IFN-γ +874 T/A Is Associated with High Levels of Sera CPK in Patients with Inflammatory Myopathies
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Laboratory Studies
2.3. Genotyping
2.4. Statistical Analysis
3. Results
3.1. Demographic Distribution and Time with the Disease
3.2. Prevalence of the Genotype and Alleles
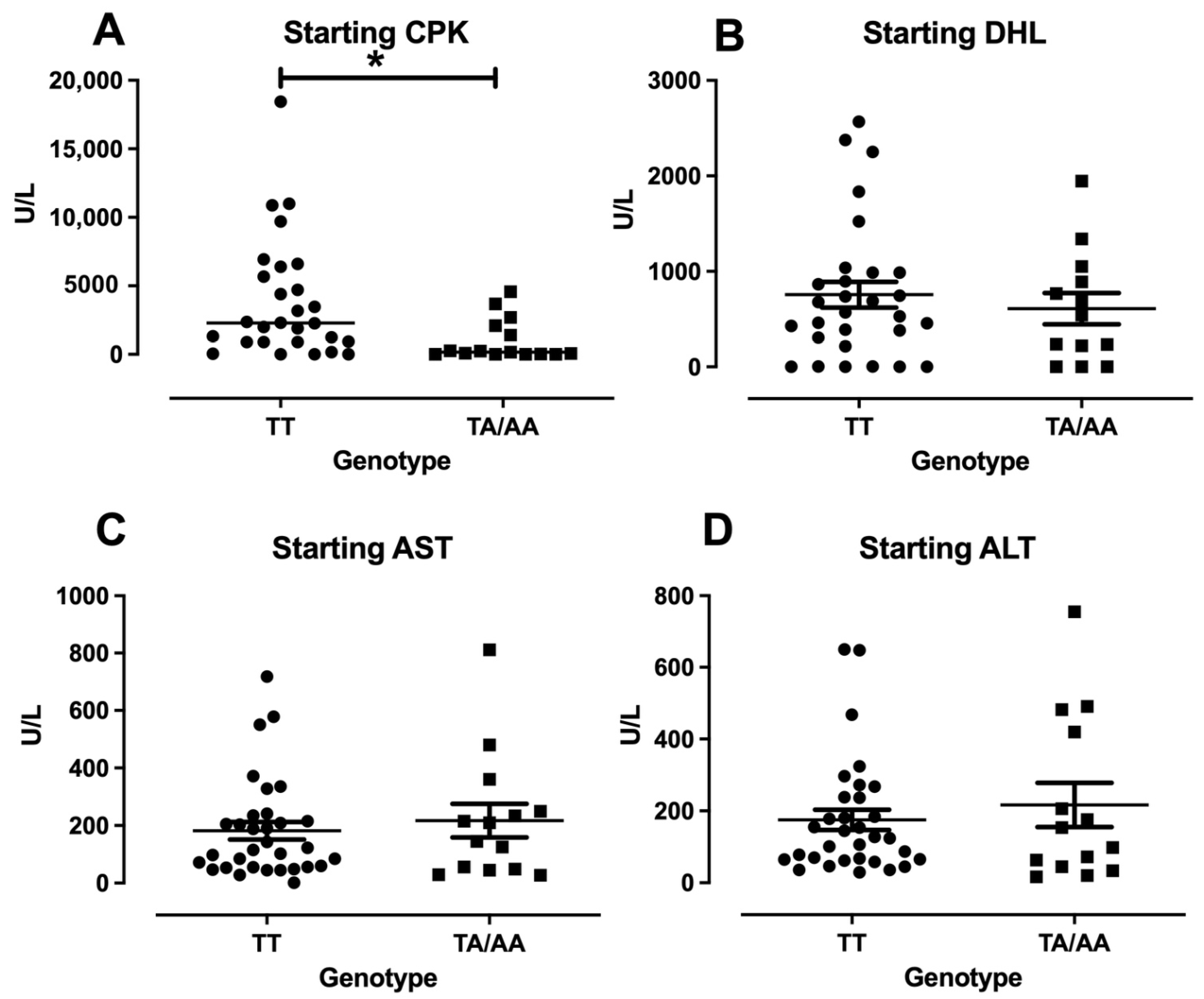
3.3. Enzymes Levels and Clinical Features in DM/PM
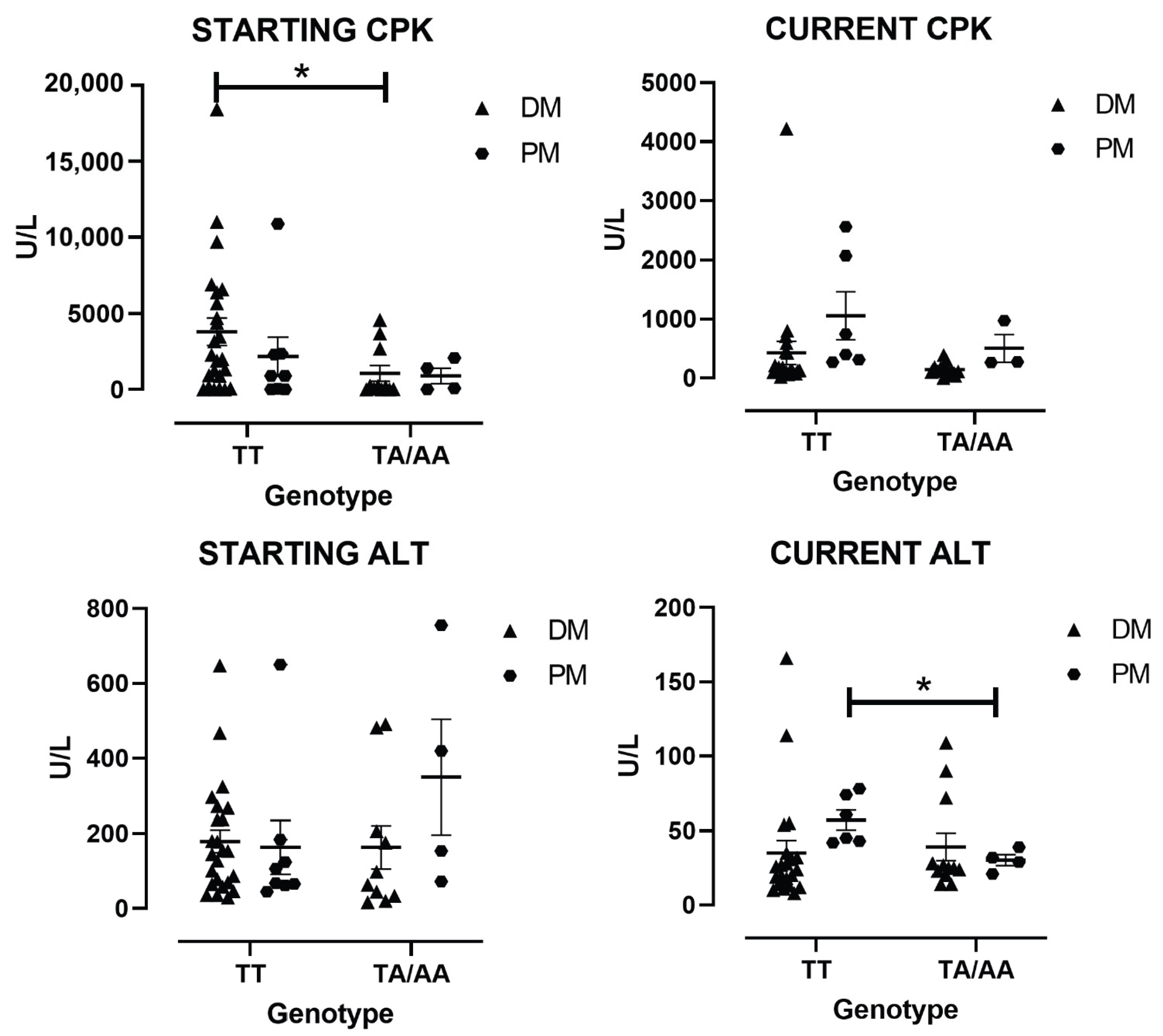
3.4. Enzymes Levels by the Type of IIM
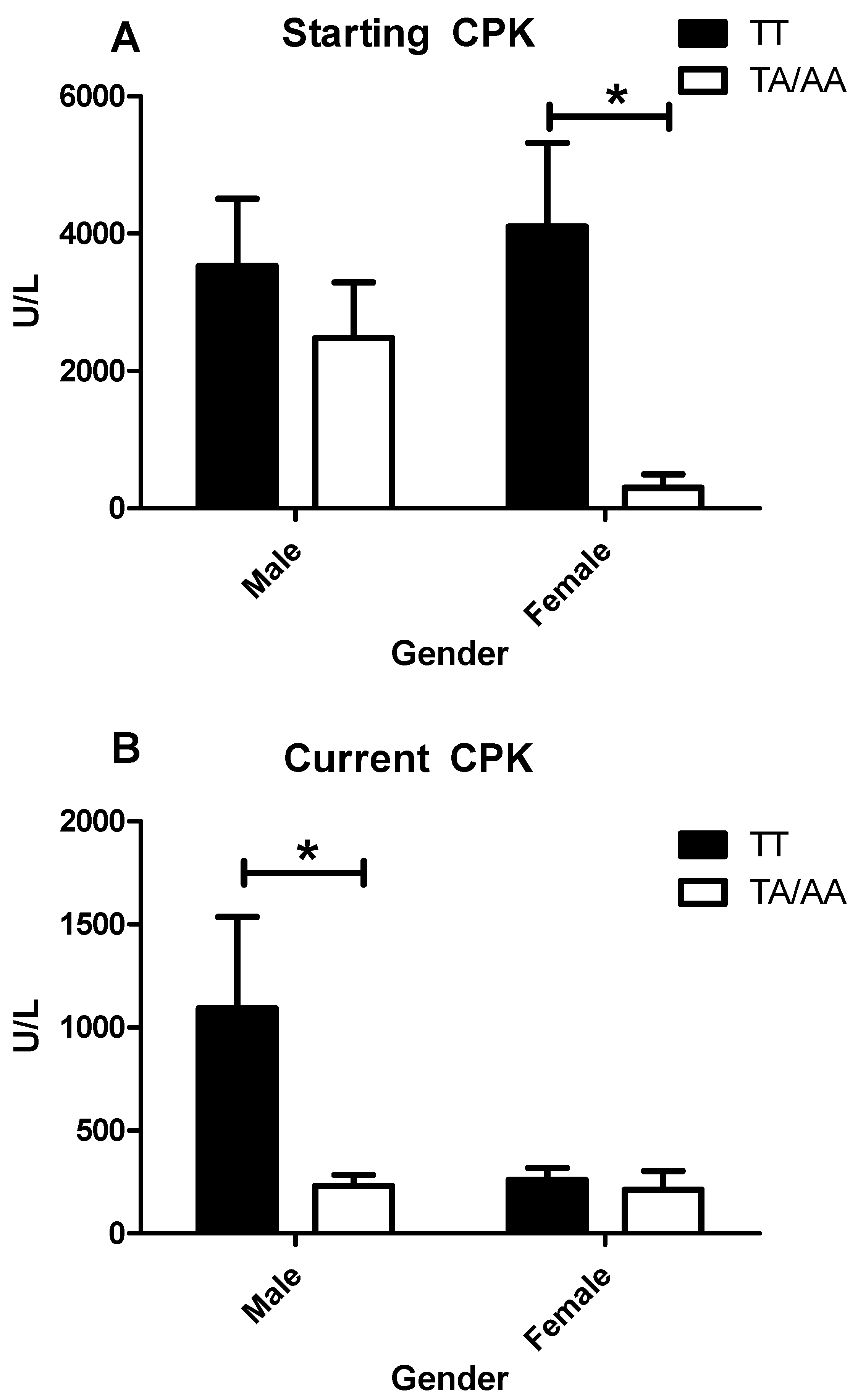
3.5. Enzymes Levels and Clinical Features in IIM by Sex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vazquez Del Mercado Espinosa, M.; Arana Argaez, V.; Heron Petri, M.; Aguilar Arreola, J. Histological and Molecular Alterations in Inflammatory Myopathies. Reumatol. Clin. 2009, 5, 20–22. [Google Scholar] [PubMed]
- Bohan, A.; Peter, J.B. Polymyositis and Dermatomyositis (First of Two Parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; Tjarnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Allenbach, Y.; Mammen, A.L.; Benveniste, O.; Stenzel, W.; Immune-Mediated Necrotizing Myopathies Working, G. 224th ENMC International Workshop:: Clinico-Sero-Pathological Classification of Immune-Mediated Necrotizing Myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul. Disord. 2018, 28, 87–99. [Google Scholar] [CrossRef]
- Hoogendijk, J.E.; Amato, A.A.; Lecky, B.R.; Choy, E.H.; Lundberg, I.E.; Rose, M.R.; Vencovsky, J.; de Visser, M.; Hughes, R.A. 119th ENMC International Workshop: Trial Design in Adult Idiopathic Inflammatory Myopathies, with the Exception of Inclusion Body Myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. 2004, 14, 337–345. [Google Scholar] [CrossRef]
