Targeting Exosomal PD-L1 as a New Frontier in Cancer Immunotherapy
Abstract
1. Introduction
2. Biogenesis and Molecular Composition of Exosomes
2.1. Exosome Formation: Mechanisms of Exosome Biogenesis
2.2. Exosome Release and Uptake
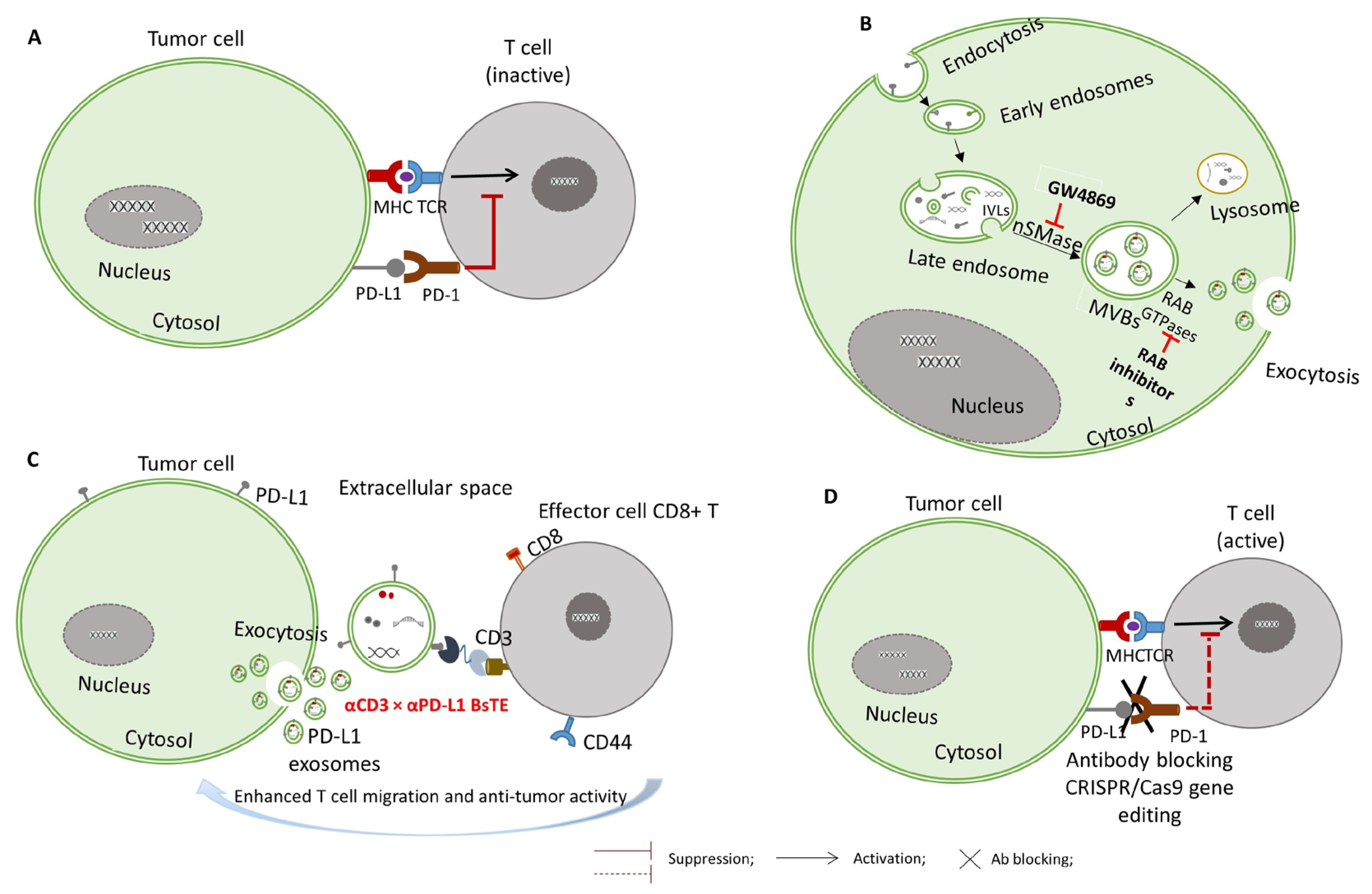
3. Mechanisms of Exosomal PD-L1 in Immune Suppression
3.1. Inhibition of T Cell Proliferation and Cytokine Production
3.2. Suppression of Dendritic Cell Maturation and Antigen Presentation
3.3. Polarization of Macrophages Toward the M2 Phenotype and Downregulation of NK Cell Cytotoxicity
4. Exosomal PD-L1 Contribution to Therapy Resistance
5. Therapeutic Strategies Targeting Exosomal PD-L1
5.1. Targeting Exosome Biogenesis and Secretion
5.2. Neutralizing Exosomal PD-L1 with Antibodies
5.3. Silencing PD-L1 Expression via RNAi and CRISPR
6. Challenges and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Stefanska, K.; Jozkowiak, M.; Angelova Volponi, A.; Shibli, J.A.; Golkar-Narenji, A.; Antosik, P.; Bukowska, D.; Piotrowska-Kempisty, H.; Mozdziak, P.; Dziegiel, P.; et al. The Role of Exosomes in Human Carcinogenesis and Cancer Therapy-Recent Findings from Molecular and Clinical Research. Cells 2023, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B. Function and therapeutic development of exosomes for cancer therapy. Arch. Pharmacal Res. 2022, 45, 295–308. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Yang, L.; Lei, L.; He, B.; Cao, J.; Gao, H. Challenges Coexist with Opportunities: Spatial Heterogeneity Expression of PD-L1 in Cancer Therapy. Adv. Sci. 2024, 11, e2303175. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e413. [Google Scholar] [CrossRef]
- Liu, J.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Zhang, S.; He, G.; Zheng, H.; Fan, Q.; et al. Extracellular vesicle PD-L1 in reshaping tumor immune microenvironment: Biological function and potential therapy strategies. Cell Commun. Signal. 2022, 20, 14. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Lee, J.E.; Sung, K.J.; Sung, Y.H.; Pack, C.G.; Jung, M.K.; Han, B.; et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.W.; Chan, L.C.; Wei, Y.; Hsu, J.M.; Xia, W.; Cha, J.H.; Hou, J.; Hsu, J.L.; Sun, L.; et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018, 28, 862–864. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Zhang, W.; Sun, W.; Huang, J. Colorectal Cancer-Derived Exosomes Impair CD4+ T Cell Function and Accelerate Cancer Progression via Macrophage Activation. Cancer Biother. Radiopharm. 2025, 40, 185–195. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef] [PubMed]
