Abstract
Postpartum metritis in dairy cows compromises reproductive performance and leads to substantial economic losses. This study investigated the molecular mechanisms underlying metritis by integrating high-throughput circulating microRNA (miRNA) profiling with systems-level bioinformatics. Previously, 30 differentially expressed miRNAs, 16 upregulated and 14 downregulated, were identified in metritis-affected cows compared to healthy controls. Building on these findings, this study predicted miRNA target genes and constructed regulatory networks involving miRNAs, mRNAs, circRNAs, lncRNAs, and snRNAs, alongside protein–protein interaction networks. Functional annotation and KEGG pathway analysis revealed that upregulated miRNAs influenced genes involved in immune activation, apoptosis, and metabolism, while downregulated miRNAs were associated with angiogenesis, immune suppression, and tissue repair. Hub genes such as AKT3, VEGFA, and HIF1A were central to immune and angiogenic signaling, whereas UBE3A and ZEB1 were linked to immune inhibition. Interferon-stimulated genes (e.g., ISG15, RSAD2, CXCL chemokines) were shown to regulate solute carriers, contributing to immune dysregulation. Key pathways included PI3K-Akt, NF-κB, JAK-STAT, insulin resistance, and T cell receptor signaling. Noncoding RNAs such as NEAT1, KCNQ1OT1, and XIST, along with miRNAs like bta-miR-15b and bta-miR-148a, emerged as pro-inflammatory regulators, while bta-miR-199a-3p appeared to exert immunosuppressive effects. These findings offer new insights into the complex regulatory networks driving metritis and suggest potential targets for improving fertility in dairy cows.
Keywords:
postpartum cows; uterus; inflammation; immune function; blood; microRNAs; mRNAs; in silico analysis 1. Introduction
Postpartum uterine diseases are categorized as puerperal metritis, clinical metritis, clinical endometritis, and subclinical endometritis based on the time of occurrence after calving and presence of clinical or subclinical signs [1,2,3,4]. Puerperal metritis was defined by an enlarged, flaccid uterus with a foul smelling, watery red-brown discharge, along with pyrexia and systemic illness occurring within 10 days of calving [4]. Other clinical signs include anorexia, depression, and decreased milk yield and feed intake [4,5,6].
In general, postpartum uterine diseases, irrespective of the type, inflict financial losses on dairy farms, mostly due to treatment costs and disposal of milk, decreased milk production, poor reproductive performance, and increased culling [2,5,6]. Although reproductive performance is compromised in dairy cows experiencing postpartum uterine disease, the exact mechanism by which the fertility is affected is not fully understood.
Gene expression of key inflammatory cytokines in circulation varied between normal cows versus cows experiencing uterine disease [7,8]. Similarly, gene expression of cytokines also differed in an inflamed versus normal endometrium of postpartum cows [9,10]. We recently reported the influence of uterine inflammation negatively affecting the length of gestational day 16 conceptus [11]. Although several studies have considered genetic components of uterine inflammation [12,13,14,15], few have elucidated epigenetic changes such as altered expression of regulatory RNAs and their integration with coding genes involved in bovine metritis. The potential regulatory role of microRNAs (miRNAs) in the development and progression of bovine subclinical endometritis has been investigated by studying expression of miRNAs in uterine endometrial samples [13,14].
Recent studies have highlighted the role of noncoding RNAs (ncRNAs), including long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), and small noncoding RNAs (sncRNAs), in the regulation of immune responses, inflammation, and tissue repair [15]. The altered expression of these ncRNAs has been associated with disrupted cellular processes in the uterus [16,17]. Understanding how specific ncRNAs are dysregulated during uterine disease provides valuable insight into the molecular mechanisms underlying infertility and may guide the development of diagnostic or therapeutic strategies.
In our previous study, 84 circulating miRNAs were profiled in postpartum dairy cows, identifying 30 differentially expressed (DE) miRNAs, 16 upregulated and 14 downregulated, in cows with metritis compared to healthy controls [18]. Building on these findings, the present study employed integrative bioinformatics analyses to predict target genes, protein–protein interactions, and regulatory networks involving miRNAs, circRNAs, lncRNAs, snRNAs, and mRNAs associated with metritis.
2. Materials and Methods
MicroRNA data from our previous study [18] were used, in which Holstein dairy cows with metritis (n = 4) and healthy controls (n = 4) were selected. Blood samples were collected via coccygeal venipuncture for serum miRNA profiling using RT-PCR. For clarity, we have included a brief description of the methodology employed. Briefly, serum samples were processed, small RNAs were extracted and reverse transcribed, and mature miRNA expression was profiled using the Qiagen miScript PCR array, which targets 84 bovine miRNAs. Normalization was performed using cel-miR-39-3p and the global CT mean. Data analysis included CT quality control, normalization, and calculation of ΔCT values, fold changes, and statistical significance using a web-based tool.
In the current study we employed integrative bioinformatics analyses to predict target genes, protein–protein interactions, and regulatory networks involving miRNAs, circRNAs, lncRNAs, snRNAs, and mRNAs associated with metritis, which are presented below.
2.1. Conserved Nucleotide Sequences
Nucleotide sequences of DE-miRNAs were retrieved from miRBase, (www.mirbase.org accessed on 10 January 2025) and compared for sequence conservation between human and cattle [19,20]. Bovine sequences were very similar to human nucleotide sequences. Therefore, human miRNA IDs were used to construct miRNA-–mRNA interaction network and functional enrichment analysis.
2.2. Prediction and Analysis of Target Genes of Differentially Expressed miRNAs
The target genes of DE-miRNAs were predicted using miRNet (http://www.mirnet.ca/ accessed on 10 January 2025) [21]. This tool integrated data from multiple miR databases including TarBase, miRTarBase, and miRecords. The target prediction analysis was performed separately for upregulated and downregulated DE-miRNAs.
2.3. Construction of Protein–Protein Interaction Network and Screening of Hub Gene
The protein–protein interaction (PPI) network for predicted target genes of DE-miRNAs’ was generated the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) online database (http://string-db.org/ accessed on 10 January 2025) [22]. Gene Ontology (GO) functional annotations and Kyoto Encyclopedia of Genes and Genomes (KEGGs) pathway enrichment analyses were conducted on these predicted targets. A p-value < 0.05 was regarded as statistically significant. The PPI network was exported to Cytoscape (version 3.9, accessed on 10 January 2025) for visualization [23]. Hub genes were identified as the top 30 nodes in the PPI network using the Maximal Clique Centrality (MCC) method [24], which is known for high precision in identifying essential proteins. Further analysis was conducted using ClueGO [25] (accessed on 10 January 2025) to integrate GO terms and KEGG pathways, generating functionally organized term networks (k score = 3). This tool supports the analysis of single or multiple gene lists and provides comprehensive visualization of functionally related terms.
2.4. Gene Ontology and Functional Annotation Analysis
To explore the biological significance of DE-miRNAs and their associated genes, biological processes were analyzed using the PANTHER (Protein ANalysis THrough Evolutionary Relationships) Classification System (https://pantherdb.org accessed on 13 January 2025). Additional GO terms and their co-occurring terms were investigated using QuickGO (https://www.ebi.ac.uk/QuickGO accessed on 13 January 2025).
Further analysis of hub genes included their roles, human tissue expression, and protein–protein interactions (up to 6 closely related genes) using data from STRING (http://string-db.org/ accessed on 13 January 2025) and the human protein atlas (https://www.proteinatlas.org accessed on 13 January 2025). Interferon-stimulated genes associated with up- and downregulated miRNAs in cows with metritis were extracted from miRNet’s target prediction output (http://www.mirnet.ca/ accessed on 13 January 2025).
2.5. miRNA, circRNA, lncRNA, snRNA, and mRNA Interaction Network
Interaction networks among miRNA, circRNA, lncRNA, snRNA, and mRNA were generated using miRNet (http://www.mirnet.ca/ accessed on 29 May 2025), separately for upregulated and downregulated miRNAs. Specific interaction maps, including miRNA–circRNA–mRNA, miRNA–lncRNA–mRNA, and miRNA–snRNA–mRNA networks, were also constructed.
3. Results
3.1. miRNA, Gene, and Protein–Protein Interactions
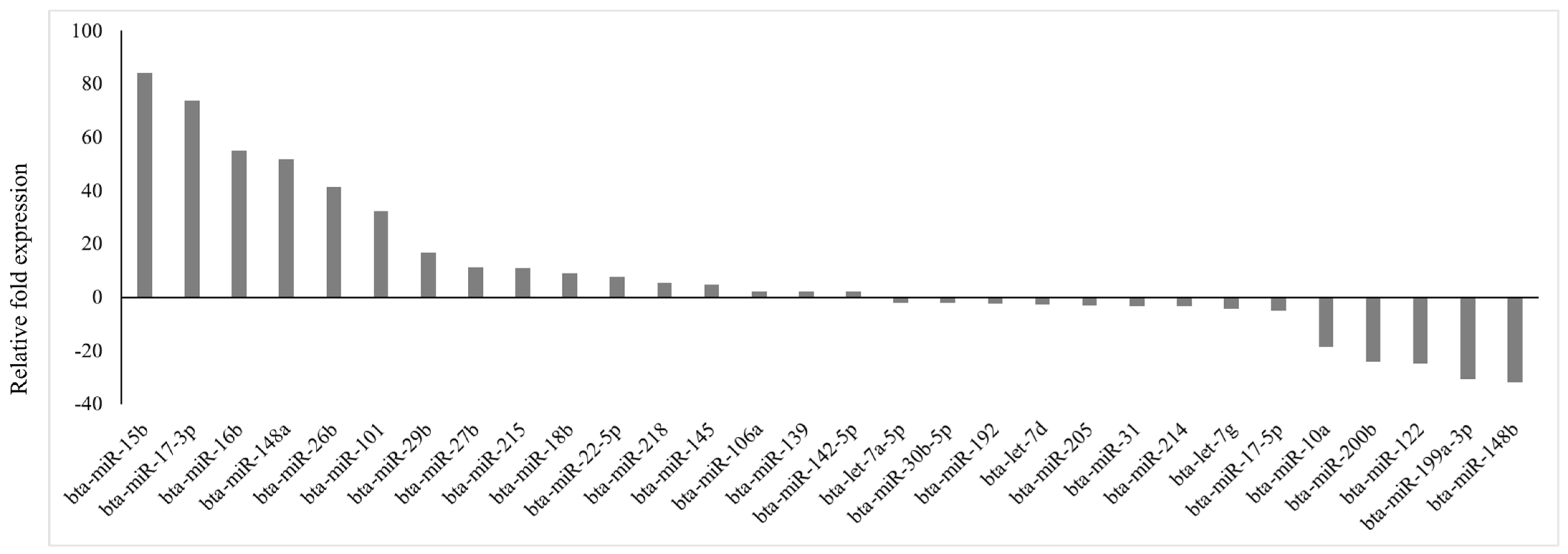
Of 84 prioritized miRNA previously profiled, 30 miRNAs were differentially expressed (p < 0.05; fold regulation ≥ 2), 16 upregulated and 14 downregulated (Figure 1), in circulation in cows with metritis compared to normal cows. These DE-miRNAs were analyzed to predict their target genes. Among the 16 upregulated miRNAs, 10 predicted 74 genes (Supplementary File S1), while 10 of the 14 downregulated miRNAs predicted 123 genes (Supplementary File S2).
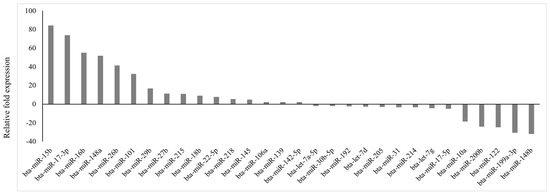
Figure 1.
Fold regulation of differentially expressed miRNAs in Holstein cows with metritis of 84 bovine-specific well-characterized miRNAs investigated, 16 were upregulated (p ≤ 0.05; fold ≥ 2) and 14 were downregulated (p ≤ 0.05; fold ≤ −2) in circulation.
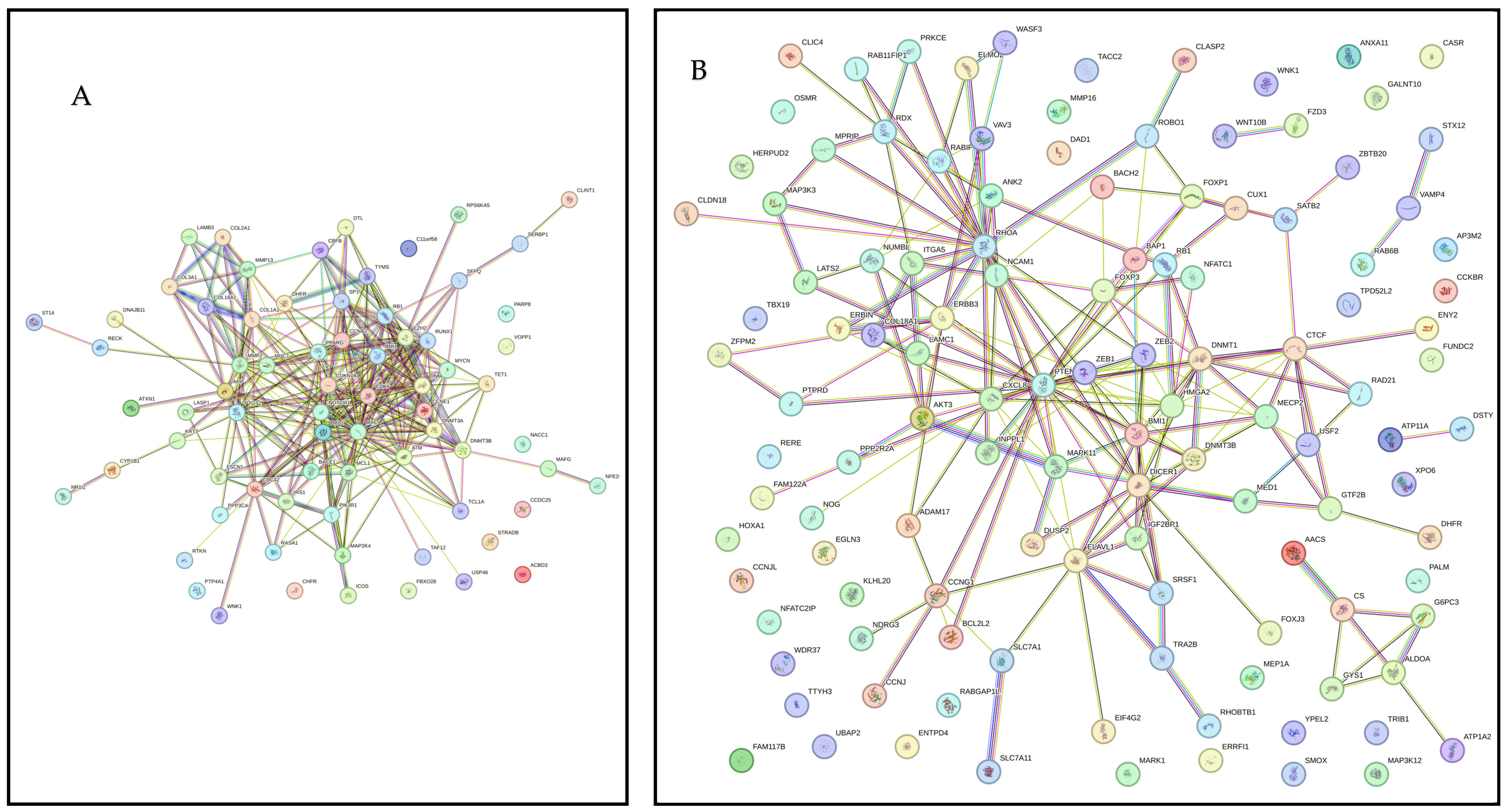
Figure 2A presents PPI network for target genes of upregulated miRNAS (72 nodes; 281 edges; PPI enrichment p < 1.0 × 10−16) revealing 511 significantly (False Discovery Rate (FDR) p < 0.05) enriched GO biological processes and 77 significantly (FDR p < 0.05) enriched KEGG pathways (Supplementary File S3). Figure 2B shows PPI network for the downregulated miRNAs (120 nodes and 189 edges, PPI enrichment p < 1.11 × 10−14) with 299 significantly (FDR p < 0.05) enriched GO biological processes and 41 significant (FDR p < 0.05) KEGG pathways (Supplementary File S4).
