Pharmacological Evaluation of Polygoni Multiflori Radix Praeparata Extract: Inhibition of PANoptosis in Alleviating Premature Ovarian Insufficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PMRP
2.3. Component Analysis of PMRP
2.4. POI Rat Model and Treatment
2.5. Estrous Cycle Evaluation and Ovarian Index Measurement
2.6. ELISA
2.7. Hematoxylin–Eosin (HE) Staining
2.8. Transmission Electron Microscopy (TEM)
2.9. Immunofluorescence
2.10. Western Blot
2.11. Statistical Analysis
3. Results
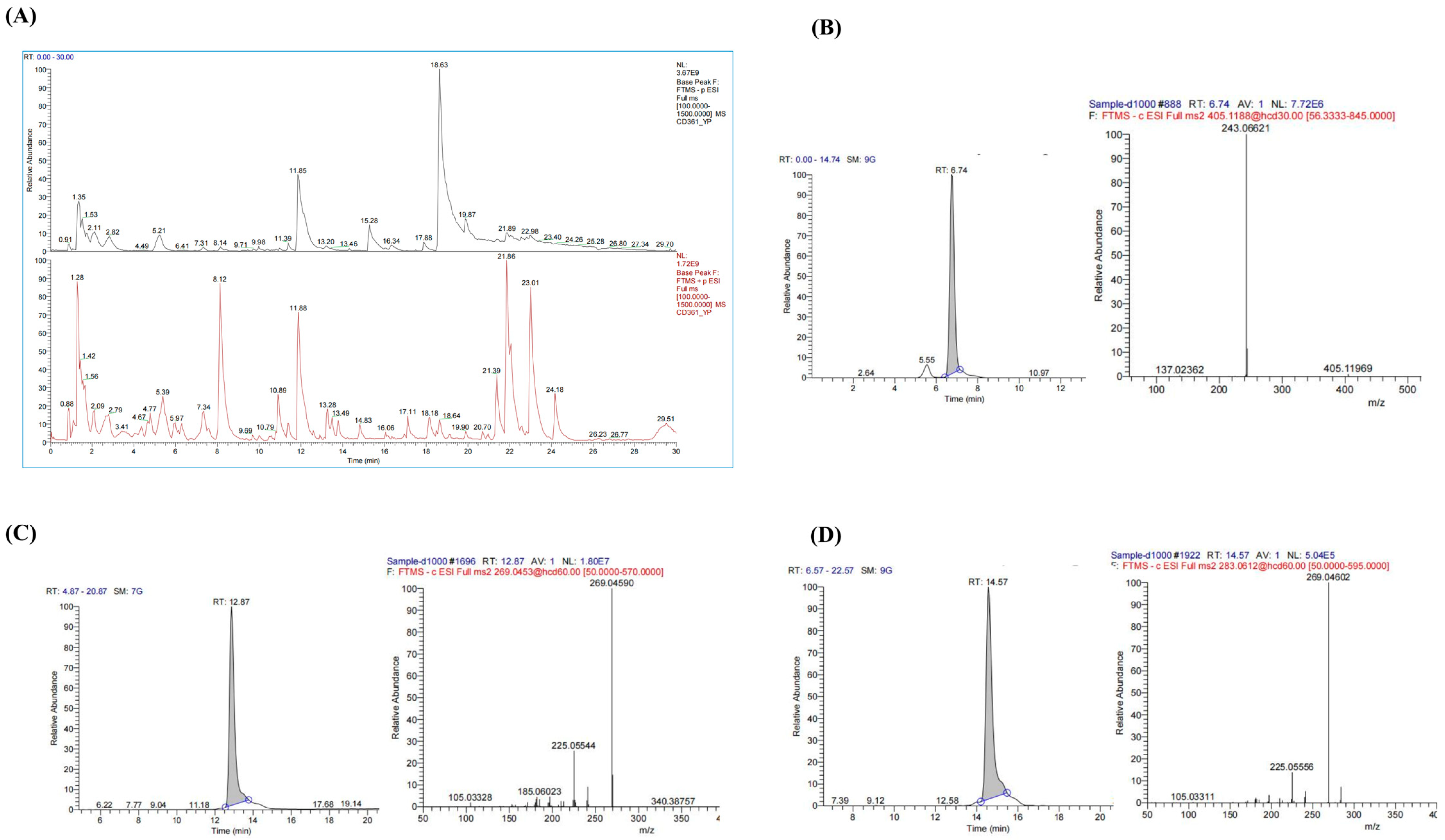
3.1. Component Analysis of PMRP
3.2. PMRP Improves Estrous Cycle and Ovarian Index in POI Rats
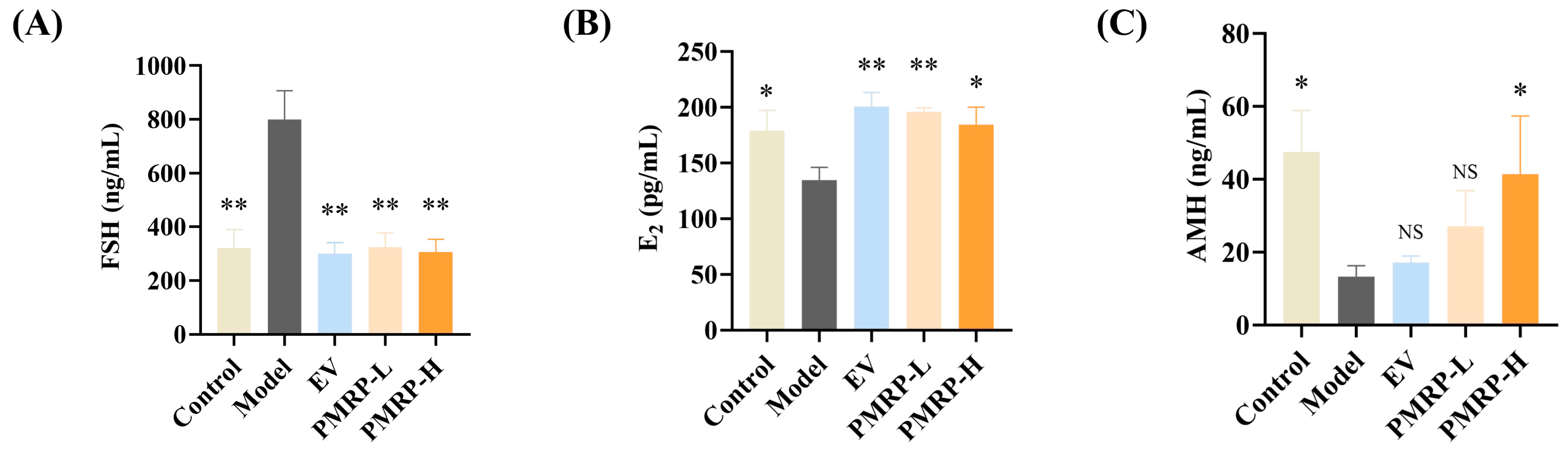
3.3. PMRP Regulates Serum Sex Hormone Levels in POI Rats
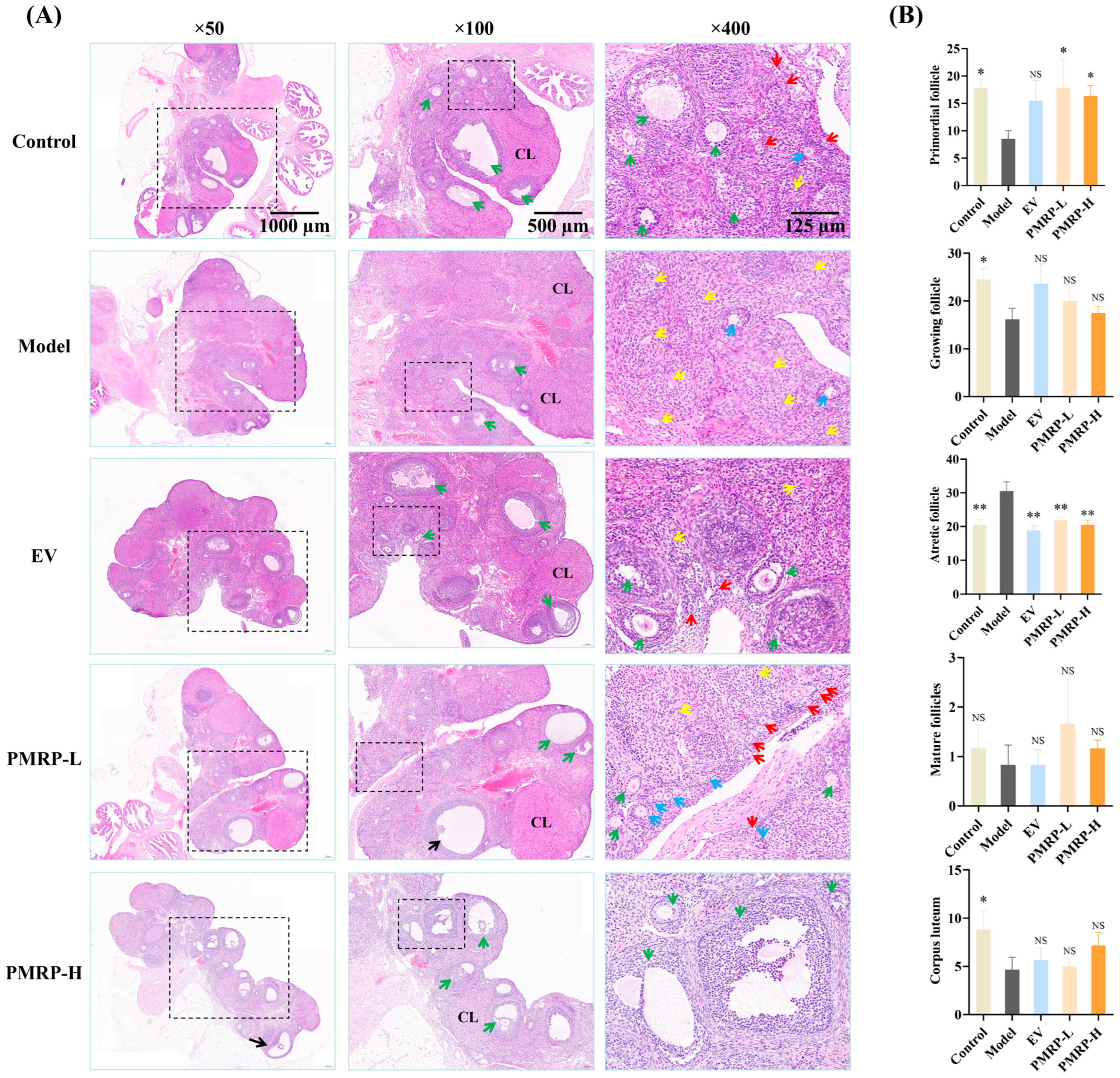
3.4. PMRP Improves Follicular Development in POI Rats
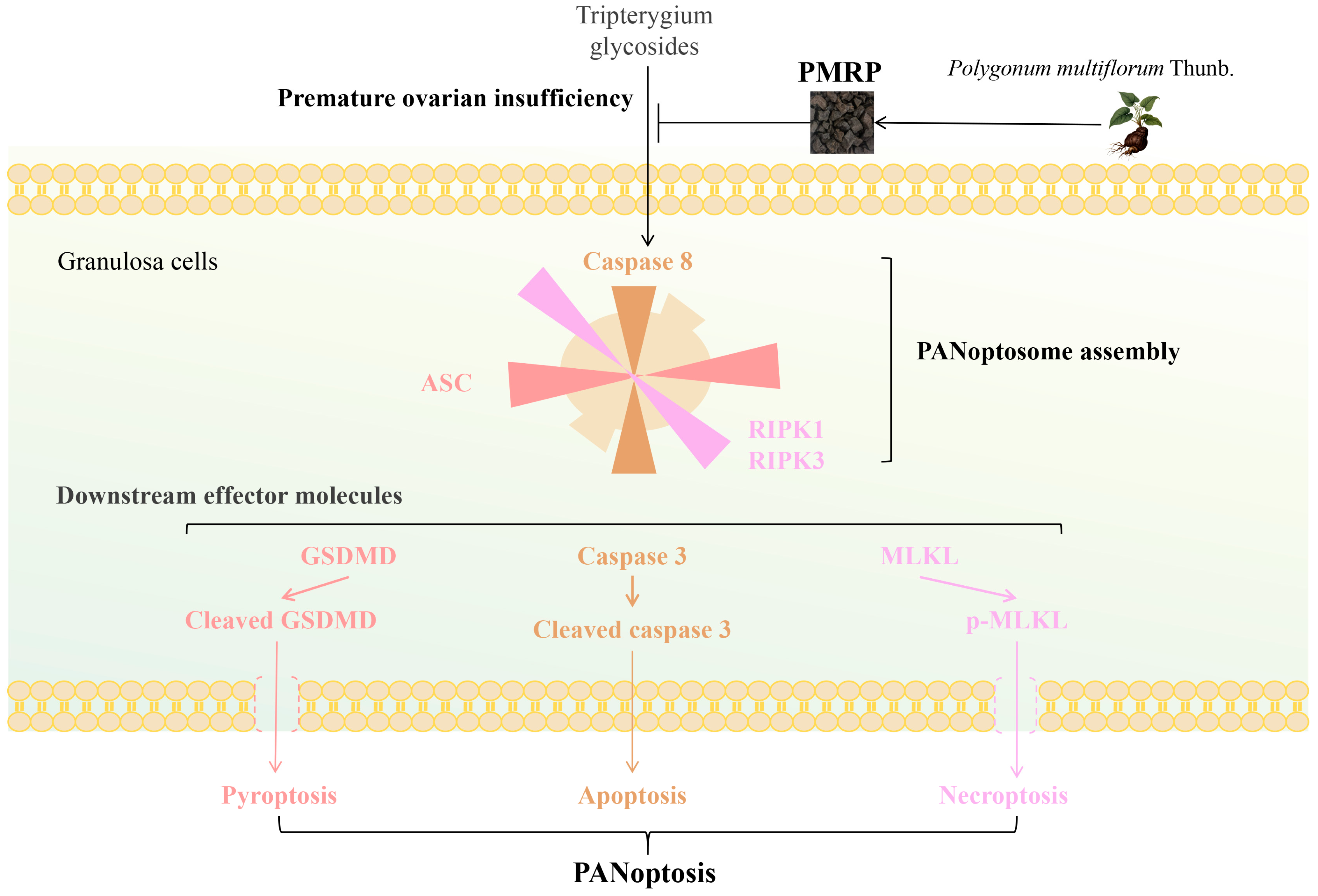
3.5. PMRP Mitigates PANoptosis-like Death of Granulosa Cells in POI Rats
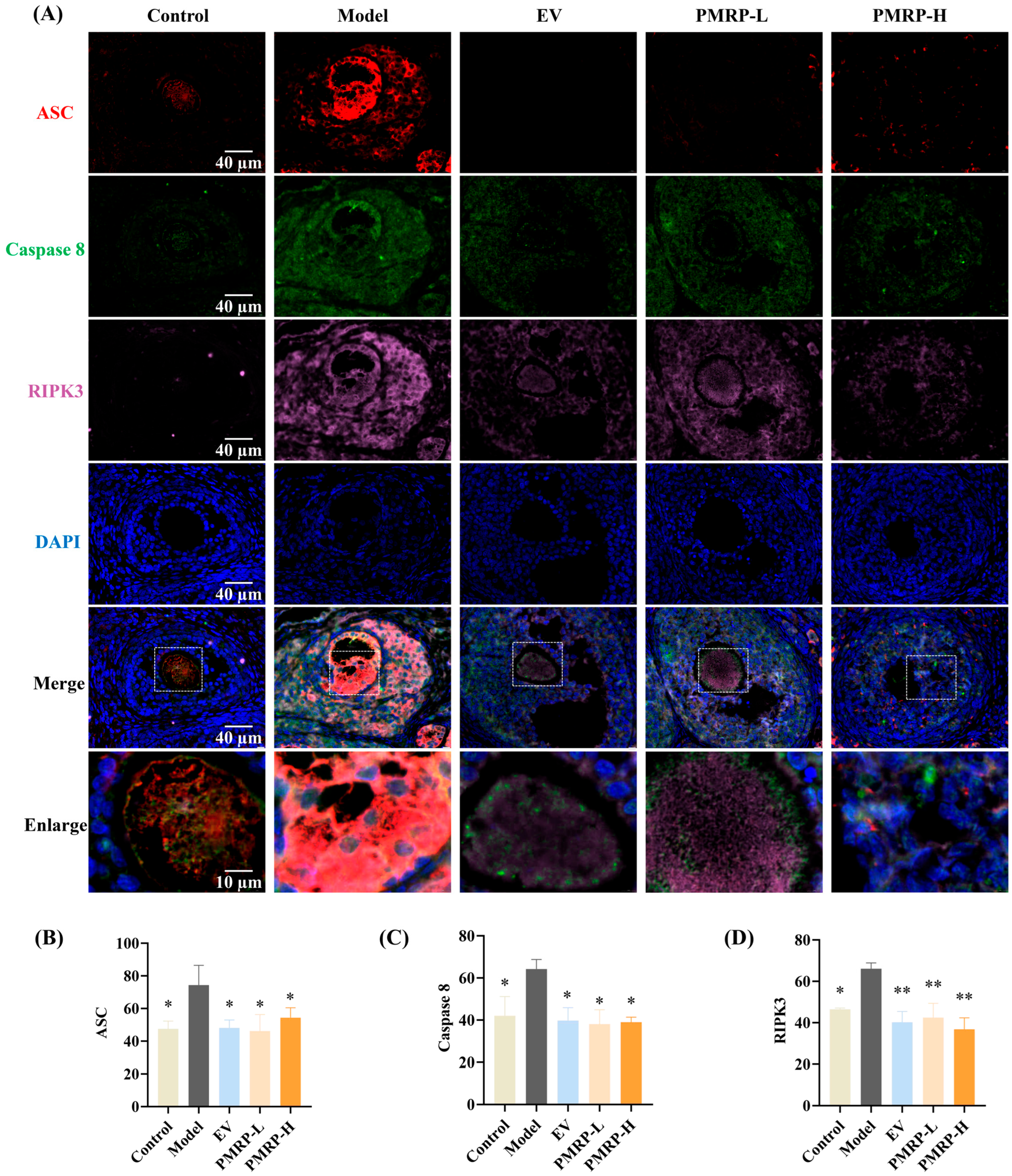
3.6. PMRP Inhibits PANoptosome Assembly in POI Rat Follicles
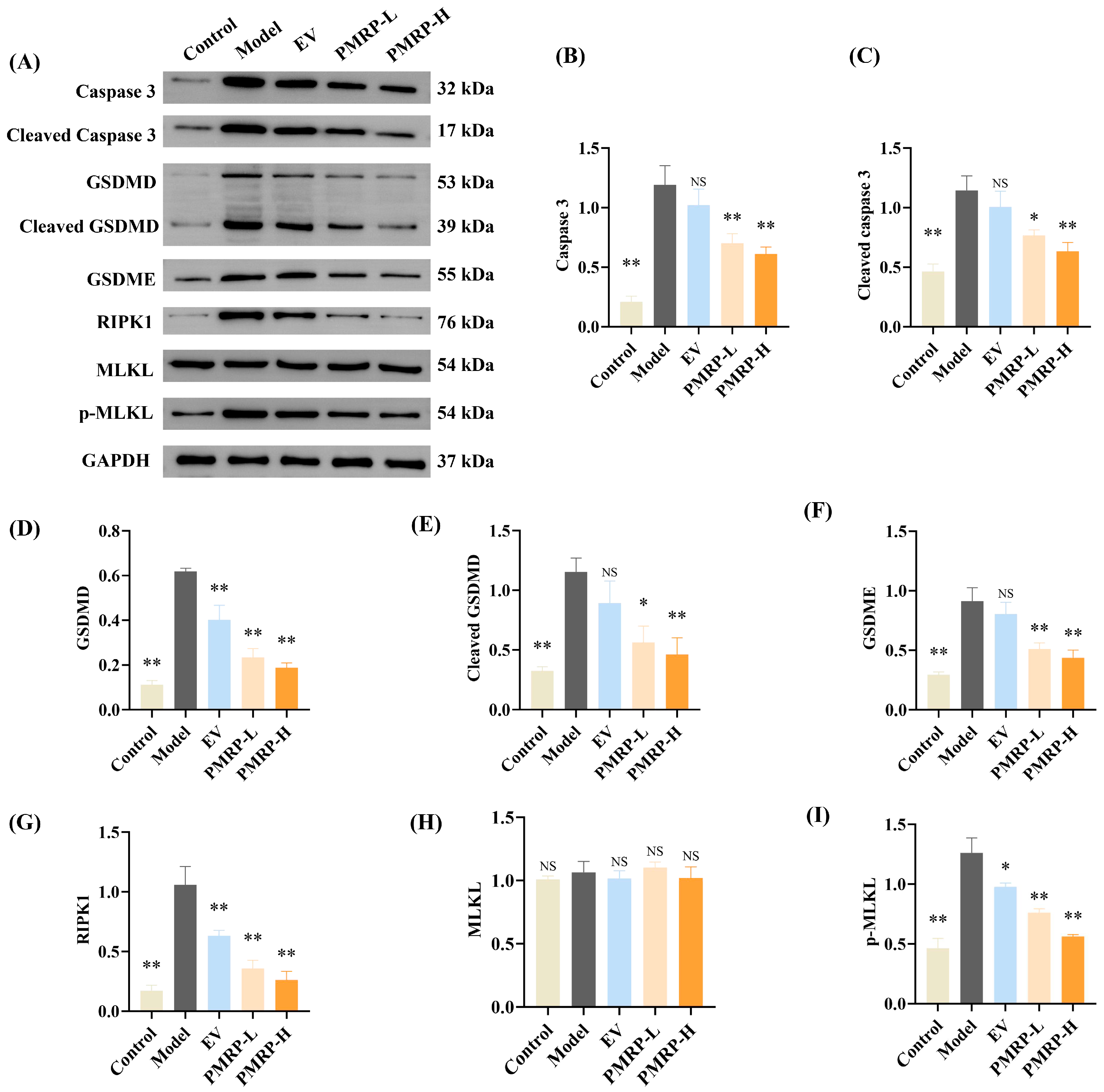
3.7. PMRP Suppresses PANoptosis Effector Proteins in POI Rat Ovaries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMH | Anti-Müllerian hormone |
| AIM2 | Absent in melanoma 2 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| E2 | Estrogen |
| FSH | Follicle-stimulating hormone |