- Gevensleben, H.; Dietrich, D.; Golletz, C.; Steiner, S.; Jung, M.; Thiesler, T.; Majores, M.; Stein, J.; Uhl, B.; Muller, S.; et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, Q.; Liu, L.; Huang, J.; Chang, F. Correlation between exosomal PD-L1 and prognosis in patients with cancer: A systematic review and meta-analysis. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2025, 27, 1288–1298. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, W.; Guo, D.; Zhang, C.; Wang, S.; Lu, X.; Huang, X.; Wang, P.; Zhang, G.; et al. Tumor extracellular vesicles mediate anti-PD-L1 therapy resistance by decoying anti-PD-L1. Cell. Mol. Immunol. 2022, 19, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics. Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Monypenny, J.; Milewicz, H.; Flores-Borja, F.; Weitsman, G.; Cheung, A.; Chowdhury, R.; Burgoyne, T.; Arulappu, A.; Lawler, K.; Barber, P.R.; et al. ALIX Regulates Tumor-Mediated Immunosuppression by Controlling EGFR Activity and PD-L1 Presentation. Cell Rep. 2018, 24, 630–641. [Google Scholar] [CrossRef]
- Yu, Z.L.; Liu, J.Y.; Chen, G. Small extracellular vesicle PD-L1 in cancer: The knowns and unknowns. NPJ Precis. Oncol. 2022, 6, 42. [Google Scholar] [CrossRef]
- Ye, Z.; Xiong, Y.; Peng, W.; Wei, W.; Huang, L.; Yue, J.; Zhang, C.; Lin, G.; Huang, F.; Zhang, L.; et al. Manipulation of PD-L1 Endosomal Trafficking Promotes Anticancer Immunity. Adv. Sci. 2023, 10, e2206411. [Google Scholar] [CrossRef]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Dadashzadeh, A.; Sousa, M.J.; Vlieghe, H.; Yang, J.; Leon-Felix, C.M.; Amorim, C.A. Extracellular vesicles in nanomedicine and regenerative medicine: A review over the last decade. Bioact. Mater. 2024, 36, 126–156. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Li, D.; Zhou, X.; Xu, W.; Chen, Y.; Mu, C.; Zhao, X.; Yang, T.; Wang, G.; Wei, L.; Ma, B. Prostate cancer cells synergistically defend against CD8+ T cells by secreting exosomal PD-L1. Cancer Med. 2023, 12, 16405–16415. [Google Scholar] [CrossRef]
- Moon, J.W.; Kong, S.K.; Kim, B.S.; Kim, H.J.; Lim, H.; Noh, K.; Kim, Y.; Choi, J.W.; Lee, J.H.; Kim, Y.S. IFNgamma induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci. Rep. 2017, 7, 17810. [Google Scholar] [CrossRef]
- Lu, M.M.; Yang, Y. Exosomal PD-L1 in cancer and other fields: Recent advances and perspectives. Front. Immunol. 2024, 15, 1395332. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, A.; Jana, S.; Dey, S.; Roy, H.; Das, M.K.; Alam, J.; Adhikary, A.; Chowdhury, A.; Biswas, A.; et al. Transforming growth factor beta orchestrates PD-L1 enrichment in tumor-derived exosomes and mediates CD8 T-cell dysfunction regulating early phosphorylation of TCR signalome in breast cancer. Carcinogenesis 2021, 42, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.R.; Rahman, M.M.; Li, Z. The link between intracellular calcium signaling and exosomal PD-L1 in cancer progression and immunotherapy. Genes Dis. 2024, 11, 321–334. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, X.; Jiang, D.; Zheng, Z.; Ma, X.; Wu, S.; Li, X.; Lu, J.; Fu, M. Exosomal PD-L1 derived from hypoxia nasopharyngeal carcinoma cell exacerbates CD8+ T cell suppression by promoting PD-L1 upregulation in macrophages. Cancer Immunol. Immunother. 