.; Meier-Staude, R.; Sudhop, S.; Clausen-Schaumann, H.; Schieker, M.; Huber, R. A perfusion bioreactor system for cell seeding and oxygen-controlled cultivation of three-dimensional cell cultures. Tissue Eng. Part C Methods 2018, 24, 585–595. [Google Scholar] [CrossRef]
- De Dobbeleer, C.; Cloutier, M.; Fouilland, M.; Legros, R.; Jolicoeur, M. A high-rate perfusion bioreactor for plant cells. Biotechnol. Bioeng. 2006, 95, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.M.; McNulty, M.J.; Macharoen, K.; McDonald, K.A.; Nandi, S. Technoeconomic analysis of semicontinuous bioreactor production of biopharmaceuticals in transgenic rice cell suspension cultures. Biotechnol. Bioeng. 2020, 117, 3053–3065. [Google Scholar] [CrossRef]
- Yıldırım, K.; Miladinović, D.; Sweet, J.; Akin, M.; Galović, V.; Kavas, M.; Zlatković, M.; de Andrade, E. Genome editing for healthy crops: Traits, tools and impacts. Front. Plant Sci. 2023, 14, 1231013. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.F.; Mohd Noor, S.N.; Abd-Aziz, N.; Pua, T.-L.; Tan, B.C. Green revolution to gene revolution: Technological advances in agriculture to feed the world. Plants 2022, 11, 1297. [Google Scholar] [CrossRef]
- Jacquemart, R.; Vandersluis, M.; Zhao, M.; Sukhija, K.; Sidhu, N.; Stout, J. A single-use strategy to enable manufacturing of affordable biologics. Comput. Struct. Biotechnol. J. 2016, 14, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Dianat, M.; Straaten, S.; Maritato, A.; Wibberg, D.; Busche, T.; Blank, L.M.; Ebert, B.E. Exploration of in situ extraction for enhanced triterpenoid production by Saccharomyces cerevisiae. Microb. Biotechnol. 2024, 17, e70061. [Google Scholar] [CrossRef]
- Santoyo-Garcia, J.H.; Walls, L.E.; Nowrouzi, B.; Galindo-Rodriguez, G.R.; Ochoa-Villarreal, M.; Loake, G.J.; Dimartino, S.; Rios-Solis, L. In situ solid-liquid extraction enhances recovery of taxadiene from engineered Saccharomyces cerevisiae cell factories. Sep. Purif. Technol. 2022, 290, 120880. [Google Scholar] [CrossRef]
- Wu, W.; Maravelias, C.T. Synthesis and techno-economic assessment of microbial-based processes for terpenes production. Biotechnol. Biofuels 2018, 11, 294. [Google Scholar] [CrossRef]
- Pinu, F.R.; Beale, D.J.; Paten, A.M.; Kouremenos, K.; Swarup, S.; Schirra, H.J.; Wishart, D. Systems biology and multi-omics integration: Viewpoints from the metabolomics research community. Metabolites 2019, 9, 76. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant metabolomics: An indispensable system biology tool for plant science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Chen, B.-S.; Wu, C.-C. Systems biology as an integrated platform for bioinformatics, systems synthetic biology, and systems metabolic engineering. Cells 2013, 2, 635–688. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Jiang, Z.; Hong, X.; Zhang, S.; Yao, R.; Xiao, Y. CRISPR base editing and prime editing: DSB and template-free editing systems for bacteria and plants. Synth. Syst. Biotechnol. 2020, 5, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, M.C.; Celik Altunoglu, Y.; Baloglu, P.; Yildiz, A.B.; Türkölmez, N.; Özden Çiftçi, Y. Gene-editing technologies and applications in Legumes: Progress, evolution, and future prospects. Front. Genet. 2022, 13, 859437. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Fang, H.; Roberts, N.; Zhang, L.; Vakulskas, C.A.; Niedz, R.P.; Culver, J.N.; Qi, Y. Highly Efficient genome editing in plant protoplasts by ribonucleoprotein delivery of CRISPR-Cas12a nucleases. Front. Genome Ed. 2022, 4, 780238. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.T.; Yuan, Y.H.; Lin, Y.C.; Lin, S.; Cheng, Q.W.; Wu, F.H.; Sheen, J.; Shih, M.C.; Lin, C.S. Efficient and economical targeted insertion in plant genomes via protoplast regeneration. Cris. J. 2021, 4, 752–760. [Google Scholar] [CrossRef]
- Madhavan, A.; Arun, K.B.; Sindhu, R.; Binod, P.; Kim, S.H.; Pandey, A. Tailoring of microbes for the production of high value plant-derived compounds: From pathway engineering to fermentative production. Biochim. Biophys. Acta. Proteins Proteom. 2019, 1867, 140262. [Google Scholar] [CrossRef]