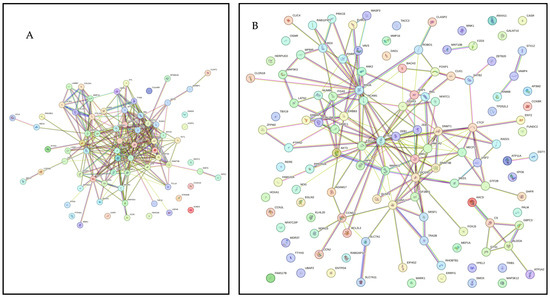
Figure 2.
STRING protein–protein interaction (PPI) network. (A) PPI network for the upregulated miRNAS-predicted 74 genes (72 nodes and 281 edges, PPI enrichment p < 1.0 × 10−16). (B) PPI network for the downregulated miRNAS-predicted 123 genes (120 nodes and 189 edges, PPI enrichment p < 1.11 × 10−14).
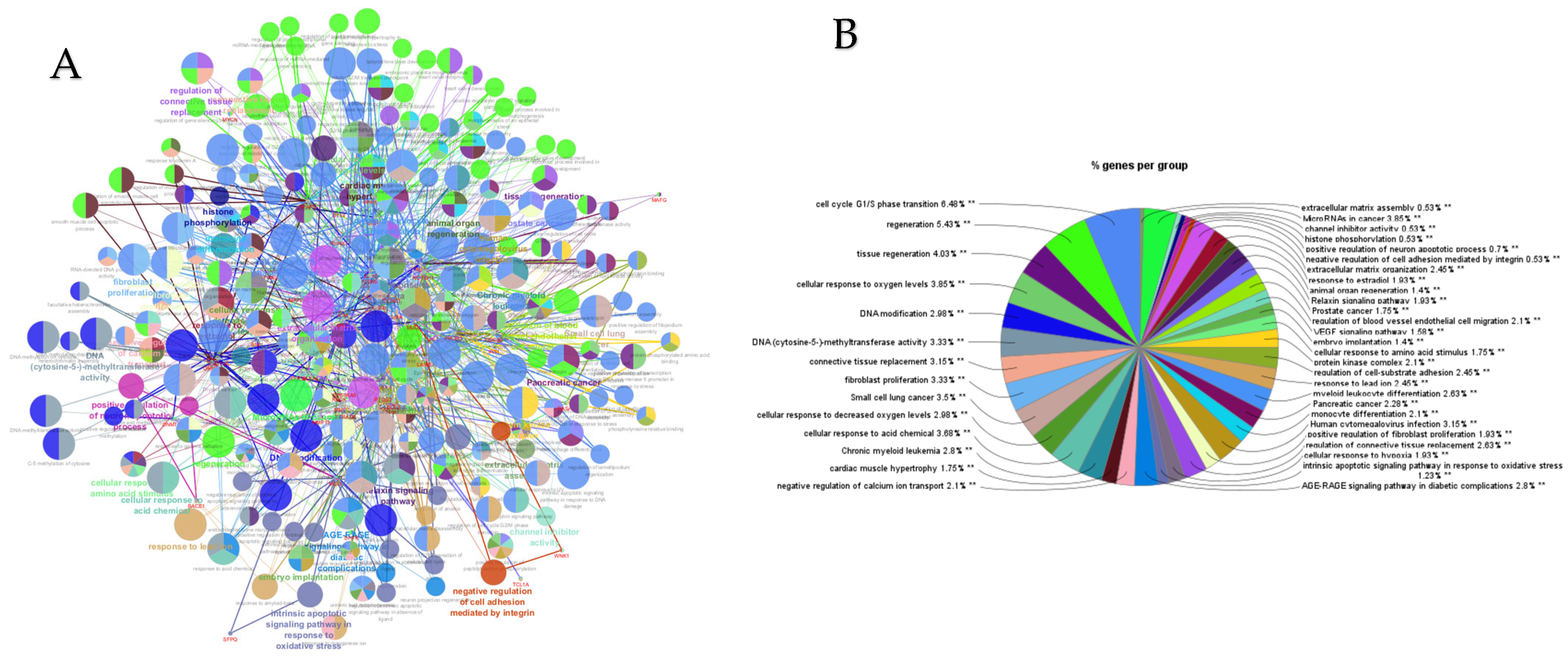
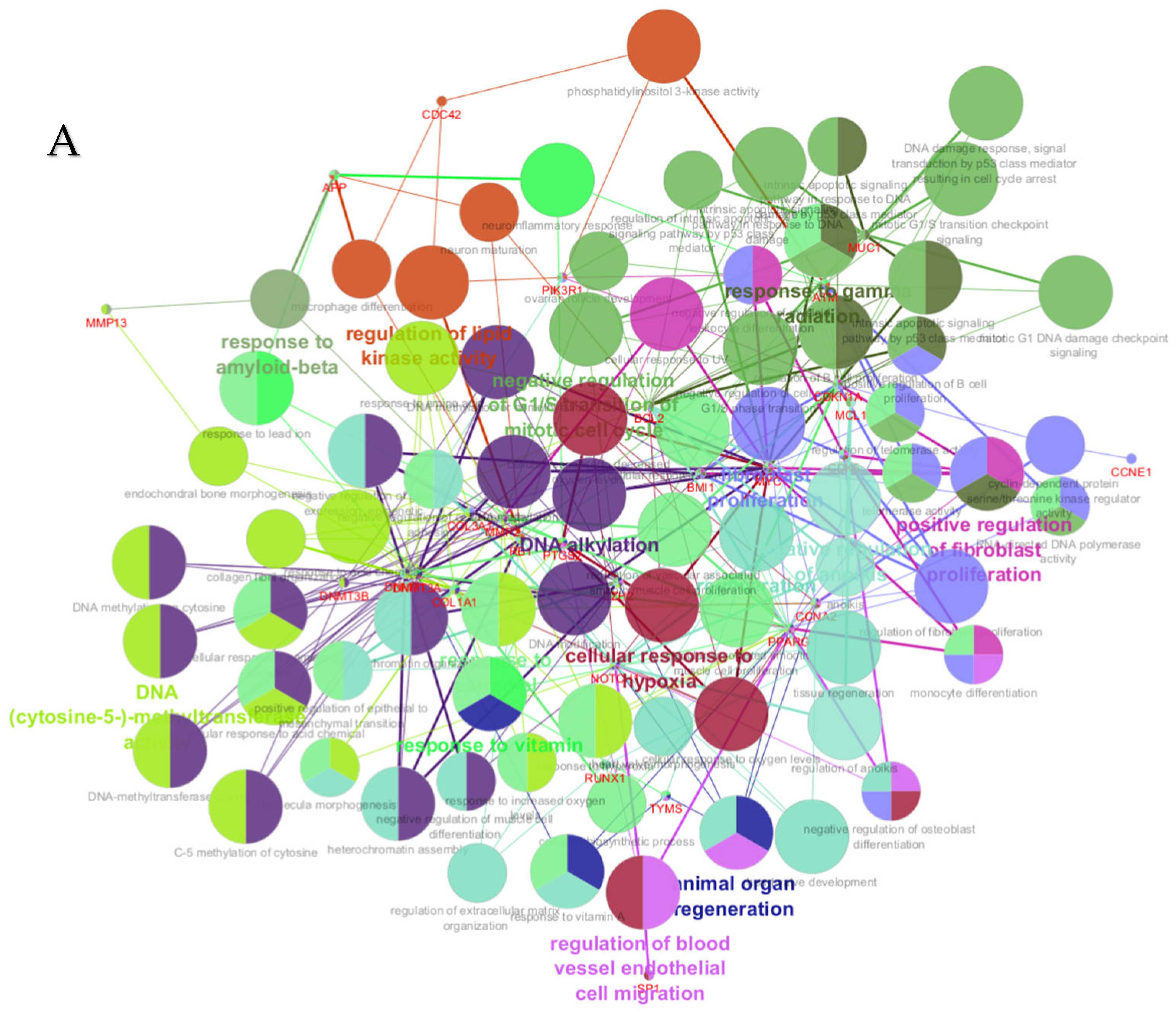
PPI networks were constructed using the STRING database and visualized in Cytoscape. To interpret the functionally nested ontology and pathway annotation networks for genes targeted by up- and downregulated DE-miRNAs in cows with metritis, ClueGO analyses were performed and visualized in Figure 3A–C and Figure 4A–C, respectively.
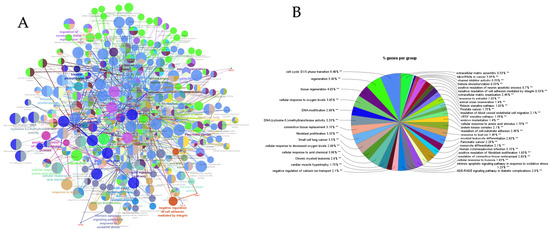
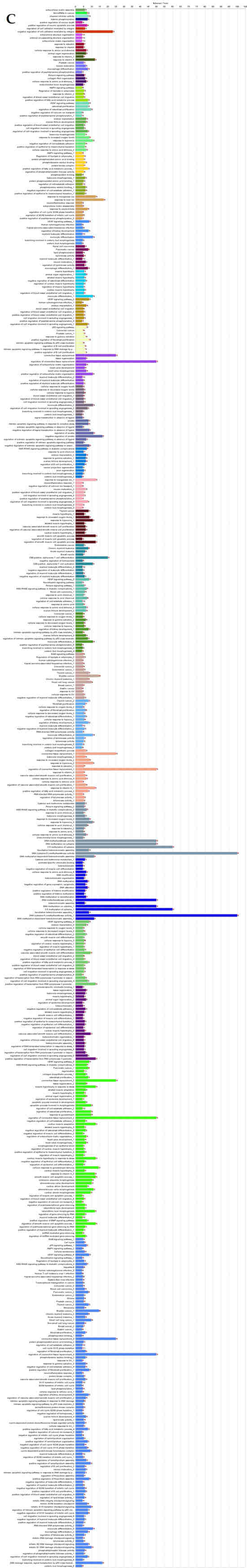
Figure 3.
