Depletion of IGFALS Serum Level up to 3 Months After Cardiac Surgery, with Exploration of Potential Relationships to Surrogates of Organ Failures and Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Consent Process
2.2. Collection of the Electronic Data
2.3. Measurements of Biological Variables
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Studied Population
3.2. Changes of IGFALS and IGF After Surgery
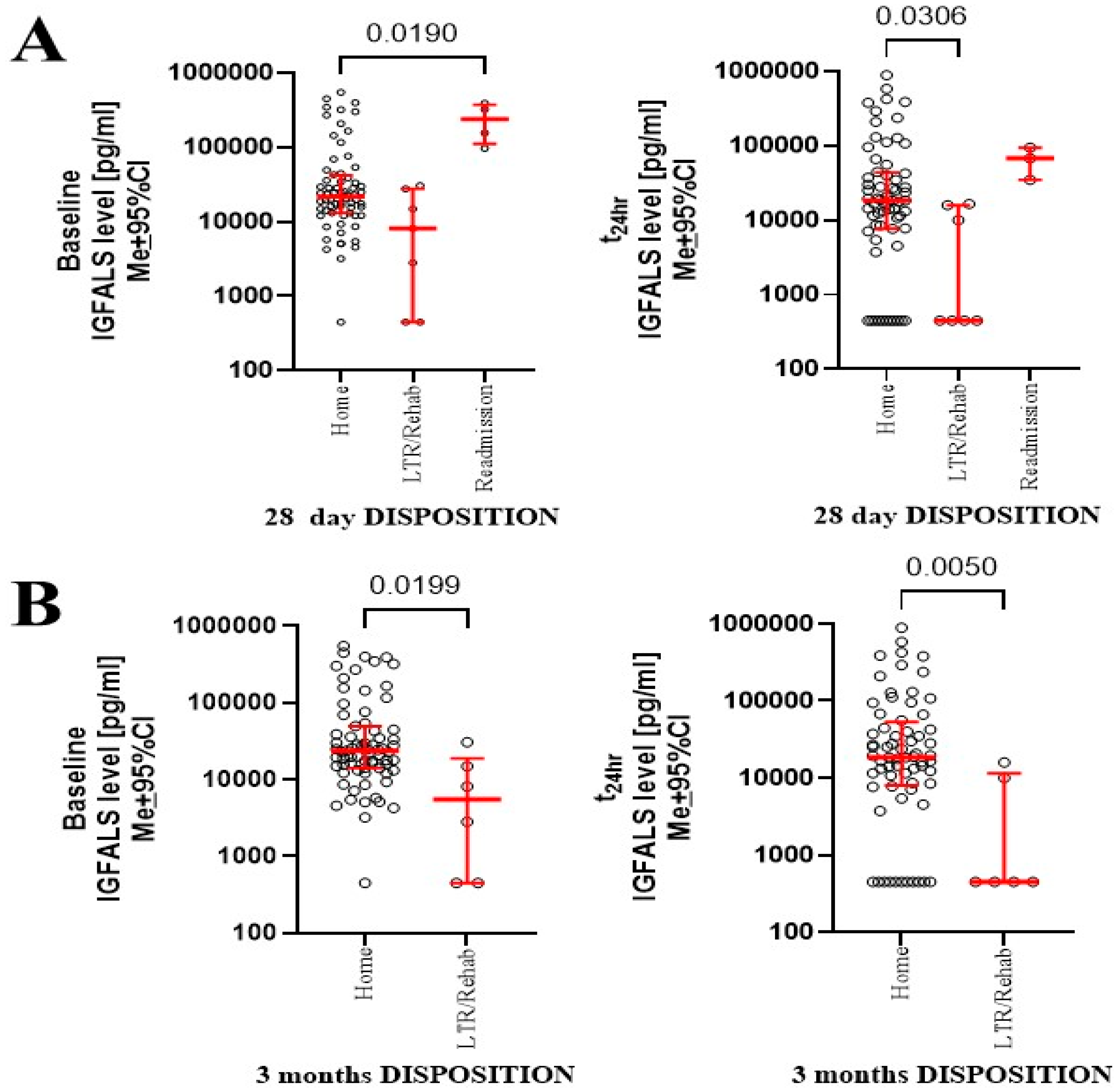
3.3. Postsurgical Clinical Recovery and IGFALS Dynamics
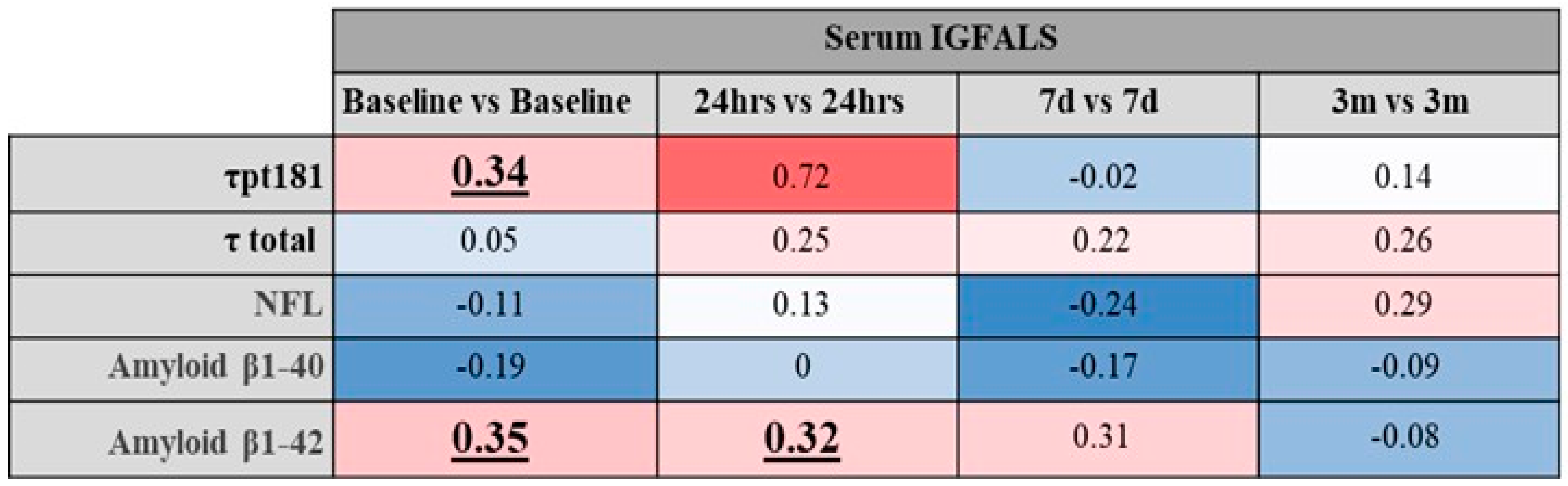
3.4. Relationship Between Perioperative Serum IGFALS and Serum Markers for Inflammation and Tissue Destruction
3.5. Relationship Between Perioperative IGFALs and Functional Immunological Responses
3.6. Influence of Known Factors Affecting IGFALS Expression During the Perioperative Period
3.7. Changes of IGFALS with Acute Kidney Injury, Heart Failure, and Neurodegeneration Surrogates
3.8. Correlation of IGFALS and Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | FULL FORM | Abbreviation | FULL FORM |
| ACS | Acute Coronary Syndrome | IRK | Inwardly Rectifying Potassium Channel |
| AKI | Acute Kidney Injury | Jak2 | Janus kinase 2 |
| ALS | Acid Labile Unit | KLK6 | Kallikrein 6 |
| APACHE | Acute Physiology and Chronic Health Evaluation | KW | Kruskal–Wallis Test |
| BMI | Body Mass Index | Me | Median |
| CCI | Charlson Comorbidity Index | MI | Myocardial Infarction |
| COPD | Chronic Obstructive Pulmonary Disease | MO | Monocytes |
| COX-1 | Cyclooxygenase 1 | NCAM-1 | Neural Cell Adhesion Molecule 1 |
| CVA | Cerebrovascular Accident | NF-L | Neurofilament Light |
| DM | Diabetes Mellitus | NRG | Neurogranin |
| EHR | Electronic Health Records | NT-BNP | Brain Natriuretic Peptide |
| ELISA | Enzyme-Linked Immunosorbent Assay | p181τ | Tau Protein Phosphorylated at Thr181 |
| GH | Growth Hormone | PVD | Peripheral Vascular Disease |
| Hb1ac | Glycated Hemoglobin | RIFLE | Risk, Injury, Failure, Loss, End-Stage Renal Disease |
| HMGB-1 | High-Mobility Group Box | X ± SD | Mean ± Standard Deviation |
| Hsp-60 | Heat Shock Protein 70 | SOCS3 | Suppressor of Cytokine Signaling 3 |
| IGF | Insulin Growth Factor | STAT5a | Signal Transducer and Activator of Transcription 5A |
| IGFALS | Insulin-like Growth Factor Binding Protein | STAT5b | Signal Transducer and Activator of Transcription 5B |
| IGFBP | Insulin Growth Factor Binding Protein | τ | Tau Protein (or Total Tau Protein) |
| IL-1β | Interleukin 1β | TIA | Transient Ischemic Attack |
| IL-6 | Interleukin 6 | Tn I | Troponin I |
| IL-8 | Interleukin 8 | TNFα | Tumor Necrosis Factor Alpha |
| IR | Interquartile Range | TRAF-6 | TNF Receptor Associated Factor 6 |
References
