The Clock and the Brain: Circadian Rhythm and Alzheimer’s Disease
Abstract
1. Introduction
2. Effect of Aging on Circadian Rhythms in the Brain
3. Evidence of CRDs in AD Patients
4. Sex Differences in CRDs in AD
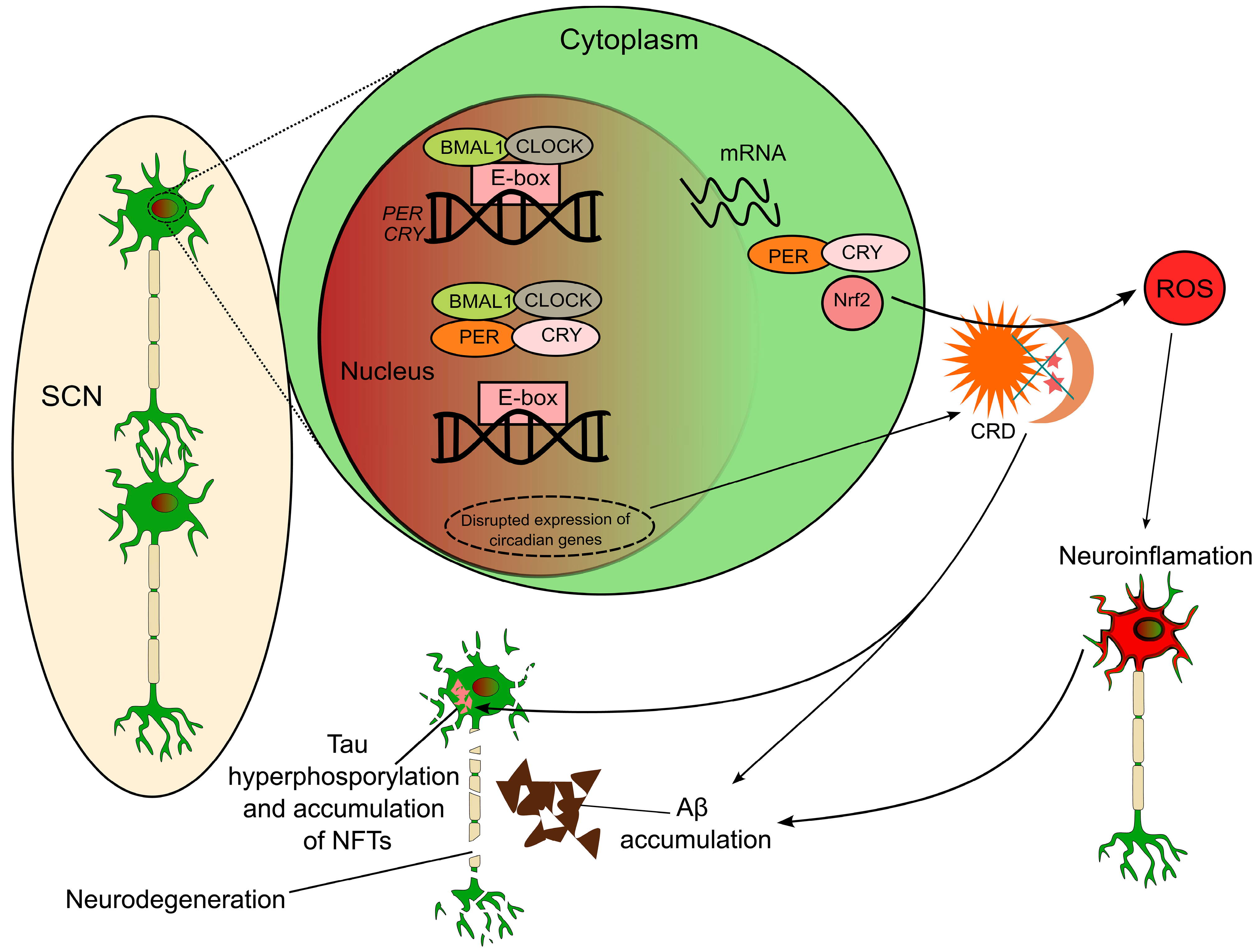
5. Molecular Mechanisms Linking AD and Circadian Rhythm
5.1. Expression of Clock Genes in AD
5.2. Circadian Clock Gene Polymorphisms Associated with AD
5.3. Impact of Amyloid-Beta and Tau on Circadian Regulation
5.4. Neuroinflammation and Oxidative Stress as Mediators of CRDs in AD
6. Bidirectional Relationship Between AD and Circadian Rhythms
7. Peripheral Circadian Clock and AD
8. Therapeutic Implications
8.1. Potential Interventions Targeting Circadian Rhythms
8.1.1. Light Therapy
8.1.2. Therapeutic Targeting of Casein Kinase 1δ/ε
8.2. Chronotherapeutics
9. Limitations
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamel, R.; Köhler, S.; Sistermans, N.; Koene, T.; Pijnenburg, Y.; van der Flier, W.; Scheltens, P.; Aalten, P.; Verhey, F.; Visser, P.J.; et al. The trajectory of cognitive decline in the pre-dementia phase in memory clinic visitors: Findings from the 4C-MCI study. Psychol. Med. 2015, 45, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Silbert, L.C.; Quinn, J.F.; Moore, M.M.; Corbridge, E.; Ball, M.J.; Murdoch, G.; Sexton, G.; Kaye, J.A. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology 2003, 61, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Xu, Z.Q.; Wang, Y.J. White Matter “Matters” in Alzheimer’s Disease. Neurosci. Bull. 2022, 38, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Schneider, J.A.; Wilson, R.S.; Bienias, J.L.; Arnold, S.E. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch. Neurol. 2004, 61, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Gouras, G.K.; Tsai, J.; Naslund, J.; Vincent, B.; Edgar, M.; Checler, F.; Greenfield, J.P.; Haroutunian, V.; Buxbaum, J.D.; Xu, H.; et al. Intraneuronal Abeta42 accumulation in human brain. Am. J. Pathol. 2000, 156, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Lee, J.H.; Yang, E.J. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer’s disease animal model. J. Neuroinflamm. 2019, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Coomans, E.M.; van Westen, D.; Binette, A.P.; Strandberg, O.; Spotorno, N.; Serrano, G.E.; Beach, T.G.; Palmqvist, S.; Stomrud, E.; Ossenkoppele, R.; et al. Interactions between vascular burden and amyloid-β pathology on trajectories of tau accumulation. Brain 2024, 147, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Lockley, S.W.; Thapan, K.; Arendt, J. Effects of light on human circadian rhythms. Reprod. Nutr. Dev. 1999, 39, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.J.; Knutson, K.L.; Pereira, A.C.; von Schantz, M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep. Med. Rev. 2017, 33, 70–78. [Google Scholar] [CrossRef] [PubMed]
- vanderLeest, H.T.; Rohling, J.H.; Michel, S.; Meijer, J.H. Phase shifting capacity of the circadian pacemaker determined by the SCN neuronal network organization. PLoS ONE 2009, 4, e4976. [Google Scholar] [CrossRef] [PubMed]