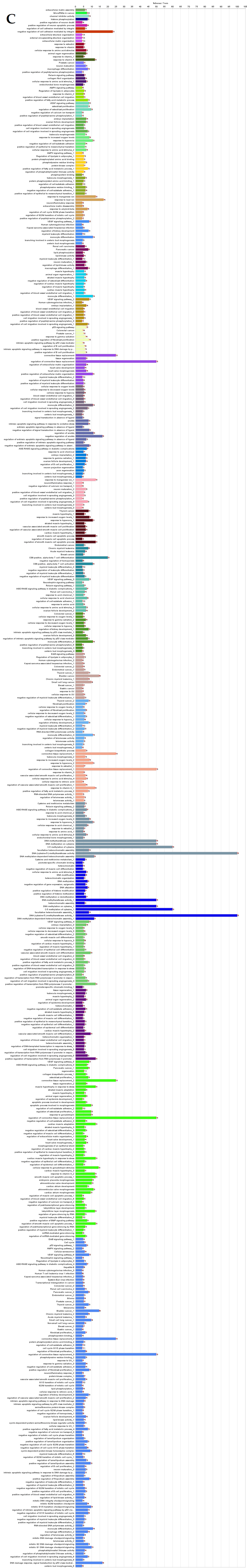
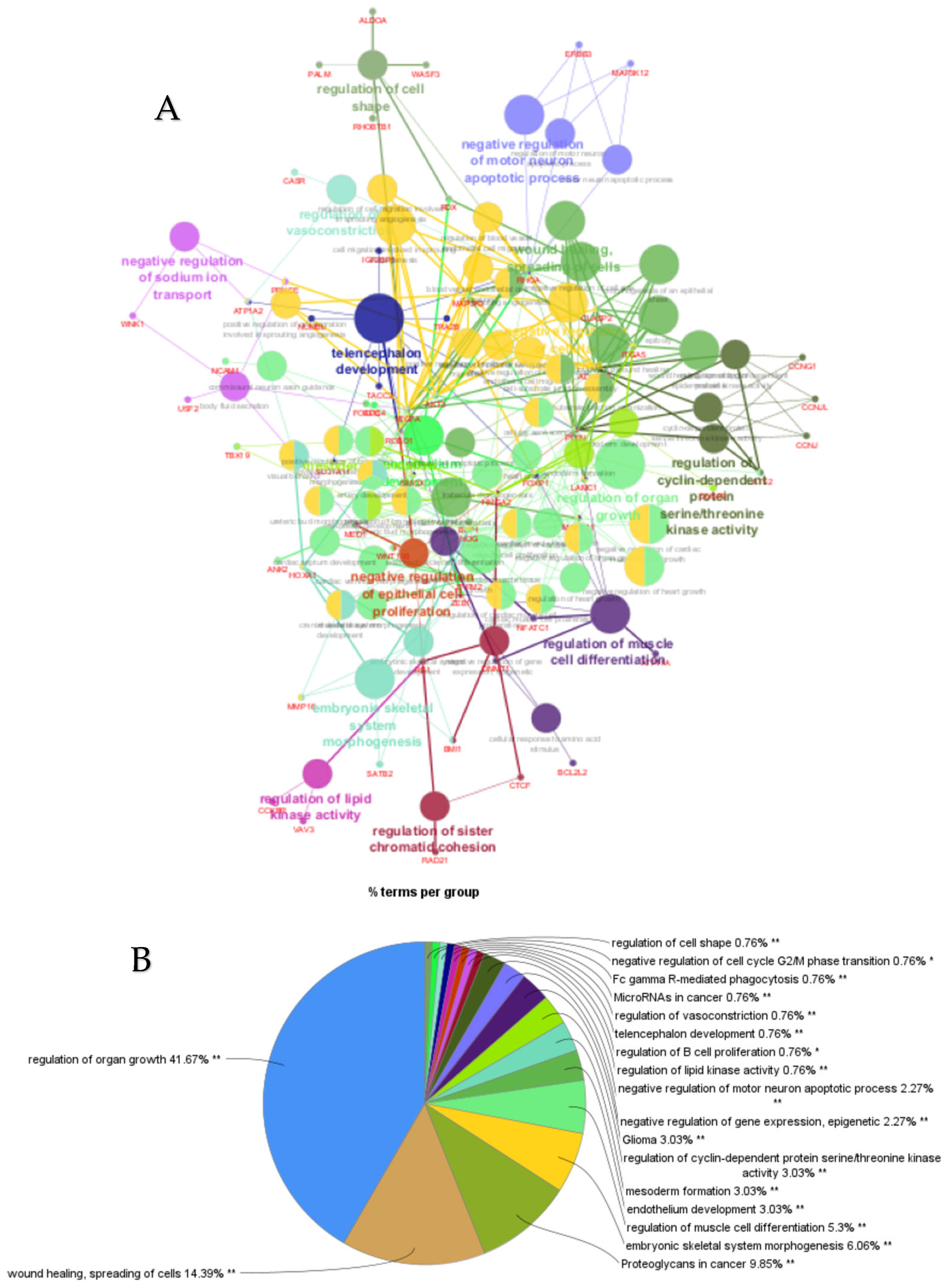
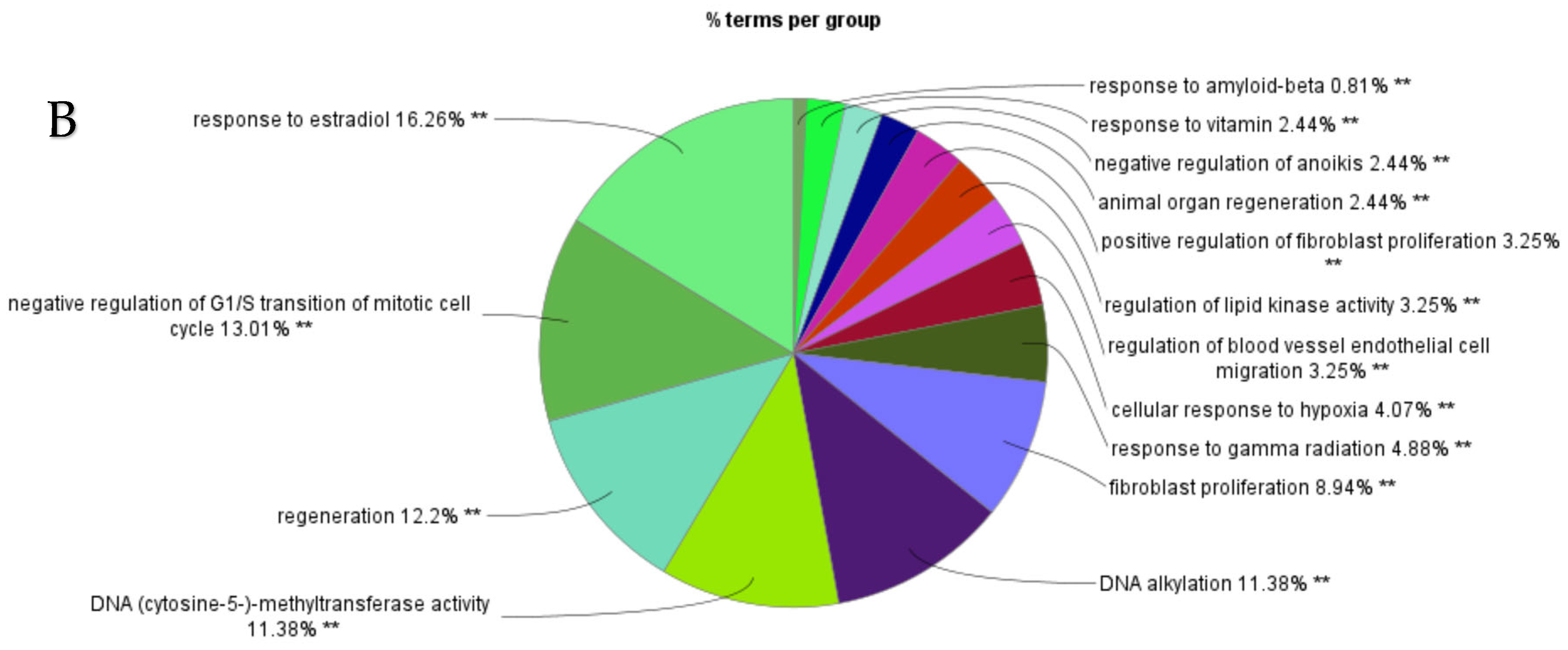
ClueGO analysis of upregulated genes in circulation in cows with metritis. (A) Functionally grouped network with terms as nodes linked based on their kappa score level (≥0.4), where only the label of the most significant term per group is shown. The node size represents the term enrichment significance. Functionally related groups partially overlap. The color gradient shows the gene proportion of each cluster associated with the term. (B) Overview chart with functional groups including specific terms for upregulated genes. The percentage of genes per term is shown as term label. ** p < 0.001; (C) GO/pathway terms specific for upregulated genes. The percentage of genes per term is shown as bar label.
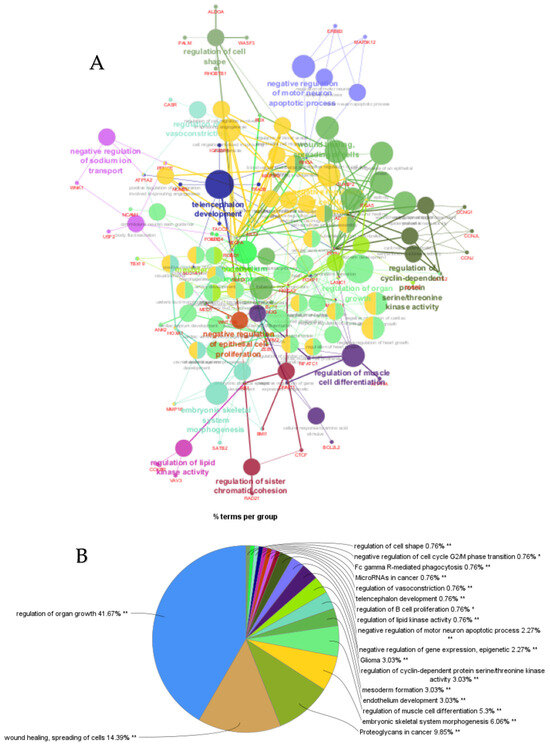
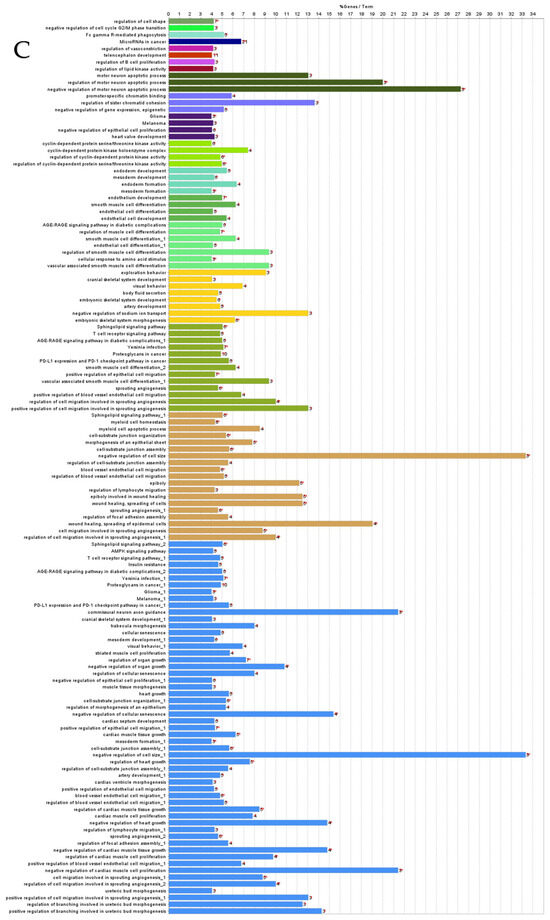
Figure 4.
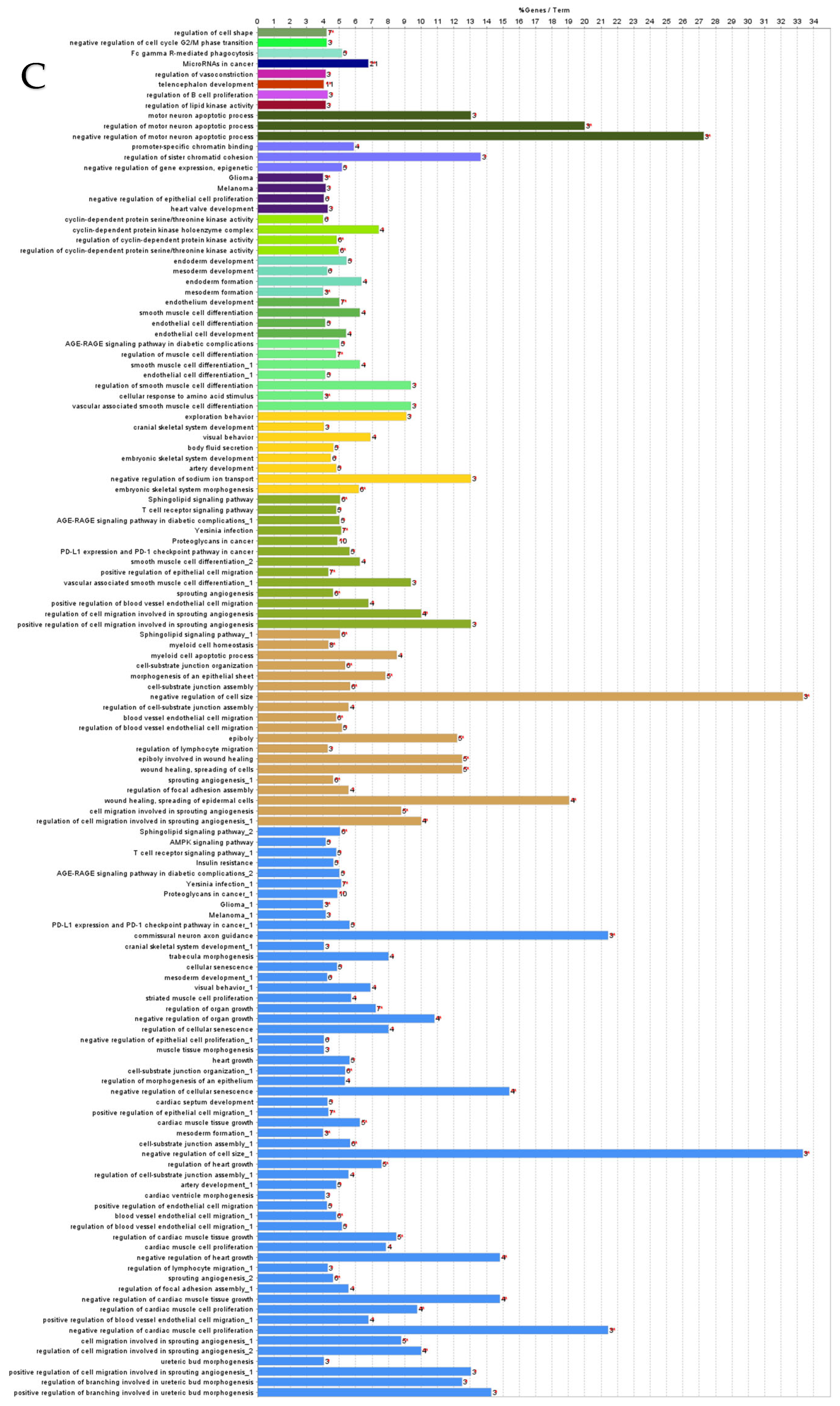
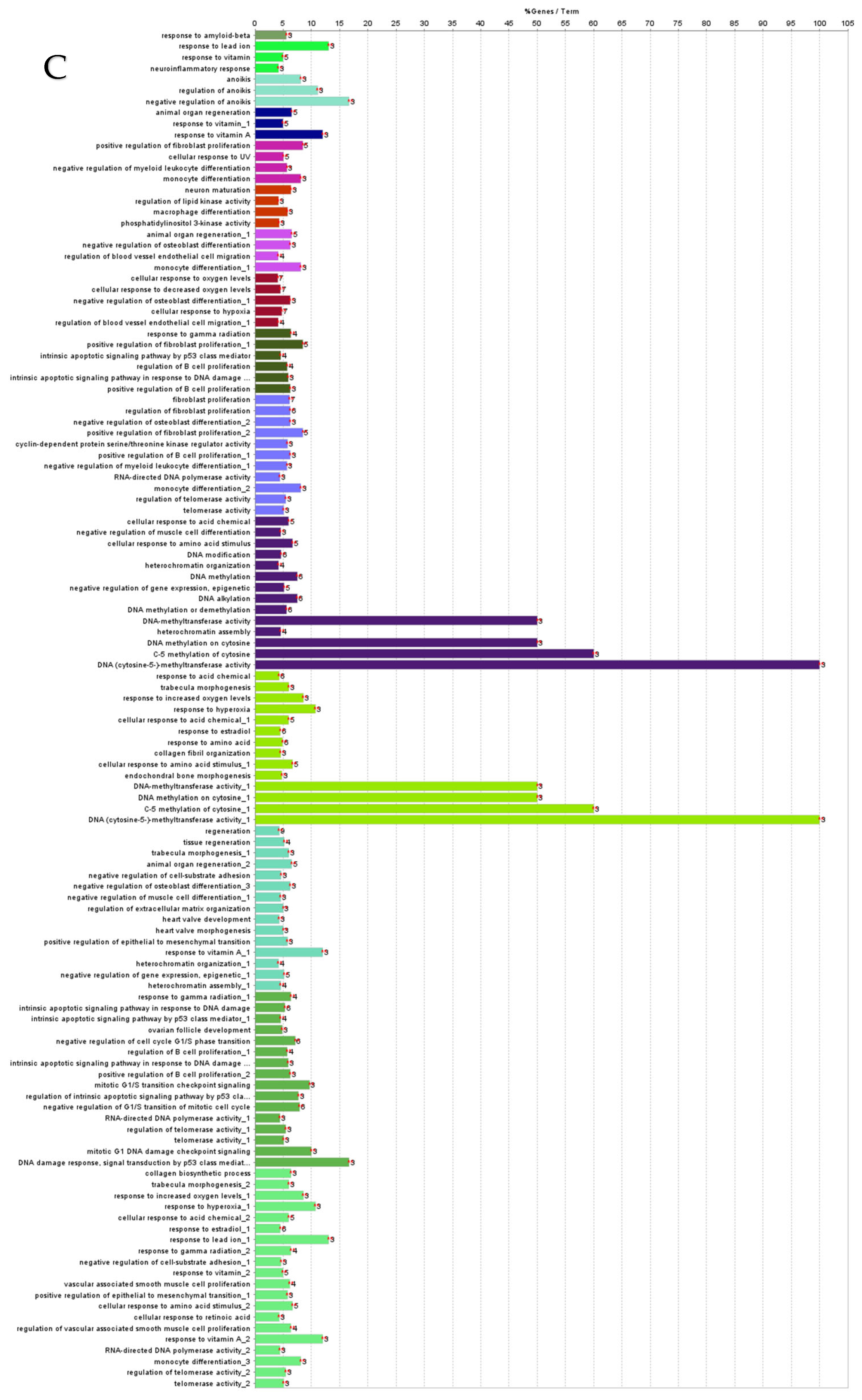
ClueGO analysis of downregulated genes in circulation in cows with metritis. (A) Functionally grouped network with terms as nodes linked based on their kappa score level (≥0.4), where only the label of the most significant term per group is shown. The node size represents the term enrichment significance. The color gradient shows the gene proportion of each cluster associated with the term. (B) Overview chart with functional groups including specific terms for downregulated genes. The percentage of genes per term is shown as term label. ** p < 0.001, * p < 0.01. (C) GO/pathway terms specific for downregulated genes. The percentage of genes per term is shown as bar label.