- Allenbach, Y.; Benveniste, O.; Stenzel, W.; Boyer, O. Immune-Mediated Necrotizing Myopathy: Clinical Features and Pathogenesis. Nat. Rev. Rheumatol. 2020, 16, 689–701. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic Inflammatory Myopathies. Nat. Rev. Dis. Primers 2021, 7, 86. [Google Scholar] [CrossRef]
- Gallay, L.; Mouchiroud, G.; Chazaud, B. Interferon-Signature in Idiopathic Inflammatory Myopathies. Curr. Opin. Rheumatol. 2019, 31, 634–642. [Google Scholar] [CrossRef]
- Thuner, J.; Coutant, F. IFN-Gamma: An Overlooked Cytokine in Dermatomyositis with Anti-MDA5 Antibodies. Autoimmun. Rev. 2023, 22, 103420. [Google Scholar] [CrossRef]
- Liao, A.P.; Salajegheh, M.; Nazareno, R.; Kagan, J.C.; Jubin, R.G.; Greenberg, S.A. Interferon Beta Is Associated with Type 1 Interferon-Inducible Gene Expression in Dermatomyositis. Ann. Rheum. Dis. 2011, 70, 831–836. [Google Scholar] [CrossRef]
- Krol, P.; Krystufkova, O.; Polanska, M.; Mann, H.; Klein, M.; Beran, O.; Vencovsky, J. Serum Levels of Interferon Alpha Do Not Correlate with Disease Activity in Patients with Dermatomyositis/Polymyositis. Ann. Rheum. Dis. 2011, 70, 879–880. [Google Scholar] [CrossRef] [PubMed]
- Goebels, N.; Michaelis, D.; Engelhardt, M.; Huber, S.; Bender, A.; Pongratz, D.; Johnson, M.A.; Wekerle, H.; Tschopp, J.; Jenne, D.; et al. Differential Expression of Perforin in Muscle-Infiltrating T Cells in Polymyositis and Dermatomyositis. J. Clin. Investig. 1996, 97, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Kalovidouris, A.E.; Plotkin, Z.; Graesser, D. Interferon-Gamma Inhibits Proliferation, Differentiation, and Creatine Kinase Activity of Cultured Human Muscle Cells. II. A Possible Role in Myositis. J. Rheumatol. 1993, 20, 1718–1723. [Google Scholar] [PubMed]
- Oliver, J.A.; Stolberg, V.R.; Chensue, S.W.; King, P.D. IL-4 Acts as a Potent Stimulator of IFN-Gamma Expression in CD8+ T Cells Through STAT6-Dependent and Independent Induction of Eomesodermin and T-Bet. Cytokine 2012, 57, 191–199. [Google Scholar] [CrossRef]
