Rewiring the Brain Through the Gut: Insights into Microbiota–Nervous System Interactions
Abstract
1. Introduction
2. Materials and Methods
3. Gut-Brain Crosstalk: The Gut-Brain Axis and Microbiota-Gut-Brain Axis Concepts
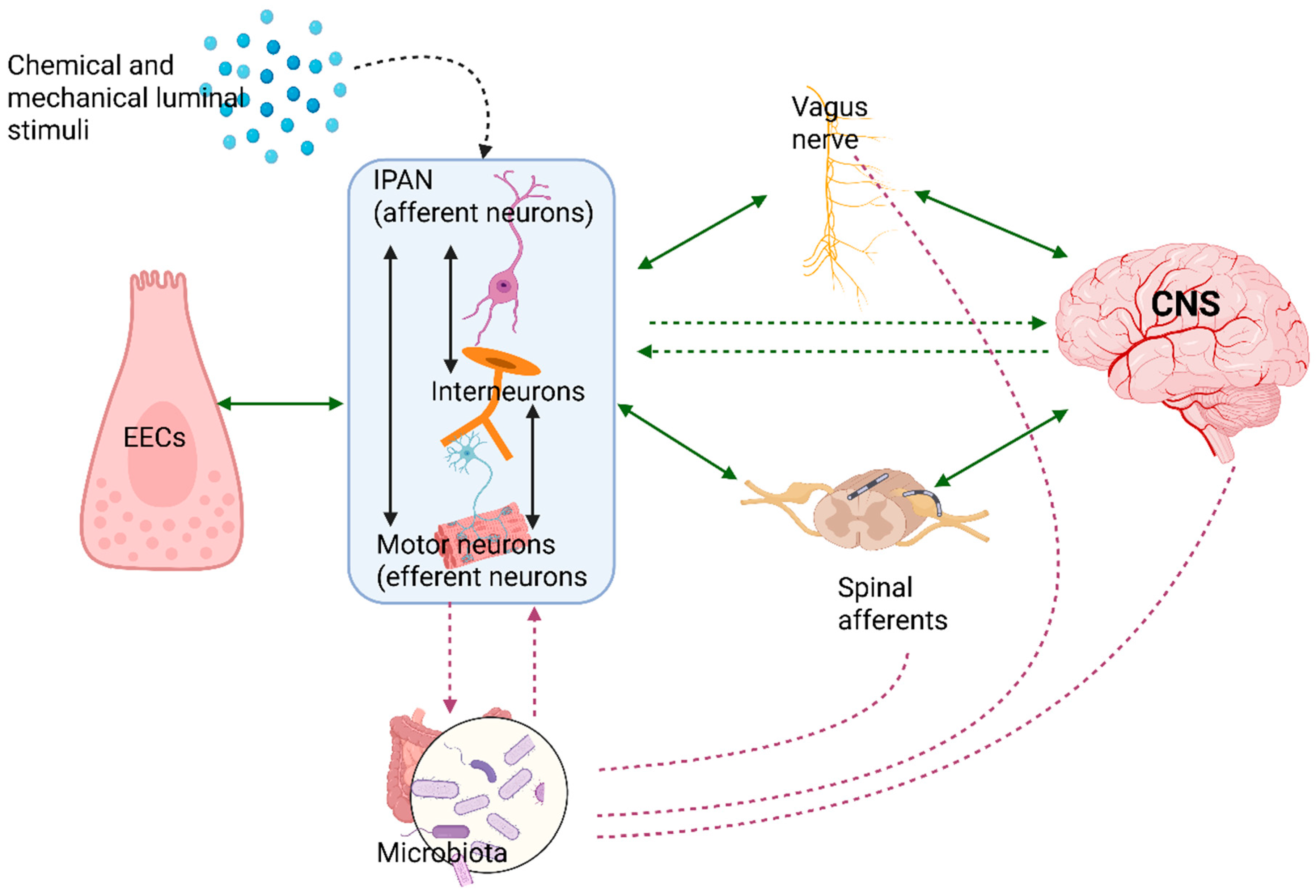
4. The Nervous Pathways in Gut-Brain Bidirectional Communication: Bottom-Up and Top-Down Signaling
5. The Leading Players in the Microbiota-Gut-Brain Axis
5.1. The Enteric Nervous System: “The Second Brain”
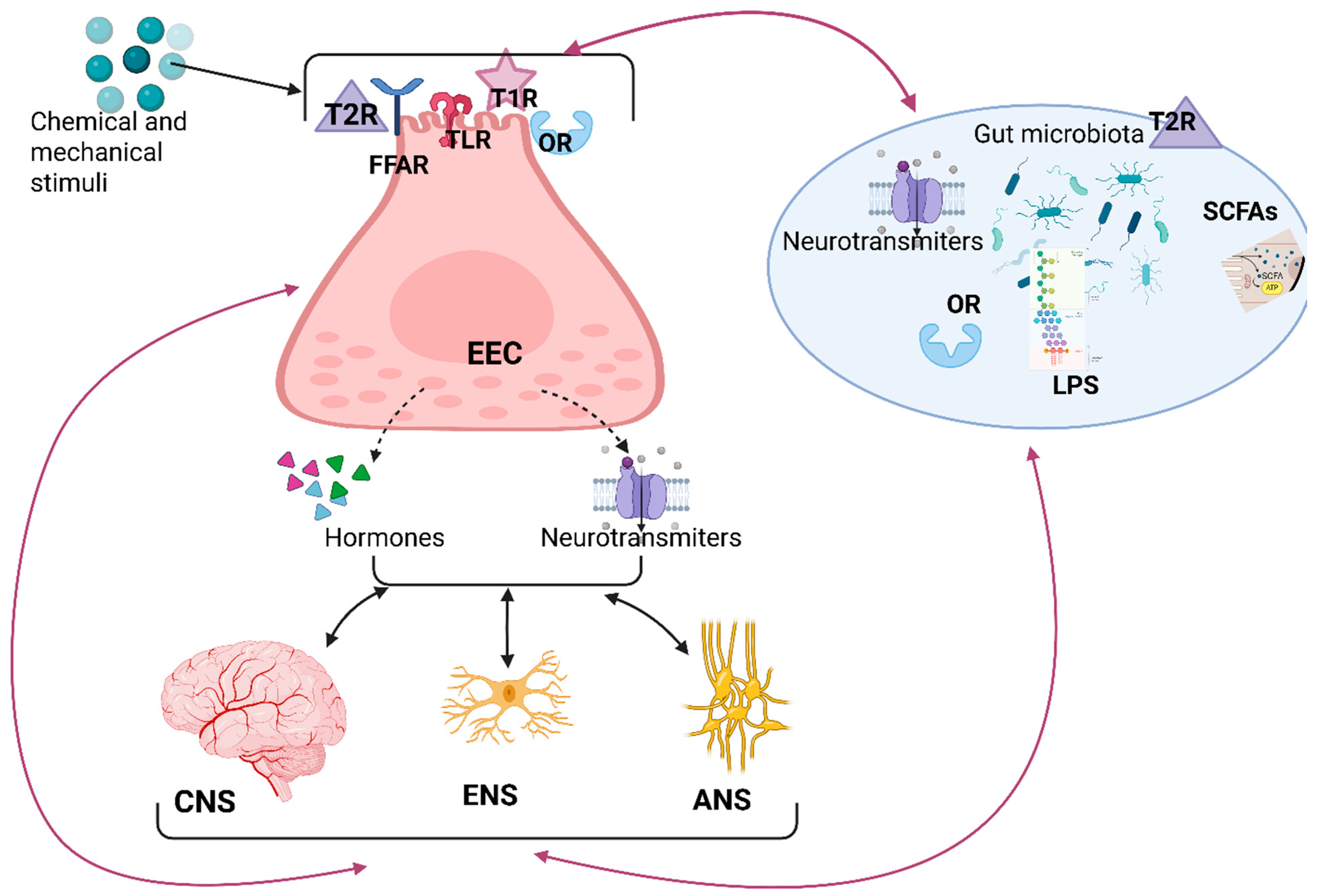
5.2. The Enteroendocrine Cells
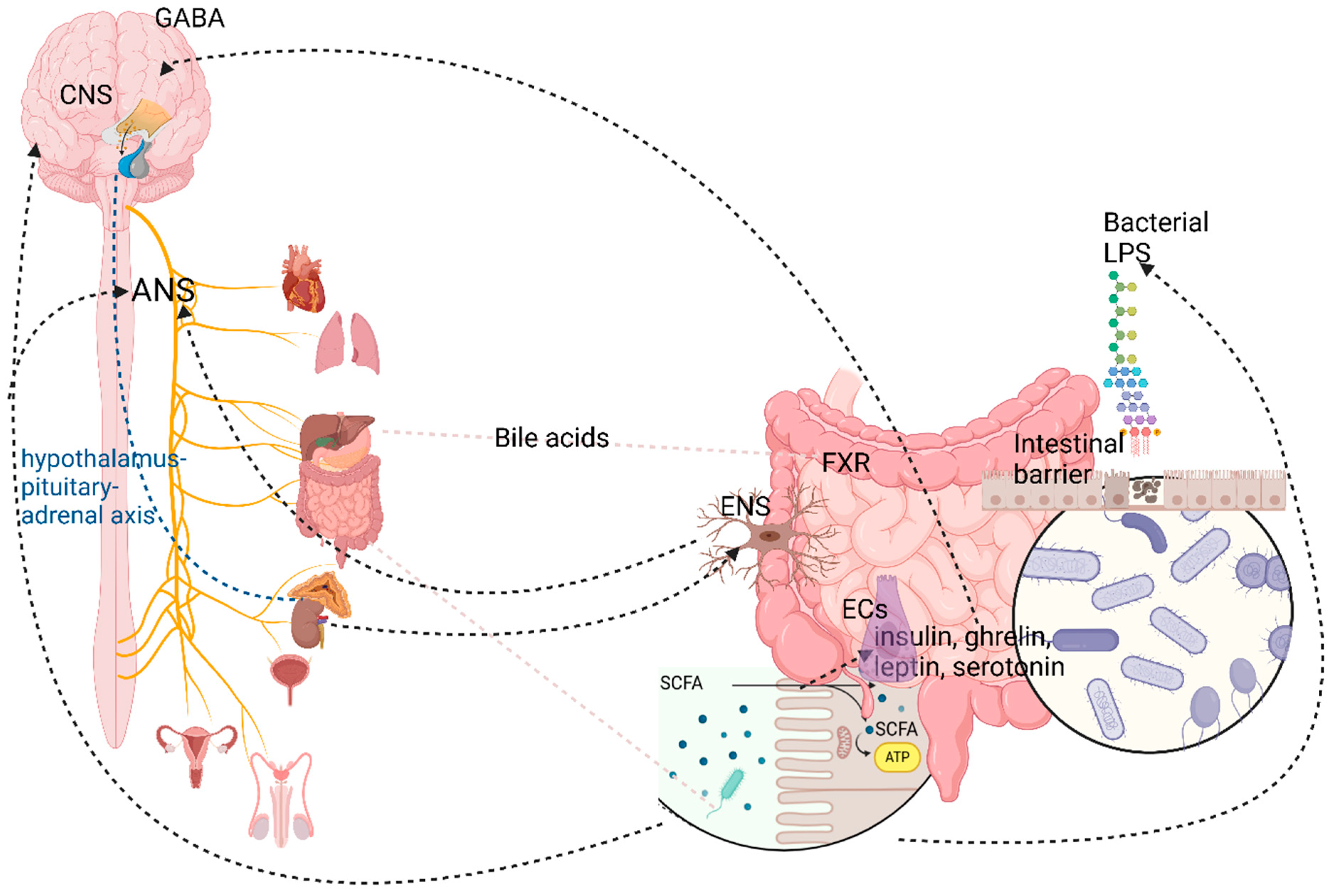
5.3. The Autonomic Nervous System
6. The Gut Microbiome (Microbiota)
7. miRNAs’ Roles
8. Methodological Limitations
8.1. Differences Between Animal and Human Models
8.2. Small Cohort Sizes and Lack of Power
8.3. Variability in Microbiome Sequencing Technologies
8.4. CNS Outcome Measurement
8.5. Environmental and Lifestyle Confounders
9. Future Directions and Outstanding Questions
- -
- Temporal causality: Do the shifts in the gut microbiome precede, follow, or co-occur with neural or behavioral changes in disorders like depression, autism, and dementia? Can the dysbiosis be reversed in order to improve the outcomes of patients [310]?
- -
- Biomarker validity: Which microbial metabolites or immune markers reliably predict CNS health or disease [316]?
- -
- Clinical translation capacity: Are interventions like fecal microbiota transplants, targeted probiotics, diet, or molecular therapies effective and safe in neuropsychiatric or neurodegenerative disease? Can these interventions improve the well-being of humans [317]?
- -
- Personalized interventions: How does inter-individual variation (baseline microbiome, diet, and genetics) influence the response to psychobiotics or fecal microbiota transplant, and can we tailor therapies accordingly [317]?