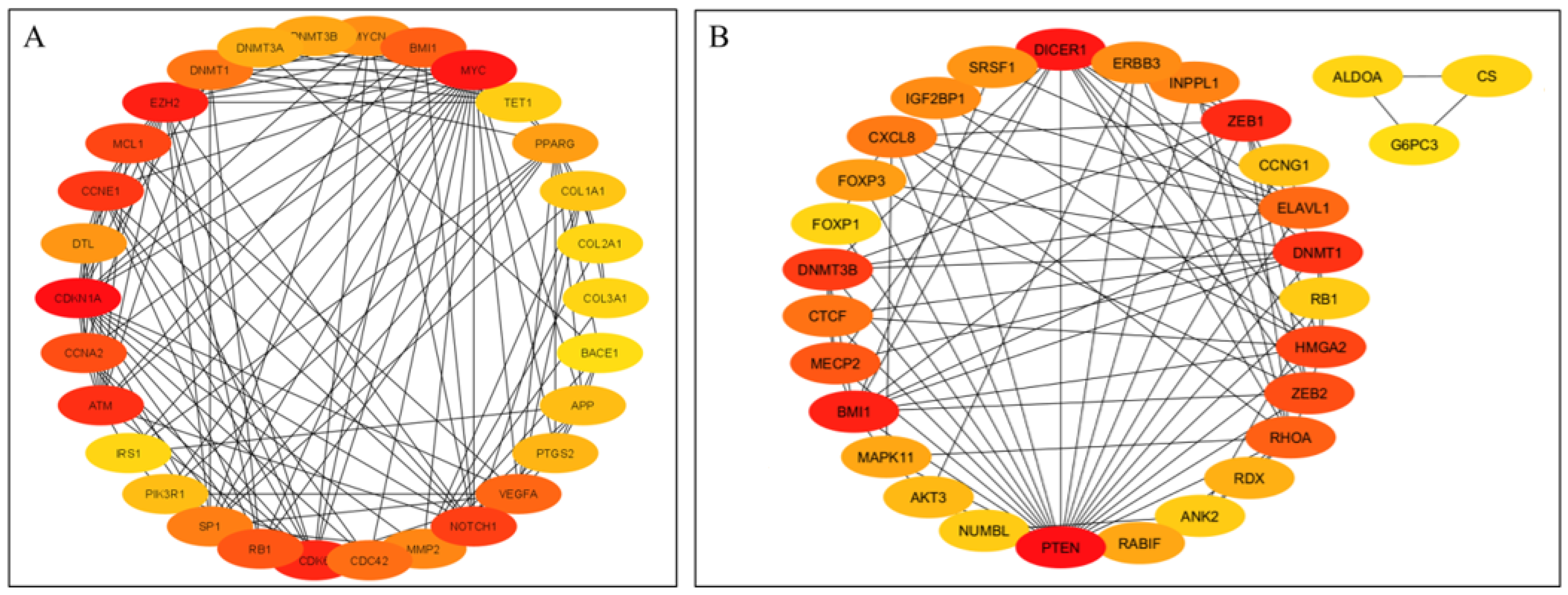
The top 30 hub genes identified using Maximal Clique Centrality (MCC) method for upregulated and downregulated DE-miRNAs are presented in Figure 5A and Figure 5B, respectively. Functional annotation and nested network analysis of these hub genes via ClueGO are shown in Figure 6 and Figure 7. Table 1A,B list the hub genes, their roles, and human tissue expression PPI (up to six closely related genes) for up- and downregulated miRNAs. Table 2 details interferon-stimulated genes targeted by DE-miRNAs in cows with metritis.
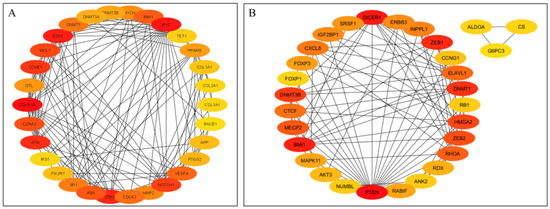
Figure 5.
Protein–protein interaction (PPI) network of hub genes of DE-miRNAs. (A) PPI network of top genes for highly upregulated DE-miRNAs. (B) PPI network of the top genes for downregulated DE-miRNAs. DE-miRNAs differentially expressed microRNAs; black lines indicate interactions between genes. The PPIs among hub genes for upregulated DE-miRNAs were greater compared with hub genes for downregulated DE-miRNAs. A complete list of hub genes are provided in Table 1.
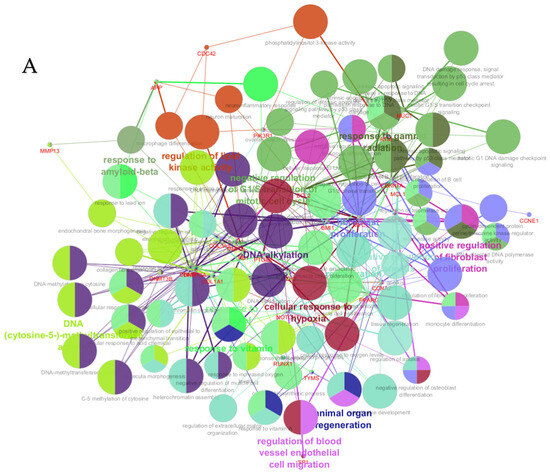
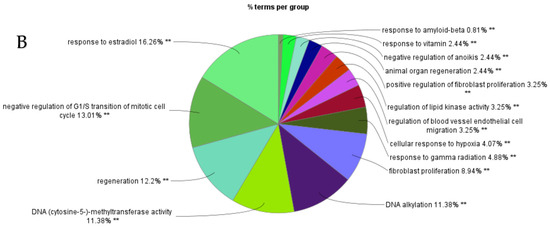
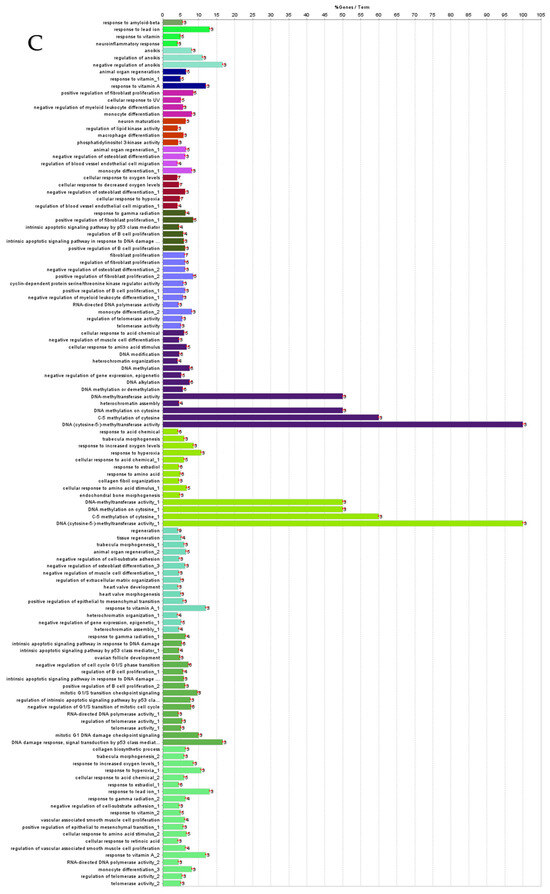
Figure 6.
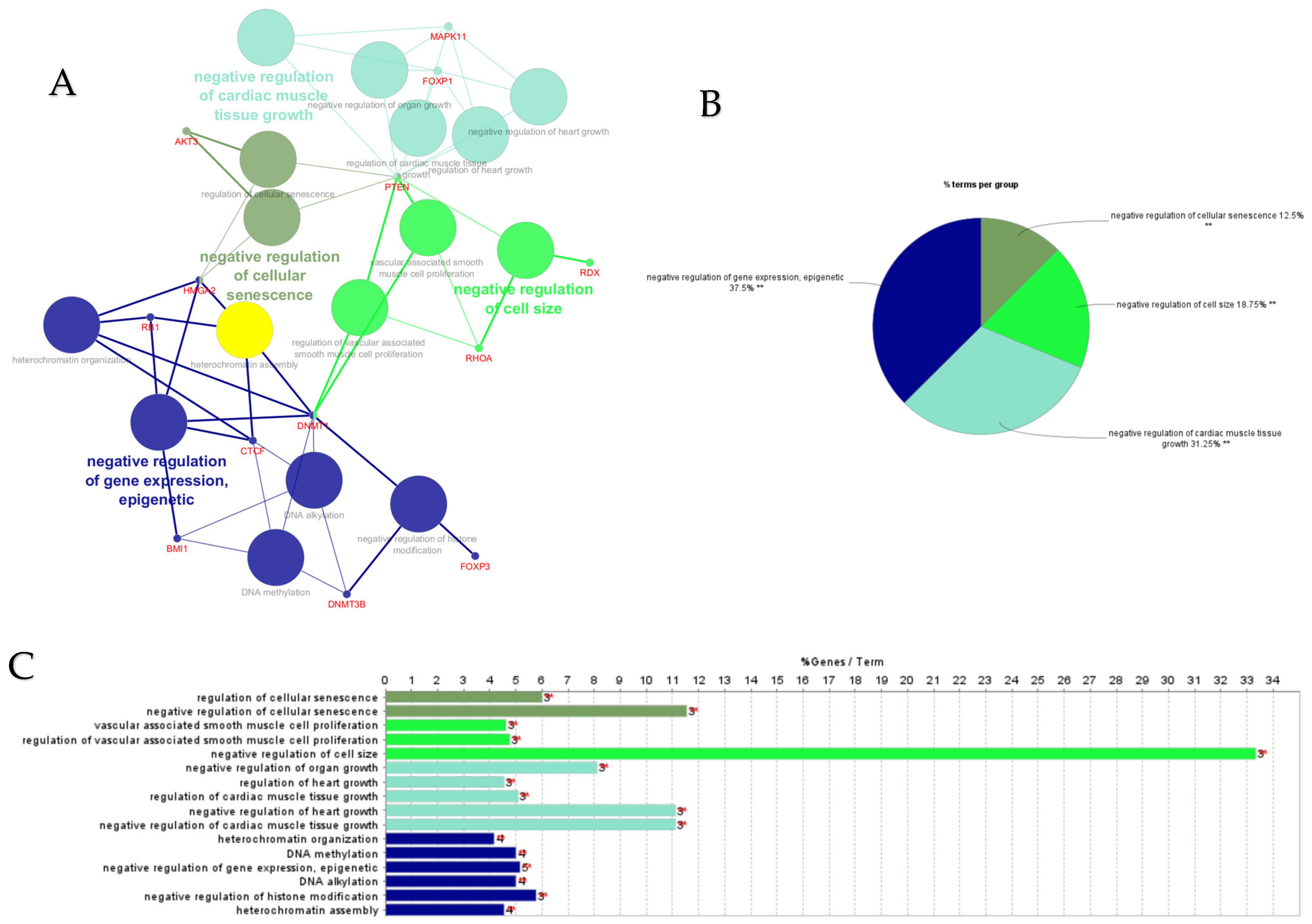
ClueGO analysis of upregulated hub genes in circulation in cows with metritis. (A) GO/pathway terms specific for upregulated hub genes. The bars represent the number of genes associated with the terms. The number of genes per term is shown as bar label. (B) Functionally grouped network with terms as nodes linked based on their kappa score level (≥0.4), where only the label of the most significant term per group is shown. The node size represents the term enrichment significance. The color gradient shows the gene proportion of each cluster associated with the term. (C) Overview chart with functional groups including specific terms for upregulated genes. The percentage of genes per term is shown as term label. ** p < 0.001.