- Ishii, W.; Matsuda, M.; Shimojima, Y.; Itoh, S.; Sumida, T.; Ikeda, S. Flow Cytometric Analysis of Lymphocyte Subpopulations and TH1/TH2 Balance in Patients with Polymyositis and Dermatomyositis. Intern. Med. 2008, 47, 1593–1599. [Google Scholar] [CrossRef]
- Jarjour, W.N.; Jeffries, B.D.; Davis, J.S.t.; Welch, W.J.; Mimura, T.; Winfield, J.B. Autoantibodies to Human Stress Proteins. A Survey of Various Rheumatic and Other Inflammatory Diseases. Arthritis Rheum. 1991, 34, 1133–1138. [Google Scholar] [CrossRef]
- Aleksza, M.; Szegedi, A.; Antal-Szalmas, P.; Irinyi, B.; Gergely, L.; Ponyi, A.; Hunyadi, J.; Sipka, S.; Zeher, M.; Szegedi, G.; et al. Altered Cytokine Expression of Peripheral Blood Lymphocytes in Polymyositis and Dermatomyositis. Ann. Rheum. Dis. 2005, 64, 1485–1489. [Google Scholar] [CrossRef]
- Villalta, S.A.; Deng, B.; Rinaldi, C.; Wehling-Henricks, M.; Tidball, J.G. IFN-Gamma Promotes Muscle Damage in the Mdx Mouse Model of Duchenne Muscular Dystrophy by Suppressing M2 Macrophage Activation and Inhibiting Muscle Cell Proliferation. J. Immunol. 2011, 187, 5419–5428. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Association Between the Interferon-Gamma +874 T/A Polymorphism and Susceptibility to Systemic Lupus Erythematosus and Rheumatoid Arthritis: A Meta-Analysis. Int. J. Immunogenet. 2022, 49, 365–371. [Google Scholar] [CrossRef]
- Hirankarn, N.; Tangwattanachuleeporn, M.; Wongpiyabovorn, J.; Wongchinsri, J.; Avihingsanon, Y. Association of IL-18 Gene Polymorphism (-137C) with Arthritis Manifestations in SLE: Combined Effect with IFN Gamma Gene Polymorphism (+874A). Clin. Rheumatol. 2009, 28, 219–223. [Google Scholar] [CrossRef]
- Nakkuntod, J.; Wongsurawat, T.; Charoenwongse, P.; Snabboon, T.; Sridama, V.; Hirankarn, N. Association of TNF-Alpha, TNF-Beta, IFN-Gamma and IL-1Ra Gene Polymorphisms with Graves’ Disease in the Thai Population. Asian Pac. J. Allergy Immunol. 2006, 24, 207–211. [Google Scholar] [PubMed]