| GSDMD/E | gasdermin D/E |
| HE | Hematoxylin–eosin |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| MLKL | Mixed lineage kinase domain-like protein |
| NLRP12 | NOD-like receptors family pyrin domain containing 12 |
| POI | Premature ovarian insufficiency |
| POF | Premature ovarian failure |
| PMRP | Polygoni Multiflori Radix Praeparata |
| RIPK1/3 | Receptor-interacting protein kinase 1/3 |
| SEM | Standard error of the mean |
| TSG | 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside |
| TG | Tripterygium glycosides |
| TEM | Transmission electron microscopy |
References
- Stuenkel, C.A.; Gompel, A. Primary Ovarian Insufficiency. N. Engl. J. Med. 2023, 388, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Panay, N.; Anderson, R.A.; Bennie, A.; Cedars, M.; Davies, M.; Ee, C.; Gravholt, C.H.; Kalantaridou, S.; Kallen, A.; Kim, K.Q.; et al. Evidence-based guideline: Premature Ovarian Insufficiency. Fertil. Steril. 2025, 123, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.M.; Alesi, L.R.; Winship, A.L.; Hutt, K.J. Beyond apoptosis: Evidence of other regulated cell death pathways in the ovary throughout development and life. Hum. Reprod. Update 2023, 29, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Li, F.; Yu, H.; Xiong, Q.; Hou, Q.; Meng, Y. Honokiol alleviates radiation-induced premature ovarian failure via enhancing Nrf2. Am. J. Reprod. Immunol. 