IL-4 and Brentuximab Vedotin in Mycosis Fungoides: A Perspective on Potential Therapeutic Interactions and Future Research Directions
Abstract
1. Introduction
2. Methods
3. Results
3.1. IL-4 in MF Pathogenesis
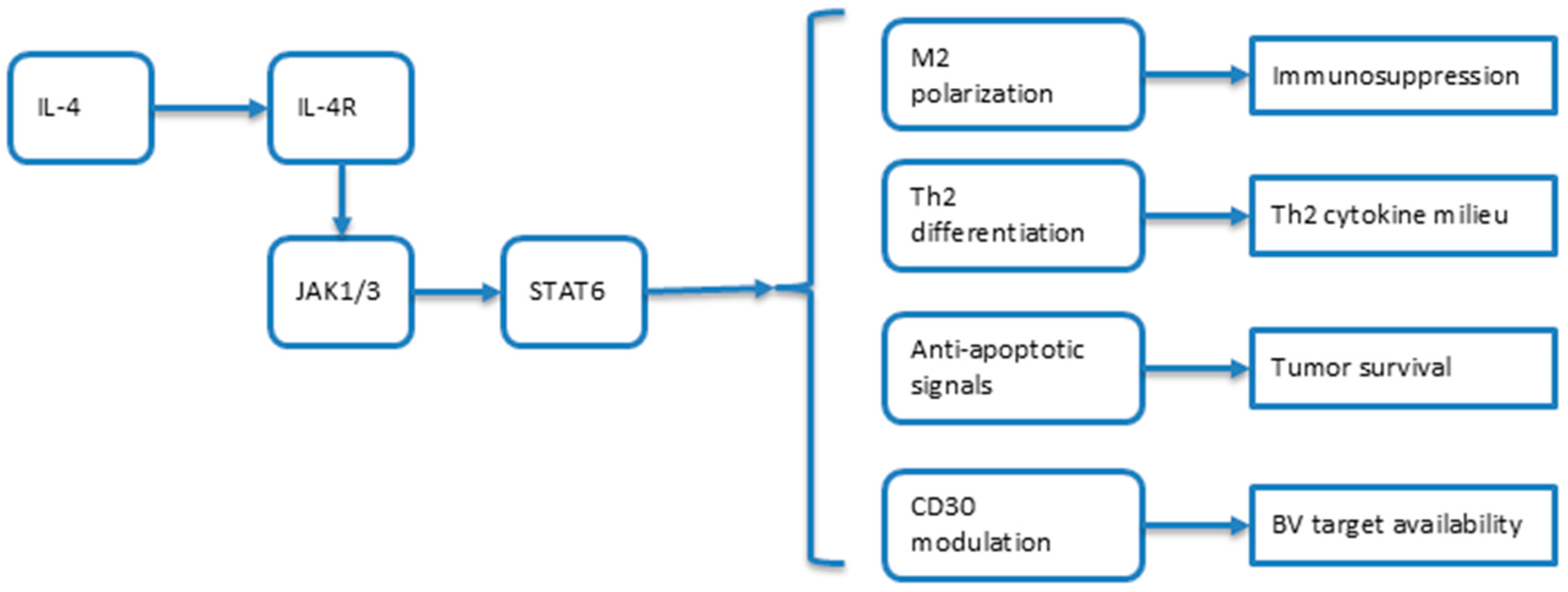
3.1.1. IL-4 in General Cancer Biology
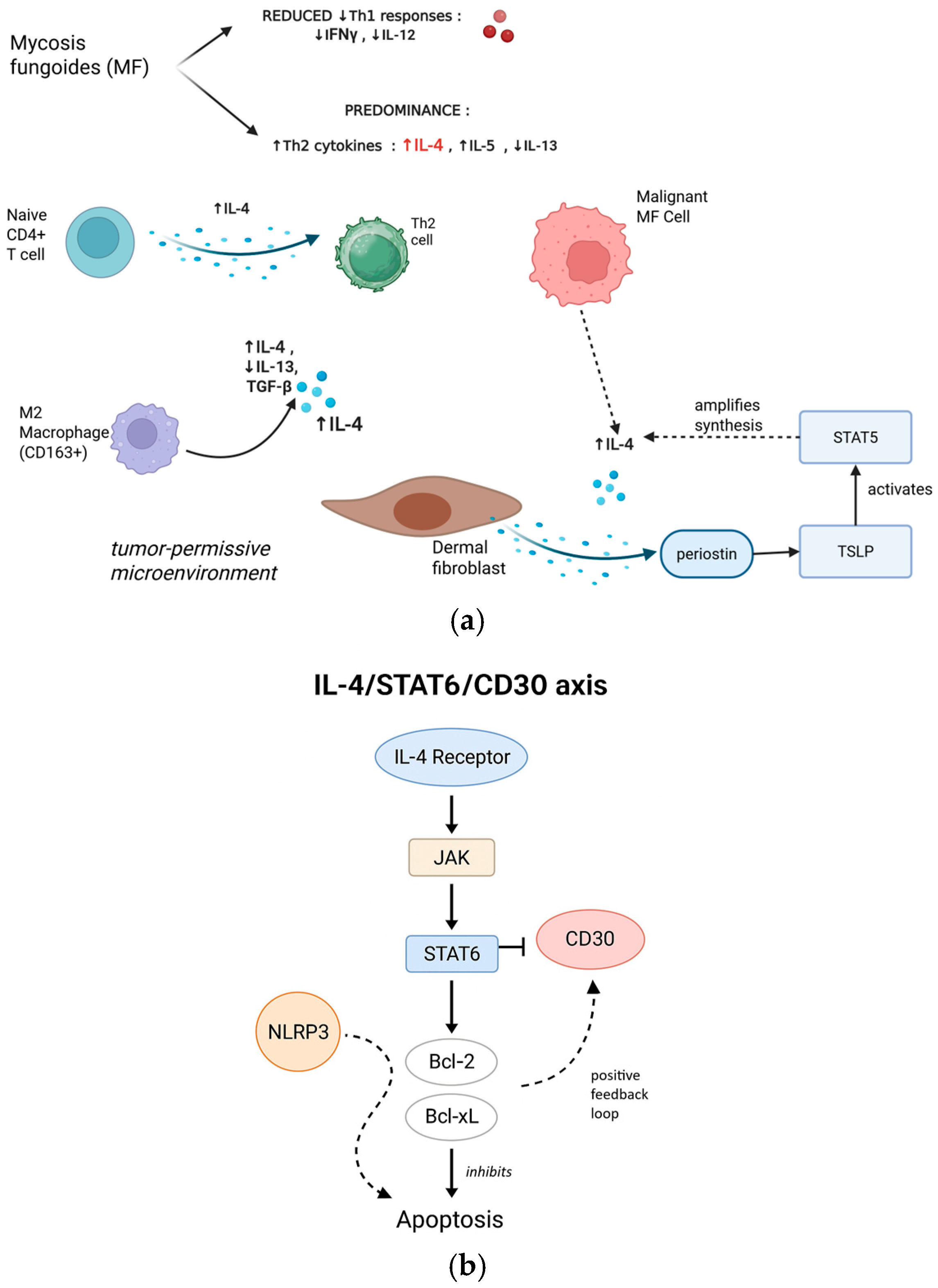
3.1.2. IL-4 in MF-Specific Pathogenesis
3.1.3. Molecular Mechanisms: The IL-4/STAT6/CD30 Axis
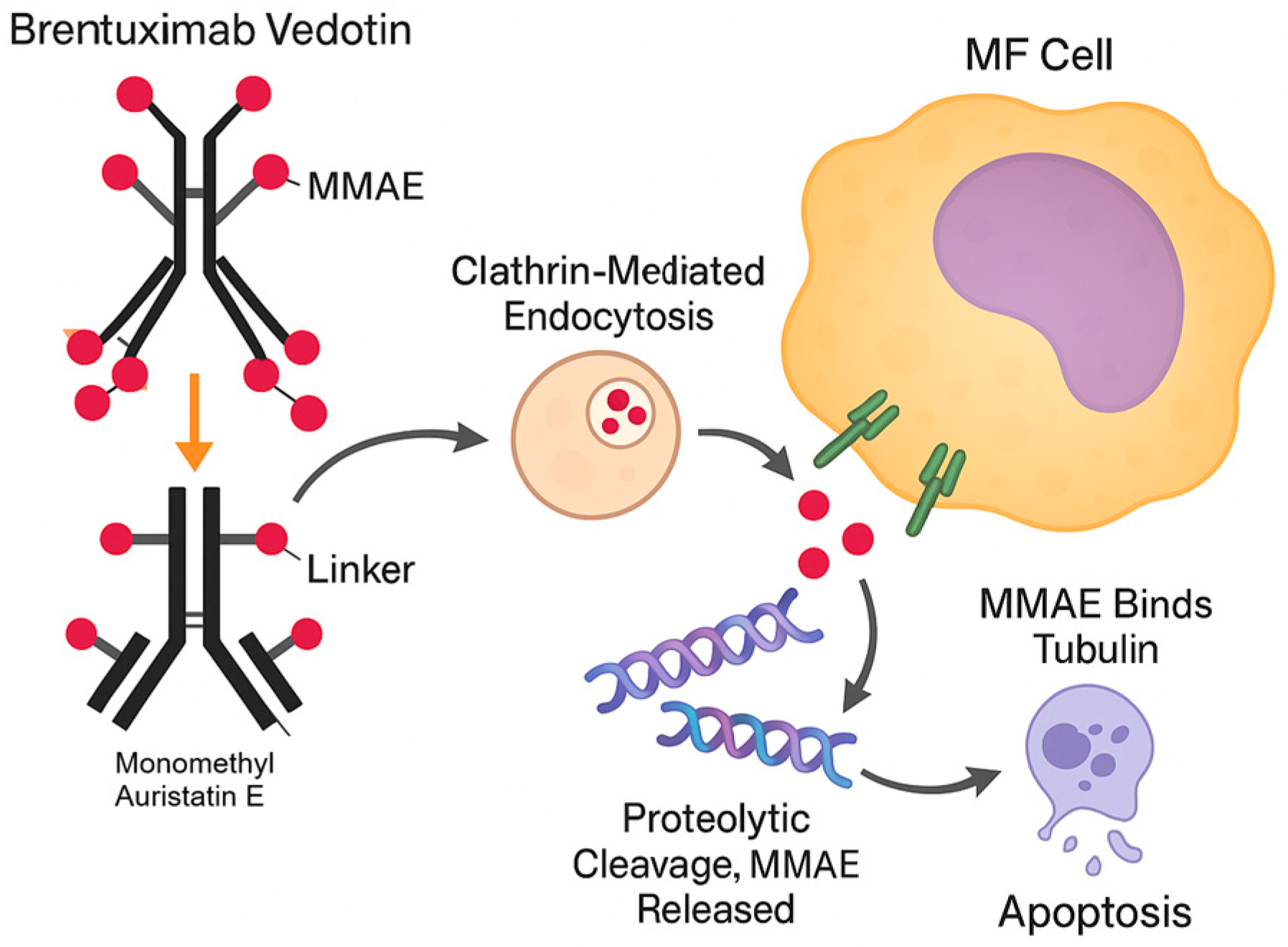
3.2. Brentuximab Vedotin in MF
3.3. Theoretical Framework for Combination Therapy
- BV + checkpoint inhibitors: Phase II trials combining BV with pembrolizumab show ORR of 71% in relapsed CTCL;