- Rekha, P.L.; Ishaq, M.; Valluri, V. A Differential Association of Interferon-Gamma High-Producing Allele T and Low-Producing Allele A (+874 A/T) with Hashimoto’s Thyroiditis and Graves’ Disease. Scand. J. Immunol. 2006, 64, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Prabhu Anand, S.; Harishankar, M.; Selvaraj, P. Interferon Gamma Gene +874A/T Polymorphism and Intracellular Interferon Gamma Expression in Pulmonary Tuberculosis. Cytokine 2010, 49, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Kordi Tamandani, M.K.; Sobti, R.C.; Shekari, M.; Mukesh, M.; Suri, V. Expression and Polimorphism of IFN-Gamma Gene in Patients with Cervical Cancer. Exp. Oncol. 2008, 30, 224–229. [Google Scholar]
- Liu, F.; Li, B.; Wei, Y.G.; Chen, X.; Ma, Y.; Yan, L.N.; Wen, T.F.; Xu, M.Q.; Wang, W.T.; Yang, J.Y. IFN-Gamma+874 A/T Polymorphism and Cancer Risk: An Updated Analysis Based on 32 Case-Control Studies. Cytokine 2011, 56, 200–207. [Google Scholar] [CrossRef]
- Karaoglan, I.; Pehlivan, S.; Namiduru, M.; Pehlivan, M.; Kilincarslan, C.; Balkan, Y.; Baydar, I. TNF-Alpha, TGF-Beta, IL-10, IL-6 and IFN-Gamma Gene Polymorphisms as Risk Factors for Brucellosis. New Microbiol. 2009, 32, 173–178. [Google Scholar]
- Seyyed Rezaei, S.A.; Asgharzadeh, V.; Mahdavi Poor, B.; Asgharzadeh, M.; Poorghani, A.; Asghari Ozma, M.; Khalili, A.A.; Jalaei Nobari, H.; Raeisi, M.; Rashedi, J. Association Between IFN-Gamma +874T/A SNP and COVID-19 Severity. Iran. J. Allergy Asthma Immunol. 2025, 24, 254–258. [Google Scholar]