2023, 90, e13769. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jing, Y.; Qu, X.; Yang, J.; Pan, P.; Liu, X.; Gao, H.; Pei, X.; Zhang, C.; Yang, Y. The Activation of Reticulophagy by ER Stress through the ATF4-MAP1LC3A-CCPG1 Pathway in Ovarian Granulosa Cells Is Linked to Apoptosis and Necroptosis. Int. J. Mol. Sci. 2023, 24, 2749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kanneganti, T.D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021, 19, 4641–4657. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, A.; Kanneganti, T.D. Therapeutic potential of PANoptosis: Innate sensors, inflammasomes, and RIPKs in PANoptosomes. Trends Mol. Med. 2024, 30, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Lei, J.; Li, F.; Wang, X.; Fu, H.; Yan, Z.; Zhu, Y. Short-Chain Chlorinated Paraffins May Induce Ovarian Damage in Mice via AIM2- and NLRP12-PANoptosome. Environ. Sci. Technol. 2025, 59, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Del Mastro, L.; Bruzzone, M.; Ceppi, M.; Razeti, M.G.; Fregatti, P.; Ruelle, T.; Pronzato, P.; Massarotti, C.; Franzoi, M.A.; et al. Safety of systemic hormone replacement therapy in breast cancer survivors: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2022, 191, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Skeith, L.; Le Gal, G.; Rodger, M.A. Oral contraceptives and hormone replacement therapy: How strong a risk factor for venous thromboembolism? Thromb. Res. 2021, 202, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China 2020; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Xu, W.; Li, X.J.; Zhong, Y.S.; He, J.Q.; Xie, W.; Kang, Y.K.; Ying, H.Z.; Yu, C.H. Structural characterizations and antiaging activities of hydrolyzed low-molecular-weight polysaccharides from Polygoni Multiflori Radix Praeparata. Carbohydr. Polym. 2025, 356, 123381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, J.; Tang, W.; Li, Y.; Lin, C.; Peng, D.; Yang, C. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside (TSG) from Polygonum multiflorum Thunb.: A Systematic Review on Anti-Aging. Int. J. Mol. Sci. 2025, 26, 3381. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Feng, C.; Wu, Z.; Yang, Y.; Gao, X.; Zhu, L.; Liu, Y.; Gao, Y. Phytochemistry, pharmacology, toxicology and detoxification of Polygonum multiflorum Thunb.: A comprehensive review. Front. Pharmacol. 2024, 15, 1427019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, Y.F.; Peng, F.; Yang, C.F.; Chen, G.Q.; Lu, T.; Liu, J. Effect of polygoni multiflori radix Preparata on diminished ovarian reserve in rats. J. Med. Postgra. 2018, 31, 602–607. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Y.F.; Peng, F.; Yang, C.F.; Fu, Y.Y.; Tian, M. Study on Phytoestrogen-like Effect and Mechanism of Polygoni Multiflori Radix Preparata. Chin. Arch. Tradit. Chin. Med. 2020, 38, 216–220. [Google Scholar] [CrossRef]