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schaub, A.-C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: A randomized controlled trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Bagga, D.; Reichert, J.L.; Koschutnig, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schöpf, V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018, 9, 486–496. [Google Scholar] [CrossRef]
- Goral, O.; Wald, I.Y.; Maimon, A.; Snir, A.; Golland, Y.; Goral, A.; Amedi, A. Enhancing interoceptive sensibility through exteroceptive–interoceptive sensory substitution. Sci. Rep. 2024, 14, 14855. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Interoception and Emotion: A Neuroanatomical Perspective—The Handbook of Emotion, 3rd ed.; Lewis, L.-J., Barrett, Eds.; Chapter 16; Cameron: Petaluma, CA, USA, 2009; pp. 1–22. [Google Scholar]
- Chahwan, B.; Kwan, S.; Isik, A.; Van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef]
- Tomaszek, N.; Urbaniak, A.D.; Bałdyga, D.; Chwesiuk, K.; Modzelewski, S.; Waszkiewicz, N. Unraveling the Connections: Eating Issues, Microbiome, and Gastrointestinal Symptoms in Autism Spectrum Disorder. Nutrients 2025, 17, 486. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tapia, E.; Almeida-Toledano, L.; Sebastiani, G.; Serra-Delgado, M.; García-Algar, Ó.; Andreu-Fernández, V. Effects of Microbiota Imbalance in Anxiety and Eating Disorders: Probiotics as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 2351. [Google Scholar] [CrossRef]
- Damasio, A.R.; Grabowski, T.J.; Bechara, A.; Damasio, H.; Ponto, L.L.B.; Parvizi, J.; Hichwa, R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000, 3, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Lyyra, P.; Parviainen, T. Behavioral Inhibition Underlies the Link Between Interoceptive Sensitivity and Anxiety-Related Temperamental Traits. Front. Psychol. 2018, 9, 1026. [Google Scholar] [CrossRef]
- Critchley, H.D.; Garfinkel, S.N. Interoception and emotion. Curr. Opin. Psychol. 2017, 17, 7–14. [Google Scholar] [CrossRef]
- Weiss, S.; Sack, M.; Henningsen, P.; Pollatos, O. On the Interaction of Self-Regulation, Interoception and Pain Perception. Psychopathology 2014, 47, 377–382. [Google Scholar] [CrossRef]
- Monti, A.; Porciello, G.; Panasiti, M.S.; Aglioti, S.M. The inside of me: Interoceptive constraints on the concept of self in neuroscience and clinical psychology. Psychol. Res. 2022, 86, 2468–2477. [Google Scholar] [CrossRef]
- Barrett, L.F.; Mesquita, B.; Ochsner, K.N.; Gross, J.J. The Experience of Emotion. Annu. Rev. Psychol. 2007, 58, 373–403. [Google Scholar] [CrossRef] [PubMed]
- Pace-Schott, E.F.; Amole, M.C.; Aue, T.; Balconi, M.; Bylsma, L.M.; Critchley, H.; Demaree, H.A.; Friedman, B.H.; Gooding, A.E.K.; Gosseries, O.; et al. Physiological feelings. Neurosci. Biobehav. Rev. 2019, 103, 267–304. [Google Scholar] [CrossRef] [PubMed]
- Karaivazoglou, K.; Aggeletopoulou, I.; Triantos, C. Interoceptive Processing in Functional Gastrointestinal Disorders. Int. J. Mol. Sci. 2024, 25, 7633. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Schoen, S. Interoception: A Multi-Sensory Foundation of Participation in Daily Life. Front. Neurosci. 2022, 16, 875200. [Google Scholar] [CrossRef]
- Prescott, S.L.; Liberles, S.D. Internal senses of the vagus nerve. Neuron 2022, 110, 579–599. [Google Scholar] [CrossRef]
- Davey, S.; Halberstadt, J.; Bell, E. Where is emotional feeling felt in the body? An integrative review. PLoS ONE 2021, 16, e0261685. [Google Scholar] [CrossRef]
- Garfinkel, S.N.; Manassei, M.F.; Hamilton-Fletcher, G.; In Den Bosch, Y.; Critchley, H.D.; Engels, M. Interoceptive dimensions across cardiac and respiratory axes. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160014. [Google Scholar] [CrossRef]
- Gibson, J. Mindfulness, Interoception, and the Body: A Contemporary Perspective. Front. Psychol. 2019, 10, 2012. [Google Scholar] [CrossRef]
- Ferentzi, E.; Bogdány, T.; Szabolcs, Z.; Csala, B.; Horváth, Á.; Köteles, F. Multichannel Investigation of Interoception: Sensitivity Is Not a Generalizable Feature. Front. Hum. Neurosci. 2018, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Vianna, E.P.M.; Tranel, D. Gastric myoelectrical activity as an index of emotional arousal. Int. J. Psychophysiol. 2006, 61, 70–76. [Google Scholar] [CrossRef]
- Hughes, P.A.; Zola, H.; Penttila, I.A.; Blackshaw, A.L.; Andrews, J.M.; Krumbiegel, D. Immune Activation in Irritable Bowel Syndrome: Can Neuroimmune Interactions Explain Symptoms? Am. J. Gastroenterol. 2013, 108, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Gut Signals and Gut Feelings: Science at the Interface of Data and Beliefs. Front. Behav. Neurosci. 2022, 16, 929332. [Google Scholar] [CrossRef] [PubMed]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Manaye, K.F.; Semendeferi, K.; Erwin, J.M.; Park, S.; Goubert, V.; Hof, P.R. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct. Funct. 2010, 214, 495–517. [Google Scholar] [CrossRef]
- Richards, P.; Thornberry, N.A.; Pinto, S. The gut–brain axis: Identifying new therapeutic approaches for type 2 diabetes, obesity, and related disorders. Mol. Metab. 2021, 46, 101175. [Google Scholar] [CrossRef]
- Muller, P.A.; Matheis, F.; Schneeberger, M.; Kerner, Z.; Jové, V.; Mucida, D. Microbiota-modulated CART+ enteric neurons autonomously regulate blood glucose. Science 2020, 370, 314–321. [Google Scholar] [CrossRef]
- Zimmerman, C.A.; Huey, E.L.; Ahn, J.S.; Beutler, L.R.; Tan, C.L.; Kosar, S.; Bai, L.; Chen, Y.; Corpuz, T.V.; Madisen, L.; et al. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 2019, 568, 98–102. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Furness, J.B.; Rivera, L.R.; Cho, H.-J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Castro, A.J.; González-Cervantes, R.M.; Bravo-Ruiseco, G.; Pacheco-López, G. The Microbiota-Gut-Brain Axis: Neurobehavioral Correlates, Health and Sociality. Front. Integr. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef]
- Browning, K.N.; Travagli, R.A. Central Nervous System Control of Gastrointestinal Motility and Secretion and Modulation of Gastrointestinal Functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Gómez-Martinez, S.; González-Soltero, R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms 2022, 10, 1368. [Google Scholar] [CrossRef]
- Glenny, E.M.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.M.; Carroll, I.M. Eating Disorders and the Intestinal Microbiota: Mechanisms of Energy Homeostasis and Behavioral Influence. Curr. Psychiatry Rep. 2017, 19, 51. [Google Scholar] [CrossRef]
- Hockley, J.R.F.; Taylor, T.S.; Callejo, G.; Wilbrey, A.L.; Gutteridge, A.; Bach, K.; Winchester, W.J.; Bulmer, D.C.; McMurray, G.; Smith, E.S.J. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 2019, 68, 633–644. [Google Scholar] [CrossRef]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Harrison, N.A. Visceral Influences on Brain and Behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef]
- Centanni, S.W.; Janes, A.C.; Haggerty, D.L.; Atwood, B.; Hopf, F.W. Better living through understanding the insula: Why subregions can make all the difference. Neuropharmacology 2021, 198, 108765. [Google Scholar] [CrossRef]
- Bassi, J.K.; Connelly, A.A.; Butler, A.G.; Liu, Y.; Ghanbari, A.; Farmer, D.G.S.; Jenkins, M.W.; Melo, M.R.; McDougall, S.J.; Allen, A.M. Analysis of the distribution of vagal afferent projections from different peripheral organs to the nucleus of the solitary tract in rats. J. Comp. Neurol. 2022, 530, 3072–3103. [Google Scholar] [CrossRef] [PubMed]
- McGovern, A.E.; Ajayi, I.E.; Farrell, M.J.; Mazzone, S.B. A neuroanatomical framework for the central modulation of respiratory sensory processing and cough by the periaqueductal grey. J. Thorac. Dis. 2017, 9, 4098–4107. [Google Scholar] [CrossRef]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Delay, E.R.; Roper, S.D. Umami Taste Signaling from the Taste Bud to Cortex. In Umami; San Gabriel, A., Rains, T.M., Beauchamp, G., Eds.; (Food and Health); Springer International Publishing: Cham, Swizerland, 2024; pp. 43–71. [Google Scholar] [CrossRef]
- Prinster, A.; Cantone, E.; Verlezza, V.; Magliulo, M.; Sarnelli, G.; Iengo, M.; Cuomo, R.; Di Salle, F.; Esposito, F. Cortical representation of different taste modalities on the gustatory cortex: A pilot study. PLoS ONE 2017, 12, e0190164. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Torrico, T.J.; Abdijadid, S. Neuroanatomy, Limbic System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538491/ (accessed on 30 March 2025).