- BV + lenalidomide demonstrates synergistic activity through immunomodulation;
- Mogamulizumab combinations: anti-CCR4 therapy depletes Tregs, potentially complementing IL-4 blockade;
- CAR-T approaches: CD30-targeted CAR-T cells are in development, raising questions about sequencing with BV.
3.4. Evidence Gaps and Research Priorities
3.4.1. Knowledge Gaps
- MF-derived cell lines (e.g., HH, MJ, HuT-78) treated with IL-4 ± anti-IL-4Rα antibodies to assess CD30’s expression dynamics;
- Patient-derived xenograft (PDX) models testing combinations’ efficacy;
- Ex vivo cytotoxicity assays using patient samples to measure ADCC/ADCP under IL-4 modulation.
- Serum markers: IL-4 (>50 pg/mL), IL-13 (>100 pg/mL), and soluble IL-4Rα (>500 ng/mL) measured by validated ELISA platforms;
- Tissue markers: STAT6 phosphorylation (pSTAT6) by immunohistochemistry (H-score >100), GATA3 expression (>30% nuclear positivity), and CD163+ M2 macrophage density (>40% of infiltrate);
- Genetic signatures: the 15-gene IL-4 response signature by NanoString or RT-PCR, with scores >75th percentile indicating high pathway activity;
- Functional assays: ex vivo IL-4-induced proliferation index >2.0 in patients’ T cells;
- CD30 thresholds: minimum 10% expression by IHC for BV eligibility, with exploration of whether IL-4 inhibition could rescue patients with 5–10% expression.