- Bokenberger, K.; Sjölander, A.; Dahl Aslan, A.K.; Karlsson, I.K.; Åkerstedt, T.; Pedersen, N.L. Shift work and risk of incident dementia: A study of two population-based cohorts. Eur. J. Epidemiol. 2018, 33, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.J.; Moffitt, T.E.; Gregory, A.M.; Goldman-Mellor, S.; Nolan, P.M.; Poulton, R.; Caspi, A. Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. Int. J. Obes. 2015, 39, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Fliers, E.; Partiman, T.S. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985, 342, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Investig. 2017, 127, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Warman, G.R.; Cheeseman, J.F. The functional changes of the circadian system organization in aging. Ageing Res. Rev. 2019, 52, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Keihani, A.; Mayeli, A.; Ferrarelli, F. Circadian Rhythm Changes in Healthy Aging and Mild Cognitive Impairment. Adv. Biol. 2023, 7, e2200237. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.J.; Nakamura, W.; Yamazaki, S.; Kudo, T.; Cutler, T.; Colwell, C.S.; Block, G.D. Age-related decline in circadian output. J. Neurosci. 2011, 31, 10201–10205. [Google Scholar] [CrossRef] [PubMed]
- Hooghiemstra, A.M.; Eggermont, L.H.; Scheltens, P.; van der Flier, W.M.; Scherder, E.J. The rest-activity rhythm and physical activity in early-onset dementia. Alzheimer Dis. Assoc. Disord. 2015, 29, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Bhimasani, M.; Zangrilli, M.A.; Morris, J.C.; Holtzman, D.M.; Ju, Y.S. Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol. 2018, 75, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Tranah, G.J.; Blackwell, T.; Stone, K.L.; Ancoli-Israel, S.; Paudel, M.L.; Ensrud, K.E.; Cauley, J.A.; Redline, S.; Hillier, T.A.; Cummings, S.R.; et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann. Neurol. 2011, 70, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Peng, X.D.; Li, Y.H.; Wang, Z.R.; Chang-quan, H.; Hui, W.; Liu, Q.X. The polymorphism of CLOCK gene 3111T/C C>T is associated with susceptibility of Alzheimer disease in Chinese population. J. Investig. Med. 2013, 61, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Rogers-Soeder, T.S.; Blackwell, T.; Yaffe, K.; Ancoli-Israel, S.; Redline, S.; Cauley, J.A.; Ensrud, K.E.; Paudel, M.; Barrett-Connor, E.; LeBlanc, E.; et al. Rest-Activity Rhythms and Cognitive Decline in Older Men: The Osteoporotic Fractures in Men Sleep Study. J. Am. Geriatr. Soc. 2018, 66, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Valentinuzzi, V.S.; Scarbrough, K.; Takahashi, J.S.; Turek, F.W. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am. J. Physiol. 1997, 273, R1957–R1964. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.D.; Strauss, M.; Patterson, M.B. Sleep disturbances in community-dwelling patients with Alzheimer’s disease. Clin. Gerontol. 1996, 16, 35–49. [Google Scholar] [CrossRef]
- Prinz, P.N.; Peskind, E.R.; Vitaliano, P.P.; Raskind, M.A.; Eisdorfer, C.; Zemcuznikov, N.; Gerber, C.J. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J. Am. Geriatr. Soc. 1982, 30, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Himali, J.J.; Baril, A.A.; Cavuoto, M.G.; Yiallourou, S.; Wiedner, C.D.; Himali, D.; DeCarli, C.; Redline, S.; Beiser, A.S.; Seshadri, S.; et al. Association Between Slow-Wave Sleep Loss and Incident Dementia. JAMA Neurol. 2023, 80, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Feenstra, M.G.; Zhou, J.N.; Liu, R.Y.; Toranõ, J.S.; Van Kan, H.J.; Fischer, D.F.; Ravid, R.; Swaab, D.F. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: Alterations in preclinical and clinical stages. J. Clin. Endocrinol. Metab. 2003, 88, 5898–5906. [Google Scholar] [CrossRef] [PubMed]