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. In Comprehensive Physiology, 1st ed.; Prakash, Y.S., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 603–621. [Google Scholar] [CrossRef]
- Kinlein, S.A.; Karatsoreos, I.N. The hypothalamic-pituitary-adrenal axis as a substrate for stress resilience: Interactions with the circadian clock. Front. Neuroendocrinol. 2020, 56, 100819. [Google Scholar] [CrossRef]
- Beutler, L.R.; Chen, Y.; Ahn, J.S.; Lin, Y.-C.; Essner, R.A.; Knight, Z.A. Dynamics of Gut-Brain Communication Underlying Hunger. Neuron 2017, 96, 461–475.e5. [Google Scholar] [CrossRef]
- Bufkin, J.L.; Luttrell, V.R. Neuroimaging Studies of Aggressive and Violent Behavior: Current Findings and Implications for Criminology and Criminal Justice. Trauma Violence Abus. 2005, 6, 176–191. [Google Scholar] [CrossRef]
- Cardinal, R.N.; Parkinson, J.A.; Hall, J.; Everitt, B.J. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002, 26, 321–352. [Google Scholar] [CrossRef]
- Sah, P.; Faber, E.S.L.; Lopez De Armentia, M.; Power, J. The Amygdaloid Complex: Anatomy and Physiology. Physiol. Rev. 2003, 83, 803–834. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, C.T.; Khan, M.R. Symbiotic microbes from the human gut. In Microbial Symbionts; Elsevier: Amsterdam, The Netherlands, 2023; pp. 533–549. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780323993340000037 (accessed on 26 March 2025).
- Ganci, M.; Suleyman, E.; Butt, H.; Ball, M. The role of the brain–gut–microbiota axis in psychology: The importance of considering gut microbiota in the development, perpetuation, and treatment of psychological disorders. Brain Behav. 2019, 9, e01408. [Google Scholar] [CrossRef] [PubMed]
- Marklund, U. Diversity, development and immunoregulation of enteric neurons. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Guyer, R.A.; Mueller, J.L.; Goldstein, A.M. Applications of Single-Cell Sequencing Technology to the Enteric Nervous System. Biomolecules 2022, 12, 452. [Google Scholar] [CrossRef]
- Michel, K.; Kuch, B.; Dengler, S.; Demir, I.E.; Zeller, F.; Schemann, M. How big is the little brain in the gut? Neuronal numbers in the enteric nervous system of mice, Guinea pig, and human. Neurogastroenterol. Motil. 2022, 34, e14440. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Mawe, G.M. The enteric nervous system. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Sharkey, K.A. Purinergic Neuron-to-Glia Signaling in the Enteric Nervous System. Gastroenterology 2009, 136, 1349–1358. [Google Scholar] [CrossRef]
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Rosenberg, H.J.; Rao, M. Enteric glia in homeostasis and disease: From fundamental biology to human pathology. iScience 2021, 24, 102863. [Google Scholar] [CrossRef]
- Sanders, K.M.; Kito, Y.; Hwang, S.J.; Ward, S.M. Regulation of Gastrointestinal Smooth Muscle Function by Interstitial Cells. Physiology 2016, 31, 316–326. [Google Scholar] [CrossRef]
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Advances in Experimental Medicine and Biology; Springer: New Yor, NY, USA, 2014; Volume 817, pp. 39–71. [Google Scholar] [CrossRef]
- Furness, J.B.; Jones, C.; Nurgali, K.; Clerc, N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 2004, 72, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Ryskalin, L.; Morucci, G.; Lazzeri, G.; Frati, A.; Fornai, F. The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease. Life 2021, 11, 732. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.M.; Bornstein, J.C.; Young, H.M. Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP mice. J. Comp. Neurol. 2013, 521, 3358–3370. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.S.; Lee, S.J.; Barlow-Anacker, A.J.; Druckenbrod, N.R.; Epstein, M.L.; Gosain, A. Appearance of cholinergic myenteric neurons during enteric nervous system development: Comparison of different Ch AT fluorescent mouse reporter lines. Neurogastroenterol. Motil. 2014, 26, 874–884. [Google Scholar] [CrossRef]
- Brehmer, A. Classification of human enteric neurons. Histochem. Cell Biol. 2021, 156, 95–108. [Google Scholar] [CrossRef]
- Furness, J.B.; Di Natale, M.; Hunne, B.; Oparija-Rogenmozere, L.; Ward, S.M.; Sasse, K.C.; Powley, T.L.; Stebbing, M.J.; Jaffey, D.; Fothergill, L.J. The identification of neuronal control pathways supplying effector tissues in the stomach. Cell Tissue Res. 2020, 382, 433–445. [Google Scholar] [CrossRef]
- Neuhuber, W.L.; Wörl, J. Enteric co-innervation of striated muscle in the esophagus: Still enigmatic? Histochem. Cell Biol. 2016, 146, 721–735. [Google Scholar] [CrossRef]
- Costa, M.; Brookes, S.H. Architecture of enteric neural circuits involved in intestinal motility. Eur. Rev. Med. Pharmacol. Sci. 2008, 12 (Suppl. S1), 3–19. [Google Scholar]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef]
- Burman, A.; Kaji, I. Luminal Chemosensory Cells in the Small Intestine. Nutrients 2021, 13, 3712. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.E.; Schneider, C.; Locksley, R.M. Tuft Cells—Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu. Rev. Immunol. 2019, 37, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft Cells and Their Role in Intestinal Diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef]
- Cheng, X.; Voss, U.; Ekblad, E. Tuft cells: Distribution and connections with nerves and endocrine cells in mouse intestine. Exp. Cell Res. 2018, 369, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Barajon, I.; Serrao, G.; Arnaboldi, F.; Opizzi, E.; Ripamonti, G.; Balsari, A.; Rumio, C. Toll-like Receptors 3, 4, and 7 Are Expressed in the Enteric Nervous System and Dorsal Root Ganglia. J. Histochem. Cytochem. 2009, 57, 1013–1023. [Google Scholar] [CrossRef]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; Van Der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef]
- Drokhlyansky, E.; Smillie, C.S.; Van Wittenberghe, N.; Ericsson, M.; Griffin, G.K.; Eraslan, G.; Dionne, D.; Cuoco, M.S.; Goder-Reiser, M.N.; Sharova, T.; et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020, 182, 1606–1622.e23. [Google Scholar] [CrossRef] [PubMed]
- Hoogerwerf, W.A.; Hellmich, H.L.; Cornélissen, G.; Halberg, F.; Shahinian, V.B.; Bostwick, J.; Savidge, T.C.; Cassone, V.M. Clock Gene Expression in the Murine Gastrointestinal Tract: Endogenous Rhythmicity and Effects of a Feeding Regimen. Gastroenterology 2007, 133, 1250–1260. [Google Scholar] [CrossRef]