2025, 74, 220. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, Y.; Che, X.; Hou, K.; Zhang, C.; Li, C.; Wen, T.; Wang, S.; Cheng, Y.; Liu, Y.; et al. 5-FU-Induced Upregulation of Exosomal PD-L1 Causes Immunosuppression in Advanced Gastric Cancer Patients. Front. Oncol. 2020, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, H.F.; Li, S. PD-1-mediated inhibition of T cell activation: Mechanisms and strategies for cancer combination immunotherapy. Cell Insight 2024, 3, 100146. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, Q.; Liu, Q.; Li, H.; Zhang, W.; Sun, C. Focus on immune checkpoint PD-1/PD-L1 pathway: New advances of polyphenol phytochemicals in tumor immunotherapy. Biomed. Pharmacother. = Biomed. Pharmacother. 2022, 154, 113618. [Google Scholar] [CrossRef]
- Catakovic, K.; Klieser, E.; Neureiter, D.; Geisberger, R. T cell exhaustion: From pathophysiological basics to tumor immunotherapy. Cell Commun. Signal. 2017, 15, 1. [Google Scholar] [CrossRef]
- Nair, R.; Somasundaram, V.; Kuriakose, A.; Krishn, S.R.; Raben, D.; Salazar, R.; Nair, P. Deciphering T-cell exhaustion in the tumor microenvironment: Paving the way for innovative solid tumor therapies. Front. Immunol. 2025, 16, 1548234. [Google Scholar] [CrossRef]
- Ma, F.; Liu, X.; Zhang, Y.; Tao, Y.; Zhao, L.; Abusalamah, H.; Huffman, C.; Harbison, R.A.; Puram, S.V.; Wang, Y.; et al. Tumor extracellular vesicle-derived PD-L1 promotes T cell senescence through lipid metabolism reprogramming. Sci. Transl. Med. 2025, 17, eadm7269. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Liang, H.; Yu, Y. Nasopharyngeal cancer cell-derived exosomal PD-L1 inhibits CD8+ T-cell activity and promotes immune escape. Cancer Sci. 2022, 113, 3044–3054. [Google Scholar] [CrossRef]
- Morrissey, S.M.; Yan, J. Exosomal PD-L1: Roles in Tumor Progression and Immunotherapy. Trends Cancer 2020, 6, 550–558. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Zheng, S.; Han, B.; Zhang, X.; Zheng, X.; Lu, Y.; Sun, Q.; Hu, X.; Wu, J. The expansion of MDSCs induced by exosomal PD-L1 promotes the progression of gastric cancer. J. Transl. Med. 2024, 22, 821. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes in Cancer: Another Mechanism of Tumor-Induced Immune Suppression. Adv. Exp. Med. Biol. 2017, 1036, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Dominiak, A.; Zolnierzak, A.; Kubiak-Tomaszewska, G.; Lorenc, T. Tumor-Derived Exosomes in Immunosuppression and Immunotherapy. J. Immunol. Res. 2020, 2020, 6272498. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Hao, H.; Chen, L.; Lv, T.; Zhang, X.; Qi, Y.; Wang, Z. Esophageal cancer cell-derived small extracellular vesicles decrease circulating Tfh/Tfr via sEV-PDL1 to promote immunosuppression. Cancer Immunol. Immunother. 2023, 72, 4249–4259. [Google Scholar] [CrossRef]