- Satlin, A.; Volicer, L.; Stopa, E.G.; Harper, D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol. Aging 1995, 16, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Pollak, C.P.; Perlick, D. Sleep problems and institutionalization of the elderly. J. Geriatr. Psychiatry Neurol. 1991, 4, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Jagust, W.J.; Walker, M.P. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016, 39, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Matsuo, R.; Kurogi, K.; Miyamoto, S.; Morita, T.; Shinozuka, M.; Taniguchi, F.; Ikegami, K.; Yasuo, S. Sex-dependent effects of chronic jet lag on circadian rhythm and metabolism in mice. Biol. Sex. Differ. 2024, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Britz, J.; Ojo, E.; Haque, N.; Dhukhwa, A.; Hascup, E.R.; Hascup, K.N.; Tischkau, S.A. Sex-Dependent Effects of Chronic Circadian Disruption in AβPP/PS1 Mice. J. Alzheimers Dis. 2024, 97, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, P.; Gaba, A.; Musiek, E.; Ju, Y.S.; Hu, K. Fractal motor activity regulation and sex differences in preclinical Alzheimer’s disease pathology. Alzheimers Dement. 2021, 13, e12211. [Google Scholar] [CrossRef] [PubMed]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Y.; Zhou, J.N.; Hoogendijk, W.J.; van Heerikhuize, J.; Kamphorst, W.; Unmehopa, U.A.; Hofman, M.A.; Swaab, D.F. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J. Neuropathol. Exp. Neurol. 2000, 59, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Oyegbami, O.; Collins, H.M.; Pardon, M.C.; Ebling, F.J.P.; Heery, D.M.; Moran, P.M. Abnormal Clock Gene Expression and Locomotor Activity Rhythms in Two Month-Old Female APPSwe/PS1dE9 Mice. Curr. Alzheimer Res. 2017, 14, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Y.; Zhou, J.N.; van Heerikhuize, J.; Hofman, M.A.; Swaab, D.F. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J. Clin. Endocrinol. Metab. 1999, 84, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Fischer, D.F.; Kalsbeek, A.; Garidou-Boof, M.L.; van der Vliet, J.; van Heijningen, C.; Liu, R.Y.; Zhou, J.N.; Swaab, D.F. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J. 2006, 20, 1874–1876. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Guo, L.; Chen, N.; Guo, Q.; Xie, Y.; Wang, Y.; Wang, E. CRY2 mediates the cognitive decline induced by sleep deprivation in 5xFAD mice. PLoS ONE 2024, 19, e0306930. [Google Scholar] [CrossRef] [PubMed]
- Kress, G.J.; Liao, F.; Dimitry, J.; Cedeno, M.R.; FitzGerald, G.A.; Holtzman, D.M.; Musiek, E.S. Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med. 2018, 215, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Niu, L.; Tian, L.; Hu, Y.; Cheng, C.; Li, S.; Le, W. Circadian rhythm disturbances in Alzheimer’s disease: Insights from plaque-free and plaque-burdened stages in APP(SWE)/PS1(dE9) mice. Alzheimer’s Res. Ther. 2025, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Huang, C.Q.; You, C.; Wang, Z.R.; Si-qing, H. Polymorphism of CLOCK gene rs 4580704 C > G is associated with susceptibility of Alzheimer’s disease in a Chinese population. Arch. Med. Res. 2013, 44, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, C.Q.; Hu, X.Y.; Li, S.B.; Zhang, X.M. Functional CLOCK gene rs1554483 G/C polymorphism is associated with susceptibility to Alzheimer’s disease in the Chinese population. J. Int. Med. Res. 2013, 41, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Bacalini, M.G.; Palombo, F.; Garagnani, P.; Giuliani, C.; Fiorini, C.; Caporali, L.; Stanzani Maserati, M.; Capellari, S.; Romagnoli, M.; De Fanti, S.; et al. Association of rs3027178 polymorphism in the circadian clock gene PER1 with susceptibility to Alzheimer’s disease and longevity in an Italian population. Geroscience 2022, 44, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Huang, C.; Guo, Q.; Liu, Q.; Xiong, G. Association of Per3 length polymorphism with susceptibility of Alzheimer disease (AD) in Chinese population. Biol. Rhythm. Res. 2019, 50, 447–453. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, X.D.; Huang, C.Q.; Hu, X.Y.; Zhang, X.M. Association between ARNTL (BMAL1) rs2278749 polymorphism T >C and susceptibility to Alzheimer disease in a Chinese population. Genet. Mol. Res. 2015, 14, 18515–18522. [Google Scholar] [CrossRef] [PubMed]
- Qing-Xiu, L.; Chang-Quan, H.; Qian, C.; Xue-Mei, Z.; Xiu-Ying, H.; Song-Bing, L. The polymorphism of ARNTL2 (BMAL2) gene rs2306074 C>T is associated with susceptibility of Alzheimer disease in Chinese population. Neurol. Sci. 2014, 35, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-D.; Huang, C.-Q.; Chen, L.; Wei, X.-M.; Liu, Q.-X.; Wang, Z.-R. The polymorphism of CLOCK gene rs4864548 A> G is associated with susceptibility of Alzheimer’s disease in Chinese population. Biol. Rhythm. Res. 2018, 49, 451–457. [Google Scholar] [CrossRef]
- Li, J.; Chang, Y.; Zhao, C.; Wang, T.; Xue, J.; Cai, Y. The ARNTL polymorphism rs900147 is associated with the risk of Alzheimer’s disease and amnestic mild cognitive impairment in a Chinese population. Biol. Rhythm. Res. 2021, 52, 146–152. [Google Scholar] [CrossRef]
- Lozano-Tovar, S.; Rodríguez-Agudelo, Y.; Dávila-Ortiz de Montellano, D.J.; Pérez-Aldana, B.E.; Ortega-Vázquez, A.; Monroy-Jaramillo, N. Relationship between APOE, PER2, PER3 and OX2R Genetic Variants and Neuropsychiatric Symptoms in Patients with Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2023, 20, 4412. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Kobayashi, E.; Ushijima, K.; Ando, H.; Maekawa, T.; Takuma, M.; Furukawa, Y.; Fujimura, A. Reduced histone H3K9 acetylation of clock genes and abnormal glucose metabolism in ob/ob mice. Chronobiol. Int. 2012, 29, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ogawa, T.; Hitosugi, S.; Ichihashi, Y.; Nakadaira, Y.; Kobayashi, M.; Tezuka, M.; Kosuge, Y.; Ishige, K.; Ito, Y.; et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE 2011, 6, e25231. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.L.; Husse, J.; Bode, B.; Naujokat, N.; Meyer-Kovac, J.; Schmid, S.M.; Lehnert, H.; Oster, H. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS ONE 2012, 7, e37150. [Google Scholar] [CrossRef] [PubMed]
- Lozić, M.; Šarenac, O.; Murphy, D.; Japundžić-Žigon, N. Vasopressin, Central Autonomic Control and Blood Pressure Regulation. Curr. Hypertens. Rep. 2018, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Japundžić-Žigon, N. Vasopressin and oxytocin in control of the cardiovascular system. Curr. Neuropharmacol. 2013, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Carraway, R.; Leeman, S.E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem. 1973, 248, 6854–6861. [Google Scholar] [CrossRef] [PubMed]
- St-Gelais, F.; Jomphe, C.; Trudeau, L.E. The role of neurotensin in central nervous system pathophysiology: What is the evidence? J. Psychiatry Neurosci. 2006, 31, 229–245. [Google Scholar] [PubMed]