- Högler, W.; Martin, D.D.; Crabtree, N.; Nightingale, P.; Tomlinson, J.; Metherell, L.; Rosenfeld, R.; Hwa, V.; Rose, S.; Walker, J.; et al. IGFALS gene dosage effects on serum IGF-I and glucose metabolism, body composition, bone growth in length and width, and the pharmacokinetics of recombinant human IGF-I administration. J. Clin. Endocrinol. Metab. 2014, 99, E703–E712. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. Endocrine and cellular physiology and pathology of the insulin-like growth factor acid-labile subunit. Nat. Rev. Endocrinol. 2024, 20, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, A.; Vigo, V.; Anile, C.; De Bonis, P.; Marziali, G.; Lofrese, G. Role and Importance of IGF-1 in Traumatic Brain Injuries. Biomed. Res. Int. 2015, 2015, 736104. [Google Scholar] [CrossRef] [PubMed]
- Annabi, M.S.; Clisson, M.; Fleury, M.A.; Voisine, M.; Hervault, M.; Shen, M.; Boilard, A.J.; Marette, A.; Ong, G.; Cote, N.; et al. Sex-differences in echocardiographic assessment of aortic valve in young adult LDLr(-/-)/ApoB(100/100)/IGF-II(+/-) mice. Exp. Gerontol. 2020, 140, 111075. [Google Scholar] [CrossRef] [PubMed]
- Trefely, S.; Khoo, P.S.; Krycer, J.R.; Chaudhuri, R.; Fazakerley, D.J.; Parker, B.L.; Sultani, G.; Lee, J.; Stephan, J.P.; Torres, E.; et al. Kinome Screen Identifies PFKFB3 and Glucose Metabolism as Important Regulators of the Insulin/Insulin-like Growth Factor (IGF)-1 Signaling Pathway. J. Biol. Chem. 2015, 290, 25834–25846. [Google Scholar] [CrossRef] [PubMed]
- Araya, P.; Kinning, K.T.; Coughlan, C.; Smith, K.P.; Granrath, R.E.; Enriquez-Estrada, B.A.; Worek, K.; Sullivan, K.D.; Rachubinski, A.L.; Wolter-Warmerdam, K.; et al. IGF1 deficiency integrates stunted growth and neurodegeneration in Down syndrome. Cell Rep. 2022, 41, 111883. [Google Scholar] [CrossRef] [PubMed]
- Ueki, I.; Ooi, G.T.; Tremblay, M.L.; Hurst, K.R.; Bach, L.A.; Boisclair, Y.R. Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin-like growth factor system. Proc. Natl. Acad. Sci. USA 2000, 97, 6868–6873. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pereira, C.; Penedo, M.A.; Rivera-Baltanás, T.; Pérez-Márquez, T.; Alves-Villar, M.; Fernández-Martínez, R.; Veiga, C.; Salgado-Barreira, Á.; Prieto-González, J.M.; Ortolano, S.; et al. Protein Plasma Levels of the IGF Signalling System Are Altered in Major Depressive Disorder. Int. J. Mol. Sci. 2023, 24, 15254. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C.; Martin, J.L.; Beniac, V.A. High molecular weight insulin-like growth factor binding protein complex. Purification and properties of the acid-labile subunit from human serum. J. Biol. Chem. 1989, 264, 11843–11848. [Google Scholar] [CrossRef] [PubMed]