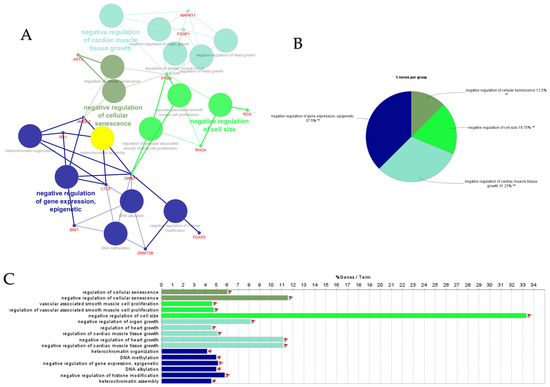
Figure 7.
ClueGO analysis of downregulated hub genes in circulation in cows with metritis. (A) Functionally grouped network with terms as nodes linked based on their kappa score level (≥0.4), where only the label of the most significant term per group is shown. The node size represents the term enrichment significance. Functionally related groups partially overlap. The color gradient shows the gene proportion of each cluster associated with the term. (B) Overview chart with functional groups including specific terms for downregulated genes. The percentage of genes per term is shown as term label. ** p < 0.001. (C) GO/pathway terms specific for downregulated hub genes. The bars represent the number of genes associated with the terms. The number of genes per term is shown as bar label.

Table 1.
(A) Upregulated top 30 hub genes and their roles, human tissue expression, and protein–protein interactions (up to 6 closely related genes). (B) Downregulated top 30 hub genes and their roles, human tissue expressions, and protein–protein interactions (up to 6 closely related genes).

Table 2.
Interferon-stimulated genes and associated up- and downregulated miRNAs.
3.2. Interaction Network Among miRNA, circRNA, lncRNA, snRNA, and mRNA
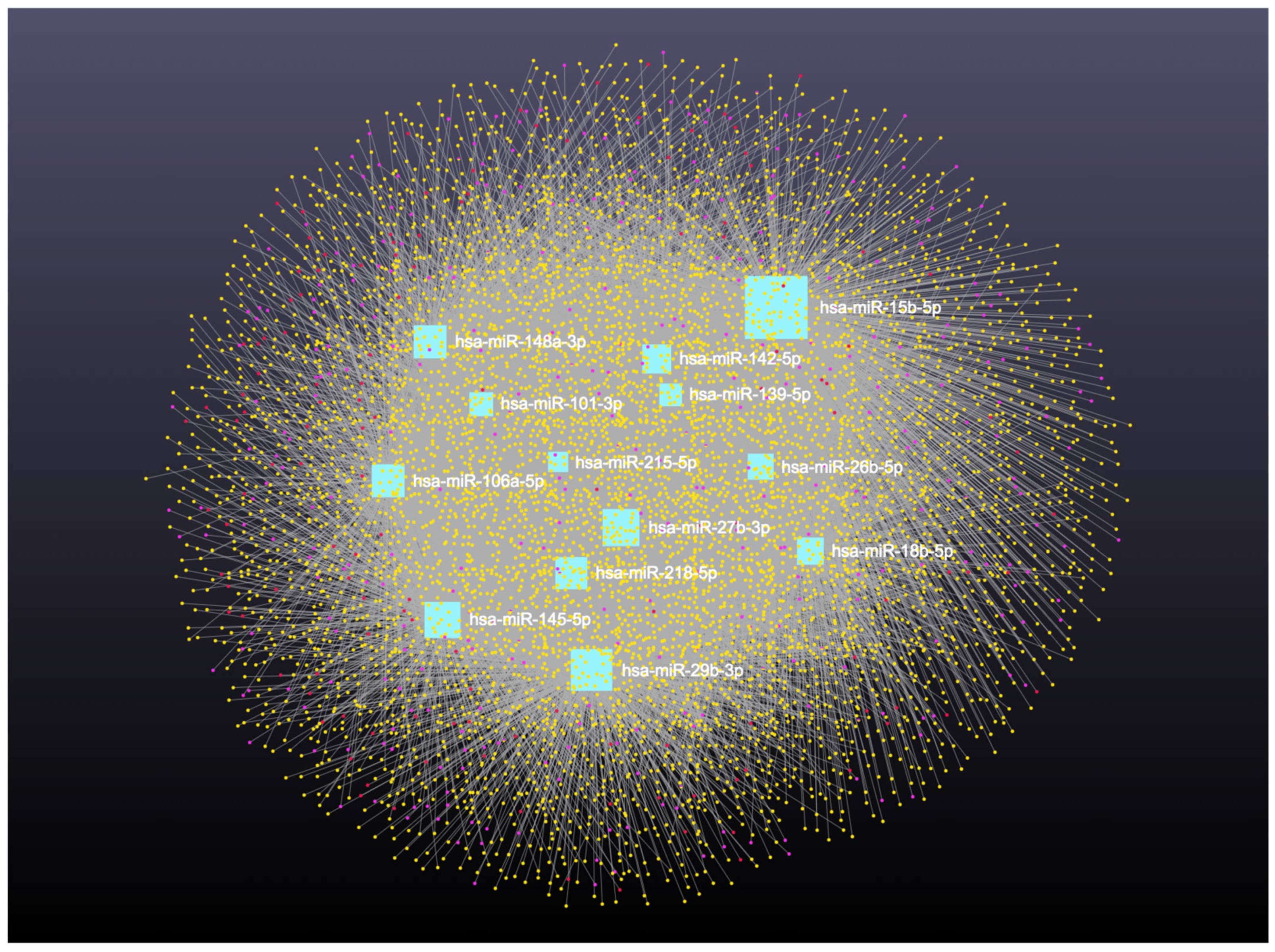
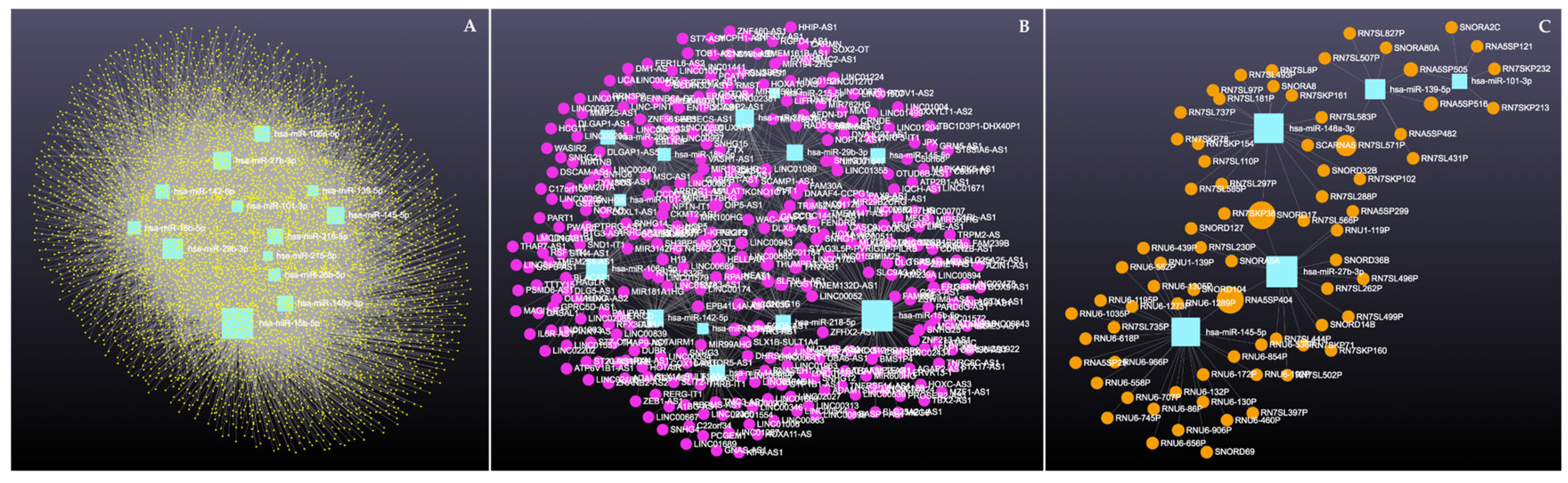
Network analysis of the 16 upregulated miRNAs, including circRNA, lncRNA, snRNA, and mRNA interactions, revealed connections with 6490 unique circRNAs, 135 snRNAs, 357 lncRNAs, and 2169 genes (Supplementary File S5). The full interaction network is illustrated in Figure 8. In addition, specific miRNA to RNA interactions are detailed in Figure 9A (miRNA to circRNA), Figure 9B (miRNA to lncRNA), and Figure 9C (miRNA to snRNA).
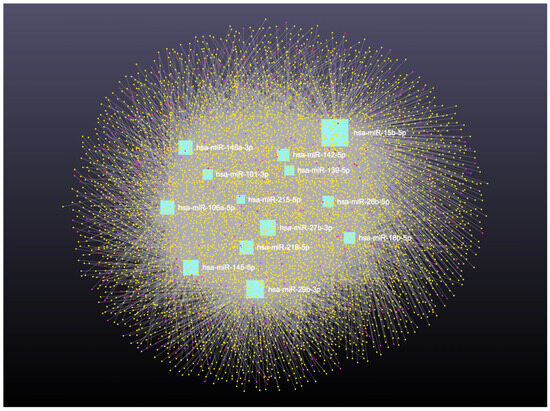
Figure 8.
Upregulated circulating miRNAs in Holstein dairy cows with metritis. Network of interactions among upregulated miRNAs and their interacting circRNAs, lncRNAs, and snRNAs. Blue squares represent upregulated miRNAs, yellow circles represent circRNAs, purple circles represent lncRNAs, and orange circles represent scRNAs. A complete list of downregulated miRNAs, circRNAs, lncRNAs, and snRNAs, interactions are provided in Supplementary File S5.

Figure 9.
Circulating upregulated miRNAs in Holstein dairy cows with metritis. (A) Interaction network of upregulated miRNAs and circRNAs. Blue squares represent miRNAs and yellow circles represent circRNAs. (B) Interaction network of upregulated miRNAs and lncRNAs. Blue squares represent miRNAs, and purple circles represent lncRNAs. (C) Interaction network of upregulated miRNAs and snRNAs. Blue squares represent miRNAs and orange circles represent snRNAs. A complete list of upregulated miRNAs, circRNAs, lncRNAs, snRNAs, and mRNAs interactions are provided in Supplementary File S5.
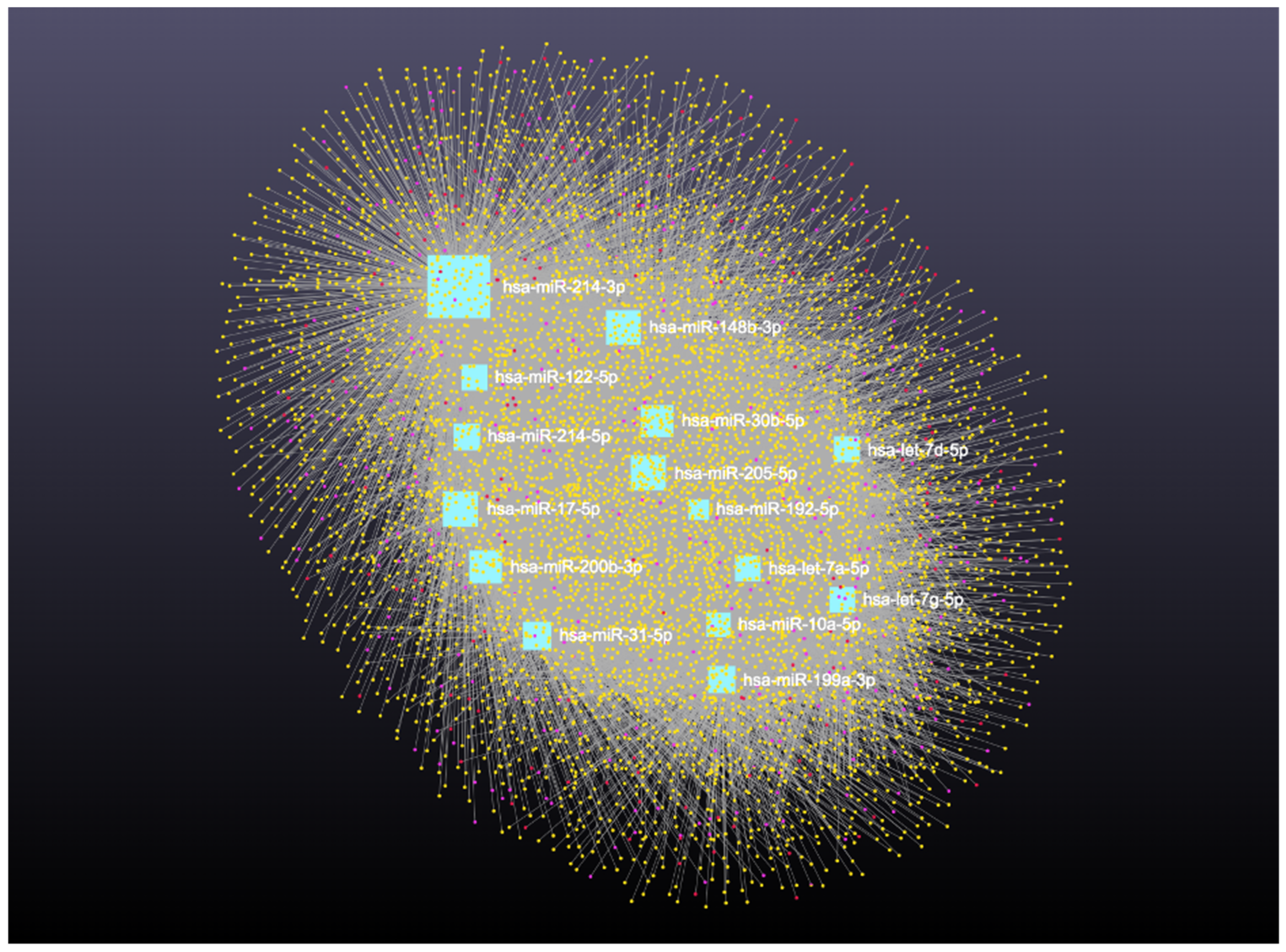
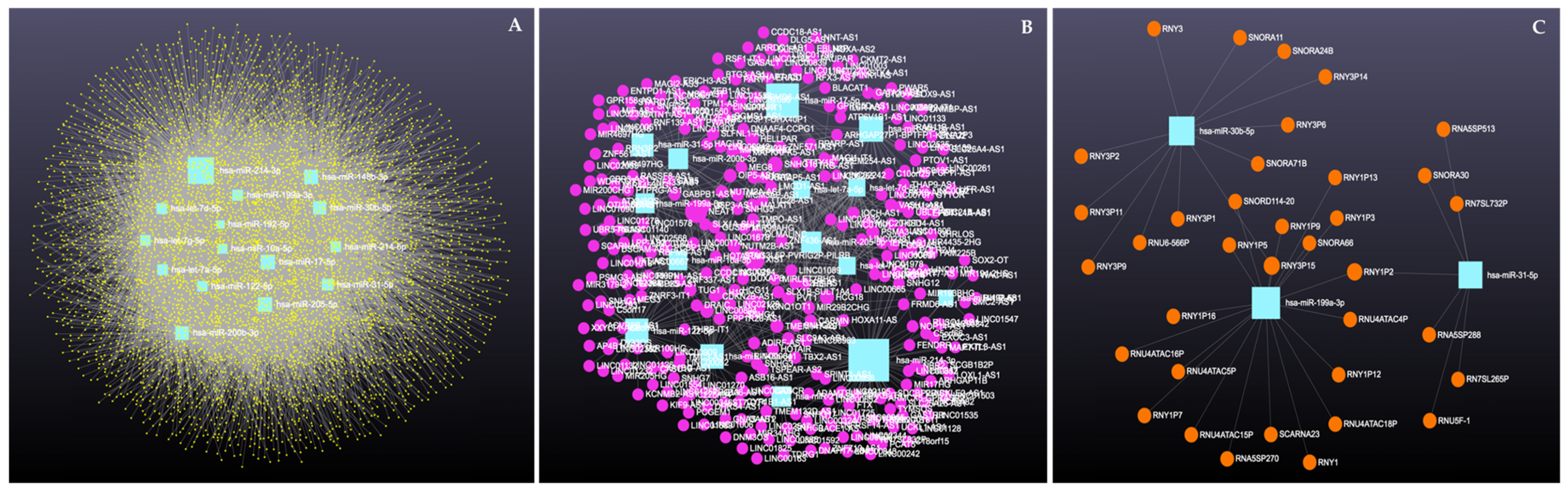
Similarly, analysis of the 14 downregulated miRNAs revealed interactions with 6313 unique circRNAs, 144 snRNAs, 337 lncRNAs, and 3401 genes (Supplementary File S6). The network is shown in Figure 10. In addition, miRNA to circRNA, miRNA to lncRNA, and miRNA to snRNA interactions are shown in Figure 11A, Figure 11B, and Figure 11C, respectively.