- Lin, H.Y.; Yang, Y.N.; Chen, Y.F.; Huang, T.Y.; Crawford, D.R.; Chuang, H.Y.; Chin, Y.T.; Chu, H.R.; Li, Z.L.; Shih, Y.J.; et al. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-Glucoside improves female ovarian aging. Front. Cell Dev. Biol. 2022, 10, 862045. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, N.; Peng, Z.; Pang, X.; Ning, N.; Du, Q.; Guo, Q.; Huang, Q. Zishen Yutai pill ameliorates ovarian reserve by mediating PI3K-Akt pathway and apoptosis level in ovary. J. Ovarian Res. 2025, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Pouladvand, N.; Azarnia, M.; Zeinali, H.; Fathi, R.; Tavana, S. An overview of different methods to establish a murine premature ovarian failure model. Animal Model. Exp. Med. 2024, 7, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.C.; Zeng, Y.S.; Zhou, Z.M.; Xiao, L. Histology and Embryology; People’s Medical Publishing House: Beijing, China, 2015; pp. 298–304. [Google Scholar]
- Fiorentino, G.; Cimadomo, D.; Innocenti, F.; Soscia, D.; Vaiarelli, A.; Ubaldi, F.M.; Gennarelli, G.; Garagna, S.; Rienzi, L.; Zuccotti, M. Biomechanical forces and signals operating in the ovary during folliculogenesis and their dysregulation: Implications for fertility. Hum. Reprod. Update 2023, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, J.; Oh, J.; Yu, G.; Ryu, H.; Kim, D.; Lee, S. Integrated NLRP3, AIM2, NLRC4, Pyrin inflammasome activation and assembly drive PANoptosis. Cell Mol. Immunol. 