- Sneppen, S.B.; Lange, M.; Pedersen, L.M.; Kristensen, L.L.; Main, K.M.; Juul, A.; Skakkebaek, N.E.; Feldt-Rasmussen, U. Total and free insulin-like growth factor I, insulin-like growth factor binding protein 3 and acid-labile subunit reflect clinical activity in acromegaly. Growth Horm. IGF Res. 2001, 11, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Deng, F.; Zuo, Q.; Liu, L.; Dou, K.; Cheng, Z.; Cao, W.; Luo, C.; Yu, C.; Liu, S.; et al. Virus-inducible IGFALS facilitates innate immune responses by mediating IRAK1 and TRAF6 activation. Cell Mol. Immunol. 2021, 18, 1587–1589. [Google Scholar] [CrossRef] [PubMed]
- Işık, E.; Haliloglu, B.; van Doorn, J.; Demirbilek, H.; Scheltinga, S.A.; Losekoot, M.; Wit, J.M. Clinical and biochemical characteristics and bone mineral density of homozygous, compound heterozygous and heterozygous carriers of three novel IGFALS mutations. Eur. J. Endocrinol. 2017, 176, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Heath, K.E.; Argente, J.; Barrios, V.; Pozo, J.; Díaz-González, F.; Martos-Moreno, G.A.; Caimari, M.; Gracia, R.; Campos-Barros, A. Primary acid-labile subunit deficiency due to recessive IGFALS mutations results in postnatal growth deficit associated with low circulating insulin growth factor (IGF)-I, IGF binding protein-3 levels, and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2008, 93, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Domené, H.M.; Scaglia, P.A.; Lteif, A.; Mahmud, F.H.; Kirmani, S.; Frystyk, J.; Bedecarrás, P.; Gutiérrez, M.; Jasper, H.G. Phenotypic effects of null and haploinsufficiency of acid-labile subunit in a family with two novel IGFALS gene mutations. J. Clin. Endocrinol. Metab. 2007, 92, 4444–4450. [Google Scholar] [CrossRef] [PubMed]
- Twickler, T.B.; Prinsen, B.H.; de Sain-van der Velden, M.G. Components of the IGF system and not insulin itself are strongly associated with apoB100 kinetics in ESRD. Kidney Int. 2004, 65, 1116–1117. [Google Scholar] [CrossRef]
- Schreiter, T.; Gieseler, R.K.; Vílchez-Vargas, R.; Jauregui, R.; Sowa, J.-P.; Klein-Scory, S.; Broering, R.; Croner, R.S.; Treckmann, J.W.; Link, A. Transcriptome-wide analysis of human liver reveals age-related differences in the expression of select functional gene clusters and evidence for a PPP1R10-Governed ‘Aging Cascade’. Pharmaceutics 2021, 13, 2009. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Desert, R.; Ge, X.; Han, H.; Song, Z.; Das, S.; Athavale, D.; You, H.; Nieto, N. The matrisome genes from hepatitis B–related hepatocellular carcinoma unveiled. Hepatol. Commun. 2021, 5, 1571–1585. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kwak, I.; Chung, C.; Choi, W.; Simmen, R.; Simmen, F. Molecular cloning of the porcine acid-labile subunit (ALS) of the insulin-like growth factor-binding protein complex and detection of ALS gene expression in hepatic and non-hepatic tissues. J. Mol. Endocrinol. 2001, 26, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ooi, G.T.; Hurst, K.R.; Poy, M.N.; Rechler, M.M.; Boisclair, Y.R. Binding of STAT5a and STAT5b to a single element resembling a γ-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol. Endocrinol. 1998, 12, 675–687. [Google Scholar] [PubMed]
- Sos, B.C.; Harris, C.; Nordstrom, S.M.; Tran, J.L.; Balázs, M.; Caplazi, P.; Febbraio, M.; Applegate, M.A.; Wagner, K.-U.; Weiss, E.J. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J. Clin. Investig. 2011, 121, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C.; Dai, J. Purification and characterization of the acid-labile subunit of rat serum insulin-like growth factor binding protein complex. Endocrinology 1994, 134, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Gargosky, S.E.; Tapanainen, P.; Rosenfeld, R.G. Administration of growth hormone (GH), but not insulin-like growth factor-I (IGF-I), by continuous infusion can induce the formation of the 150-kilodalton IGF-binding protein-3 complex in GH-deficient rats. Endocrinology 1994, 134, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Barreca, A.; Ketelslegers, J.-M.; Arvigo, M.; Minuto, F.; Thissen, J.-P. Decreased acid-labile subunit (ALS) levels by endotoxin in vivo and by interleukin-1β in vitro. Growth Horm. IGF Res. 1998, 8, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Hawker, F.; To, C.; Stewart, P.; Holman, S. Thrity-day monitoring of insulin-like growth factors and their binding proteins in intensive care unit patients. Growth Horm. IGF Res. 1998, 8, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Baxter, R.; Weekers, F.; Wouters, P.; Bowers, C.; Veldhuis, J. A paradoxical gender dissociation within the growth hormone/insulin-like growth factor I axis during protracted critical illness. J. Clin. Endocrinol. Metab. 2000, 85, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.M.; Amuzie, C.J.; Pestka, J.J. Evaluation of insulin-like growth factor acid-labile subunit as a potential biomarker of effect for deoxynivalenol-induced proinflammatory cytokine expression. Toxicology 2013, 304, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanmartín, A.; Ribas, V.; Suñol, D.; Chiscano-Camón, L.; Palmada, C.; Bajaña, I.; Larrosa, N.; González, J.J.; Canela, N.; Ferrer, R. Characterization of a proteomic profile associated with organ dysfunction and mortality of sepsis and septic shock. PLoS ONE 2022, 17, e0278708. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Nakai, Y.; Shin, J.; Hara, M.; Takeda, Y.; Kubo, S.; Jeremiah, S.S.; Ino, Y.; Akiyama, T.; Moriyama, K. Identification of serum prognostic biomarkers of severe COVID-19 using a quantitative proteomic approach. Sci. Rep. 2021, 11, 20638. [Google Scholar] [CrossRef] [PubMed]