- Stenkamp-Strahm, C.M.; Nyavor, Y.E.A.; Kappmeyer, A.J.; Horton, S.; Gericke, M.; Balemba, O.B. Prolonged high fat diet ingestion, obesity, and type 2 diabetes symptoms correlate with phenotypic plasticity in myenteric neurons and nerve damage in the mouse duodenum. Cell Tissue Res. 2015, 361, 411–426. [Google Scholar] [CrossRef]
- Reichardt, F.; Chassaing, B.; Nezami, B.G.; Li, G.; Tabatabavakili, S.; Mwangi, S.; Uppal, K.; Liang, B.; Vijay-Kumar, M.; Jones, D.; et al. Western diet induces colonic nitrergic myenteric neuropathy and dysmotility in mice via saturated fatty acid- and lipopolysaccharide-induced TLR4 signalling. J. Physiol. 2017, 595, 1831–1846. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Vitari, F.; Bosi, G.; Savoini, G.; Domeneghini, C. The Chemical Code of Porcine Enteric Neurons and the Number of Enteric Glial Cells Are Altered by Dietary Probiotics. Neurogastroenterol. Motil. 2010, 22, e271–e278. [Google Scholar] [CrossRef] [PubMed]
- Khoshdel, A.; Verdu, E.F.; Kunze, W.; McLean, P.; Bergonzelli, G.; Huizinga, J.D. Bifidobacterium longum NCC 3001 Inhibits AH Neuron Excitability. Neurogastroenterol. Motil. 2013, 25, e478–e484. [Google Scholar] [CrossRef]
- Nurgali, K.; Qu, Z.; Hunne, B.; Thacker, M.; Pontell, L.; Furness, J.B. Morphological and functional changes in guinea-pig neurons projecting to the ileal mucosa at early stages after inflammatory damage. J. Physiol. 2011, 589, 325–339. [Google Scholar] [CrossRef]
- Liu, M.-T.; Kuan, Y.-H.; Wang, J.; Hen, R.; Gershon, M.D. 5-HT4 Receptor-Mediated Neuroprotection and Neurogenesis in the Enteric Nervous System of Adult Mice. J. Neurosci. 2009, 29, 9683–9699. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S.S.; Kulkarni, S.; Saha, M.; Sylvia, K.E.; Sears, C.L.; Pasricha, P.J. Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology 2020, 159, 200–213.e8. [Google Scholar] [CrossRef]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.-B.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef]
- McVey Neufeld, K.A.; Mao, Y.K.; Bienenstock, J.; Foster, J.A.; Kunze, W.A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013, 25, 183. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-K.; Kasper, D.L.; Wang, B.; Forsythe, P.; Bienenstock, J.; Kunze, W.A. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat. Commun. 2013, 4, 1465. [Google Scholar] [CrossRef]
- Kunze, W.A.; Mao, Y.; Wang, B.; Huizinga, J.D.; Ma, X.; Forsythe, P.; Bienenstock, J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell. Mol. Med. 2009, 13, 2261–2270. [Google Scholar] [CrossRef]
- Wang, B.; Mao, Y.-K.; Diorio, C.; Wang, L.; Huizinga, J.D.; Bienenstock, J.; Kunze, W. Lactobacillus reuteri ingestion and IKCa channel blockade have similar effects on rat colon motility and myenteric neurones. Neurogastroenterol. Motil. 2010, 22, 98. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e16. [Google Scholar] [CrossRef]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Sternini, C.; De Giorgio, R.; Greenwood-Van Meerveld, B. Enteroendocrine cells: A review of their role in brain–gut communication. Neurogastroenterol. Motil. 2016, 28, 620–630. [Google Scholar] [CrossRef]
- Bohórquez, D.V.; Shahid, R.A.; Erdmann, A.; Kreger, A.M.; Wang, Y.; Calakos, N.; Wang, F.; Liddle, R.A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 2015, 125, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef]
- MacEachern, S.J.; Keenan, C.M.; Papakonstantinou, E.; Sharkey, K.A.; Patel, B.A. Alterations in melatonin and 5-HT signalling in the colonic mucosa of mice with dextran-sodium sulfate-induced colitis. Br. J. Pharmacol. 2018, 175, 1535–1547. [Google Scholar] [CrossRef]
- Chandra, R.; Hiniker, A.; Kuo, Y.-M.; Nussbaum, R.L.; Liddle, R.A. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2017, 2, e92295. [Google Scholar] [CrossRef]
- Ford, M.J.; Burton, L.J.; Morris, R.J.; Hall, S.M. Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience 2002, 113, 177–192. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C.; Franceschetti, L.; Gianò, M.; Favero, G. A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role. Int. J. Mol. Sci. 2022, 23, 3758. [Google Scholar] [CrossRef]
- Braun, T.; Voland, P.; Kunz, L.; Prinz, C.; Gratzl, M. Enterochromaffin Cells of the Human Gut: Sensors for Spices and Odorants. Gastroenterology 2007, 132, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Hata, T.; Asano, Y.; Yoshihara, K.; Kimura-Todani, T.; Miyata, N.; Zhang, X.-T.; Takakura, S.; Aiba, Y.; Koga, Y.; Sudo, N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE 2017, 12, e0180745. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Salmonson, C.E.; Iii, J.F.R.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Lafferty, R.A.; Flatt, P.R.; Irwin, N. Emerging therapeutic potential for peptide YY for obesity-diabetes. Peptides 2018, 100, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Billing, L.J.; Smith, C.A.; Larraufie, P.; Goldspink, D.A.; Galvin, S.; Kay, R.G.; Howe, J.D.; Walker, R.; Pruna, M.; Glass, L.; et al. Co-storage and release of insulin-like peptide-5, glucagon-like peptide-1 and peptideYY from murine and human colonic enteroendocrine cells. Mol. Metab. 2018, 16, 65–75. [Google Scholar] [CrossRef]
- Arora, T.; Vanslette, A.M.; Hjorth, S.A.; Bäckhed, F. Microbial regulation of enteroendocrine cells. Med 2021, 2, 553–570. [Google Scholar] [CrossRef]
- Suzuki, K.; Iwasaki, K.; Murata, Y.; Harada, N.; Yamane, S.; Hamasaki, A.; Shibue, K.; Joo, E.; Sankoda, A.; Fujiwara, Y.; et al. Distribution and hormonal characterization of primary murine L cells throughout the gastrointestinal tract. J. Diabetes Investig. 2018, 9, 25–32. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Gehart, H.; Van Es, J.H.; Hamer, K.; Beumer, J.; Kretzschmar, K.; Dekkers, J.F.; Rios, A.; Clevers, H. Identification of Enteroendocrine Regulators by Real-Time Single-Cell Differentiation Mapping. Cell 2019, 176, 1158–1173.e16. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef]
- Zhang, C.; Kaye, J.A.; Cai, Z.; Wang, Y.; Prescott, S.L.; Liberles, S.D. Area Postrema Cell Types that Mediate Nausea-Associated Behaviors. Neuron 2021, 109, 461–472.e5. [Google Scholar] [CrossRef]
- Silva, A.D.; Bloom, S.R. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver 2012, 6, 10–20. [Google Scholar] [CrossRef]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and Characterization of GLP-1 Receptor–Expressing Cells Using a New Transgenic Mouse Model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Li, J.; Kim, G.; Stewart, A.; Urban, J.F.; Huang, Y.; Chen, S.; Wu, L.-G.; Chesler, A.; et al. Interleukin-33 Promotes Serotonin Release from Enterochromaffin Cells for Intestinal Homeostasis. Immunity 2021, 54, 151–163.e6. [Google Scholar] [CrossRef] [PubMed]