- Lio, D.; Scola, L.; Crivello, A.; Bonafe, M.; Franceschi, C.; Olivieri, F.; Colonna-Romano, G.; Candore, G.; Caruso, C. Allele Frequencies of +874T-->A Single Nucleotide Polymorphism at the First Intron of Interferon-Gamma Gene in a Group of Italian Centenarians. Exp. Gerontol. 2002, 37, 315–319. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A Simple Salting out Procedure for Extracting DNA from Human Nucleated Cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Petri, M.H.; Satoh, M.; Martin-Marquez, B.T.; Vargas-Ramirez, R.; Jara, L.J.; Saavedra, M.A.; Cruz-Gonzalez, C.; Andrade-Ortega, L.; Vera-Lastra, O.; Salazar-Paramo, M.; et al. Implications in the Difference of Anti-Mi-2 and -p155/140 Autoantibody Prevalence in Two Dermatomyositis Cohorts from Mexico City and Guadalajara. Arthritis Res. Ther. 2013, 15, R48. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Oddis, C.V.; Aggarwal, R. Approach to Asymptomatic Creatine Kinase Elevation. Cleve Clin. J. Med. 2016, 83, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, T.; Zhang, C. The Relationship Between Expression Level and Gene Polymorphism of Inflammatory Factors and Sepsis Risk. Sci. Rep. 2025, 15, 6701. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, B.D.R.; Vieira, M.; Silva, M.J.A.; da Silva, L.; Gurrao, E.; Dos Santos, E.C.; Cabral, J.G.; Souza, A.B.; Sardinha, D.M.; Marinho, R.L.; et al. Study of TNF-Alpha, IFN-Gamma, IL-10, TGF-Beta and IL-6 Gene Polymorphisms in a Cohort of Professionals Who Worked in the First Pandemic Wave in the Brazilian Amazon. Crit. Rev. Immunol. 2025, 45, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Carlo-Stella, N.; Badulli, C.; De Silvestri, A.; Bazzichi, L.; Martinetti, M.; Lorusso, L.; Bombardieri, S.; Salvaneschi, L.; Cuccia, M. A First Study of Cytokine Genomic Polymorphisms in CFS: Positive Association of TNF-857 and IFNgamma 874 Rare Alleles. Clin. Exp. Rheumatol. 2006, 24, 179–182. [Google Scholar]
| Geographic Distribution of IIM Patients and HC | |||||
|---|---|---|---|---|---|
| Male | Time with Disease (Months) Mean ± SEM | Female | Time with Disease (Months) Mean ± SEM | Age Mean ± SEM | |