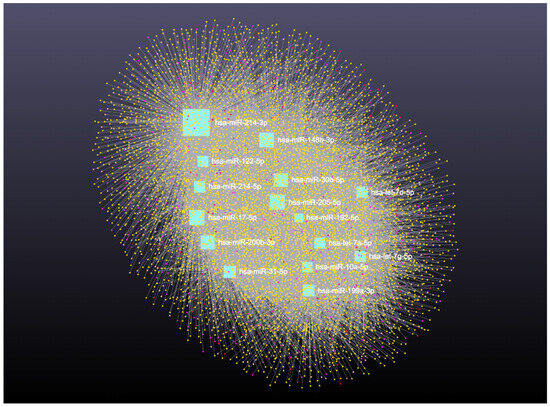
Figure 10.
Downregulated circulating miRNAs in Holstein dairy cows with metritis. Network of interactions among downregulated miRNAs and their interacting circRNAs, lncRNAs, and snRNAs. Blue squares represent upregulated miRNAs, yellow circles represent circRNAs, purple circles represent lncRNAs, and orange circles represent snRNAs. A complete list of downregulated miRNAs, circRNAs, lncRNAs, and snRNAs, interactions are provided in Supplementary File S6.

Figure 11.
Circulating downregulated miRNAs in Holstein dairy cows with metritis. (A) Interaction network of downregulated miRNAs and circRNAs. Blue squares represent miRNAs and yellow circles represent circRNAs. (B) Interaction network of downregulated miRNAs and lncRNAs. Blue squares represent miRNAs, and purple circles represent lncRNAs. (C) Interaction network of downregulated miRNAs and snRNAs. Blue squares represent miRNAs and orange circles represent snRNAs. A complete list of downregulated miRNAs, circRNAs, lncRNAs, and snRNAs, interactions are provided in Supplementary File S6.
Table 3 summarizes the top five coding and noncoding RNAs, ranked by high degree and betweenness centrality, targeted by the DE-miRNAs along with their endometrial expressions and potential functional roles.

Table 3.
Top 5 coding and noncoding RNAs with high degree and betweenness centrality targeted by up- and downregulated miRNAs, their endometrial expressions, and potential roles.
4. Discussion
Recent advances in high-throughput techniques have enabled the representation of experimental data as networks, where nodes (proteins, transcripts, or metabolites) are connected by edges to illustrate interactions among them. Network analysis helps to elucidate the role of individual proteins and their interactions, the protein–protein interactions. Centrality, a network ranking of biological components, has been widely used to identify influential nodes in complex biological networks [26]. These observed nodes with higher degrees (i.e., more connections) are more likely to represent essential proteins that significantly influence the biological process.
Using these network-based methods, key biological mechanisms contributing to metritis and reduced reproductive performance in dairy cows were identified. In general, abnormal metabolic profile during the periparturient period interferes with immune function and predisposes the cow to postpartum uterine disease and subsequent infertility. Analysis of upregulated miRNAs and their associated genes in cows with metritis revealed a wide range of predicted biological processes both positively and negatively. Key predicted processes included regulation of cellular activities (metabolic process, biosynthetic process, protein modification, multicellular organismal), cell death, proliferation, stress responses, mitosis, cell cycle regulation, cell cycle arrest, adhesion, communication, endogenous stimuli, differentiation, maturation, motility, angiogenesis, receptor signaling, intracellular signal transduction, cellular component organization, development, and response to hypoxia, lipids, vitamins and ions, and hormone stimuli. In addition, processes such as immune response-regulating receptor signaling and regulation of T cells and leukocytes were also implicated. These biological processes are critical for timely uterine involution and any disruptions in these biological processes may result in delayed and impaired uterine recovery after calving.
Interestingly, differentially upregulated miRNAs were associated with the cellular response to vitamins A (GO:0033189 and GO:0071300) and E (GO:0071306). In cows with metritis, higher lipid peroxidation and lower plasma concentrations of vitamin A and vitamin E were observed during the first 3 weeks postpartum compared to healthy cows [27]. Beta-carotene, a precursor to vitamin A, is essential for cellular health. Improved reproductive efficiency has been associated with increased postpartum β-carotene concentration in plasma [28]. Although dietary β-carotene supplementation during the dry period did not affect ovarian activity, progesterone production, or the diameters of the cervix and uterine horns [29], its association with hydroxyproline, a key component in uterine tissue repair, suggests that it may help reduce uterine inflammation and accelerate uterine involution after calving.
In contrast, prepartum supplementation with Selenium (Se), alpha-tocopherol, or both, did not improve postpartum ovarian activity, uterine involution, or reduce clinical abnormalities [30]. However, vitamin E and Se supplementation decreased the incidence of metritis, reduced the number of services per conception, and shortened days open, though they had no effect on retained fetal membranes [31]. Notably, prepartum Se treatment, administered 3 weeks before calving, significantly hastened uterine involution in cows diagnosed with metritis [32]. Collectively, vitamins A and E appear to reduce the incidence of uterine diseases, promote uterine recovery, and support earlier resumption of ovarian cyclicity in postpartum dairy cows.
Additional biological processes predicted from DE-miRNAs included cellular response to lipids (GO:0033993), regulation of ovarian follicle development (GO:0001541) embryo development (GO:0009790, GO:0043009), and embryo implantation (GO:0007566). The positive effects of fatty acid supplementation around calving on reproductive performance in dairy cows have been well documented [33,34].
For example, dairy cows supplemented with calcium salts of safflower oil (SO) from 30 days prepartum to 35 days postpartum exhibited improved innate immunity. Neutrophil functions, phagocytosis, and oxidative burst at 4 days postpartum (dpp), and oxidative burst at 7 dpp, were significantly higher in SO-fed cows compared to those fed palm oil (PO). Neutrophil surface expression of L-selectin and cytokine production (TNF-α, IL-1β) at 35 dpp were also increased in SO-fed cows [34]. These immunomodulatory effects may enhance the cow’s ability to combat bacterial challenges during the postpartum period.
Furthermore, supplementation with unsaturated fatty acids (UFAs) from flaxseed reduced pregnancy losses [35]. Diets enriched with UFAs from flaxseed or sunflower sources promoted embryo development [36], while linolenic acid supplementation led to the development of significantly larger ovarian follicles [35]. Additionally, long-chain fatty acid supplementation postpartum was shown to hasten the resumption of ovarian cyclicity and reduce the incidence of cystic ovarian degeneration [37]. These findings indicate that lipid supplementation not only supports immune function but also improves uterine health, follicular development, and embryo viability.
KEGG pathway analysis of genes associated with upregulated miRNAs revealed enrichment in several key signaling pathways, including PI3K-Akt, relaxin, p53, AMPK/MAPK, ErbB, JAK-STAT, IGF receptor, T cell receptor, HIF-1, TNF, FoxO, estrogen, GnRH, NF-κB, C-type lectin receptor, and Ras pathways. These networks involve genes and their products that mediate cellular responses to intrinsic and extrinsic stimuli. They regulate essential processes such as DNA replication, chromosome segregation, cell division, metabolism, proliferation, survival, growth, and angiogenesis, processes that are critically involved in postpartum uterine recovery and reproductive function.
The PI3K-Akt signaling pathway is a key intracellular signal transduction pathway that regulates metabolism, cell proliferation, survival, growth, and angiogenesis in response to extracellular stimuli. Dysregulation of this pathway has been implicated in endometriosis in women, where elevated levels of PI3K and phosphorylated AKT (Ser473) contribute to abnormal cell proliferation [38,39,40]. It should be noted that hormones could play an essential role in these regulatory pathways; for instance, estradiol (E2) promotes endometriotic cell proliferation through the reduced expression of PTEN and the subsequent activation of AKT [41].
In cows, pathogenic Escherichia coli lipopolysaccharide induces endometrial inflammation via the Toll-Like Receptor (TLR)4-IRAK-TRAF4-NF-κB signaling axis. Activation of interleukin-1 receptor-associated kinase (IRAK) and TNF receptor-associated factor 4 (TRAF4) by LPS leads to nuclear translocation of NF-κB, triggering inflammation and apoptosis in bovine endometrial cells [42].
Analysis of upregulated miRNAs and their associated genes in metritis cows also predicted enrichment of insulin receptor binding (GO:0005158), insulin resistance (KEGG pathway), and insulin-like growth factor receptor (IGFR) signaling (GO:0048009). Insulin plays a critical role in homeorhesis during the transition period. Both insulin secretion and tissue responsiveness to insulin are altered postpartum. A disrupted metabolic profile—specifically impaired PMN function—has been linked to increased susceptibility to postpartum uterine diseases and infertility [43]. Cows that developed uterine disease had lower circulating glucose and reduced glycogen concentrations in their PMNs [41]. Reduced glycogen storage likely impairs the oxidative burst capacity of PMNs, weakening immune defense mechanisms and predisposing cows to uterine infections [44].
Key miRNAs and Their Functional Roles
The top five highly upregulated miRNAs in cows with metritis were bta-miR-15b, bta-miR-17-3p, bta-miR-16b, bta-miR-148a, and bta-miR-26b. MicroRNAs are involved in the regulation of several biological functions and pathways. The proposed functions of miR-15b include hypoxia, angiogenesis, and apoptosis; miR-16 is associated with hypoxia, angiogenesis, inflammation, cell growth, and apoptosis; miR-26 is involved in cell proliferation, myogenesis, cell cycle progression, BMP signaling, and cell differentiation; miR-142 is linked to immune suppression. Increased expression of miR-15b reduced activities of anti-apoptotic protein BCL2 [45], whereas miR-145 inhibits endometriotic cell proliferation, invasiveness, and stemness through regulation of cytoskeletal elements, cell adhesion molecules, and proteolytic factors [46]. Notably, bta-miR-26b, derived from intrauterine extracellular vesicles, contributes to suppression of maternal neutrophil-mediated immunity, thereby possibly promoting uterine inflammation [47]. Despite the generally pro-inflammatory profile of the upregulated miRNAs, miR-148a appears to have a compensatory anti-inflammatory role, countering uterine inflammation through the TLR4–NF-κB axis [48].
In contrast, the top five downregulated miRNAs were bta-miR-148b-5p, bta-miR-199a-3p, bta-miR-122-5p, bta-miR-200b-3p, and bta-miR-10a-5p in cows with metritis. miR-148b target genes TNFRSF10B are involved in regulation of T cell function and apoptosis and enhanced bactericidal activity [49]. MicroRNA-199a-3p suppresses high glucose-induced inflammation by regulating the IKKβ/NF-κB signaling pathway in epithelial cells [50]. Further, miR-199a-3p is significantly lower in women with endometriosis compared to normal women [51]. MicroRNA-200 family regulates cellular proliferation and migration in the endometrium, and downregulation may interfere with regulation of the postpartum immune response in women [52,53]. The downregulation of these miRNAs likely impairs immune function, cellular repair, and inflammatory resolution, potentially contributing to persistent uterine inflammation and delayed involution.
GO/pathway terms specific for upregulated hub genes included regulation of transcription involved in G1/S transition of mitotic cell cycle to prevent mitotic catastrophe, beta-catenin binding, phosphatidylinositol phosphate kinase activity, filopodium, and negative regulation of vasculature development and poly-purine tract binding. Regulatory mechanisms including crosstalk between TLR and Wnt/β-catenin signaling pathways may elicit both pro- and anti-inflammatory functions that show involvement in both normal and pathological conditions [54]. Phosphatidylinositol phosphate kinase is essential for the activation of the signaling pathways regulating cytokine production, cell cycle progression, survival, T cell metabolism, and focal adhesions [55,56]. Filopodia have roles in sensing, migration, and cell–cell interaction. In macrophages, filopodia act as phagocytic tentacles and pull bound objects towards the cell for phagocytosis [57]. Both innate and adaptive immune cells are involved in the mechanisms of endothelial cell proliferation, migration, and activation. Anti-angiogenic cytokines such as IFN-gamma and IL-12 [58] may be involved in negative regulation of vasculature development and tissue repair in the uterus of postpartum cows.