- Oh, S.A.; Wu, D.C.; Cheung, J.; Navarro, A.; Xiong, H.; Cubas, R.; Totpal, K.; Chiu, H.; Wu, Y.; Comps-Agrar, L.; et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 2020, 1, 681–691. [Google Scholar] [CrossRef]
- Ning, Y.; Shen, K.; Wu, Q.; Sun, X.; Bai, Y.; Xie, Y.; Pan, J.; Qi, C. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol. Lett. 2018, 199, 36–43. [Google Scholar] [CrossRef]
- Pritchard, A.; Tousif, S.; Wang, Y.; Hough, K.; Khan, S.; Strenkowski, J.; Chacko, B.K.; Darley-Usmar, V.M.; Deshane, J.S. Lung Tumor Cell-Derived Exosomes Promote M2 Macrophage Polarization. Cells 2020, 9, 1303. [Google Scholar] [CrossRef]
- Lu, X.; Shen, J.; Huang, S.; Liu, D.; Wang, H. Tumor cells-derived exosomal PD-L1 promotes the growth and invasion of lung cancer cells in vitro via mediating macrophages M2 polarization. Eur. J. Histochem. 2023, 67, 3784. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, J.; Yin, M.; Liu, H.; Zhang, X.; Li, J.; Yan, B.; Guo, Y.; Zhou, J.; Tao, J.; et al. Inhibition of xCT suppresses the efficacy of anti-PD-1/L1 melanoma treatment through exosomal PD-L1-induced macrophage M2 polarization. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 2321–2334. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, F.; He, L.; Wang, X.; Song, L.; Wang, H.; Sun, S.; Guo, Z.; Ma, K.; Xu, J.; et al. AML cell-derived exosomes suppress the activation and cytotoxicity of NK cells in AML via PD-1/PD-L1 pathway. Cell Biol. Int. 2024, 48, 1588–1598. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Qu, X. ASO Author Reflections: The Prognostic Role of Exosomal PD-L1 in Patients with Gastric Cancer. Ann. Surg. Oncol. 2019, 26, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.; Kahlert, C.; Grutzmann, R.; Pilarsky, C. c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 3305. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, M.; Nardin, C.; Chanteloup, G.; Derangere, V.; Algros, M.P.; Arnould, L.; Garrido, C.; Aubin, F.; Gobbo, J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J. Extracell. Vesicles 2020, 9, 1710899. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology 2019, 8, 1593805. [Google Scholar] [CrossRef]
- Smith, S.; Kao, S.; Boyer, M.; Franco, M.; Moore, M. Treatment selection and real-world analysis of immunotherapy with or without chemotherapy in PD-L1-high metastatic non-small cell lung cancer. Intern. Med. J. 2024, 54, 1337–1343. [Google Scholar] [CrossRef]
- Hektoen, H.H.; Tsuruda, K.M.; Brustugun, O.T.; Neumann, K.; Andreassen, B.K. Real-world comparison of pembrolizumab alone and combined with chemotherapy in metastatic lung adenocarcinoma patients with PD-L1 expression ≥50%. ESMO Open 2025, 10, 105073. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, M.; Ma, T.; Zhang, C.; Huang, S.; Karimzadeh, M.R.; Momtazi-Borojeni, A.A.; Chen, S. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: A key role of exosomal PD-L1. J. Immunother. Cancer 2021, 9, e001698. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Zhao, C.; Zhang, S.; Zhu, J. Recent Advancement of PD-L1 Detection Technologies and Clinical Applications in the Era of Precision Cancer Therapy. J. Cancer 2023, 14, 850–873. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.K.; Duan, X.; Zhang, H.J.; Xiao, B.L.; Wang, K.M.; Chen, G. Targeted inhibition of tumor-derived exosomes as a novel therapeutic option for cancer. Exp. Mol. Med. 2022, 54, 1379–1389. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef]
- Wang, L.; Shen, K.; Gao, Z.; Ren, M.; Wei, C.; Yang, Y.; Li, Y.; Zhu, Y.; Zhang, S.; Ding, Y.; et al. Melanoma Derived Exosomes Amplify Radiotherapy Induced Abscopal Effect via IRF7/I-IFN Axis in Macrophages. Adv. Sci. 2024, 11, e2304991. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, M.; Hu, Y.; Guo, H.; Zhang, Y.; Huang, Y.; Zhao, L.; Chai, Y.; Wang, Z. Blockade of exosome generation by GW4869 inhibits the education of M2 macrophages in prostate cancer. BMC Immunol. 2022, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Irep, N.; Inci, K.; Tokgun, P.E.; Tokgun, O. Exosome inhibition improves response to first-line therapy in small cell lung cancer. J. Cell. Mol. Med. 2024, 28, e18138. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Ramadass, M.; He, J.; Brown, S.J.; Zhang, J.; Abgaryan, L.; Biris, N.; Gavathiotis, E.; Rosen, H.; Catz, S.D. Identification of Neutrophil Exocytosis Inhibitors (Nexinhibs), Small Molecule Inhibitors of Neutrophil Exocytosis and Inflammation: DRUGGABILITY OF THE SMALL GTPase Rab27a. J. Biol. Chem. 2016, 291, 25965–25982. [Google Scholar] [CrossRef]
- Martin, L.A.; Head, J.E.; Pancholi, S.; Salter, J.; Quinn, E.; Detre, S.; Kaye, S.; Howes, A.; Dowsett, M.; Johnston, S.R. The farnesyltransferase inhibitor R115777 (tipifarnib) in combination with tamoxifen acts synergistically to inhibit MCF-7 breast cancer cell proliferation and cell cycle progression in vitro and in vivo. Mol. Cancer Ther. 2007, 6, 2458–2467. [Google Scholar] [CrossRef]
- Liu, X.; Wills, C.A.; Chen, L.; Zhang, J.; Zhao, Y.; Zhou, M.; Sundstrom, J.M.; Schell, T.; Spiegelman, V.S.; Young, M.M.; et al. Small extracellular vesicles induce resistance to anti-GD2 immunotherapy unveiling tipifarnib as an adjunct to neuroblastoma immunotherapy. J. Immunother. Cancer 2022, 10, e004399. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Liu, J.; Zhang, G.; Lu, A. Advances in the discovery of exosome inhibitors in cancer. J. Enzym. Inhib. Med. Chem. 2020, 35, 1322–1330. [Google Scholar] [CrossRef]
- Cho, J.; Tae, N.; Song, Y.; Kim, C.W.; Lee, S.J.; Ahn, J.H.; Lee, K.H.; Lee, B.H.; Kim, B.S.; Chang, S.Y.; et al. The expression of PD-L1 on tumor-derived exosomes enhances infiltration and anti-tumor activity of alphaCD3 x alphaPD-L1 bispecific antibody-armed T cells. Cancer Immunol. Immunother. 2024, 73, 196. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef]
- Saha, S.; Roix, J.J.; Welsch, D. Bispecific Antibodies. U.S. Patent Application No. 14/900,757, 26 May 2016. [Google Scholar]