| DM | 10 | 3.7 ± 0.3 | 26 | 17.2 ± 1.3 | 42 ± 6 |
| PM | 7 | 2.54 ± 0.5 | 5 | 23.2 ± 9.5 | 47 ± 4 |
| HC | 31 | 44 | 36 ± 5 | ||
| Genotype and Frequencies of IFN-γ +874 Polymorphism in IIM Patients and HC | |||||
|---|---|---|---|---|---|
| Genotype | DM/PM | DM | PM | HC | |
| N = 48 (%) | N = 36 (%) | N = 12(%) | N = 75 (%) | p Value | |
| TT | 32 (66.6) | 24 (66.8) | 8 (66.7) | 39 (52) | NS |
| TA | 15 (31.3) | 11 (30.5) | 4 (33.3) | 31 (41.3) | NS |
| AA | 1 (2.1) | 1 (2.7) | 0 (0) | 5 (6.7) | NS |
| Allele | |||||
| T | 79 (82) | 60 (81) | 20 (83) | 109 (73) | NS |
| A | 17 (18) | 14 (19) | 4 (17) | 41 (27) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Del Mercado, M.; Martín-Márquez, B.T.; Martínez-García, E.A.; Petri, M.H. IFN-γ +874 T/A Is Associated with High Levels of Sera CPK in Patients with Inflammatory Myopathies. Curr. Issues Mol. Biol. 2025, 47, 492. https://doi.org/10.3390/cimb47070492
Vázquez-Del Mercado M, Martín-Márquez BT, Martínez-García EA, Petri MH. IFN-γ +874 T/A Is Associated with High Levels of Sera CPK in Patients with Inflammatory Myopathies. Current Issues in Molecular Biology. 2025; 47(7):492. https://doi.org/10.3390/cimb47070492
Chicago/Turabian StyleVázquez-Del Mercado, Mónica, Beatriz Teresita Martín-Márquez, Erika Aurora Martínez-García, and Marcelo Heron Petri. 2025. "IFN-γ +874 T/A Is Associated with High Levels of Sera CPK in Patients with Inflammatory Myopathies" Current Issues in Molecular Biology 47, no. 7: 492. https://doi.org/10.3390/cimb47070492
APA StyleVázquez-Del Mercado, M., Martín-Márquez, B. T., Martínez-García, E. A., & Petri, M. H. (2025). IFN-γ +874 T/A Is Associated with High Levels of Sera CPK in Patients with Inflammatory Myopathies. Current Issues in Molecular Biology, 47(7), 492. https://doi.org/10.3390/cimb47070492