3.4.2. Proposed Research Framework: A Phased Approach to Clinical Translation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IL | Interleukin |
| MF | Mycosis Fungoides |
| BV | Brentuximab Vedotin |
| CTCL | Cutaneous T Cell Lymphoma |
| ADC | Antibody–Drug Conjugate |
| TME | Tumor Microenvironment |
| IFN | Interferon |
| JAK | Janus Kinase |
| SOCS | Suppressor of Cytokine Signaling |
| MMAE | Monomethyl Auristatin E |
| ADCC | Antibody-Dependent Cellular Cytotoxicity |
| ADCP | Antibody-Dependent Cellular Phagocytosis |
| ORR | Overall Response Rate |
| CAR | Chimeric Antigen Receptor |
| PDX | Patient-Derived Xenograft |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| RT | Reverse Transcription |
| PCR | Polymerase Chain Reaction |
| IHC | Immunohistochemistry |
| HDAC | Histone Deacetylase |
| STAT | Signal Transducer and Activator of Transcription |
References
- Kaufman, A.E.; Patel, K.; Goyal, K.; O’Leary, D.; Rubin, N.; Pearson, D.; Bohjanen, K.; Goyal, A. Mycosis fungoides: Developments in incidence, treatment and survival. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T. Diagnosis of early mycosis fungoides. Diagnostics 2021, 11, 1721. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Ferreri, A.J.; Cerroni, L. Mycosis fungoides. Crit. Rev. Oncol. Hematol. 2008, 65, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Hodak, E.; Amitay-Laish, I. Mycosis fungoides: A great imitator. Clin. Dermatol. 2019, 37, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Krejci-Manwaring, J.; McCarty, M.A.; Camacho, F.; Carroll, C.L.; Johnson, K.; Manuel, J.; Balkrishnan, R.; Hartle, J.; Fleischer, A.; Feldman, S.R. Adherence with topical treatment is poor compared with adherence with oral agents: Implications for effective clinical use of topical agents. J. Am. Acad. Dermatol. 2006, 54 (Suppl. 5), S235–S236. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Szepietowski, J.C.; Bagot, M.; Sandhu, S.; Patil, A.; Grabbe, S.; Goldust, M. Mycosis fungoides in pediatric population: Comprehensive review on epidemiology, clinical presentation, and management. Int. J. Dermatol. 2022, 61, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.L.J.; Tan, E.S.T.; Tee, S.I.; Ho, Z.Y.; Boey, J.J.J.; Tan, W.P.; Tang, M.; Shen, L.; Chan, Y.; Tan, S. Epidemiology and prognostic factors for mycosis fungoides and Sézary syndrome in a multi-ethnic Asian cohort: A 12-year review. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Wohl, Y.; Tur, E. Environmental risk factors for mycosis fungoides. Curr. Probl. Dermatol. 2007, 35, 52–64. [Google Scholar] [PubMed]
- Teras, L.R.; DeSantis, C.E.; Cerhan, J.R.; Morton, L.M.; Jemal, A.; Flowers, C.R. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J. Clin. 2016, 66, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, T.; Badri, T. Mycosis Fungoides. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519572/ (accessed on 10 June 2025).