GO/pathway terms specific for downregulated hub genes were angiogenesis, endoderm formation, morphogenesis, regulation of cell differentiation, macrophage differentiation, and regulation of cyclin-dependent protein serine/threonine kinase activity, essential to drive cell cycle progression and transition into different phases [59]. Chemokine family CXL ligand (CXCL) mRNAs are upregulated in dairy cows with endometritis [9,10]. Further, upregulated expression of bta-miR-101 may be associated with TLR2. The overexpression of TLR2 is associated with the activation of the NF-κB-mitogen-activated protein kinase B (MAP3KB) complex and consequent upregulation of pro-inflammatory cytokines and chemokines [60]. This overexpression of these inflammatory cytokines and chemokines can induce severe endometrial tissue damage postpartum and adversely prolong uterine involution and negatively impact future pregnancy outcomes. We observed that interferon-stimulated genes were downregulated in cows with subclinical endometritis, which may adversely affect embryo elongation [9,10]. Differentially expressed (DE) miRNAs and their associated ISG targets are listed in Table 2.
Insights from cancer biology have expanded our understanding of the role of noncoding RNAs in uterine diseases of dairy cows. Among upregulated lncRNAs, KCNQ1OT1 and NEAT1 are of particular interest. KCNQ1OT1 is involved in epigenetic regulation and may influence immune-related gene expression [61,62]. NEAT1 is essential for paraspeckle formation and amplifies inflammatory signaling [63]. Its overexpression in uterine tissue has been linked to chronic inflammation and impaired healing. The expression of TUG1 was significantly decreased in HUVECS following lipopolysaccharide treatment in a time-dependent manner [64]. Other lncRNAs such as XIST, HELLPAR, and TUG1 are also linked to immune modulation and epithelial cell function; their dysregulation may contribute to uterine dysfunction [65,66,67,68,69].
Upregulated circRNAs such as RANBP2, RGPD4, and NBPF10 may influence intracellular transport and immune signaling, potentially exacerbating inflammation [70,71,72,73,74]. Meanwhile, sncRNAs including SNORD17 regulate RNA modification and protein synthesis, with elevated levels indicating cellular stress responses [75,76].
Conversely, the downregulation of lncRNAs like SNHG16, HCG18, and NEAT1 may impair immune responses and placental development [77,78,79,80]. Similarly, decreased expression of circRNAs (KPNA6, MACF1) and sncRNAs (SNORA66) may disrupt in cytoskeletal organization and RNA processing [81,82,83].
It should be noted that some lncRNAs such as KCNQ1OT1, NEAT1, and XIST appear to have dual roles. KCNQ1OT1 affects gene expression and several cellular functions including cell proliferation, migration, epithelial–mesenchymal transition (EMT), apoptosis, viability, autophagy, and inflammation [84]. NEAT1 functions as an miRNA sponge, regulating gene expression involved in cell growth, invasion, EMT, stemness, and resistance to chemotherapy and radiotherapy [85]. •XIST, while originally known for X-chromosome inactivation, also influences immune gene expression and participates in multiple signaling pathways, including TGF-β, PI3K/AKT, Wnt/β-catenin, FOXO, NF-κB, mTOR, MAPK, Toll-like receptor, JAK-STAT, and T/B cell receptor pathways [86,87]. Upregulation of XIST may lead to aberrant gene silencing and immune dysregulation, whereas its downregulation may impair chromosomal regulation and immune homeostasis, worsening uterine dysfunction.
In our network analysis, KCNQ1OT1 exhibited both upregulation and downregulation in different contexts, suggesting its role in immune homeostasis. When upregulated, it may suppress immune-related gene expression and prolong inflammation. When downregulated, it may interfere with genomic imprinting and immune regulation, hindering uterine recovery and increasing infection susceptibility. NEAT1, essential for paraspeckle formation, modulates inflammatory gene expression. Its upregulation may enhance innate immune responses but also cause chronic inflammation and tissue damage. Conversely, NEAT1 downregulation may impair protective immunity and delay healing—both conditions negatively affecting uterine health and fertility. XIST is key to X-chromosome inactivation but also regulates immune gene expression. Its upregulation may cause abnormal gene silencing and immune imbalance, while downregulation can impair chromosomal function and immune regulation, further aggravating uterine disorders.
We observed that the regulatory roles of noncoding RNAs vary widely across biological processes. However, these RNAs often exert their effects by interacting with miRNAs to modulate downstream gene expression. Although the role of noncoding RNAs in many signaling pathways remains underexplored, their functional significance is becoming increasingly evident. Continued research into their mechanisms will likely uncover new insights and therapeutic targets in uterine disease.
5. Conclusions
This study used high-throughput miRNA profiling and integrative network analyses to explore molecular mechanisms underlying postpartum uterine disease, particularly metritis in dairy cows. Differentially expressed miRNAs and their target genes were implicated in key biological processes and signaling pathways involved in immune regulation, inflammation, metabolism, cellular homeostasis, and reproduction.
Protein–protein interaction and ClueGO analyses linked these miRNAs to numerous enriched GO terms and KEGG pathways, highlighting roles in cellular stress, angiogenesis, immune signaling, and hormonal regulation—critical for uterine involution and reproductive recovery. Centrality analyses identified hub genes associated with inflammation resolution and tissue remodeling.
Interaction networks involving miRNAs and noncoding RNAs (circRNAs, lncRNAs, snRNAs) revealed complex regulatory mechanisms. Notably, NEAT1, XIST, and KCNQ1OT1 exhibited dual roles in modulating inflammatory responses, potentially influencing disease progression or recovery.
The study also identified miRNA-regulated pathways tied to vitamins A and E, lipid metabolism, insulin resistance, and immune function, factors linked to postpartum health. These findings suggest that metritis involves widespread dysregulation of immune and metabolic pathways, possibly worsened by nutritional deficiencies.
In summary, this integrative transcriptomic analysis provides a detailed molecular view of postpartum uterine health, highlighting miRNA, and noncoding RNA networks plays a role in disease development and recovery, offering promising biomarkers and therapeutic targets for improving fertility in dairy cows.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cimb47080643/s1.
Author Contributions
Conceptualization, R.K. and V.K.; methodology, R.K. and V.K.; software, R.K., V.K. and J.F.; validation, R.K., V.K. and J.F.; formal analysis, R.K., V.K. and J.F.; investigation, R.K. and V.K.; resources, R.K. and V.K.; data curation, R.K., V.K. and J.F.; writing—original draft preparation, R.K.; writing—review and editing, R.K.,V.K. and J.F.; visualization, R.K., V.K. and J.F.; supervision, R.K. and V.K.; project administration, R.K. and V.K. All authors have read and agreed to the published version of the manuscript.
Funding
Joao Ferriera was supported by the São Paulo Research Foundation (CAPES #001), FAPESP, Brazil and FMVZ-UNESP, Botucatu, Brazil.
Institutional Review Board Statement
Not applicable, as this study utilized data from a previously published study (doi: 10.1038/srep29509) for further bioinformatic analysis. Our previous study was exempt from IACUC review per WSU IACUC Tissue Use Policy #21 as it utilized blood samples collected and archived from another approved study (WSU ASAF 4017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article or Supplementary Materials herein.
Acknowledgments
The authors thank the College of Veterinary medicine, Washington State University for the support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bondurant, R.H. Inflammation in the bovine female reproductive tract. J. Anim. Sci. 1999, 77 (Suppl. S2), 101–110. [Google Scholar] [CrossRef]
- Bartlett, P.C.; Kirk, J.H.; Wilke, M.A.; Kaneene, J.B.; Mather, E.C. Metritis complex in Michigan Holstein-Friesian cattle: Incidence, descriptive epidemiology and estimated economic impact. Prev. Vet. Med. 1986, 4, 235–248. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 2004, 62, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.S.; Celestino, M.L.; Oliveira, E.B.; Lima, F.S.; Ballou, M.A.; Galvão, K.N. The association of cow-related factors assessed at metritis diagnosis with metritis cure risk, reproductive performance, milk yield, and culling for untreated and ceftiofur-treated dairy cows. J. Dairy Sci. 2020, 103, 9261–9276. [Google Scholar] [CrossRef] [PubMed]
- Piccardi, M.; Romero, G.; Veneranda, G.; Castello, E.; Romero, D.; Balzarini, M.; Bó, G.A. Effect of puerperal metritis on reproductive and productive performance in dairy cows in Argentina. Theriogenology 2016, 85, 887–893, Erratum in Theriogenology 2020, 148, 249. [Google Scholar] [CrossRef]
- Credille, B.C.; Woolums, A.R.; Overton, M.W.; Hurley, D.J.; Giguère, S. Expression of inflammation-associated genes in circulating leukocytes and activity of indoleamine-2,3-dioxygenase in dairy cattle with acute puerperal metritis and bacteremia. Res. Vet. Sci. 2015, 101, 6–10. [Google Scholar] [CrossRef]
- Magata, F.; Kitaoka, R.; Morino, I.; Teramura, M.; Kawashima, C.; Haneda, S.; Shimizu, T. Long-term impact of puerperal metritis on the profiles of peripheral blood leukocytes in peripartum dairy cows. Anim. Sci. 2016, 87, 151–155. [Google Scholar] [CrossRef]
- Kasimanickam, R.K.; Kasimanickam, V.R. IFNT, ISGs, PPARs, RXRs and MUC1 in day 16 embryo and endometrium of repeat-breeder cows, with or without subclinical endometritis. Theriogenology 2020, 158, 39–49. [Google Scholar] [CrossRef]
- Kasimanickam, R.K.; Kasimanickam, V.R. mRNA expressions of candidate genes in gestational day 16 conceptus and corresponding endometrium in repeat breeder dairy cows with suboptimal uterine environment following transfer of different quality day 7 embryos. Animals 2021, 11, 1092. [Google Scholar] [CrossRef]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Kumar, N.; Reisenauer, C. Day 7 embryo quality and suboptimal uterine environment influence morphometry of Day 16 conceptus in dairy cows. Theriogenology 2021, 163, 10–17. [Google Scholar] [CrossRef]
- Chegini, N. Uterine microRNA signature and consequence of their dysregulation in uterine disorders. Anim. Reprod. 2010, 7, 117–128. [Google Scholar]
- Hailemariam, D.; Ibrahim, S.; Hoelker, M.; Drillich, M.; Heuwieser, W.; Looft, C.; Cinar, M.U.; Tholen, E.; Schellander, K.; Tesfaye, D. MicroRNA-regulated molecular mechanism underlying bovine subclinical endometritis. Reprod. Fertil. Dev. 2014, 26, 898–913. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Ibrahim, S.; Gebremedhn, S.; Tesfaye, D.; Heppelmann, M.; Bollwein, H.; Pfarrer, C.; Tholen, E.; Neuhoff, C.; Schellander, K.; et al. Clinical and subclinical endometritis induced alterations in bovine endometrial transcriptome and miRNome profile. BMC Genom. 2016, 17, 218. [Google Scholar] [CrossRef]
- Oyelami, F.O.; Usman, T.; Suravajhala, P.; Ali, N.; Do, D.N. Emerging Roles of Noncoding RNAs in Bovine Mastitis Diseases. Pathogens 2022, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Panir, K.; Schjenken, J.E.; Robertson, S.A.; Hull, M.L. Non-coding RNAs in endometriosis: A narrative review. Hum. Reprod. Update 2018, 24, 497–515. [Google Scholar] [CrossRef]
- Aljubran, F.; Nothnick, W.B. Long non-coding RNAs in endometrial physiology and pathophysiology. Mol. Cell. Endocrinol. 2021, 525, 111190. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kastelic, J. Circulating cell-free mature microRNAs and their target gene prediction in bovine metritis. Sci. Rep. 2016, 6, 29509. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Gustavsen, J.A.; Pai, S.; Isserlin, R.; Demchak, B.; Pico, A.R. RCy3: Network biology using Cytoscape from within R. F1000Res 2019, 8, 1774. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. Cytohubba: Identifying hub objects and sub-networks from complex interactome. BMC Sys. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Jeong, H.; Mason, S.P.; Barabási, A.L.; Oltvai, Z.N. Lethality and centrality in protein networks. Nature 2001, 411, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Mikulková, K.; Kadek, R.; Filípek, J.; Illek, J. Evaluation of oxidant/antioxidant status, metabolic profile and milk production in cows with metritis. Ir. Vet. J. 2020, 73, 8. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.C.; Guerreiro, B.M.; Morais Junior, N.N.; Araujo, R.L.; Pereira, R.A.; Pereira, M.N. Supplementation of prepartum dairy cows with β-carotene. J. Dairy Sci. 2015, 98, 6304–6314, Erratum in J. Dairy Sci. 2015, 98, 7419. [Google Scholar] [CrossRef] [PubMed]
- Kaewlamun, W.; Okouyi, M.; Humblot, P.; Techakumphu, M.; Ponter, A.A. Does supplementing dairy cows with β-carotene during the dry period affect postpartum ovarian activity, progesterone, and cervical and uterine involution? Theriogenology 2011, 75, 1029–1038. [Google Scholar] [CrossRef]
- Wichtel, J.J.; Craigie, A.L.; Thompson, K.G.; Williamson, N.B. Effect of selenium and a-tocopherol supplementation on postpartum reproductive function of dairy heifers at pasture. Theriogenology 1996, 46, 491–502. [Google Scholar] [CrossRef]
