Oral Squamous Cell Carcinoma Exosomes Upregulate PIK3/AKT, PTEN, and NOTCH Signaling Pathways in Normal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Analyses
MicroRNA Expression and GEO Dataset Analysis
2.2. Cell Cultures
2.3. Exosome Isolation
2.4. Exosome Characterization
2.5. RNA Isolation and Reverse Transcription
2.6. TaqMan microRNA Assay
2.7. Statistical Analysis
3. Results
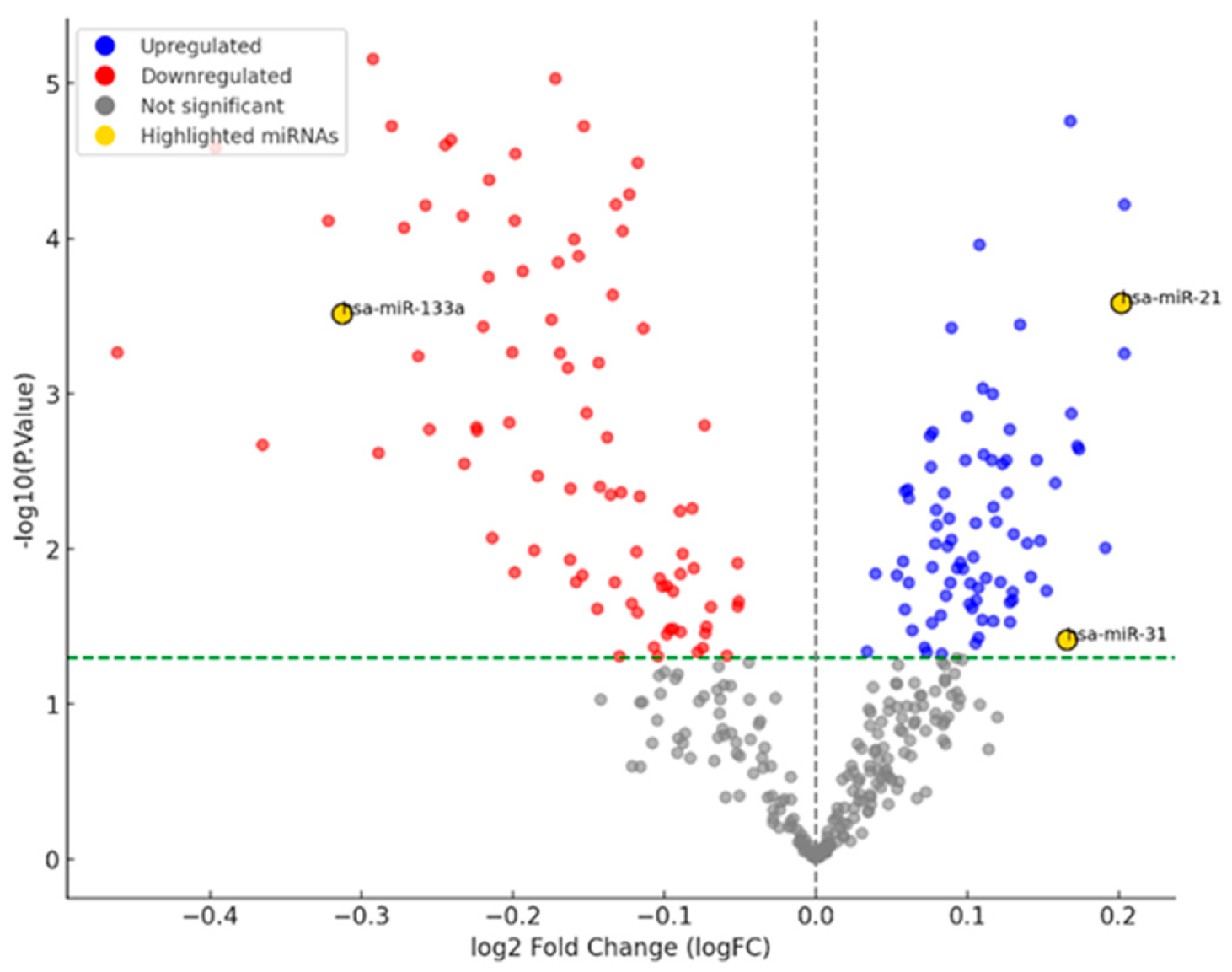
3.1. miRNA Network Analysis in OSCC
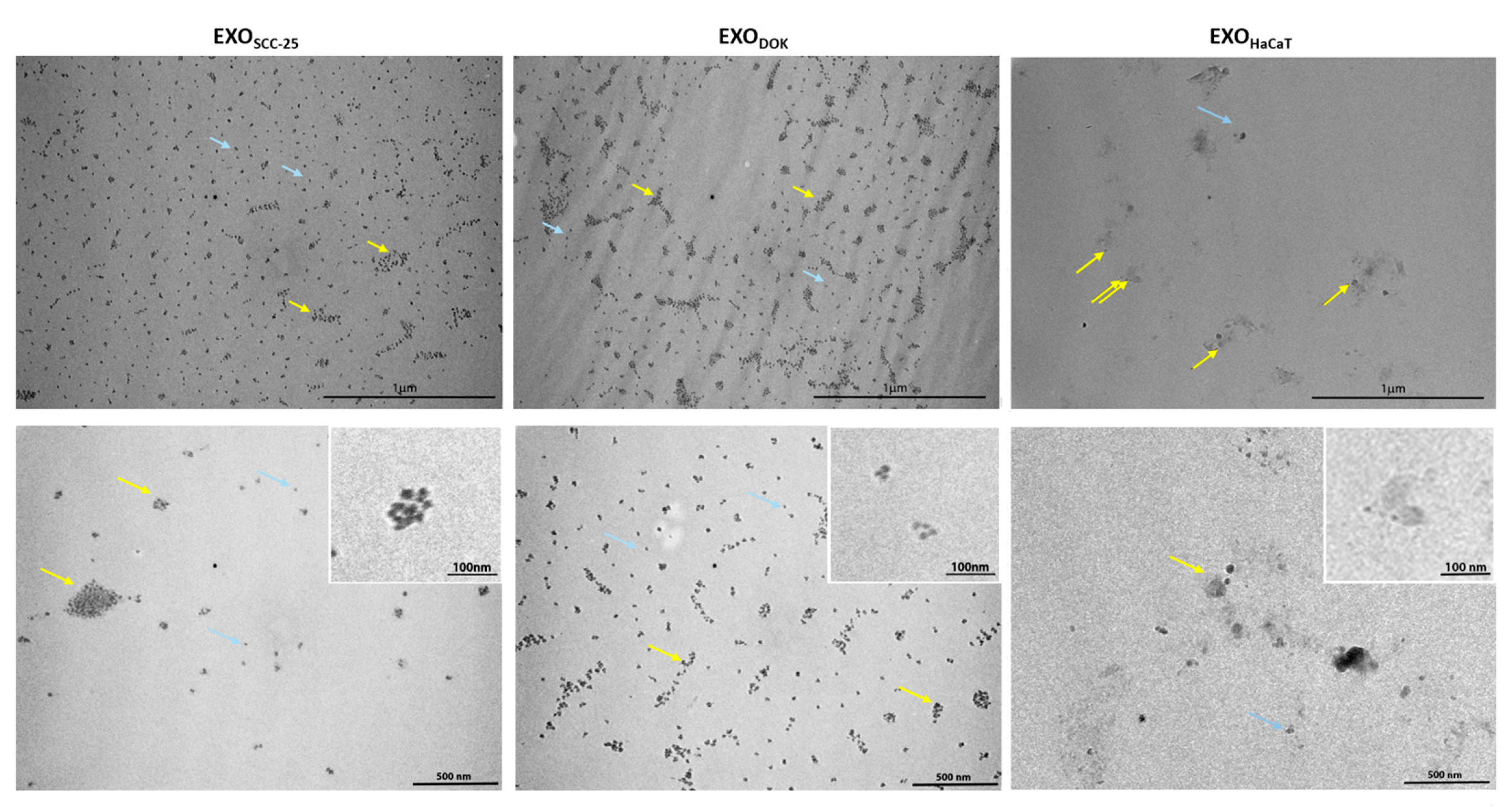
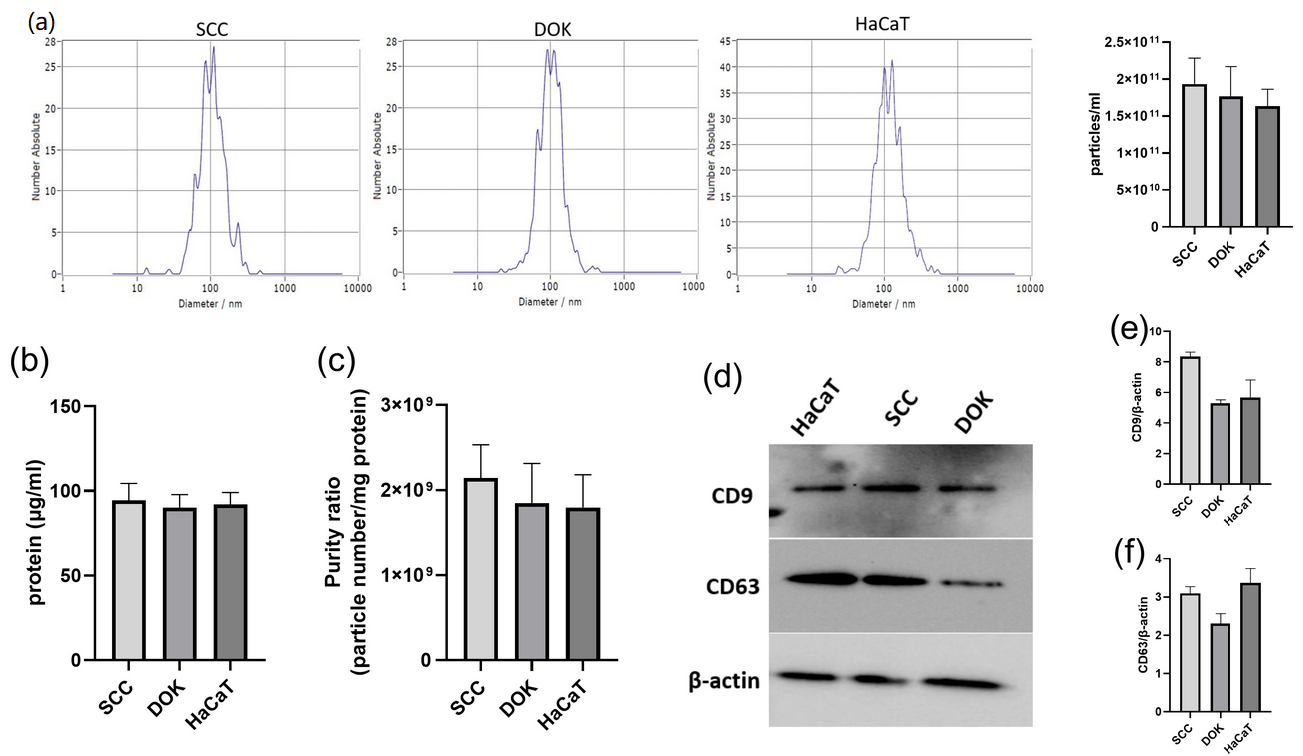
3.2. Characterization of Exosomes Isolated from SCC, DOK, and HaCaT Cell Lines
3.3. MicroRNA Expression in SCC, DOK, and HaCaT Cell Lines
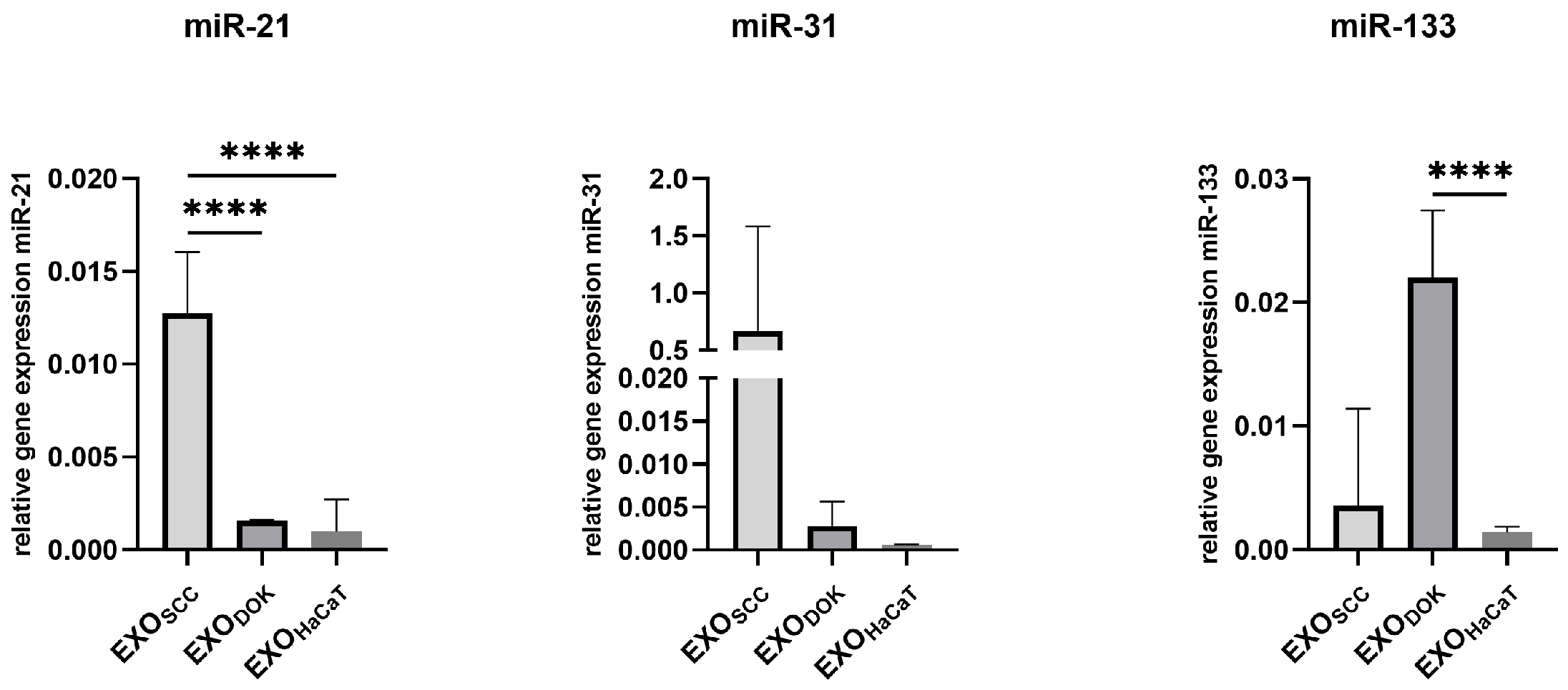
3.4. MicroRNA Expression in SCC, DOK, and HaCaT Exosomes
3.5. SCC, DOK, and HaCaT Exosomes and Conditioned Medium Effects on Fibroblasts
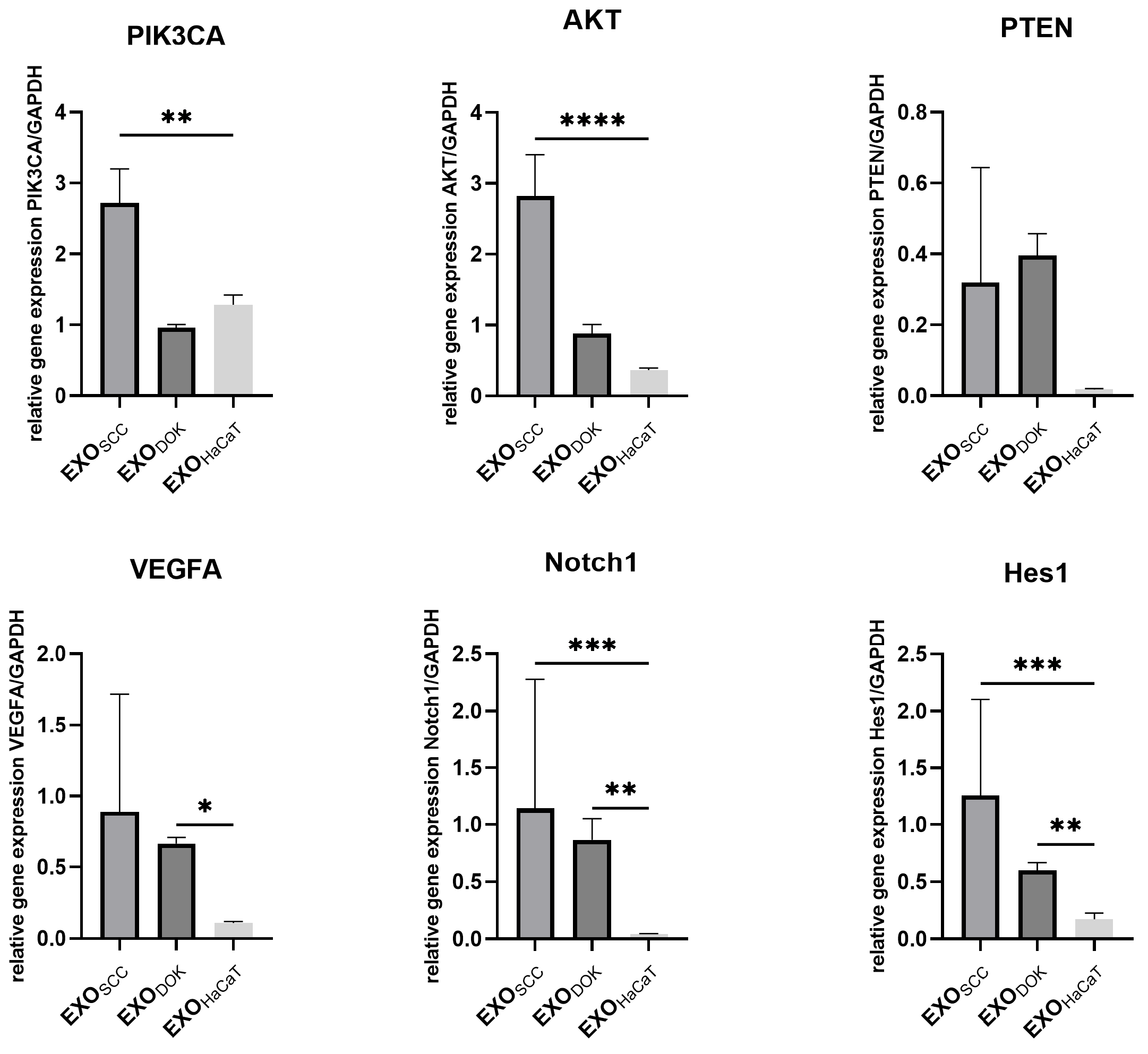
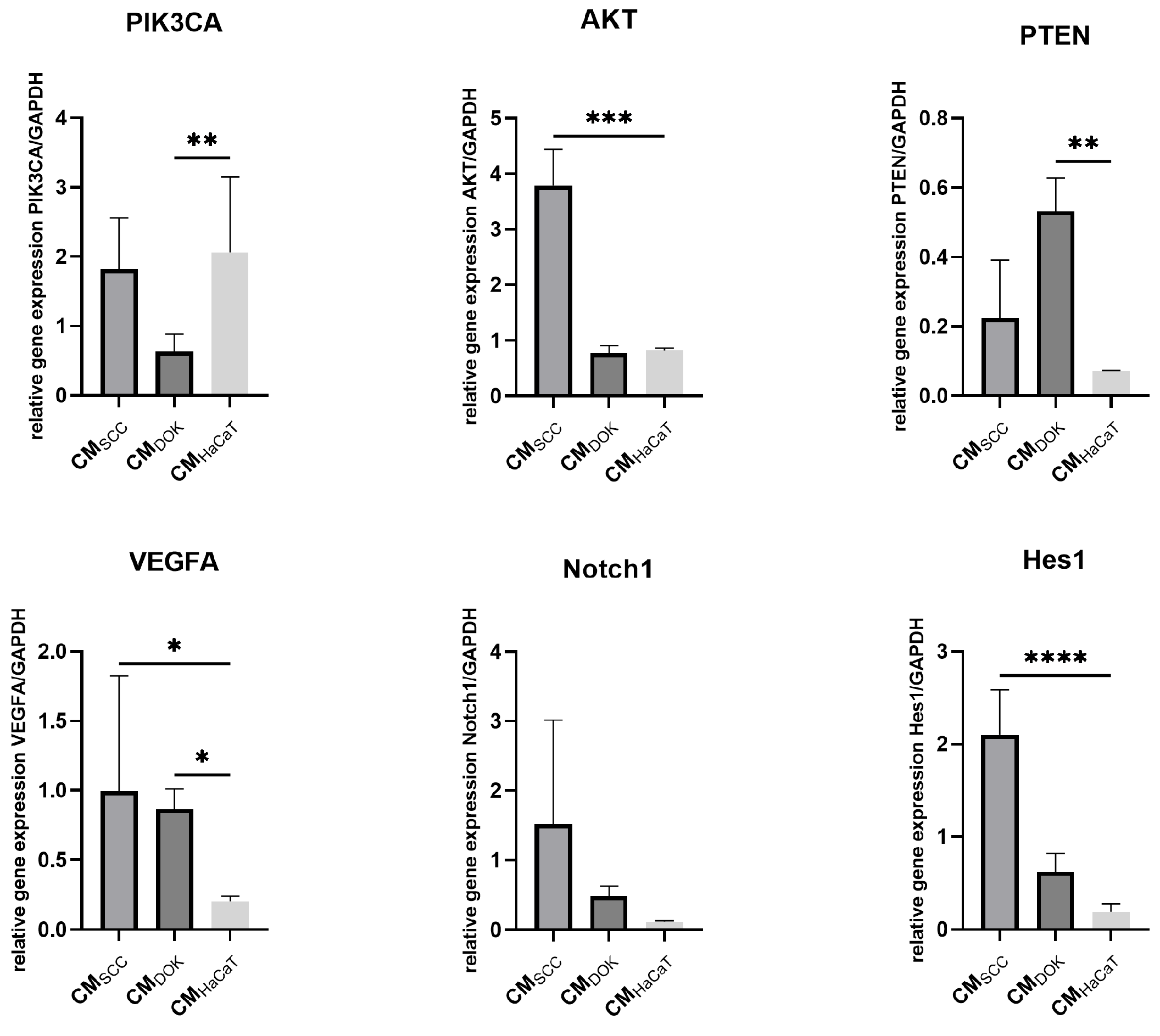
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOK | dysplastic oral keratinocyte |
| SCC | squamous cell carcinoma |
| NF | normal fibroblast |
| CAF | cancer-associated fibroblast |
| CM | conditioned medium |
| GEO | gene expression omnibus |
| EMT | epithelial-to-mesenchymal transition |
| CSC | cancer stem cell |
| OSCC | oral squamous cell carcinoma |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Dou, W.; Liu, W.; Li, J.; Han, X.; Li, S.; Wu, X.; Wang, F.; Xu, X.; Li, J. Global, Regional, and National Burden of Oral Cancer and Its Attributable Risk Factors from 1990 to 2019. Cancer Med. 2023, 12, 13811–13820. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.W.Y.; Lim, K.P.; Cheong, S.C. Translational Genomics and Recent Advances in Oral Squamous Cell Carcinoma. Semin. Cancer Biol. 2020, 61, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Cillo, A.R.; Kürten, C.H.L.; Tabib, T.; Qi, Z.; Onkar, S.; Wang, T.; Liu, A.; Duvvuri, U.; Kim, S.; Soose, R.J.; et al. Immune Landscape of Viral-and Carcinogen-Driven Head and Neck Cancer. Immunity 2020, 52, 183–199.e9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Xiang, J. Mechanisms of Cellular Communication through Intercellular Protein Transfer. J. Cell. Mol. Med. 2011, 15, 1458–1473. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular Vesicles: Masters of Intercellular Communication and Potential Clinical Interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Lavotshkin, S.; Lyden, D. The Secreted Factors Responsible for Pre-Metastatic Niche Formation: Old Sayings and New Thoughts. Semin. Cancer Biol. 2011, 21, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular Vesicle Isolation and Characterization: Toward Clinical Application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular Vesicles in Cancer—Implications for Future Improvements in Cancer Care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.K.; Greening, D.W.; Rai, A.; Chen, M.; Xu, R.; Shafiq, A.; Mathias, R.A.; Zhu, H.J.; Simpson, R.J. Extracellular Vesicles: Their Role in Cancer Biology and Epithelial–Mesenchymal Transition. Biochem. J. 2017, 474, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; McAndrews, K.M. The Role of Extracellular Vesicles in Cancer. Cell 2023, 186, 1610–1626. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Guha, A. Extracellular Vesicles–Vehicles That Spread Cancer Genes. BioEssays 2012, 34, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.R.; Dias, M.S.; Hainaut, P. Tumor-Cell-Derived Microvesicles as Carriers of Molecular Information in Cancer. Curr. Opin. Oncol. 2013, 25, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Cully, M. Exosome-Based Candidates Move into the Clinic. Nat. Rev. Drug Discov. 2021, 20, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.T.; Sloane, H.S.; Kester, M.; Kelly, K.A. Formation and Role of Exosomes in Cancer. Cell. Mol. Life Sci. 2015, 72, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 74, pp. 103–141. ISBN 978-0-12-804689-0. [Google Scholar]