- Stopa, E.G.; Volicer, L.; Kuo-Leblanc, V.; Harper, D.; Lathi, D.; Tate, B.; Satlin, A. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J. Neuropathol. Exp. Neurol. 1999, 58, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Furio, A.M.; Cutrera, R.A.; Castillo Thea, V.; Pérez Lloret, S.; Riccio, P.; Caccuri, R.L.; Brusco, L.L.; Cardinali, D.P. Effect of melatonin on changes in locomotor activity rhythm of Syrian hamsters injected with beta amyloid peptide 25-35 in the suprachiasmatic nuclei. Cell Mol. Neurobiol. 2002, 22, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.; Sözer-Topcular, N.; Jockers, R.; Ravid, R.; Angeloni, D.; Fraschini, F.; Eckert, A.; Müller-Spahn, F.; Savaskan, E. Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer’s disease. Eur. J. Histochem. 2006, 50, 311–316. [Google Scholar] [PubMed]
- Pappolla, M.A.; Matsubara, E.; Vidal, R.; Pacheco-Quinto, J.; Poeggeler, B.; Zagorski, M.; Sambamurti, K. Melatonin Treatment Enhances Aβ Lymphatic Clearance in a Transgenic Mouse Model of Amyloidosis. Curr. Alzheimer Res. 2018, 15, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Warfield, A.E.; Gupta, P.; Ruhmann, M.M.; Jeffs, Q.L.; Guidone, G.C.; Rhymes, H.W.; Thompson, M.I.; Todd, W.D. A brainstem to circadian system circuit links Tau pathology to sundowning-related disturbances in an Alzheimer’s disease mouse model. Nat. Commun. 2023, 14, 5027. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Spanetta, M.; Izzi, F.; Franchini, F.; Nuccetelli, M.; Sancesario, G.M.; Di Santo, S.; Bernardini, S.; Mercuri, N.B.; Placidi, F. Sleep-Wake Cycle in Alzheimer’s Disease Is Associated with Tau Pathology and Orexin Dysregulation. J. Alzheimer’s Dis. 2020, 74, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Lucey, B.P.; McCullough, A.; Landsness, E.C.; Toedebusch, C.D.; McLeland, J.S.; Zaza, A.M.; Fagan, A.M.; McCue, L.; Xiong, C.; Morris, J.C.; et al. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci. Transl. Med. 2019, 11, eaau6550. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Yokota, A.; Shiraishi, T.; Yamada, S.; Haraguchi, A.; Shinozaki, A.; Shibata, S. In vitro and in vivo phase changes of the mouse circadian clock by oxidative stress. J. Circadian Rhythm. 2016, 14, 4. [Google Scholar] [CrossRef]
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife 2018, 7, e31656. [Google Scholar] [CrossRef] [PubMed]
- LeVault, K.R.; Tischkau, S.A.; Brewer, G.J. Circadian Disruption Reveals a Correlation of an Oxidative GSH/GSSG Redox Shift with Learning and Impaired Memory in an Alzheimer’s Disease Mouse Model. J. Alzheimer’s Dis. 2016, 49, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Yang, M.; Zhang, H.; Zhang, L.; Song, H.; Liu, Y.; Zeng, Y.; Yang, B.; Wang, X.; Chen, Y.; et al. Microglia activation mediates circadian rhythm disruption-induced cognitive impairment in mice. J. Neuroimmunol. 2023, 379, 578102. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Kitt, M.M.; Gaudet, A.D.; Barrientos, R.M.; Watkins, L.R.; Maier, S.F. Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol. Aging 2016, 47, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Cavadini, G.; Petrzilka, S.; Kohler, P.; Jud, C.; Tobler, I.; Birchler, T.; Fontana, A. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc. Natl. Acad. Sci. USA 2007, 104, 12843–12848. [Google Scholar] [CrossRef] [PubMed]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Cummins, D.J.; Paul, S.M. Neuroinflammation-induced acceleration of amyloid deposition in the APPV717F transgenic mouse. Eur. J. Neurosci. 