- You, G.; Won, J.; Lee, Y.; Moon, D.; Park, Y.; Lee, S.H.; Lee, S.W. Bispecific Antibodies: A Smart Arsenal for Cancer Immunotherapies. Vaccines 2021, 9, 724. [Google Scholar] [CrossRef]
- Mirfakhraie, R.; Dehaghi, B.K.; Ghorbi, M.D.; Ghaffari-Nazari, H.; Mohammadian, M.; Salimi, M.; Ardakani, M.T.; Parkhideh, S. All about blinatumomab: The bispecific T cell engager immunotherapy for B cell acute lymphoblastic leukemia. Hematol. Transfus. Cell Ther. 2024, 46, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.Y.; Yang, C.; Whilding, L.M.; Parente-Pereira, A.C.; Maher, J.; George, A.J.; Hedrick, J.L.; Yang, Y.Y.; Ghaem-Maghami, S. Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: Strategies to enhance T cell killing. Adv. Healthc. Mater. 2015, 4, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, M.A.; Pico de Coana, Y.; Shmushkovich, T.; Yoshimoto, Y.; Truxova, I.; Yang, Y.; Betancur-Boissel, M.; Eliseev, A.V.; Wolfson, A.D.; Kiessling, R. Self-Delivering RNAi Targeting PD-1 Improves Tumor-Specific T Cell Functionality for Adoptive Cell Therapy of Malignant Melanoma. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Abounar, S.A.; El-Nikhely, N.A.; Turkowski, K.; Savai, R.; Saeed, H. CRISPR/Cas-Mediated Knockdown of PD-L1 and KRAS in Lung Cancer Cells. Int. J. Mol. Sci. 2024, 25, 9086. [Google Scholar] [CrossRef]
- Fierro, J., Jr.; DiPasquale, J.; Perez, J.; Chin, B.; Chokpapone, Y.; Tran, A.M.; Holden, A.; Factoriza, C.; Sivagnanakumar, N.; Aguilar, R.; et al. Dual-sgRNA CRISPR/Cas9 knockout of PD-L1 in human U87 glioblastoma tumor cells inhibits proliferation, invasion, and tumor-associated macrophage polarization. Sci. Rep. 2022, 12, 2417. [Google Scholar] [CrossRef]
- Deng, H.; Tan, S.; Gao, X.; Zou, C.; Xu, C.; Tu, K.; Song, Q.; Fan, F.; Huang, W.; Zhang, Z. Cdk5 knocking out mediated by CRISPR-Cas9 genome editing for PD-L1 attenuation and enhanced antitumor immunity. Acta Pharm. Sin. B 2020, 10, 358–373. [Google Scholar] [CrossRef]
- Wei, S.; Shao, X.; Liu, Y.; Xiong, B.; Cui, P.; Liu, Z.; Li, Q. Genome editing of PD-L1 mediated by nucleobase-modified polyamidoamine for cancer immunotherapy. J. Mater. Chem. B 2022, 10, 1291–1300. [Google Scholar] [CrossRef]
- Cheng, W.J.; Chen, L.C.; Ho, H.O.; Lin, H.L.; Sheu, M.T. Stearyl polyethylenimine complexed with plasmids as the core of human serum albumin nanoparticles noncovalently bound to CRISPR/Cas9 plasmids or siRNA for disrupting or silencing PD-L1 expression for immunotherapy. Int. J. Nanomed. 2018, 13, 7079–7094. [Google Scholar] [CrossRef]
- Gondaliya, P.; Sayyed, A.A.; Yan, I.K.; Driscoll, J.; Ziemer, A.; Patel, T. Targeting PD-L1 in cholangiocarcinoma using nanovesicle-based immunotherapy. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 2762–2777. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Y.; Alves, C.S.; Shi, X. Efficient delivery of therapeutic siRNA into glioblastoma cells using multifunctional dendrimer-entrapped gold nanoparticles. Nanomedicine 2016, 11, 3103–3115. [Google Scholar] [CrossRef]