- Kumar, S. Engineering cytochrome P450 biocatalysts for biotechnology, medicine and bioremediation. Expert. Opin. Drug Metab. Toxicol. 2010, 6, 115–131. [Google Scholar] [CrossRef]
- Otey, C.R.; Bandara, G.; Lalonde, J.; Takahashi, K.; Arnold, F.H. Preparation of human metabolites of propranolol using laboratory-evolved bacterial cytochromes P450. Biotechnol. Bioeng. 2006, 93, 494–499. [Google Scholar] [CrossRef]
- Zhang, F.; Neik, T.X.; Thomas, W.J.W.; Batley, J. CRISPR-based genome editing tools: An accelerator in crop breeding for a changing future. Int. J. Mol. Sci. 2023, 24, 8623. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Li, C. Engineering electron transfer pathway of cytochrome P450s. Molecules 2024, 29, 2480. [Google Scholar] [CrossRef]
- Boynton, J.E.; Gillham, N.W.; Harris, E.H.; Hosler, J.P.; Johnson, A.M.; Jones, A.R.; Randolph-Anderson, B.L.; Robertson, D.; Klein, T.M.; Shark, K.B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 1988, 240, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Oey, M.; Lohse, M.; Kreikemeyer, B.; Bock, R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 2009, 57, 436–445. [Google Scholar] [CrossRef]
- Jackson, H.O.; Taunt, H.N.; Mordaka, P.M.; Smith, A.G.; Purton, S. The algal chloroplast as a testbed for synthetic biology designs aimed at radically rewiring plant metabolism. Front. Plant Sci. 2021, 12, 708370. [Google Scholar] [CrossRef]
- Sakamoto, W.; Miyagishima, S.Y.; Jarvis, P. Chloroplast biogenesis: Control of plastid development, protein import, division and inheritance. Arab. Book 2008, 6, e0110. [Google Scholar] [CrossRef] [PubMed]
- Agapakis, C.M.; Niederholtmeyer, H.; Noche, R.R.; Lieberman, T.D.; Megason, S.G.; Way, J.C.; Silver, P.A. Towards a synthetic chloroplast. PLoS ONE 2011, 6, e18877. [Google Scholar] [CrossRef] [PubMed]
- Karkute, S.G.; Singh, A.K.; Gupta, O.P.; Singh, P.M.; Singh, B. CRISPR/Cas9 mediated genome engineering for improvement of horticultural crops. Front. Plant Sci. 2017, 8, 1635. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, A.; Partap, M.; Thakur, M.; Bhargava, B. CRISPR-Cas: A robust technology for enhancing consumer-preferred commercial traits in crops. Front. Plant Sci. 2023, 14, 1122940. [Google Scholar] [CrossRef]
- De Paola, C. Enhancing Nicotiana benthamiana as Chassis for Molecular Farming: Targeting Flowering Time for Increased Biomass and Recombinant Protein Production. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2024. [Google Scholar]
- Uetz, P.; Melnik, S.; Grünwald-Gruber, C.; Strasser, R.; Stoger, E. CRISPR/Cas9-mediated knockout of a prolyl-4-hydroxylase subfamily in Nicotiana benthamiana using DsRed2 for plant selection. Biotechnol. J. 2022, 17, e2100698. [Google Scholar] [CrossRef] [PubMed]
- Bat-Erdene, U.; Billingsley, J.M.; Turner, W.C.; Lichman, B.R.; Ippoliti, F.M.; Garg, N.K.; O’Connor, S.E.; Tang, Y. Cell-free total biosynthesis of plant terpene natural products using an orthogonal cofactor regeneration system. ACS Catal. 2021, 11, 9898–9903. [Google Scholar] [CrossRef]
- He, C.; Zhang, C.; Bian, T.; Jiao, K.; Su, W.; Wu, K.-J.; Su, A. A review on artificial intelligence enabled design, synthesis, and process optimization of chemical products for industry 4.0. Processes 2023, 11, 330. [Google Scholar] [CrossRef]
- Zheng, S.; Zeng, T.; Li, C.; Chen, B.; Coley, C.W.; Yang, Y.; Wu, R. Deep learning driven biosynthetic pathways navigation for natural products with BioNavi-NP. Nat. Commun. 2022, 13, 3342. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Xu, J.; Sui, C.; Wei, J. Application of CRISPR/Cas9 in plant biology. Acta Pharm. Sin. B 2017, 7, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Luo, L.; Jin, B.; Liu, J.; Chen, T.; Tang, J.; Shen, Y.; Zhang, H.; Guo, J.; Zhang, H.; et al. Highly efficient Agrobacterium rhizogenes-mediated gene editing system in Salvia miltiorrhiza inbred line bh2-7. Plant Biotechnol. J. 2025, 23, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fu, X.; Pan, Q.; Tang, Y.; Shen, Q.; Lv, Z.; Yan, T.; Shi, P.; Li, L.; Zhang, L.; et al. Overexpression of AaWRKY1 leads to an enhanced content of artemisinin in Artemisia annua. Biomed Res. Int. 2016, 2016, 7314971. [Google Scholar] [CrossRef]