- Liz, J.; Esteller, M. lncRNAs and microRNAs with a Role in Cancer Development. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2016, 1859, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, T.; Chen, J.; Li, R.; Chen, H.; Luo, S.; Chen, D.; Cai, C.; Li, W. The Role of Exosomal miRNAs in Cancer. J. Transl. Med. 2022, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F. Exosomal microRNAs in Cancer: Potential Biomarkers and Immunotherapeutic Targets for Immune Checkpoint Molecules. Front. Genet. 2023, 14, 1052731. [Google Scholar] [CrossRef] [PubMed]
- Nedaeinia, R.; Najafgholian, S.; Salehi, R.; Goli, M.; Ranjbar, M.; Nickho, H.; Javanmard, S.H.; Ferns, G.A.; Manian, M. The Role of Cancer-Associated Fibroblasts and Exosomal miRNAs-Mediated Intercellular Communication in the Tumor Microenvironment and the Biology of Carcinogenesis: A Systematic Review. Cell Death Discov. 2024, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.Z.; Marković, Z.M.; Budimir, M.D.; Zdravković, N.M.; Trišić, D.D.; Bugárová, N.; Danko, M.; Kozyrovska, N.O.; Špitalský, Z.; Kleinová, A.; et al. Photoactive and Antioxidant Nanochitosan Dots/Biocellulose Hydrogels for Wound Healing Treatment. Mater. Sci. Eng. C 2021, 122, 111925. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Chen, S.; Chen, X.; Lin, W.R.; Li, W.; Ma, J.; Wu, T.; Cui, X.; Ji, H.; Li, Y.; et al. Cancer-Associated Fibroblasts Enhance Pancreatic Cancer Cell Invasion by Remodeling the Metabolic Conversion Mechanism. Oncol. Rep. 2017, 37, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, E.; Bozbeyoglu, N.; Gursel, I.; Korkusuz, F.; Bakan Misirlioglu, F.; Korkusuz, P. Comparative Analysis of Magnetically Activated Cell Sorting and Ultracentrifugation Methods for Exosome Isolation. PLoS ONE 2023, 18, e0282238. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal Stromal Cell-Derived Exosomes Attenuate Myocardial Ischaemia-Reperfusion Injury through miR-182-Regulated Macrophage Polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Jaksic Karisik, M.; Lazarevic, M.; Mitic, D.; Milosevic Markovic, M.; Riberti, N.; Jelovac, D.; Milasin, J. MicroRNA-21 as a Regulator of Cancer Stem Cell Properties in Oral Cancer. Cells 