- Alcaino, C.; Knutson, K.R.; Treichel, A.J.; Yildiz, G.; Strege, P.R.; Linden, D.R.; Li, J.H.; Leiter, A.B.; Szurszewski, J.H.; Farrugia, G.; et al. A Population of Gut Epithelial Enterochromaffin Cells is Mechanosensitive and Requires Piezo2 to Convert Force into Serotonin Release. Proc. Natl. Acad. Sci. USA 2018, 115, E7632–E7641. [Google Scholar] [CrossRef]
- Treichel, A.J.; Finholm, I.; Knutson, K.R.; Alcaino, C.; Whiteman, S.T.; Brown, M.R.; Matveyenko, A.; Wegner, A.; Kacmaz, H.; Mercado-Perez, A.; et al. Specialized Mechanosensory Epithelial Cells in Mouse Gut Intrinsic Tactile Sensitivity. Gastroenterology 2022, 162, 535–547.e13. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Bae, M.; Cassilly, C.D.; Jabba, S.V.; Thorpe, D.W.; Martin, A.M.; Lu, H.-Y.; Wang, J.; Thompson, J.D.; Lickwar, C.R.; et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 2021, 29, 179–196.e9. [Google Scholar] [CrossRef]
- Szőke, H.; Kovács, Z.; Bókkon, I.; Vagedes, J.; Szabó, A.E.; Hegyi, G.; Sterner, M.-G.; Kiss, Á.; Kapócs, G. Gut dysbiosis and serotonin: Intestinal 5-HT as a ubiquitous membrane permeability regulator in host tissues, organs, and the brain. Rev. Neurosci. 2020, 31, 415–425. [Google Scholar] [CrossRef]
- Foster, J.A.; Lyte, M.; Meyer, E.; Cryan, J.F. Gut Microbiota and Brain Function: An Evolving Field in Neuroscience: Table 1. Int. J. Neuropsychopharmacol. 2016, 19, pyv114. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.K.; Chang, R.B.; Strochlic, D.E.; Umans, B.D.; Lowell, B.B.; Liberles, S.D. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 2016, 166, 209–221. [Google Scholar] [CrossRef]
- Lyte, J.M. Eating for 3.8 × 1013: Examining the Impact of Diet and Nutrition on the Microbiota-Gut-Brain Axis Through the Lens of Microbial Endocrinology. Front. Endocrinol. 2019, 9, 796. [Google Scholar] [CrossRef]
- Koopman, N.; Remijas, L.; Seppen, J.; Setlow, P.; Brul, S. Mechanisms and Applications of Bacterial Sporulation and Germination in the Intestine. Int. J. Mol. Sci. 2022, 23, 3405. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Sternini, C.; Anselmi, L.; Rozengurt, E. Enteroendocrine cells: A site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 73–78. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.-J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef]
- Depoortere, I. Taste receptors in the gut tune the release of peptides in response to nutrients. Peptides 2015, 66, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Laermans, J.; Verhulst, P.-J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2012, 302, R1119–R1133. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ueji, K. Brain Mechanisms of Flavor Learning. Front. Syst. Neurosci. 2011, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Huynh, J.; Mazzoni, M.; Gupta, A.; Bonora, E.; Clavenzani, P.; Chang, L.; Mayer, E.A.; De Giorgio, R.; Sternini, C. Expression of the Bitter Taste Receptor, T2R38, in Enteroendocrine Cells of the Colonic Mucosa of Overweight/Obese vs. Lean Subjects. PLoS ONE 2016, 11, e0147468. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Arthurs, J.; Reilly, S. Conditioned taste aversions: From poisons to pain to drugs of abuse. Psychon. Bull. Rev. 2017, 24, 335–351. [Google Scholar] [CrossRef]
- Overduin, J.; Gibbs, J.; Cummings, D.E.; Reeve, J.R. CCK-58 elicits both satiety and satiation in rats while CCK-8 elicits only satiation. Peptides 2014, 54, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, H.; Gao, X.; Zeng, X.; Zheng, S.; Wang, L.; Yuan, F.; Xu, S.; Yin, Z.; Hu, G. Cholecystokinin (CCK) Is a Mediator Between Nutritional Intake and Gonadal Development in Teleosts. Cells 2025, 14, 78. [Google Scholar] [CrossRef]
- Miyamoto, J.; Hasegawa, S.; Kasubuchi, M.; Ichimura, A.; Nakajima, A.; Kimura, I. Nutritional Signaling via Free Fatty Acid Receptors. Int. J. Mol. Sci. 2016, 17, 450. [Google Scholar] [CrossRef]
- Schlatterer, K.; Peschel, A.; Kretschmer, D. Short-Chain Fatty Acid and FFAR2 Activation—A New Option for Treating Infections? Front. Cell. Infect. Microbiol. 2021, 11, 785833. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Avery, J.A.; Kerr, K.L.; Ingeholm, J.E.; Burrows, K.; Bodurka, J.; Simmons, W.K. A common gustatory and interoceptive representation in the human mid-insula: Gustatory-Interoceptive Overlap. Hum. Brain Mapp. 2015, 36, 2996–3006. [Google Scholar] [CrossRef]
- Powley, T.L.; Phillips, R.J.I. Morphology and topography of vagal afferents innervating the GI tract. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G1217–G1225. [Google Scholar] [CrossRef] [PubMed]
- De Lartigue, G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J. Physiol. 2016, 594, 5791–5815. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.-W.; Gao, X.-B.; et al. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.Y.; Musser, M.A.; Pinho-Ribeiro, F.A.; Baral, P.; Jacobson, A.; Ma, P.; Potts, D.E.; Chen, Z.; Paik, D.; Soualhi, S.; et al. Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 2020, 180, 33–49.e22. [Google Scholar] [CrossRef]
- Farb, N.A.S.; Zuo, Z.; Price, C.J. Interoceptive Awareness of the Breath Preserves Attention and Language Networks amidst Widespread Cortical Deactivation: A Within-Participant Neuroimaging Study. Eneuro 2023, 10, ENEURO.0088-23.2023. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Zagorodnyuk, V.; Brookes, S.J.; Hibberd, T. Spinal afferent nerve endings in visceral organs: Recent advances. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 311, G1056–G1063. [Google Scholar] [CrossRef]
- Umans, B.D.; Liberles, S.D. Neural Sensing of Organ Volume. Trends Neurosci. 2018, 41, 911–924. [Google Scholar] [CrossRef]
- Sabatini, P.V.; Frikke-Schmidt, H.; Arthurs, J.; Gordian, D.; Patel, A.; Rupp, A.C.; Adams, J.M.; Wang, J.; Beck Jørgensen, S.; Olson, D.P.; et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc. Natl. Acad. Sci. USA 2021, 118, e2021357118. [Google Scholar] [CrossRef]
- Hisa, Y. (Ed.) Neuroanatomy and Neurophysiology of the Larynx; Springer: Tokyo, Japan, 2016. [Google Scholar] [CrossRef]