- Völlmy, F.; van den Toorn, H.; Chiozzi, R.Z.; Zucchetti, O.; Papi, A.; Volta, C.A.; Marracino, L.; Dalla Sega, F.V.; Fortini, F.; Demichev, V. A serum proteome signature to predict mortality in severe COVID-19 patients. Life Sci. Alliance 2021, 4, e202101099. [Google Scholar] [CrossRef] [PubMed]
- Barbu, M.; Jónsson, K.; Zetterberg, H.; Blennow, K.; Kolsrud, O.; Ricksten, S.E.; Dellgren, G.; Björk, K.; Jeppsson, A. Serum biomarkers of brain injury after uncomplicated cardiac surgery: Secondary analysis from a randomized trial. Acta Anaesthesiol. Scand. 2022, 66, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Glumac, S.; Kardum, G.; Karanovic, N. Postoperative Cognitive Decline After Cardiac Surgery: A Narrative Review of Current Knowledge in 2019. Med. Sci. Monit. 2019, 25, 3262–3270. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, K.; Zawadka, M.; Polosak, J.; Modi, J.; DiMeglio, M.; Gutsche, J.; Szeto, W.Y.; Puzianowska-Kuznicka, M. Acquired immunological imbalance after surgery with cardiopulmonary bypass due to epigenetic over-activation of PU.1/M-CSF. J. Transl. Med. 2018, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.G.; Weldon, B.C.; Garvan, C.W.; Dede, D.E.; van Der Aa, M.T.; Heilman, K.M.; Gravenstein, J.S. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008, 108, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.; Heinonen, S.E.; Gurzeler, E.; Berglund, L.M.; Dutius Andersson, A.M.; Kotova, O.; Jonsson-Rylander, A.C.; Yla-Herttuala, S.; Gomez, M.F. In vivo inhibition of nuclear factor of activated T-cells leads to atherosclerotic plaque regression in IGF-II/LDLR(-/-)ApoB(100/100) mice. Diab. Vasc. Dis. Res. 2018, 15, 302–313. [Google Scholar] [CrossRef] [PubMed]
- de Souza, K.S.; Ururahy, M.A.; da Costa Oliveira, Y.M.; Loureiro, M.B.; da Silva, H.P.; Bortolin, R.H.; Melo Dos Santos, F.; Luchessi, A.D.; Neto, J.J.; Arrais, R.F.; et al. Low bone mineral density in patients with type 1 diabetes: Association with reduced expression of IGF1, IGF1R and TGF B 1 in peripheral blood mononuclear cells. Diabetes/Metab. Res. Rev. 2016, 32, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, J.; Singh, J.; Alif, R.; Vazquez-Padron, R.I.; Gomes, S.A.; Hare, J.M.; Shehadeh, L.A. miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE-/- mice. Cardiovasc. Res. 2015, 106, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Andrzejczyk, K.; Abou Kamar, S.; van Ommen, A.-M.; Canto, E.D.; Petersen, T.B.; Valstar, G.; Akkerhuis, K.M.; Cramer, M.J.; Umans, V.; Rutten, F.H.; et al. Identifying plasma proteomic signatures from health to heart failure, across the ejection fraction spectrum. Sci. Rep. 2024, 14, 14871. [Google Scholar] [CrossRef] [PubMed]