2025, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Pérez Lanzón, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.M.; Baldini, N.; et al. Human Bone Marrow-and Adipose-Mesenchymal Stem Cells Secrete Exosomes Enriched in Distinctive miRNA and tRNA Species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Endzeliņš, E.; Berger, A.; Melne, V.; Bajo-Santos, C.; Soboļevska, K.; Ābols, A.; Rodriguez, M.; Šantare, D.; Rudņickiha, A.; Lietuvietis, V.; et al. Detection of Circulating miRNAs: Comparative Analysis of Extracellular Vesicle-Incorporated miRNAs and Cell-Free miRNAs in Whole Plasma of Prostate Cancer Patients. BMC Cancer 2017, 17, 730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Wei, F.; Zhang, X.; Yu, J.; Zhao, H.; Sun, Q.; Yan, F.; Yan, C.; Li, H.; et al. Diagnostic and Prognostic Value of Circulating miR-21 for Cancer: A Systematic Review and Meta-Analysis. Gene 2014, 533, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, B. MicroRNA-21 Identified as Predictor of Cancer Outcome: A Meta-Analysis. PLoS ONE 2014, 9, e103373. [Google Scholar] [CrossRef] [PubMed]
- Ishinaga, H.; He, F.; Hou, B.; Shah, S.; Murata, M.; Takeuchi, K. A Longitudinal Study on Circulating miR-21 as a Therapeutic Effect Marker in Head and Neck Squamous Cell Carcinoma. Carcinogenesis 2019, 40, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Takahashi, M.; Hur, K.; Nagasaka, T.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Serum miR-21 as a Diagnostic and Prognostic Biomarker in Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2013, 105, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Ishinaga, H.; Midorikawa, K.; Shah, S.A.; Nakamura, S.; Hiraku, Y.; Oikawa, S.; Murata, M.; Takeuchi, K. Circulating microRNAs as Novel Prognosis Biomarkers for Head and Neck Squamous Cell Carcinoma. Cancer Biol. Ther. 2015, 16, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.A.R.; Gomes, C.C.; Guimarães, A.L.S.; Campos, K.; Gomez, R.S. Relationship between microRNA Expression Levels and Histopathological Features of Dysplasia in Oral Leukoplakia. J. Oral. Pathol. Med. 2014, 43, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Shreya Reddy, C.S.; PP, A.S.U.; Ganapathy, D.M.; KP, A.; Sekar, D. MicroRNA-21 as a Biomarker in Terminal Stage Oral Squamous Cell Carcinoma (OSCC) in the South Indian Population. Oral. Oncol. Rep. 2024, 9, 100139. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, J.; Zheng, J.; Hsueh, C.; Guo, Y.; Zhou, L. Characterization of Selective Exosomal microRNA Expression Profile Derived from Laryngeal Squamous Cell Carcinoma Detected by next Generation Sequencing. Oncol. Rep. 2018, 40, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Y.; Yuan, Y.; Du, R.; Li, P.; Liu, F.; Tian, Y.; Wang, Y.; Zhang, R.; Zhao, B.; et al. MicroRNA-31 Can Positively Regulate the Proliferation, Differentiation and Migration of Keratinocytes. Biomed. Hub. 2020, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stepicheva, N.A.; Song, J.L. Function and Regulation of microRNA-31 in Development and Disease. Mol. Reprod. Devel 2016, 83, 654–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Kao, S.Y.; Tu, H.F.; Tsai, M.M.; Chang, K.W.; Lin, S.C. Increase of microRNA miR-31 Level in Plasma Could Be a Potential Marker of Oral Cancer. Oral. Dis. 2010, 16, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Chiang, C.Y.F.; Yang, C.C.; Liu, Y.C.; Chang, S.R.; Chang, K.W.; Lin, S.C. miR-31-NUMB Cascade Modulates Monocarboxylate Transporters to Increase Oncogenicity and Lactate Production of Oral Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 11731. [Google Scholar] [CrossRef] [PubMed]
- Laurila, E.M.; Kallioniemi, A. The Diverse Role of miR-31 in Regulating Cancer Associated Phenotypes. Genes. Chromosomes Cancer 2013, 52, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Slaby, O.; Svoboda, M.; Fabian, P.; Smerdova, T.; Knoflickova, D.; Bednarikova, M.; Nenutil, R.; Vyzula, R. Altered Expression of miR-21, miR-31, miR-143 and miR-145 Is Related to Clinicopathologic Features of Colorectal Cancer. Oncology 2007, 72, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, M.; Milosevic, M.; Jelovac, D.; Milenkovic, S.; Tepavcevic, Z.; Baldan, F.; Suboticki, T.; Toljic, B.; Trisic, D.; Dragovic, M.; et al. Marked Epithelial to Mesenchymal Transition in Surgical Margins of Oral Cancer-an In vitro Study. Oncol. Lett. 2020, 19, 3743–3750. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Rahmani, J.; Asghari, M.H.; Goel, A. The Prognostic Role of miR-31 in Colorectal Cancer: The Results of a Meta-Analysis of 4720 Patients. Epigenomics 2022, 14, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Siow, M.; Karen Ng, L.; Vincent Chong, V.; Jamaludin, M.; Abraham, M.; Abdul Rahman, Z.; Kallarakkal, T.; Yang, Y.; Cheong, S.; Zain, R. Dysregulation of Mi R-31 and Mi R-375 Expression Is Associated with Clinical Outcomes in Oral Carcinoma. Oral. Dis. 2014, 20, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.S.; Tu, H.F.; Kao, S.Y.; Yang, C.C.; Liu, C.J.; Huang, T.Y.; Chang, K.W.; Lin, S.C. miR-31 Is Upregulated in Oral Premalignant Epithelium and Contributes to the Immortalization of Normal Oral Keratinocytes. Carcinogenesis 2014, 35, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.S.; Cheng, Y.N.; Zhang, W.B.; Fan, H.; Mao, Q.H.; Xu, P. circRNA_0000140 Suppresses Oral Squamous Cell Carcinoma Growth and Metastasis by Targeting miR-31 to Inhibit Hippo Signaling Pathway. Cell Death Dis. 2020, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, Y.; Zhao, Z.; Ji, X.; Wang, X.; Jin, J.; Wang, Q.; Guo, X.; Cheng, Z.; Lu, M.; et al. Oral Mucosal Mesenchymal Stem Cell-derived Exosomes: A Potential Therapeutic Target in Oral Premalignant Lesions. Int. J. Oncol. 2019, 54, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Karisik, M.J.; Lazarevic, M.; Mitic, D.; Nikolic, N.; Markovic, M.M.; Jelovac, D.; Milasin, J. Osteogenic and Adipogenic Differentiation Potential of Oral Cancer Stem Cells May Offer New Treatment Modalities. Int. J. Mol. Sci. 2023, 24, 4704. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Li, S. Long Noncoding RNA LINC01278 Favors the Progression of Osteosarcoma via Modulating miR-133a-3p/PTHR1 Signaling. J. Cell. Physiol. 