2023, 20, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Liu, B.; Xie, Z.; He, Y.; Xue, D.; Zhao, D.; Hao, C. Global trends in PANoptosis research: Bibliometrics and knowledge graph analysis. Apoptosis 2024, 29, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Geng, Z.X.; Yi, X.; Wei, X.; Zhu, X.H.; Jiang, D.S. PANoptosis: A novel target for cardiovascular diseases. Trends Pharmacol. Sci. 2024, 45, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiao, H.; Qu, L.; Liu, H. Association Between Hormone Replacement Therapy and Development of Endometrial Cancer: Results From a Prospective US Cohort Study. Front. Med. 2021, 8, 802959. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Wang, P.; Wei, M.; Yang, Q.; Dong, X. Yu Linzhu alleviates primary ovarian insufficiency in a rat model by improving proliferation and energy metabolism of granulosa cells through hif1α/cx43 pathway. J. Ovarian Res. 2024, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Padhiar, A.A.; Wang, T.; He, L.; Chen, M.; Wu, S.; Zhou, Y.; Zhou, G. Stem Cell-Based Therapeutic Strategies for Premature Ovarian Insufficiency and Infertility: A Focus on Aging. Cells 2022, 11, 3713. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, M.; Liu, C.; Wang, L.; Wei, H.; Zhang, R.; Ren, Z.; Chen, Y.; Luo, M.; Zhao, J.; et al. Epg5 deficiency leads to primary ovarian insufficiency due to WT1 accumulation in mouse granulosa cells. Autophagy 2023, 19, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, J.H.; Chen, T.Q.; Peng, F. PANoptosis: A novel perspective on the pathogenesis of ovarian hypofunction. Chin. J. Cell Mol. Immunol. 2025, 1–9. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, T.; Wen, Y.; Li, W.; Xu, J.; Chen, Y.; Chen, D.; Zhu, Y. Oral administration of cranberry-derived exosomes attenuates murine premature ovarian failure in association with changes in the specific gut microbiota and diminution in ovarian granulosa cell PANoptosis. Food Funct. 2024, 15, 11697–11714. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, B.; Pandian, N.; Kim, H.J.; Abdelaal, H.M.; Mall, R.; Indari, O.; Sarkar, R.; Tweedell, R.E.; Alonzo, E.Q.; Klein, J.; et al. NLRC5 senses NAD(+) depletion, forming a PANoptosome and driving PANoptosis and inflammation. Cell 2024, 187, 4061–4077.e17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Karki, R.; Wang, Y.; Nguyen, L.N.; Kalathur, R.C.; Kanneganti, T.D. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature 2021, 597, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Karki, R.; Rajesh, Y.; Kanneganti, T.D. Immune regulator IRF1 contributes to ZBP1-, AIM2-, RIPK1-, and NLRP12-PANoptosome activation and inflammatory cell death (PANoptosis). J. Biol. Chem. 2023, 299, 105141. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Li, J.; Li, Y.; Chen, D.; Lin, C. Pharmacological Evaluation of Polygoni Multiflori Radix Praeparata Extract: Inhibition of PANoptosis in Alleviating Premature Ovarian Insufficiency. Curr. Issues Mol. Biol. 2025, 47, 569. https://doi.org/10.3390/cimb47070569
Zhu C, Li J, Li Y, Chen D, Lin C. Pharmacological Evaluation of Polygoni Multiflori Radix Praeparata Extract: Inhibition of PANoptosis in Alleviating Premature Ovarian Insufficiency. Current Issues in Molecular Biology. 2025; 47(7):569. https://doi.org/10.3390/cimb47070569
Chicago/Turabian StyleZhu, Can, Jinhong Li, Yaofeng Li, Daiyong Chen, and Chang Lin. 2025. "Pharmacological Evaluation of Polygoni Multiflori Radix Praeparata Extract: Inhibition of PANoptosis in Alleviating Premature Ovarian Insufficiency" Current Issues in Molecular Biology 47, no. 7: 569. https://doi.org/10.3390/cimb47070569
APA StyleZhu, C., Li, J., Li, Y., Chen, D., & Lin, C. (2025). Pharmacological Evaluation of Polygoni Multiflori Radix Praeparata Extract: Inhibition of PANoptosis in Alleviating Premature Ovarian Insufficiency. Current Issues in Molecular Biology, 47(7), 569. https://doi.org/10.3390/cimb47070569