- Tardío, J.C.; Arias, D.; Khedaoui, R. Indeterminate Cell Histiocytosis and Mycosis Fungoides: A Hitherto Unreported Association. Am. J. Dermatopathol. 2019, 41, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Stuver, R.; Geller, S. Advances in the treatment of mycoses fungoides and Sézary syndrome: A narrative update in skin-directed therapies and immune-based treatments. Front. Immunol. 2023, 14, 1284045. [Google Scholar] [CrossRef] [PubMed]
- Case, K.B.; Allen, P.B. Advances in Novel Systemic Therapies for the Management of Cutaneous T Cell Lymphoma (CTCL). Curr. Hematol. Malig. Rep. 2025, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Z. Recent Advances in Understanding the Clinical Responses of Brentuximab Vedotin in Lymphoma and the Correlation with CD30 Expression. OncoTargets Ther. 2025, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, A.; Zengarini, C.; Agostinelli, C.; Motta, G.; Sabattini, E.; Pileri, A. The Role of Cytokines in Cutaneous T Cell Lymphoma: A Focus on the State of the Art and Possible Therapeutic Targets. Cells 2024, 13, 584. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, J.; Wang, Z.; Zhang, J.; Xiao, M.; Wang, C.; Lu, Y.; Qin, Z. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. 2008, 68, 8687–8694. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, Z.J.; Shenoy, A.; Chen, A.; Heller, N.M.; Spangler, J.B. Engineering the IL-4/IL-13 axis for targeted immune modulation. Immunol. Rev. 2023, 320, 29–57. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, J.; Caruso-Neves, C.; Pinheiro, A. IL-4: An important cytokine in determining the fate of T cells. Biophys. Rev. 2014, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Mashima, T.; Yuan, X.; Yamashita, M.; Kitano, S.; Torii, S.; Migita, T.; Seimiya, H. Interleukin-4 induced 1-mediated resistance to an immune checkpoint inhibitor through suppression of CD8+ T cell infiltration in melanoma. Cancer Sci. 2024, 115, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, Y.; Yu, S.; Hu, K.; Zhang, L.; Xiong, M.; Liu, M.; Sun, X.; Li, S.; Yuan, Y.; et al. IL-4/IL-4R axis signaling drives resistance to immunotherapy by inducing the upregulation of Fcγ receptor IIB in M2 macrophages. Cell Death Dis. 2024, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Nappo, G.; Handle, F.; Santer, F.R.; McNeill, R.V.; Seed, R.I.; Collins, A.T.; Morrone, G.; Culig, Z.; Maitland, N.J.; Erb, H.H.H. The immunosuppressive cytokine interleukin-4 increases the clonogenic potential of prostate stem-like cells by activation of STAT6 signalling. Oncogenesis 2017, 6, e342. [Google Scholar] [CrossRef] [PubMed]
- Jauregui, P. IL-4 promotes immunotherapy resistance. Nat. Immunol. 2024, 25, 2169. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; Scarisbrick, J.J.; Porcu, P.; O’Connor, O.A.; Wolter, N.; Fledarko, C.; Duvic, M. Efficacy and safety of brentuximab vedotin in cutaneous T-cell lymphoma: Results from a phase 2 study. Lancet Oncol. 2015, 16, 15–27. [Google Scholar]
- Ito S-e Shirota, H.; Kasahara, Y.; Saijo, K.; Ishioka, C. IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol. Immunother. 2017, 66, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Bai, Z.; Zhou, X.; Zhao, Y.; Xie, Y.-Q.; Huang, X.; Liu, Y.; Enbar, T.; Li, R.; Wang, Y.; et al. The type 2 cytokine Fc–IL-4 revitalizes exhausted CD8+ T cells against cancer. Nature 2024, 634, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Kuttikrishnan, S.; Khan, A.Q.; Ahmad, F.; Alam, M.; Buddenkotte, J.; Ahmad, A.; Steinhoff, M.; Uddin, S. Molecular pathogenesis of Cutaneous T cell Lymphoma: Role of chemokines, cytokines, and dysregulated signaling pathways. Semin. Cancer Biol. 2022, 86, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Mazzetto, R.; Miceli, P.; Tartaglia, J.; Ciolfi, C.; Sernicola, A.; Alaibac, M. Role of IL-4 and IL-13 in Cutaneous T Cell Lymphoma. Life 2024, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Cordeiro, B.; Fredholm, S.; Ødum, N.; Zargham, H.; Huang, Y.; Zhou, Y.; Pehr, K.; Kupper, T.S.; Woetmann, A. Denis Sasseville Analysis of STAT4 expression in cutaneous T-cell lymphoma (CTCL) patients and patient-derived cell lines. Cell Cycle 2014, 13, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Economidou, J.; Psarra, A.; Kapsimali, V.; Mantzana, V.; Antoniou, C.; Limas, K.; Stratigos, A.; Stavrianeas, N.; Avgerinou, G.; et al. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sézary syndrome. Br. J. Dermatol. 2003, 148, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, R.T.; Medeiros, L.J.; Warnke, R.A.; Wood, G.S. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J. Am. Acad. Dermatol. 1995, 32, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, V.; Toonstra, J.; Bihari, I.C.; Bruijnzeel-Koomen, C.A.; van Vloten, W.A.; Thepen, T. Interleukin 4 and interferon-gamma expression of the dermal infiltrate in patients with erythroderma and mycosis fungoides. An immuno-histochemical study. J. Cutan. Pathol. 2000, 27, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Furudate, S.; Fujimura, T.; Kakizaki, A.; Hidaka, T.; Asano, M.; Aiba, S. Tumor-associated M2 macrophages in mycosis fungoides acquire immunomodulatory function by interferon alpha and interferon gamma. J. Dermatol. Sci. 2016, 83, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Tavallaee, M.; Sundram, U.; Salva, K.A.; Wood, G.S.; Li, S.; Rozati, S.; Nagpal, S.; Krathen, M.; Reddy, S.; et al. Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides and Sézary Syndrome With Variable CD30 Expression Level: A Multi-Institution Collaborative Project. J. Clin. Oncol. 2015, 33, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Saulite, I.; Ignatova, D.; Chang, Y.T.; Fassnacht, C.; Dimitriou, F.; Varypataki, E.; Anzengruber, F.; Nägeli, M.; Cozzio, A.; Dummer, R.; et al. Blockade of programmed cell death protein 1 (PD-1) in Sézary syndrome reduces Th2 phenotype of non-tumoral T lymphocytes but may enhance tumor proliferation. Oncoimmunology 2020, 9, 1738797. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Pavel, A.B.; Diaz, A.; Zhang, N.; Del Duca, E.; Estrada, Y.; King, B.; Banerjee, A.; Banfield, C.; Cox, L.A.; et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J. Allergy Clin. Immunol. 2022, 149, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Guenova, E.; Watanabe, R.; Teague, J.E.; Desimone, J.A.; Jiang, Y.; Dowlatshahi, M.; Schlapbach, C.; Schaekel, K.; Rook, A.H.; Tawa, M.; et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin. Cancer Res. 2013, 19, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Sugaya, M.; Suga, H.; Oka, T.; Kawaguchi, M.; Miyagaki, T.; Fujita, H.; Sato, S. Thymic Stromal Chemokine TSLP Acts through Th2 Cytokine Production to Induce Cutaneous T-cell Lymphoma. Cancer Res. 2016, 76, 6241–6252. [Google Scholar] [CrossRef] [PubMed]
- Jfri, A.; Smith, J.S.; Larocca, C. Diagnosis of mycosis fungoides or Sézary syndrome after dupilumab use: A systematic review. J. Am. Acad. Dermatol. 2023, 88, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ramachandran, V.; Weston, G.; Kim, R.H.; Latkowski, J.A. Diagnosing mycosis fungoides after initiation of therapy with dupilumab: A case report and literature review. Int. J. Dermatol. 2023, 62, e500–e503. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, L.; Hao, S.; Liu, Z.; Ding, S.; Zhang, W.; Yang, X.; Li, S. Activation of the IL-4/STAT6 signaling pathway promotes lung cancer progression by increasing M2 myeloid cells. Front. Immunol. 2019, 10, 2638. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.-T.; Chyuan, I.-T.; Lai, J.-H. Targeting the JAK-STAT pathway in autoimmune diseases and cancers: A focus on molecular mechanisms and therapeutic potential. Biochem. Pharmacol. 2021, 193, 114760. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Molina, A.; Akhter, A.; Lim, K.H.; Wang, Q.; Harvey, R.C.; Wenzel, S.S.; Chiu, A.; Qualls, A.E.; Park, J.; Weinberg, O.K.; et al. STAT6 mutations compensate for CREBBP mutations in follicular lymphoma by restoring IL-4-driven RRAGD/mTOR signaling. Leukemia 2025, 39, 899–908. [Google Scholar] [CrossRef]