- Bayril, T.; Yildiz, A.S.; Akdemir, F.; Yalcin, C.; Köse, M.; Yilmaz, O. The technical and financial effects of parenteral supplementation with selenium and vitamin E during late pregnancy and the early lactation period on the productivity of dairy cattle. Asian-Australas. J. Anim. Sci. 2015, 28, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.H.; Hancock, D.D.; St Pierre, N.; Conrad, H.R.; Harvey, W.R. Effect of prepartum selenium treatment on uterine involution in the dairy cow. J. Dairy. Sci. 1986, 69, 1421–1425. [Google Scholar] [CrossRef]
- Silvestre, F.T.; Carvalho, T.S.; Francisco, N.; Santos, J.E.; Staples, C.R.; Jenkins, T.C.; Thatcher, W.W. Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: I. Uterine and metabolic responses, reproduction, and lactation. J. Dairy. Sci. 2011, 94, 189–204. [Google Scholar] [CrossRef]
- Silvestre, F.T.; Carvalho, T.S.; Crawford, P.C.; Santos, J.E.; Staples, C.R.; Jenkins, T.; Thatcher, W.W. Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: II. Neutrophil fatty acids and function, and acute phase proteins. J. Dairy. Sci. 2011, 94, 2285–2301. [Google Scholar] [CrossRef]
- Ambrose, D.J.; Kastelic, J.P.; Corbett, R.; Pitney, P.A.; Petit, H.V.; Small, J.A.; Zalkovic, P. Lower pregnancy losses in lactating dairy cows fed a diet enriched in alpha-linolenic acid. J. Dairy. Sci. 2006, 89, 3066–3074. [Google Scholar] [CrossRef]
- Thangavelu, G.; Colazo, M.G.; Ambrose, D.J.; Oba, M.; Okine, E.K.; Dyck, M.K. Diets enriched in unsaturated fatty acids enhance early embryonic development in lactating Holstein cows. Theriogenology 2007, 68, 949–957. [Google Scholar] [CrossRef]
- Colazo, M.G.; Hayirli, A.; Doepel, L.; Ambrose, D.J. Reproductive performance of dairy cows is influenced by prepartum feed restriction and dietary fatty acid source. J. Dairy. Sci. 2009, 92, 2562–2571. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Canis, M.; Vaurs-Barrière, C.; Boespflug-Tanguy, O.; Dastugue, B.; Mage, G. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertil. Steril. 2005, 84 (Suppl. S2), 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Cinar, O.; Seval, Y.; Uz, Y.H.; Cakmak, H.; Ulukus, M.; AKayisli, U.; Arici, A. Differential regulation of Akt phosphorylation in endometriosis. Reprod. Biomed. Online 2009, 19, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Barrueto, F.F.; Gogusev, J.; Im, D.D.; Morin, P.J. Serial analysis of gene expression reveals differential expression between endometriosis and normal endometrium. Possible roles for AXL and SHC1 in the pathogenesis of endometriosis. Reprod. Biol. Endocrinol. 2008, 6, 59. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Liu, S.; Li, J.; Wen, Z.; Li, M. 17betaE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFkappaB-dependent pathway. Mol. Cell Endocrinol. 2010, 317, 31–43. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Song, C.; He, F.; Yang, L.; Li, X. Enforced expression of miR-92b blunts E. coli lipopolysaccharide-mediated inflammatory injury by activating the PI3K/AKT/Œ≤-catenin pathway via targeting PTEN. Int. J. Biol. Sci. 2021, 17, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Association of oxidative status and insulin sensitivity in periparturient dairy cattle: An observational study. J. Anim. Physiol. Anim. Nutr. 2016, 100, 279–286. [Google Scholar] [CrossRef]
- Galvão, K.N.; Flaminio, M.J.; Brittin, S.B.; Sper, R.; Fraga, M.; Caixeta, L.; Ricci, A.; Guard, C.L.; Butler, W.R.; Gilbert, R.O. Association between uterine disease and indicators of neutrophil and systemic energy status in lactating Holstein cows. J. Dairy. Sci. 2010, 93, 2926–2937. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949, Erratum in Proc. Natl. Acad. Sci. USA 2006, 103, 2464. [Google Scholar] [CrossRef]
- Adammek, M.; Greve, B.; Kässens, N.; Schneider, C.; Brüggemann, K.; Schüring, A.N.; Starzinski-Powitz, A.; Kiesel, L.; Götte, M. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil. Steril. 2013, 99, 1346–1355, e5. [Google Scholar] [CrossRef]
- Nakamura, K.; Kusama, K.; Hori, M.; Imakawa, K. The effect of bta-miR-26b in intrauterine extracellular vesicles on maternal immune system during the implantation period. Biochem. Biophys. Res. Commun. 2021, 573, 100–106. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Yang, C.; Zhang, T.; Shaukat, A.; Yang, X.; Dai, A.; Wu, H.; Deng, G. miR-148a suppresses inflammation in lipopolysaccharide-induced endometritis. J. Cell Mol. Med. 2020, 24, 405–417. [Google Scholar] [CrossRef] [PubMed]
- van de Vosse, E.; Hoeve, M.A.; Ottenhoff, T.H. Human genetics of intracellular infectious diseases: Molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect. Dis. 2004, 4, 739–749. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, L.; Shi, J. MicroRNA-199a-3p suppresses high glucose-induced apoptosis and inflammation by regulating the IKKβ/NF-κB signaling pathway in renal tubular epithelial cells. Int. J. Mol. Med. 2020, 46, 2161–2171. [Google Scholar] [CrossRef]
- Papari, E.; Noruzinia, M.; Kashani, L.; Foster, W.G. Identification of candidate microRNA markers of endometriosis with the use of next-generation sequencing and quantitative real-time polymerase chain reaction. Fertil. Steril. 2020, 113, 1232–1241. [Google Scholar] [CrossRef]
- Nothnick, W.B. The role of micro-RNAs in the female reproductive tract. Reproduction 2012, 143, 559–576. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, Y.A.; Choi, J.J.; Lee, Y.Y.; Kim, C.J.; Choi, C.; Kim, T.J.; Lee, N.W.; Kim, B.G.; Bae, D.S. The expression of the miRNA-200 family in endometrial endometrioid carcinoma. Gynecol. Oncol. 2011, 120, 56–62. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Tadagavadi, R.K.; Swafford, D.; Manicassamy, S. Modulation of inflammatory responses by Wnt/β-Catenin signaling in dendritic cells: A novel immunotherapy target for autoimmunity and cancer. Front. Immunol. 2016, 7, 460. [Google Scholar] [CrossRef]
- Ling, K.; Doughman, R.L.; Firestone, A.J.; Bunce, M.W.; Anderson, R.A. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 2002, 420, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Porciello, N.; Kunkl, M.; Viola, A.; Tuosto, L. Phosphatidylinositol 4-phosphate 5-kinases in the regulation of T cell activation. Front. Immunol. 2016, 7, 186. [Google Scholar] [CrossRef]
- Lillico, D.; Pemberton, J.G.; Stafford, J.L. Selective regulation of cytoskeletal dynamics and filopodia formation by teleost leukocyte immune-type receptors differentially contributes to target capture during the phagocytic process. Front. Immunol. 2018, 9, 1144. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Immune cells and angiogenesis. J. Cell Mol. Med. 2009, 13, 2822–2833. [Google Scholar] [CrossRef] [PubMed]
- Giannone, G.; Tuninetti, V.; Ghisoni, E.; Genta, S.; Scotto, G.; Mittica, G.; Valabrega, G. Role of cyclin-dependent kinase inhibitors in endometrial cancer. Int. J. Mol. Sci. 2019, 20, 2353. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, C.F.; Cheng, Z.; Abudureyimu, A.; Fouladi-Nashta, A.A.; Wathes, D.C. Global transcriptomic profiling of bovine endometrial immune response in vitro. I. Effect of lipopolysaccharide on innate immunity. Biol. Reprod. 2015, 93, 100. [Google Scholar] [CrossRef][Green Version]
- Xia, F.; Wang, Y.; Xue, M.; Zhu, L.; Jia, D.; Shi, Y.; Gao, Y.; Li, L.; Li, Y.; Chen, S.; et al. LncRNA KCNQ1OT1: Molecular mechanisms and pathogenic roles in human diseases. Genes Dis. 2021, 9, 1556–1565. [Google Scholar] [CrossRef]
- Yue, T.; Li, J.; Liang, M.; Yang, J.; Ou, Z.; Wang, S.; Ma, W.; Fan, D. Identification of the KCNQ1OT1/ miR-378a-3p/ RBMS1 axis as a novel prognostic biomarker associated with immune cell infiltration in gastric cancer. Front. Genet. 2022, 13, 928754. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, T.; Zhao, Z.; Wei, W.; Yang, X.; Wang, X.; Xin, W. Novel Insights into the emerging role of Neat1 and its effects downstream in the regulation of inflammation. J. Inflamm. Res. 2022, 15, 557–571. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, G.; Li, Y.; Zhou, Q. TUG1 Represses Apoptosis, Autophagy, and Inflammatory Response by Regulating miR-27a-3p/SLIT2 in Lipopolysaccharide-Treated Vascular Endothelial Cells. J. Surg. Res. 2020, 256, 345–354. [Google Scholar] [CrossRef]
- Yu, B.; Qi, Y.; Li, R.; Shi, Q.; Satpathy, A.T.; Chang, H.Y. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell 2021, 184, 1790–1803.e17. [Google Scholar] [CrossRef]
- Ganapathy, K.; Ngo, C.; Andl, T.; Coppola, D.; Park, J.; Chakrabarti, R. Anticancer function of microRNA-30e is mediated by negative regulation of HELLPAR, a noncoding macroRNA, and genes involved in ubiquitination and cell cycle progression in prostate cancer. Mol. Oncol. 2022, 16, 2936–2958. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.; Visser, A.; Buabeng, K.M.; Poutsma, A.; van der Schors, R.C.; Oudejans, C.B. Mutations within the LINC-HELLP non-coding RNA differentially bind ribosomal and RNA splicing complexes and negatively affect trophoblast differentiation. Hum. Mol. Genet. 2015, 24, 5475–5485. [Google Scholar] [CrossRef]
- Guo, C.; Qi, Y.; Qu, J.; Gai, L.; Shi, Y.; Yuan, C. Pathophysiological functions of the lncRNA TUG1. Curr. Pharm. Des. 2020, 26, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, L.; Wan, F. Molecular mechanisms of TUG1 in the proliferation, apoptosis, migration and invasion of cancer cells. Oncol. Lett. 2019, 18, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Su, L.; Jiang, J.; Wang, Y.E.; Ling, Y.; Qiu, Y.; Yu, H.; Huang, Y.; Wu, J.; Jiang, S.; et al. RanBP2/Nup358 Mediates Sumoylation of STAT1 and Antagonizes Interferon-α-Mediated Antiviral Innate Immunity. Int. J. Mol. Sci. 2023, 25, 299. [Google Scholar] [CrossRef]
- Zang, X.; He, X.Y.; Xiao, C.M.; Lin, Q.; Wang, M.Y.; Liu, C.Y.; Kong, L.Y.; Chen, Z.; Xia, Y.Z. Circular RNA-encoded oncogenic PIAS1 variant blocks immunogenic ferroptosis by modulating the balance between SUMOylation and phosphorylation of STAT1. Mol. Cancer 2024, 23, 207. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, Q.; Sun, W.; Yang, X.; Huang, H.; Xu, Z. Exome sequencing link mutation in RGPD4 with systemic sclerosis-associated interstitial lung disease and the low level of testosterone-an exploration study. Front. Oncol. 2022, 12, 956552. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.; Chen, J. Development of an Inflammation-Related lncRNA-miRNA-mRNA Network Based on Competing Endogenous RNA in Breast Cancer at Single-Cell Resolution. Front. Cell Dev. Biol. 2022, 10, 839876. [Google Scholar] [CrossRef]
- Li, W.; Li, K.; Zhao, L.; Zou, H. Bioinformatics analysis reveals disturbance mechanism of MAPK signaling pathway and cell cycle in Glioblastoma multiforme. Gene 2014, 547, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, G.; Liao, J.; Huang, Z.; Wen, J.; Wang, Y.; Chen, Z.; Cai, G.; Xu, W.; Ding, Z.; et al. Non-coding small nucleolar RNA SNORD17 promotes the progression of hepatocellular carcinoma through a positive feedback loop upon p53 inactivation. Cell Death Diff. 2022, 29, 988–1003. [Google Scholar] [CrossRef]
- Shen, L.P.; Zhang, W.C.; Deng, J.R.; Qi, Z.H.; Lin, Z.W.; Wang, Z.D. Advances in the mechanism of small nucleolar RNA and its role in DNA damage response. Mil. Med. Res. 2024, 11, 53. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Wu, M.; Zhang, Y.; Hou, S.; Wang, X.; Peng, Y. LncRNA SNHG16 drives PD-L1-mediated immune escape in colorectal cancer through regulating miR-324-3p/ELK4 Signaling. Biochem. Genet. 2024. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, X.; He, X.; Zhang, J.; Wang, X.; Lin, W.; Wang, X.; Wu, X. Long non-coding RNA SNHG16 silencing inhibits proliferation and inflammation in Mycobacterium tuberculosis-infected macrophages by targeting miR-140-5p expression. Infect. Genet. Evol. 2022, 103, 105325. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Wang, T.; Zhang, H.; Lu, X.; Liu, L.; Li, L.; Bo, C.; Kong, X.; Xu, S.; et al. Identification of the regulatory role of lncRNA HCG18 in myasthenia gravis by integrated bioinformatics and experimental analyses. J. Transl. Med. 2021, 19, 468. [Google Scholar] [CrossRef]
- Gremlich, S.; Damnon, F.; Reymondin, D.; Braissant, O.; Schittny, J.C.; Baud, D.; Gerber, S.; Roth-Kleiner, M. The long non-coding RNA NEAT1 is increased in IUGR placentas, leading to potential new hypotheses of IUGR origin/development. Placenta 2014, 35, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, R.; Yang, S.; Ma, Z.; Lin, S.; Nan, Y.; Li, Q.; Tang, Q.; Zhang, Y.J. Karyopherin alpha 6 is required for replication of Porcine Reproductive and Respiratory Syndrome virus and Zika virus. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Zhu, Y.; Duan, C.; Gui, Y.; Chen, D.; Su, X. Exosomal circMACF1 drives PI3K/AKT/mTOR-mediated autophagy suppression in laryngeal squamous cell carcinoma. Cell Mol. Biol. 2024, 70, 179–185. [Google Scholar] [CrossRef]
- Li, M.W.; Huang, F.X.; Xie, Z.C.; Hong, H.Y.; Xu, Q.Y.; Peng, Z.G. Identification of three small nucleolar RNAs (snoRNAs) as potential prognostic markers in diffuse large B-cell lymphoma. Cancer Med. 2023, 12, 3812–3829. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, Y.; Wen, Y.; Shi, L.; Zhu, Z.; Ke, G.; Gu, Y. LncRNA KCNQ1OT1 knockdown inhibits viability, migration and epithelial-mesenchymal transition in human lens epithelial cells via miR-26a-5p/ITGAV/TGF-beta/Smad3 axis. Exp. Eye Res. 2000, 200, 108251. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Kobayashi, N.; Todo, Y.; Watari, H. Long non-coding RNA NEAT1: A novel target for diagnosis and therapy in human tumors. Front. Genet. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, X.; Zeng, Y. 3D genomic regulation of lncRNA and Xist in X chromosome. Semin. Cell Dev. Biol. 2019, 90, 174–180. [Google Scholar] [CrossRef]
- Wang, W.; Min, L.; Qiu, X.; Wu, X.; Liu, C.; Ma, J.; Zhang, D.; Zhu, L. Biological function of long non-coding RNA (LncRNA) Xist. Front. Cell Dev. Biol. 2021, 9, 645647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).