- Haddick, L.; Zhang, W.; Reinhard, S.; Moller, K.; Engelke, H.; Wagner, E.; Bein, T. Particle-Size-Dependent Delivery of Antitumoral miRNA Using Targeted Mesoporous Silica Nanoparticles. Pharmaceutics 2020, 12, 505. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, W.; Tang, J.; Xu, Z.P. Devising new lipid-coated calcium phosphate/carbonate hybrid nanoparticles for controlled release in endosomes for efficient gene delivery. J. Mater. Chem. B 2017, 5, 7194–7203. [Google Scholar] [CrossRef]

| Compound/Antibody | Target | Mechanism/Recent Application (2020–2025) | Structure # | References |
|---|---|---|---|---|
| GW4869 | nSMase2/exosomes | Inhibits nSMase2; reduces ceramide biosynthesis, leading to decreased exosome release; widely used in cancer/inflammation models. | 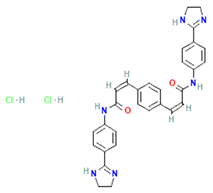 | [62,63] |
| Nexinhib20 | Rab27A | Blocks Rab27A–JFC1 interaction; inhibits EV release in cancer and immune cells; pharmacologically validated. | 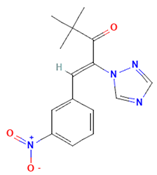 | [63,64] |
| Tipifarnib | Rab27A (modeling) | Used in molecular docking simulations targeting Rab27A ‘WF-pocket’; proposed structure-based inhibitor. | 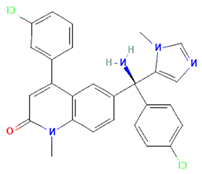 | [65,66] |
| Y-27632 | ROCK1/2 (indirect exosomes) | Inhibits ROCK kinase; decreases EV/microvesicle release in models of cancer and inflammation. |  | [67] |
| BsAb anti-PD-L1/CD63 | PD-L1 + exosomal marker CD63 | Bispecific DVD-Ig; simultaneously binds PD-L1 (immune checkpoint) and CD63 (exosome marker); enables selective targeting of exo-PD-L1. | 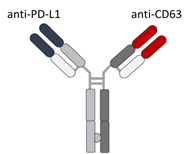 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragu, L.D.; Chivu-Economescu, M.; Pitica, I.M.; Matei, L.; Bleotu, C.; Diaconu, C.C.; Necula, L.G. Targeting Exosomal PD-L1 as a New Frontier in Cancer Immunotherapy. Curr. Issues Mol. Biol. 2025, 47, 525. https://doi.org/10.3390/cimb47070525
Dragu LD, Chivu-Economescu M, Pitica IM, Matei L, Bleotu C, Diaconu CC, Necula LG. Targeting Exosomal PD-L1 as a New Frontier in Cancer Immunotherapy. Current Issues in Molecular Biology. 2025; 47(7):525. https://doi.org/10.3390/cimb47070525
Chicago/Turabian StyleDragu, Laura Denisa, Mihaela Chivu-Economescu, Ioana Madalina Pitica, Lilia Matei, Coralia Bleotu, Carmen Cristina Diaconu, and Laura Georgiana Necula. 2025. "Targeting Exosomal PD-L1 as a New Frontier in Cancer Immunotherapy" Current Issues in Molecular Biology 47, no. 7: 525. https://doi.org/10.3390/cimb47070525
APA StyleDragu, L. D., Chivu-Economescu, M., Pitica, I. M., Matei, L., Bleotu, C., Diaconu, C. C., & Necula, L. G. (2025). Targeting Exosomal PD-L1 as a New Frontier in Cancer Immunotherapy. Current Issues in Molecular Biology, 47(7), 525. https://doi.org/10.3390/cimb47070525