- Heyse, W.; Vandewalle, V.; Marot, G.; Amouyel, P.; Bauters, C.; Pinet, F. Identification of patient subtypes based on protein expression for prediction of heart failure after myocardial infarction. Iscience 2023, 26, 106171. [Google Scholar] [CrossRef] [PubMed]
- Wysokinski, W.E.; Tafur, A.; Ammash, N.; Asirvatham, S.J.; Wu, Y.; Gosk-Bierska, I.; Grill, D.E.; Slusser, J.P.; Mruk, J.; McBane, R.D. Impact of atrial fibrillation on platelet gene expression. Eur. J. Haematol. 2017, 98, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Bereket, A.; Wilson, T.A.; Blethen, S.L.; Sakurai, Y.; Herndon, D.N.; Wolfe, R.R.; Lang, C.H. Regulation of the acid-labile subunit of the insulin-like growth factor ternary complex in patients with insulin-dependent diabetes mellitus and severe burns. Clin. Endocrinol. 1996, 44, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Li, H.-Y.; Wu, T.-J.; Jeng, C.-Y.; Chuang, L.-M. Correlation of Circulating Acid-Labile Subunit Levels with Insulin Sensitivity and Serum LDL Cholesterol in Patients with Type 2 Diabetes: Findings from a Prospective Study with Rosiglitazone. PPAR Res. 2014, 2014, 917823. [Google Scholar] [CrossRef] [PubMed]
- Lleo, A.; Alcolea, D.; Martinez-Lage, P.; Scheltens, P.; Parnetti, L.; Poirier, J.; Simonsen, A.H.; Verbeek, M.M.; Rosa-Neto, P.; Slot, R.E.R.; et al. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimers Dement. 2019, 15, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Otomo, S.; Maekawa, K.; Baba, T.; Goto, T.; Yamamoto, T. Evaluation of the risk factors for neurological and neurocognitive impairment after selective cerebral perfusion in thoracic aortic surgery. J. Anesth. 2020, 34, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.H.; Ramachandran, R.; Narayan, S.; Jani, A.B.; Vijayakumar, S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 2004, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Barie, P.S.; Hydo, L.J.; Fischer, E. Comparison of APACHE II and III scoring systems for mortality prediction in critical surgical illness. Arch. Surg. 1995, 130, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Peres Bota, D.; Melot, C.; Lopes Ferreira, F.; Nguyen Ba, V.; Vincent, J.L. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002, 28, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Fernandes, P.; Jorge, S.; Goncalves, S.; Alvarez, A.; Costa e Silva, Z.; Franca, C.; Prata, M.M. Acute kidney injury in intensive care unit patients: A comparison between the RIFLE and the Acute Kidney Injury Network classifications. Crit. Care 2008, 12, R110. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, K.; Liu, D.; Karnatovskaia, L.; Devang, S.; Mathew, A.; Szeto, W.Y. Whole Blood Reactivity to Viral and Bacterial Pathogens after Non-Emergent Cardiac Surgery during the Acute and Convalescence Periods Demonstrates a Distinctive Profile of Cytokines Production Compared to the Preoperative Baseline in Cohort of 108 Patients, Suggesting Immunological Reprogramming during the 28 Days Traditionally Recognized as the Post-Surgical Recovery Period. Biomedicines 2023, 12, 28. [Google Scholar] [CrossRef]
- Kong, S.-E.; Firth, S.M.; Baxter, R.C.; Delhanty, P.J. Regulation of the acid-labile subunit in sustained endotoxemia. Am. J. Physiol.-Endocrinol. Metab. 2002, 283, E692–E701. [Google Scholar] [CrossRef] [PubMed]
- Peacock, W.F.t.; De Marco, T.; Fonarow, G.C.; Diercks, D.; Wynne, J.; Apple, F.S.; Wu, A.H. Cardiac troponin and outcome in acute heart failure. N. Engl. J. Med. 2008, 358, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Evke, S.; Lin, Q.; Melendez, J.A.; Begley, T.J. Epitranscriptomic Reprogramming Is Required to Prevent Stress and Damage from Acetaminophen. Genes 2022, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Houglum, J.E.; Harrelson, G.L.; Seefeldt, T.M. Pharmacodynamic Principles: Mechanism of Drug Action and Therapeutic Considerations. In Principles of Pharmacology for Athletic Trainers; Routledge: Oxfordshire, UK, 2024; pp. 36–59. [Google Scholar]
- Poyrazoğlu, Ş.; Hwa, V.; Baş, F.; Dauber, A.; Rosenfeld, R.; Darendeliler, F. A Novel Homozygous Mutation of the Acid-Labile Subunit (IGFALS) Gene in a Male Adolescent. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Melly, L.; Torregrossa, G.; Lee, T.; Jansens, J.L.; Puskas, J.D. Fifty years of coronary artery bypass grafting. J. Thorac. Dis. 2018, 10, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
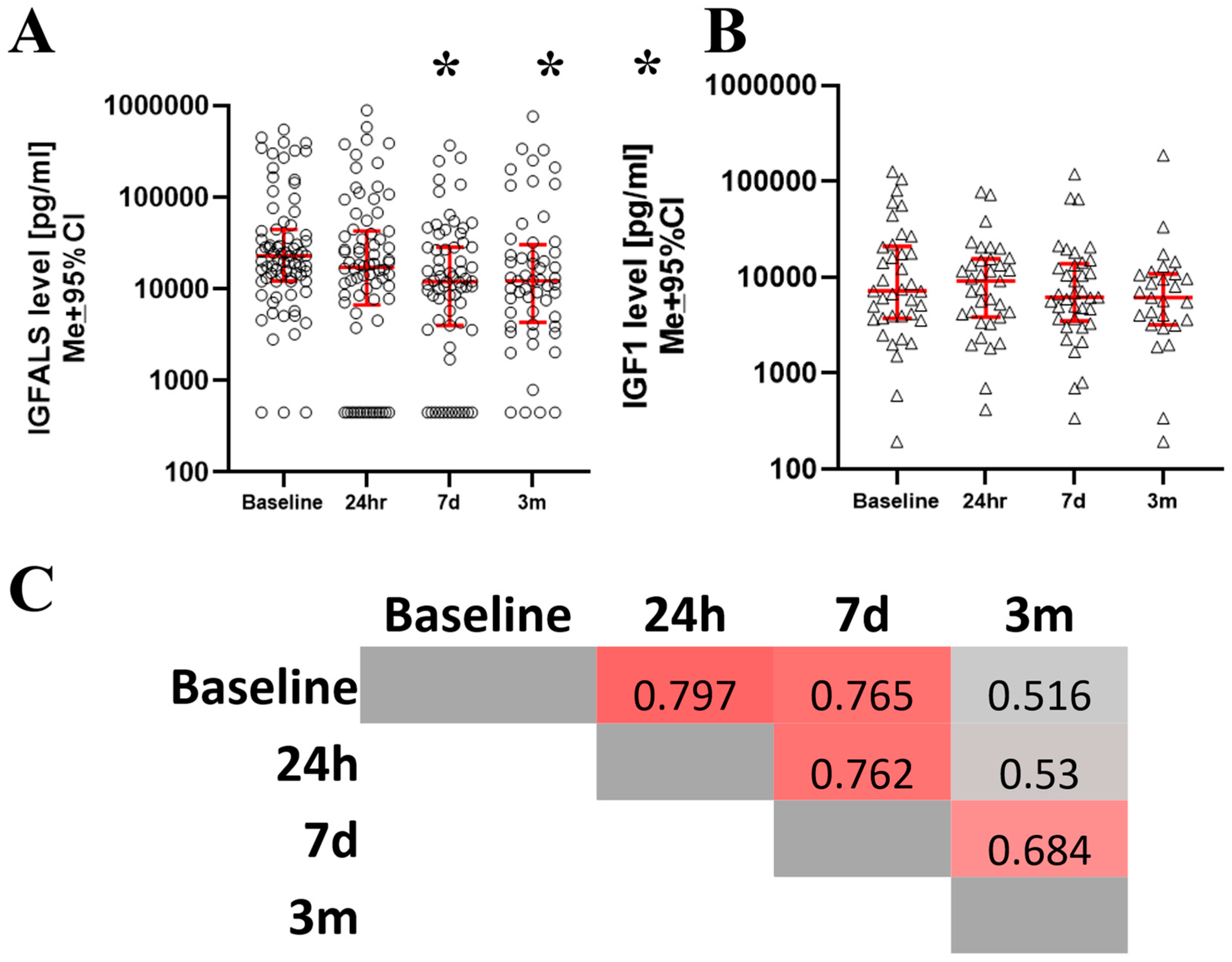
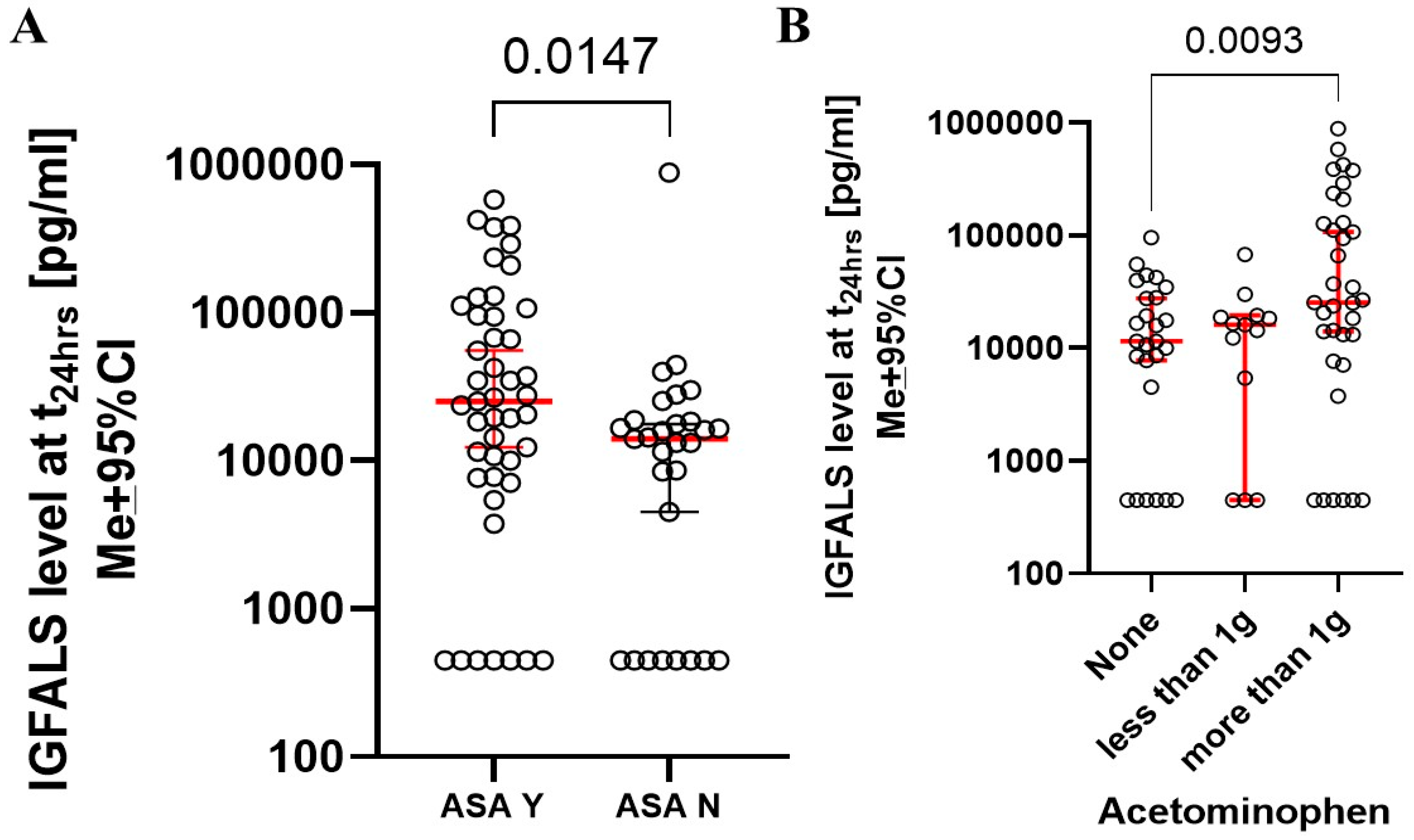
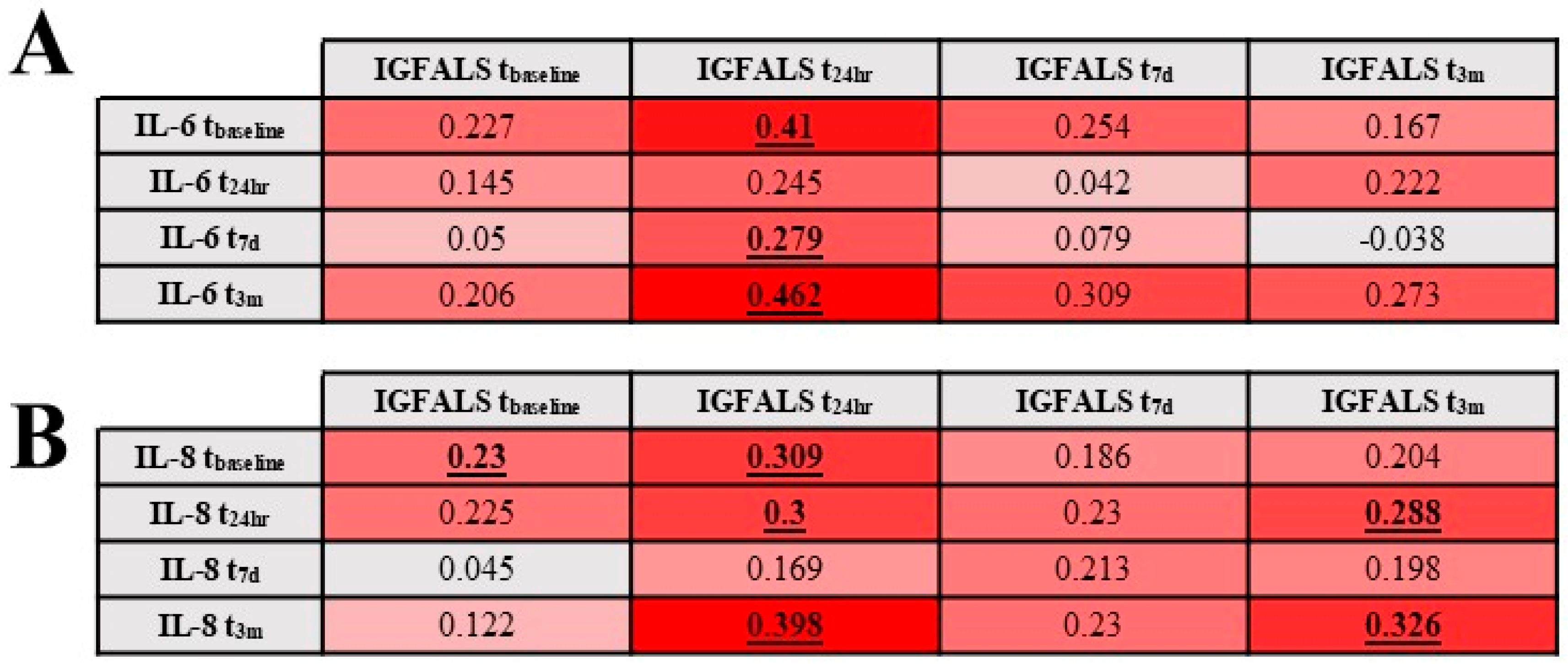
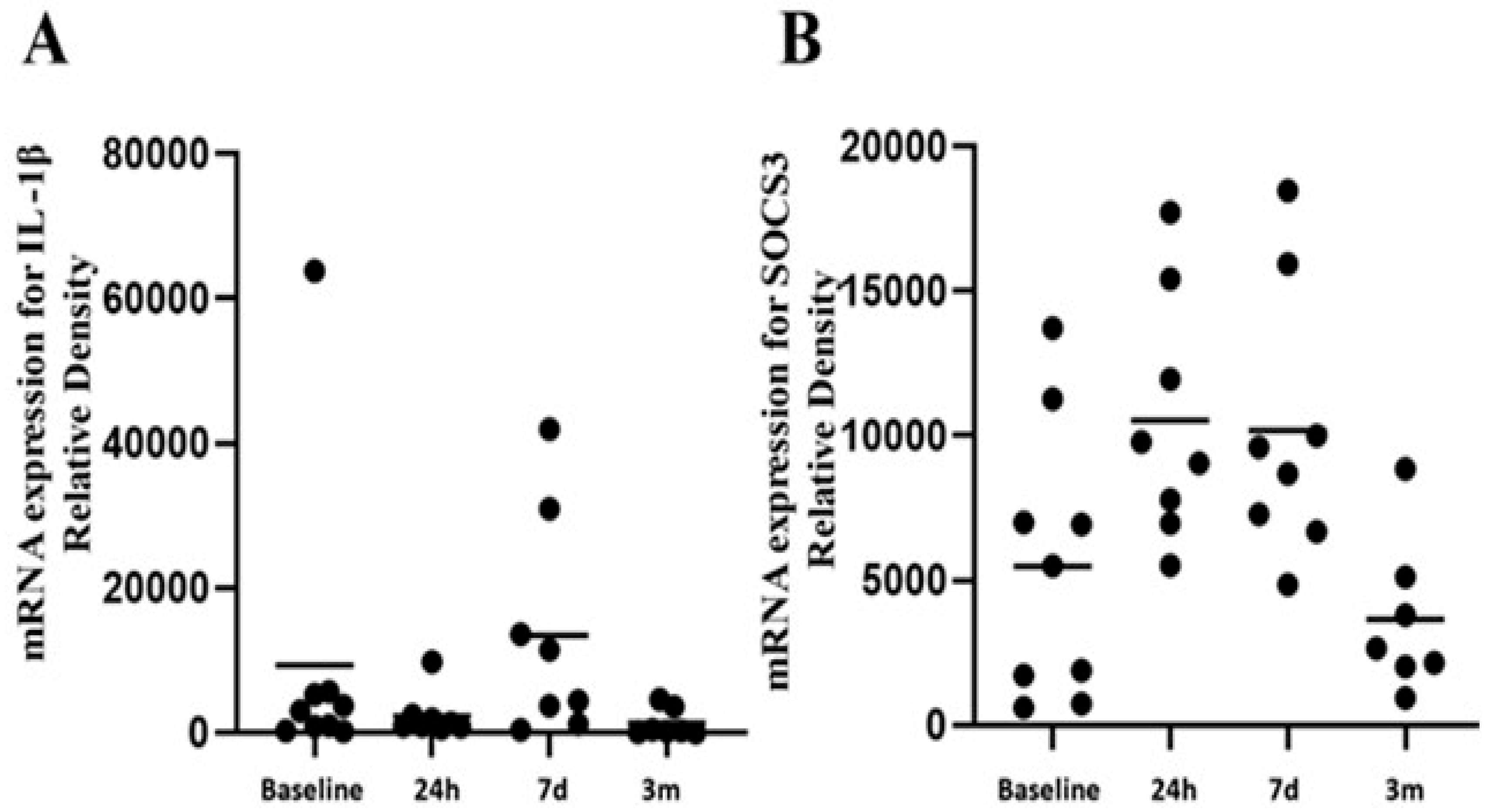
| DEMOGRAPHICS (n = 94) | |
| AGE [X ± SD] | 63.2 ± 11.08 |
| OVER 60 [%] | 68.5% |
| GENDER | |
| MALE [%] | 70.7% |
| FEMALE [%] | 29.3% |
| NOT REPORTED [%] | 0% |
| RACE | |
| WHITE [%] | 89.1% |
| OTHER/ASIAN/BLACK/HISPANIC LATINO/UNKNOWN [%] | 10.9% |
| PRE-EXISTING CONDITIONS | |
| BMI | 27.7 ± 5.73 |
| CHARLESTON COMORBIDITY INDEX [X ± SD] | 3.9 ± 2.12 |
| ACS/MI [%] | 83.7% |
| CHF [%] | 81.5% |
| PVD [%] | 88% |
| CVA/TIA [%] | 87% |
| DEMENTIA [%] | 0% |
| COPD [%] | 93.4% |
| DM [%] | 75% |
| ANESTHESIA AND SURGERY DATA | |
| DURATION OF ANESTHESIA; MEAN ± SD [MIN] | 376.9 ± 102.21 |
| DURATION OF SURGERY; MEAN ± SD [MIN] | 267.4 ± 97.35 |