- Huanosta-Murillo, E.; Alcántara-Hernández, M.; Hernández-Rico, B.; Victoria-Acosta, G.; Miranda-Cruz, P.; Domínguez-Gómez, M.A.; Jurado-Santacruz, F.; Patiño-López, G.; Pérez-Koldenkova, V.; Palma-Guzmán, A.; et al. NLRP3 regulates IL-4 expression in TOX+ CD4+ T cells of cutaneous T cell lymphoma to potentially promote disease progression. Front. Immunol. 2021, 12, 668369. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Lee, R.K.; Nam, S.Y.; Al-Ramadi, B.K.; Koni, P.A.; Bottomly, K.; Podack, E.R.; A Flavell, R. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-gamma. J. Immunol. 1997, 158, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, R.; O’Connor, O.A.; Gopal, A.K.; Ramchandren, R.; Goy, A.; Matous, J.V.; Fasanmade, A.A.; Manley, T.J.; Han, T.H. Brentuximab vedotin, an antibody-drug conjugate, in patients with CD30-positive haematologic malignancies and hepatic or renal impairment. Br. J. Clin. Pharmacol. 2016, 82, 696–705. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.; Dhimolea, E. Brentuximab vedotin. MAbs 2012, 4, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Kapniari, E.; Pappa, V.; Nikolaou, V.; Iliakis, T.; Dalamaga, M.; Jonak, C.; Porkert, S.; Engelina, S.; Quaglino, P.; et al. Multicentric EORTC retrospective study shows efficacy of brentuximab vedotin in patients who have mycosis fungoides and Sézary syndrome with variable CD30 positivity. Br. J. Dermatol. 2021, 185, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Scarisbrick, J.J.; Dummer, R.; Whittaker, S.; Duvic, M.; Kim, Y.H.; Quaglino, P.; Zinzani, P.L.; Bechter, O.; Eradat, H.; et al. Randomized phase 3 ALCANZA study of brentuximab vedotin vs physician’s choice in cutaneous T-cell lymphoma: Final data. Blood Adv. 2021, 5, 5098–5106, Erratum in Blood Adv. 2024, 8, 2243. [Google Scholar] [CrossRef] [PubMed]
- Duvic, M.; Tetzlaff, M.T.; Gangar, P.; Clos, A.L.; Sui, D.; Talpur, R. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J. Clin. Oncol. 2015, 33, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.M.; Kim, Y.H.; Horwitz, S.M.; Dummer, R.; Scarisbrick, J.; Quaglino, P.; Zinzani, P.L.; Wolter, P.; Sanches, J.A.; Ortiz-Romero, P.L.; et al. 10Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An international, open-label, randomised, phase 3, multicentre trial. Lancet 2017, 390, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Barta, S.K.; Liu, N.; DerSarkissian, M.; Chang, R.; Ye, M.; Duh, M.S.; Surinach, A.; Fanale, M.; Yu, K.S. Real-World Treatment Patterns and Clinical Outcomes With Brentuximab Vedotin or Other Standard Therapies in Patients With Previously Treated Cutaneous T-Cell Lymphoma in the United States. Clin. Lymphoma Myeloma Leuk. 2024, 24, e21–e32.e4. [Google Scholar] [CrossRef] [PubMed]
- Duvic, M.; Donato, M.L.; Dabaj, S.; Oquendo, N.D.; Chang, T.L.; Zhou, X.; Zhang, T. The Th2 cytokine profile in mycosis fungoides: A critical target for therapy. J. Immunother. Cancer 2021, 9, e002773. [Google Scholar]
- Saed, G.M.; Al-Ghoraba, M.; Gurevich, V.; Al-Mohanna, M. The role of Th1/Th2 balance in mycosis fungoides. Dermatol. Online J. 2016, 22, 13030. [Google Scholar]
- Kim, J.; Kim, H.; Kim, Y.; Kwon, K.; Lee, Y.; Kim, J. Th2 cytokine-mediated signaling pathways in cutaneous T-cell lymphoma. Front. Immunol. 2024, 15, 1472772. [Google Scholar]
- Duvic, M.; Tetzlaff, M.T.; Clos, K.; Zhang, T.; Donato, M.L. Novel Therapies for Mycosis Fungoides. Curr. Oncol. Rep. 2024, 26, 1–11. [Google Scholar]
- Deng, Q.; Zhang, Z.; Zhou, Y.; Chen, J.; Liu, K.; Li, J.; Zhao, Z.; Wang, X. Spatial genomics uncovers cytokines promoting ovarian tumour heterogeneity and immunotherapy resistance. Nat. Commun. 2025, 15, e70248. [Google Scholar]
- Beygi, S.; Naranang, P.; Khosravi-Shahi, P. Role of CD30 expression in Mycosis Fungoides: A Comprehensive Review. J. Oncol. Pharm. Pract. 2023, 29, 1373–1381. [Google Scholar]
- Li, Z.; Guo, W.; Bai, O. Mechanism of action and therapeutic targeting of CD30 molecule in lymphomas. Front. Oncol. 2023, 13, 1301437. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Hadzijusufovic, E.; Cerny-Reiterer, S.; Hoermann, G.; Reifinger, M.; Pirker, A.; Valent, P.; Willmann, M. IL-4 downregulates expression of the target receptor CD30 in neoplastic canine mast cells. Vet. Comp. Oncol. 2017, 15, 1240–1256. [Google Scholar] [CrossRef] [PubMed]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef] [PubMed]