2001, 14, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Monje, F.J.; Cicvaric, A.; Acevedo Aguilar, J.P.; Elbau, I.; Horvath, O.; Diao, W.; Glat, M.; Pollak, D.D. Disrupted Ultradian Activity Rhythms and Differential Expression of Several Clock Genes in Interleukin-6-Deficient Mice. Front. Neurol. 2017, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Lundkvist, G.B.; Brask, J.; Davidson, A.; Menaker, M.; Kristensson, K.; Block, G.D. Interferon-gamma alters electrical activity and clock gene expression in suprachiasmatic nucleus neurons. J. Biol. Rhythm. 2008, 23, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Grimm, A.; Eckert, A. Amyloid-β-Induced Changes in Molecular Clock Properties and Cellular Bioenergetics. Front. Neurosci. 2017, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Kamila, P.; Kar, K.; Chowdhury, S.; Chakraborty, P.; Dutta, R.; Sowmiya, S.; Singh, S.A.; Prajapati, B.G. Effect of neuroinflammation on the progression of Alzheimer’s disease and its significant ramifications for novel anti-inflammatory treatments. IBRO Neurosci. Rep. 2025, 18, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rangel, W.; Acuña-Vaca, S.; Padilla-Ponce, D.J.; García-Mercado, F.G.; Torres-Mendoza, B.M.; Pacheco-Moises, F.P.; Escoto-Delgadillo, M.; García-Benavides, L.; Delgado-Lara, D.L.C. Modulation of the Circadian Rhythm and Oxidative Stress as Molecular Targets to Improve Vascular Dementia: A Pharmacological Perspective. Int. J. Mol. Sci. 2024, 25, 4401. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Sachdeva, P.; Sarkar, J.; Izhaar, R. Circadian dysfunction and Alzheimer’s disease—An updated review. Aging Med. 2023, 6, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.F.; Ortiz, J.B.; Wonnacott, S.; Williams, R.J.; Rowe, R.K. The Bidirectional Relationship Between Sleep and Inflammation Links Traumatic Brain Injury and Alzheimer’s Disease. Front. Neurosci. 2020, 14, 894. [Google Scholar] [CrossRef] [PubMed]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian rhythm connections to oxidative stress: Implications for human health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Malek-Ahmadi, M.H.; Alldred, M.J.; Che, S.; Elarova, I.; Chen, Y.; Jeanneteau, F.; Kranz, T.M.; Chao, M.V.; Counts, S.E.; et al. Selective decline of neurotrophin and neurotrophin receptor genes within CA1 pyramidal neurons and hippocampus proper: Correlation with cognitive performance and neuropathology in mild cognitive impairment and Alzheimer’s disease. Hippocampus 2019, 29, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.M.; Xu, S.; Kritikou, J.S.; Marosi, K.; Brodin, L.; Mattson, M.P. Exercise and BDNF reduce Aβ production by enhancing α-secretase processing of APP. J. Neurochem. 2017, 142, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.J.; Blaine, S.; Wallace, N.K.; Karatsoreos, I.N. Brain-derived neurotrophic factor Val66Met polymorphism modulates the effects of circadian desynchronization on activity and sleep in male mice. Front. Neurosci. 2022, 16, 1013673. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.J.; Huang, C.Q. Cognitive impairment induced by circadian rhythm disorders involves hippocampal brain-derived neurotrophic factor reduction and amyloid-β deposition. Chronobiol. Int. 2024, 41, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.W.; Blurton-Jones, M.; Tu, C.H.; Feinberg, L.M.; Chabrier, M.A.; Harris, J.W.; Jeon, N.L.; Cotman, C.W. β-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol. Aging 2011, 32, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Lucey, B.P.; Hicks, T.J.; McLeland, J.S.; Toedebusch, C.D.; Boyd, J.; Elbert, D.L.; Patterson, B.W.; Baty, J.; Morris, J.C.; Ovod, V.; et al. Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann. Neurol. 2018, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wolff, S.E.C.; Korpel, N.; Milanova, I.; Sandu, C.; Rensen, P.C.N.; Kooijman, S.; Cassel, J.C.; Kalsbeek, A.; Boutillier, A.L.; et al. Deficiency of the Circadian Clock Gene Bmal1 Reduces Microglial Immunometabolism. Front. Immunol. 2020, 11, 586399. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.T.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.A.; Lee, J.; Cai, Y.; Saito, T.; Saido, T.; Musiek, E.S. Astrocytes deficient in circadian clock gene Bmal1 show enhanced activation responses to amyloid-beta pathology without changing plaque burden. Sci. Rep. 2022, 12, 1796. [Google Scholar] [CrossRef] [PubMed]
- Ooms, S.; Overeem, S.; Besse, K.; Rikkert, M.O.; Verbeek, M.; Claassen, J.A. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: A randomized clinical trial. JAMA Neurol. 2014, 71, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kojori, E.; Wang, G.J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Xie, L.; Yu, M.; Kang, H.; Feng, T.; Deane, R.; Logan, J.; Nedergaard, M.; Benveniste, H. The Effect of Body Posture on Brain Glymphatic Transport. J. Neurosci. 2015, 35, 11034–11044. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Lone, S.R.; Liu, S.; Liu, Q.; Zhang, J.; Spira, A.P.; Wu, M.N. Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr. Biol. 2015, 25, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Numano, R.; Abe, M.; Hida, A.; Takahashi, R.; Ueda, M.; Block, G.D.; Sakaki, Y.; Menaker, M.; Tei, H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000, 288, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, L.; Mou, Y.; Zhang, Y.; Ping, Y. Sleep, circadian rhythm and gut microbiota: Alterations in Alzheimer’s disease and their potential links in the pathogenesis. Gut Microbes 2021, 13, 1957407. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Blokhina, I.; Khorovodov, A.; Fedosov, I.; Yu, T.; Karandin, G.; Evsukova, A.; Elovenko, D.; Adushkina, V.; et al. Night Photostimulation of Clearance of Beta-Amyloid from Mouse Brain: New Strategies in Preventing Alzheimer’s Disease. Cells 2021, 10, 3289. [Google Scholar] [CrossRef] [PubMed]
- Cremascoli, R.; Sparasci, D.; Giusti, G.; Cattaldo, S.; Prina, E.; Roveta, F.; Bruno, F.; Ghezzi, C.; Cerri, S.; Picascia, M.; et al. Effects of Circadian Phase Tailored Light Therapy on Sleep, Mood, and Cognition in Alzheimer’s Disease: Preliminary Findings in a Pivotal Study. Front. Physiol. 2021, 12, 755322. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.; Mayne, J.; Walker, K.; Ning, Z.; Figeys, D. Therapeutic Targeting of Casein Kinase 1δ/ε in an Alzheimer’s Disease Mouse Model. J. Proteome Res. 2019, 18, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, J.; Reynolds, L.; Kleiman, R.; Tate, B.; Swanson, T.A.; Pickard, G.E. Chronic treatment with a selective inhibitor of casein kinase I delta/epsilon yields cumulative phase delays in circadian rhythms. Psychopharmacology 2010, 210, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, M.; Sun, J.; Yang, X. Casein kinase 1α: Biological mechanisms and theranostic potential. Cell Commun. Signal. 2018, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Huart, A.S.; MacLaine, N.J.; Meek, D.W.; Hupp, T.R. CK1alpha plays a central role in mediating MDM2 control of p53 and E2F-1 protein stability. J. Biol. Chem. 2009, 284, 32384–32394. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Bapat, S. Chronobiology and chronotherapeutics. Kathmandu Univ. Med. J. 2004, 2, 384–388. [Google Scholar]