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.S.; Huey, E.L.; Liu, Y.; Gray, L.A.; Aitken, T.J.; Chen, Y.; Beutler, L.R.; Ahn, J.S.; et al. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 2019, 179, 1129–1143.e23. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Zagorodnyuk, V.P.; Chen, B.N.; Costa, M.; Brookes, S.J.H. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J. Physiol. 2003, 553, 575–587. [Google Scholar] [CrossRef]
- Borgmann, D.; Ciglieri, E.; Biglari, N.; Brandt, C.; Cremer, A.L.; Backes, H.; Tittgemeyer, M.; Wunderlich, F.T.; Brüning, J.C.; Fenselau, H. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab. 2021, 33, 1466–1482.e7. [Google Scholar] [CrossRef]
- Blackshaw, L.A.; Brookes, S.J.H.; Grundy, D.; Schemann, M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol. Motil. 2007, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R.; Albaugh, V.L.; Neuhuber, W.L. Gut-brain communication and obesity: Understanding functions of the vagus nerve. J. Clin. Invest. 2021, 131, e143770. [Google Scholar] [CrossRef]
- Aklan, I.; Sayar Atasoy, N.; Yavuz, Y.; Ates, T.; Coban, I.; Koksalar, F.; Filiz, G.; Topcu, I.C.; Oncul, M.; Dilsiz, P.; et al. NTS Catecholamine Neurons Mediate Hypoglycemic Hunger via Medial Hypothalamic Feeding Pathways. Cell Metab. 2020, 31, 313–326.e5. [Google Scholar] [CrossRef]
- Bunyoz, A.H.; Christensen, R.H.B.; Orlovska-Waast, S.; Nordentoft, M.; Mortensen, P.B.; Petersen, L.V.; Benros, M.E. Vagotomy and the risk of mental disorders: A nationwide population-based study. Acta Psychiatr. Scand. 2022, 145, 67–78. [Google Scholar] [CrossRef]
- Sclafani, A.; Koepsell, H.; Ackroff, K. SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R631–R639. [Google Scholar] [CrossRef]
- Tan, H.-E.; Sisti, A.C.; Jin, H.; Vignovich, M.; Villavicencio, M.; Tsang, K.S.; Goffer, Y.; Zuker, C.S. The gut–brain axis mediates sugar preference. Nature 2020, 580, 511–516. [Google Scholar] [CrossRef]
- Grabauskas, G.; Owyang, C. Plasticity of vagal afferent signaling in the gut. Medicina 2017, 53, 73–84. [Google Scholar] [CrossRef]
- Raybould, H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010, 153, 41–46. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Margolis, K.G. The gut, its microbiome, and the brain: Connections and communications. J. Clin. Investig. 2021, 131, e143768. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [CrossRef]
- Sun, L.-J.; Li, J.-N.; Nie, Y.-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef]
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr. Physiol. 2016, 6, 1239–1278. [Google Scholar] [CrossRef]
- Davis, E.A.; Zhou, W.; Dailey, M.J. Evidence for a direct effect of the autonomic nervous system on intestinal epithelial stem cell proliferation. Physiol. Rep. 2018, 6, e13745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Human Microbiome Project (HMP) Data Portal. Available online: https://portal.hmpdacc.org/ (accessed on 31 March 2025).
- Leonard, J.M.; Toro, D.D. Defining the Microbiome Components (Bacteria, Viruses, Fungi) and Microbiome Geodiversity. Surg. Infect. 2023, 24, 208–212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baquero, F.; Nombela, C. The microbiome as a human organ. Clin. Microbiol. Infect. 2012, 18, 2–4. [Google Scholar] [CrossRef]
- Cani, P.D. Gut microbiota—At the intersection of everything? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 321–322. [Google Scholar] [CrossRef]
- Bucurica, S.; Lupanciuc, M.; Ionita-Radu, F.; Stefan, I.; Munteanu, A.E.; Anghel, D.; Jinga, M.; Gaman, E.L. Estrobolome and Hepatocellular Adenomas—Connecting the Dots of the Gut Microbial β-Glucuronidase Pathway as a Metabolic Link. Int. J. Mol. Sci. 2023, 24, 16034. [Google Scholar] [CrossRef]
- Singh, R.; Zogg, H.; Ro, S. Role of microRNAs in Disorders of Gut-Brain Interactions: Clinical Insights and Therapeutic Alternatives. J. Pers. Med. 2021, 11, 1021. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Qian, L.; Siliceo, S.L.; Long, X.; Nychas, E.; Liu, Y.; Ismaiah, M.J.; Leung, H.; Zhang, L.; Gao, Q.; et al. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023, 35, 1530–1547.e8. [Google Scholar] [CrossRef]
- Ionita-Radu, F.; Patoni, C.; Nancoff, A.S.; Marin, F.-S.; Gaman, L.; Bucurica, A.; Socol, C.; Jinga, M.; Dutu, M.; Bucurica, S. Berberine Effects in Pre-Fibrotic Stages of Non-Alcoholic Fatty Liver Disease—Clinical and Pre-Clinical Overview and Systematic Review of the Literature. Int. J. Mol. Sci. 2024, 25, 4201. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain. Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.; Jacobi, M.; Rusch, K.; Andreas, S. Association of dietary type with fecal microbiota and short chain fatty acids in vegans and omnivores. J. Int. Soc. Microbiota 2016, 1, 1. [Google Scholar]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Singh, P.; Meenatchi, R.; Ahmed, Z.H.T.; Thacharodi, A.; M, R.; Kumar, R.R.; Varthan M K, H.; Hassan, S. Implications of the gut microbiome in cardiovascular diseases: Association of gut microbiome with cardiovascular diseases, therapeutic interventions and multi-omics approach for precision medicine. Med. Microecol. 2024, 19, 100096. [Google Scholar] [CrossRef]
- Lu, X.; Shi, Z.; Jiang, L.; Zhang, S. Maternal gut microbiota in the health of mothers and offspring: From the perspective of immunology. Front. Immunol. 2024, 15, 1362784. [Google Scholar] [CrossRef]
- Al Rubaye, H.; Adamson, C.C.; Jadavji, N.M. The role of maternal diet on offspring gut microbiota development: A review. J. Neurosci. Res. 2021, 99, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef]
- Hébuterne, X. Gut changes attributed to ageing: Effects on intestinal microflora. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Fragiadakis, G.K.; Wastyk, H.C.; Robinson, J.L.; Sonnenburg, E.D.; Sonnenburg, J.L.; Gardner, C.D. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 2020, 111, 1127–1136. [Google Scholar] [CrossRef]
- Ng, K.M.; Aranda-Díaz, A.; Tropini, C.; Frankel, M.R.; Van Treuren, W.; O’Loughlin, C.T.; Merrill, B.D.; Yu, F.B.; Pruss, K.M.; Oliveira, R.A.; et al. Recovery of the Gut Microbiota after Antibiotics Depends on Host Diet, Community Context, and Environmental Reservoirs. Cell Host Microbe 2019, 26, 650–665.e4. [Google Scholar] [CrossRef]
- Dwiyanto, J.; Hussain, M.H.; Reidpath, D.; Ong, K.S.; Qasim, A.; Lee, S.W.H.; Lee, S.M.; Foo, S.C.; Chong, C.W.; Rahman, S. Ethnicity influences the gut microbiota of individuals sharing a geographical location: A cross-sectional study from a middle-income country. Sci. Rep. 2021, 11, 2618. [Google Scholar] [CrossRef]