| Aspect | Native Medicinal Plants | Microbial Chassis | Heterologous Plant Hosts |
|---|---|---|---|
| Key Advantages | Native enzymatic context for complex modifications; | Rapid growth & high cell density; Well-established genetic tools & high-throughput screening; Scalable fermentation | Eukaryotic PTMs and compartmentalization; Low-cost biomass production (agroinfiltration); Capable of complex pathways |
| Pre-existing storage structures | |||
| Major Limitations | Long growth cycles; | Cytotoxicity of intermediates; Lack of specific P450s/UGTs; Cofactor balancing issues; High substrate costs | Transient expression limitations; Metabolic competition with endogenous pathways; Scale-up challenges for extraction |
| Low yields; | |||
| Complex genetics & recalcitrance to transformation; | |||
| Ecological concerns | |||
| Max. Yields | Artemisinin: ~1.2% DW [14]; Paclitaxel: ~0.05% DW [15] | Artemisinic acid: >25 g/L (yeast) [16]; Taxadiene: >1 g/L (E. coli) [17]; Protopanaxadiol: 11 g/L (yeast) [9] Ginsenoside K: 5.74 g/L (yeast) [18] | Taxadiene: ~48 µg/g DW (chloroplast-targeted) [19]; Triterpenes: 37.9 mg/g DW [20] |
| Cost & Scalability | High agricultural land & labor cost; | Fermentation costs significant but controllable; Highly scalable to industrial bioreactors (10,000+ L) | Medium cost; Scaling requires large greenhouse space, not yet industrial |
| Difficult to scale, season-dependent | |||
| Tech. Maturity (TRL) | Medium | High | Medium-High |
| Ideal Terpenoid Targets | High-value compounds already produced by the plant; | Volatile mono/sesquiterpenes; Triterpene scaffolds; Non-natural derivatives via combinatorial biosynthesis | Complex diterpenes/triterpenes; Molecules requiring plant-specific P450s/UGTs; Rapid prototyping of pathways |
| Molecules requiring extensive, plant-specific modifications |
| Target Compound | Host | Target Organelle | Engineering Strategy | Key Targeting Signal | Outcome | Reference |
|---|---|---|---|---|---|---|
| Taxadiene | N. tabacum | Chloroplast | Plastid-targeted expression of Taxus taxadiene synthase | Chloroplast transit peptide | 5.6 μg/g DW | [64,95] |
| Valencene | S. cerevisiae | Mitochondria | Mitochondrial-targeted valencene synthase | COX4 MTS | 3-fold increase | [41,104] |
| Triterpenoids | N. tabacum | Chloroplast | Reconstitution of cytosolic MVA pathway in chloroplasts | Plastid-targeted HMGR, FPS | Significant yield enhancement | [30,105] |
| Artemisinic acid | S. cerevisiae | Endoplasmic Reticulum (ER) | ER-membrane anchoring of CYP71AV1 and CPR | Cytochrome P450 N-terminal anchor | Improved electron transfer, higher oxidation efficiency | [16] |
| limonene | S. cerevisiae | Cytoplasm | Orthogonal pathway (SlNDPS1 + LS) + ERG20 repression by HXT1 promoter | N/A (cytosolic expression) | 917.7 mg/L (6-fold increase) | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Xu, S.; Guo, X. Metabolic Engineering of Terpenoid Biosynthesis in Medicinal Plants: From Genomic Insights to Biotechnological Applications. Curr. Issues Mol. Biol. 2025, 47, 723. https://doi.org/10.3390/cimb47090723
Guo C, Xu S, Guo X. Metabolic Engineering of Terpenoid Biosynthesis in Medicinal Plants: From Genomic Insights to Biotechnological Applications. Current Issues in Molecular Biology. 2025; 47(9):723. https://doi.org/10.3390/cimb47090723
Chicago/Turabian StyleGuo, Changfeng, Si Xu, and Xiaoyun Guo. 2025. "Metabolic Engineering of Terpenoid Biosynthesis in Medicinal Plants: From Genomic Insights to Biotechnological Applications" Current Issues in Molecular Biology 47, no. 9: 723. https://doi.org/10.3390/cimb47090723
APA StyleGuo, C., Xu, S., & Guo, X. (2025). Metabolic Engineering of Terpenoid Biosynthesis in Medicinal Plants: From Genomic Insights to Biotechnological Applications. Current Issues in Molecular Biology, 47(9), 723. https://doi.org/10.3390/cimb47090723