| DURATION OF CARDIOPULMONARY BYPASS; MEAN ± SD [MIN] | 130.4 ± 64.49 |
| CORONARY ARTERY BYPASS SURGERY; NO. | 44 |
| MITRAL VALVULOPLASTY AND REPLACEMENT; NO. | 15 |
| AORTIC VALVULOPLASTY AND REPLACEMENT; NO. | 22 |
| AORTIC ANEURYSM REPAIR; NO. | 4 |
| OTHERS; NO | 7 |
| TRANSFUSIONS DURING SURGERY | |
| PACKED RED BLOOD CELLS, MEAN (IQ25; IQ75) [ML] | 159.8 [0; 0] |
| FRESH FROZEN PLASMA, MEAN (IQ25; IQ75) [ML] | 154.9 [0; 0] |
| TOTAL CRYSTALLOID DURING SURGERY[ML] | 97.3 ± 186.92 |
| CLINICAL CARE DURING 24 H POST-SURGERY | |
| PACKED RED BLOOD CELLS, MEAN; (IQ25; IQ75) [ML] | 19.8 [0;0] |
| FRESH FROZEN PLASMA, MEAN; (IQ25; IQ75) [ML] | 0 [0; 0] |
| PERIOPERATIVE MEDICATIONS | |
| CORTICOSTEROID ADMINISTRATION (% OF ALL CASES) | 1.1% |
| KETOROLAC ADMINISTRATION (% OF ALL CASES) | 5% |
| ACETAMINOPHEN ADMINISTRATION (% OF ALL CASES) | 69.6% |
| ACETYLSALICYLIC ACID ADMINISTRATION | 67.4% |
| OPIOIDS ADMINISTRATION | 90.1 ± 25.08 |
| BZD ADMINISTRATION | 3.5 ± 1.65 |
| ICU STAY | |
| APACHE SCORE AT 1 H, MEAN ± SD | 16.4 ± 5.83 |
| APACHE SCORE AT 24 H, MEAN ± SD | 9.2 ± 4.74 |
| APACHE SCORE AT 48 H, MEAN ± SD | 9.0 ± 4.28 |
| OUTCOME AT 28 DAYS | |
| LOS ICU | 9.3 ± 42.97 |
| LOS HOSPITAL | 10.5 ± 22.1 |
| DVT | 3.3% |
| PE | 0% |
| CVA | 14.1% |
| DISCHARGED/IN THE HEALTHCARE FACILITY/EXPIRED | 88%/6.5%/4.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laudanski, K.; Mahmoud, M.A.; Gad, H.; Diedrich, D.A. Depletion of IGFALS Serum Level up to 3 Months After Cardiac Surgery, with Exploration of Potential Relationships to Surrogates of Organ Failures and Clinical Outcomes. Curr. Issues Mol. Biol. 2025, 47, 581. https://doi.org/10.3390/cimb47080581
Laudanski K, Mahmoud MA, Gad H, Diedrich DA. Depletion of IGFALS Serum Level up to 3 Months After Cardiac Surgery, with Exploration of Potential Relationships to Surrogates of Organ Failures and Clinical Outcomes. Current Issues in Molecular Biology. 2025; 47(8):581. https://doi.org/10.3390/cimb47080581
Chicago/Turabian StyleLaudanski, Krzysztof, Mohamed A. Mahmoud, Hossam Gad, and Daniel A. Diedrich. 2025. "Depletion of IGFALS Serum Level up to 3 Months After Cardiac Surgery, with Exploration of Potential Relationships to Surrogates of Organ Failures and Clinical Outcomes" Current Issues in Molecular Biology 47, no. 8: 581. https://doi.org/10.3390/cimb47080581
APA StyleLaudanski, K., Mahmoud, M. A., Gad, H., & Diedrich, D. A. (2025). Depletion of IGFALS Serum Level up to 3 Months After Cardiac Surgery, with Exploration of Potential Relationships to Surrogates of Organ Failures and Clinical Outcomes. Current Issues in Molecular Biology, 47(8), 581. https://doi.org/10.3390/cimb47080581