- Whittaker, D.S.; Akhmetova, L.; Colwell, C.S.; Desplats, P. A time-restricted feeding intervention reduces alterations in circadian behaviors and pathology in a mouse model of Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, e052723. [Google Scholar]
- Kim, E.; Nohara, K.; Wirianto, M.; Escobedo, G., Jr.; Lim, J.Y.; Morales, R.; Yoo, S.H.; Chen, Z. Effects of the Clock Modulator Nobiletin on Circadian Rhythms and Pathophysiology in Female Mice of an Alzheimer’s Disease Model. Biomolecules 2021, 11, 1004. [Google Scholar] [CrossRef] [PubMed]
- Song, H.R.; Woo, Y.S.; Wang, H.R.; Jun, T.Y.; Bahk, W.M. Effect of the timing of acetylcholinesterase inhibitor ingestion on sleep. Int. Clin. Psychopharmacol. 2013, 28, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Basheer, A. The art and science of writing narrative reviews. Int. J. Adv. Med. Health Res. 2022, 9, 124–126. [Google Scholar] [CrossRef]
| Gene | Polymorphism (SNP) | Findings in AD Patients | Reference |
|---|---|---|---|
| CLOCK | rs4580704 | Higher prevalence of C allele carriers | Chen et al. [44] |
| CLOCK | rs1554483 | Higher prevalence of G allele carriers | Chen et al. [45] |
| PER1 | rs3027178 | G allele is protective against AD | Bacalini et al. [46] |
| PER3 | Five repeat homozygotes of Per3 length | Increased prevalence of five-repeat homozygotes | Xiang et al. [47] |
| BMAL1 | rs2278749 | Higher prevalence of TT genotypes | Chen et al. [48] |
| BMAL2 | rs2306074 | Higher prevalence of C allele carriers and CC genotypes | Qing-Xiu et al. [49] |
| CLOCK | 3111T/C | Higher prevalence of C allele carriers | Yang et al. [22] |
| CLOCK | rs4864548 | Higher prevalence of A allele carriers | Peng et al. [50] |
| BMAL1 | rs900147 | Higher risk of developing AD in G allele carriers | Li et al. [51] |
| PER3 | rs228697 | There may be a higher risk of CRDs in APOE ε4 carriers | Lozano-Tovar et al. [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbani Shirkouhi, S.; Karimi, A.; Khatami, S.S.; Asgari Gashtrodkhani, A.; Kamari, F.; Blaabjerg, M.; Andalib, S. The Clock and the Brain: Circadian Rhythm and Alzheimer’s Disease. Curr. Issues Mol. Biol. 2025, 47, 547. https://doi.org/10.3390/cimb47070547
Ghorbani Shirkouhi S, Karimi A, Khatami SS, Asgari Gashtrodkhani A, Kamari F, Blaabjerg M, Andalib S. The Clock and the Brain: Circadian Rhythm and Alzheimer’s Disease. Current Issues in Molecular Biology. 2025; 47(7):547. https://doi.org/10.3390/cimb47070547
Chicago/Turabian StyleGhorbani Shirkouhi, Samaneh, Ashkan Karimi, Seyed Sepehr Khatami, Ashkan Asgari Gashtrodkhani, Farzin Kamari, Morten Blaabjerg, and Sasan Andalib. 2025. "The Clock and the Brain: Circadian Rhythm and Alzheimer’s Disease" Current Issues in Molecular Biology 47, no. 7: 547. https://doi.org/10.3390/cimb47070547
APA StyleGhorbani Shirkouhi, S., Karimi, A., Khatami, S. S., Asgari Gashtrodkhani, A., Kamari, F., Blaabjerg, M., & Andalib, S. (2025). The Clock and the Brain: Circadian Rhythm and Alzheimer’s Disease. Current Issues in Molecular Biology, 47(7), 547. https://doi.org/10.3390/cimb47070547