2024, 239, e29582. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.S.; Jiang, L.P.; He, C.Y.; Tong, Y.N.; Liu, Y.Y. Up-Regulation of MicroRNA-133a Inhibits the MEK/ERK Signaling Pathway to Promote Cell Apoptosis and Enhance Radio-Sensitivity by Targeting EGFR in Esophageal Cancer In Vivo and In Vitro. J. Cell. Biochem. 2017, 118, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Q.; Han, F.; Shi, Z.L.; Yu, L.; Li, X.F.; Yu, C.; Shen, C.L.; Wan, D.W.; Zhu, X.G.; Li, R.; et al. miR-133a-3p Targets SUMO-Specific Protease 1 to Inhibit Cell Proliferation and Cell Cycle Progress in Colorectal Cancer. Oncol. Res. 2018, 26, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, F.; Liang, Y. MicroRNA-133a Inhibits the Proliferation of Non-small Cell Lung Cancer by Targeting YES1. Oncol. Lett. 2019, 18, 6759–6765. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lin, X.; Tian, F.; Yu, W.; Qiao, B. MiR-133a-3p Inhibits Oral Squamous Cell Carcinoma (OSCC) Proliferation and Invasion by Suppressing COL1A1. J. Cell. Biochem. 2018, 119, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Du, L.; Li, X.; Zhang, X.; Gao, Y. miR-133a-3p Suppresses Cell Proliferation, Migration, and Invasion and Promotes Apoptosis in Esophageal Squamous Cell Carcinoma. J. Cell. Physiol. 2019, 234, 12757–12770. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Lee, J.Y.; Park, H.R.; Kang, J.W.; Kim, Y.H.; Lee, J.H. MicroRNA-133 Targets Phosphodiesterase 1C in Drosophila and Human Oral Cancer Cells to Regulate Epithelial-Mesenchymal Transition. J. Cancer 2021, 12, 5296–5309. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, F.; Huang, G.; Ni, Y.; Zhang, T.; Zou, Z.; Meng, M. Exosomal miR-133a-3p Promotes the Growth and Metastasis of Lung Cancer Cells Following Incomplete Microwave Ablation. Int. J. Hyperth. 2023, 40, 2190065. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Hamsa, D.; Fareed, M.; Karobari, M.I. An Update on the Molecular Mechanisms Underlying the Progression of miR-21 in Oral Cancer. World J. Surg. Onc 2025, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, W.; Ying, Y.; Luo, J.; Xu, X.; Zheng, L.; Wu, W.; Yang, S.; Zhao, S. MicroRNA-31: A Pivotal Oncogenic Factor in Oral Squamous Cell Carcinoma. Cell Death Discov. 2022, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, H.; Qiu, T.; Dai, J.; Zhang, Y.; Chen, J.; Cai, H. Loss of PTEN Induces Lung Fibrosis via Alveolar Epithelial Cell Senescence Depending on NF-κB Activation. Aging Cell 2019, 18, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.Q.; Wu, H.; Huang, X.; Shen, H.; Shu, Y.Q.; Zhang, B.; Xiang, C.C.; Yu, S.M.; Guo, R.H.; Chen, L. MiR-1 Targets PIK3CA and Inhibits Tumorigenic Properties of A549 Cells. Biomed. Pharmacother. 2014, 68, 155–161. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitic, D.; Jaksic Karisik, M.; Lazarevic, M.; Carkic, J.; Zivkovic, E.; Mitrovic Ajtic, O.; Milasin, J. Oral Squamous Cell Carcinoma Exosomes Upregulate PIK3/AKT, PTEN, and NOTCH Signaling Pathways in Normal Fibroblasts. Curr. Issues Mol. Biol. 2025, 47, 568. https://doi.org/10.3390/cimb47070568
Mitic D, Jaksic Karisik M, Lazarevic M, Carkic J, Zivkovic E, Mitrovic Ajtic O, Milasin J. Oral Squamous Cell Carcinoma Exosomes Upregulate PIK3/AKT, PTEN, and NOTCH Signaling Pathways in Normal Fibroblasts. Current Issues in Molecular Biology. 2025; 47(7):568. https://doi.org/10.3390/cimb47070568
Chicago/Turabian StyleMitic, Dijana, Milica Jaksic Karisik, Milos Lazarevic, Jelena Carkic, Emilia Zivkovic, Olivera Mitrovic Ajtic, and Jelena Milasin. 2025. "Oral Squamous Cell Carcinoma Exosomes Upregulate PIK3/AKT, PTEN, and NOTCH Signaling Pathways in Normal Fibroblasts" Current Issues in Molecular Biology 47, no. 7: 568. https://doi.org/10.3390/cimb47070568
APA StyleMitic, D., Jaksic Karisik, M., Lazarevic, M., Carkic, J., Zivkovic, E., Mitrovic Ajtic, O., & Milasin, J. (2025). Oral Squamous Cell Carcinoma Exosomes Upregulate PIK3/AKT, PTEN, and NOTCH Signaling Pathways in Normal Fibroblasts. Current Issues in Molecular Biology, 47(7), 568. https://doi.org/10.3390/cimb47070568