| Knowledge Gap | Research Question | Confidence Level | Clinical Impact |
|---|---|---|---|
| Unclear whether IL-4 upregulates or downregulates CD30 in the MF context. | How does IL-4 signaling affect CD30 expression in malignant T cells from MF patients under different conditions? | Moderate | Directly affects patient selection and the likelihood of BV efficacy in combination strategies. |
| Impact of Th2 cytokines on ADC efficacy is poorly understood. | What is the effect of Th2 cytokines (IL-4, IL-13) on the internalization and cytotoxic activity of Brentuximab Vedotin in MF? | Moderate | May influence response rates and the durability of BV therapy; could necessitate adjunct immunomodulation. |
| Limited data on IL-4/IL-13 inhibitors in MF. | Do IL-4 pathway inhibitors demonstrate clinical activity or modify the disease course in MF patients? | Moderate | Limits the adoption of IL-4/IL-13 inhibitors in routine MF management or in combination regimens. |
| No evidence for synergy between IL-4 blockade and BV. | Does IL-4 pathway inhibition enhance the efficacy of BV in MF by modulating immune activity or altering CD30 expression? | Low | Prevents rational design of combination trials; synergy would justify new therapeutic protocols. |
| Resistance mechanisms in MF not fully addressed by current therapies. | Can combination therapy targeting IL-4 and CD30 overcome intrinsic or acquired resistance in MF? | Low | Novel combinations may offer solutions for refractory/relapsed MF patients. |
| Biomarker frameworks for combination strategies are conceptual. | What biomarkers can identify the MF patients likely to benefit from IL-4 and BV co-targeting? | Low | Limits personalized therapy and efficient trial enrollment; impedes biomarker-driven clinical practice. |
| Risk of MF progression with IL-4 inhibitors. | What is the mechanism behind disease acceleration in MF patients receiving dupilumab? | Requires caution | Uncertainty deters clinicians from using IL-4 blockade; may expose patients to unforeseen adverse events. |
| The neurotoxicity profile of BV in combinations is unknown. | What is the cumulative neurotoxicity risk of combining BV with IL-4 pathway inhibitors in MF patients? | Requires caution | Potential for increased or unexpected toxicity could limit the clinical use of combinations. |
| No clinical studies on IL-4 and BV combination therapy. | What are the safety and efficacy outcomes of combining IL-4 blockade and BV in MF patients? | Requires caution | Hinders clinical guideline development; necessitates preclinical and early-phase trial evidence. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreescu, M.; Tudorache, S.I.; Moldovan, C.A.; Andreescu, B. IL-4 and Brentuximab Vedotin in Mycosis Fungoides: A Perspective on Potential Therapeutic Interactions and Future Research Directions. Curr. Issues Mol. Biol. 2025, 47, 586. https://doi.org/10.3390/cimb47080586
Andreescu M, Tudorache SI, Moldovan CA, Andreescu B. IL-4 and Brentuximab Vedotin in Mycosis Fungoides: A Perspective on Potential Therapeutic Interactions and Future Research Directions. Current Issues in Molecular Biology. 2025; 47(8):586. https://doi.org/10.3390/cimb47080586
Chicago/Turabian StyleAndreescu, Mihaela, Sorin Ioan Tudorache, Cosmin Alec Moldovan, and Bogdan Andreescu. 2025. "IL-4 and Brentuximab Vedotin in Mycosis Fungoides: A Perspective on Potential Therapeutic Interactions and Future Research Directions" Current Issues in Molecular Biology 47, no. 8: 586. https://doi.org/10.3390/cimb47080586
APA StyleAndreescu, M., Tudorache, S. I., Moldovan, C. A., & Andreescu, B. (2025). IL-4 and Brentuximab Vedotin in Mycosis Fungoides: A Perspective on Potential Therapeutic Interactions and Future Research Directions. Current Issues in Molecular Biology, 47(8), 586. https://doi.org/10.3390/cimb47080586