- Moeller, A.H.; Li, Y.; Mpoudi Ngole, E.; Ahuka-Mundeke, S.; Lonsdorf, E.V.; Pusey, A.E.; Peeters, M.; Hahn, B.H.; Ochman, H. Rapid changes in the gut microbiome during human evolution. Proc. Natl. Acad. Sci. USA 2014, 111, 16431–16435. [Google Scholar] [CrossRef]
- Van Der Vossen, E.W.J.; Davids, M.; Bresser, L.R.F.; Galenkamp, H.; Van Den Born, B.-J.H.; Zwinderman, A.H.; Levin, E.; Nieuwdorp, M.; De Goffau, M.C. Gut microbiome transitions across generations in different ethnicities in an urban setting—The HELIUS study. Microbiome 2023, 11, 99. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, J.; Gao, Y.; Peng, Y.; Li, X.; Huang, W.; Zhou, H.; Liu, R.; Zhang, W. Combined analysis of cross-population healthy adult human microbiome reveals consistent differences in gut microbial characteristics between Western and non-Western countries. Comput. Struct. Biotechnol. J. 2024, 23, 87–95. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.M.; Peng, Y.; Chen, B.; Konya, T.B.; Morales-Lizcano, N.P.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; et al. Ethnicity Associations With Food Sensitization Are Mediated by Gut Microbiota Development in the First Year of Life. Gastroenterology 2021, 161, 94–106. [Google Scholar] [CrossRef]
- Mondo, E.; Marliani, G.; Accorsi, P.A.; Cocchi, M.; Di Leone, A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 2019, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Van Der Kleij, H.; O’Mahony, C.; Shanahan, F.; O’Mahony, L.; Bienenstock, J. Protective effects of Lactobacillus reuteri and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1131–R1137. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef]
- Sobko, T.; Huang, L.; Midtvedt, T.; Norin, E.; Gustafsson, L.E.; Norman, M.; Jansson, E.Å.; Lundberg, J.O. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic. Biol. Med. 2006, 41, 985–991. [Google Scholar] [CrossRef]
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E.V. Evolution of cell–cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Caspani, G.; Swann, J. Small talk: Microbial metabolites involved in the signaling from microbiota to brain. Curr. Opin. Pharmacol. 2019, 48, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T.; Hurn, D.; Hermanus, D. Gut Bacteria and Neuropsychiatric Disorders. Microorganisms 2021, 9, 2583. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; McVey Neufeld, K.-A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice: Behavior in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The role of probiotics and prebiotics in modulating of the gut-brain axis. Front. Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.T.; Sperandio, V. Inter-kingdom signalling: Communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008, 6, 111–120. [Google Scholar] [CrossRef]
- Leigh, S.; Uhlig, F.; Wilmes, L.; Sanchez-Diaz, P.; Gheorghe, C.E.; Goodson, M.S.; Kelley-Loughnane, N.; Hyland, N.P.; Cryan, J.F.; Clarke, G. The impact of acute and chronic stress on gastrointestinal physiology and function: A microbiota–gut–brain axis perspective. J. Physiol. 2023, 601, 4491–4538. [Google Scholar] [CrossRef]
- Lopes, G.V.; Ramires, T.; Kleinubing, N.R.; Scheik, L.K.; Fiorentini, Â.M.; Padilha Da Silva, W. Virulence factors of foodborne pathogen Campylobacter jejuni. Microb. Pathog. 2021, 161, 105265. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Nizer, W.S.; Inkovskiy, V.; Versey, Z.; Strempel, N.; Cassol, E.; Overhage, J. Oxidative Stress Response in Pseudomonas aeruginosa. Pathogens 2021, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Boehme, M.; Rémond-Derbez, N.; Lerond, C.; Lavalle, L.; Keddani, S.; Steinmann, M.; Rytz, A.; Dalile, B.; Verbeke, K.; Van Oudenhove, L.; et al. Bifidobacterium longum subsp. longum Reduces Perceived Psychological Stress in Healthy Adults: An Exploratory Clinical Trial. Nutrients 2023, 15, 3122. [Google Scholar] [CrossRef]
- Önning, G.; Montelius, C.; Hillman, M.; Larsson, N. Intake of Lactiplantibacillus plantarum HEAL9 Improves Cognition in Moderately Stressed Subjects: A Randomized Controlled Study. Nutrients 2023, 15, 3466. [Google Scholar] [CrossRef]
- Casertano, M.; Dekker, M.; Valentino, V.; De Filippis, F.; Fogliano, V.; Ercolini, D. Gaba-producing lactobacilli boost cognitive reactivity to negative mood without improving cognitive performance: A human Double-Blind Placebo-Controlled Cross-Over study. Brain. Behav. Immun. 2024, 122, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.-P.; Cominetti, O.; Berger, B.; Combremont, S.; Marquis, J.; Xie, G.; Jia, W.; Pinto-Sanchez, M.I.; Bercik, P.; Bergonzelli, G. Metabolome-associated psychological comorbidities improvement in irritable bowel syndrome patients receiving a probiotic. Gut Microbes 2024, 16, 2347715. [Google Scholar] [CrossRef]
- Sarkawi, M.; Raja Ali, R.A.; Abdul Wahab, N.; Abdul Rathi, N.D.; Mokhtar, N.M. A randomized, double-blinded, placebo-controlled clinical trial on Lactobacillus-containing cultured milk drink as adjuvant therapy for depression in irritable bowel syndrome. Sci. Rep. 2024, 14, 9478. [Google Scholar] [CrossRef]
- Chao, W.-C.; Huang, J.-C.; Young, S.-L.; Wu, C.-L.; Shih, J.-C.; Liao, L.-D.; Cheng, B. Interplay of yoga, physical activity, and probiotics in irritable bowel syndrome management: A double-blind randomized study. Complement. Ther. Clin. Pract. 2024, 57, 101892. [Google Scholar] [CrossRef] [PubMed]
- Ziegert, Z.; Dietz, M.; Hill, M.; McBride, M.; Painter, E.; Elias, M.H.; Staley, C. Targeting quorum sensing for manipulation of commensal microbiota. BMC Biotechnol. 2024, 24, 106. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bu, X.; Chen, D.; Wu, X.; Wu, H.; Caiyin, Q.; Qiao, J. Molecules-mediated bidirectional interactions between microbes and human cells. Npj Biofilms Microbiomes 2025, 11, 38. [Google Scholar] [CrossRef]
- Jamerlan, A.M.; An, S.S.A.; Hulme, J.P. Microbial diversity and fitness in the gut–brain axis: Influences on developmental risk for Alzheimer’s disease. Gut Microbes 2025, 17, 2486518. [Google Scholar] [CrossRef]
- Falà, A.K.; Álvarez-Ordóñez, A.; Filloux, A.; Gahan, C.G.M.; Cotter, P.D. Quorum sensing in human gut and food microbiomes: Significance and potential for therapeutic targeting. Front. Microbiol. 2022, 13, 1002185. [Google Scholar] [CrossRef]
- Lamin, A.; Kaksonen, A.H.; Cole, I.S.; Chen, X.-B. Quorum sensing inhibitors applications: A new prospect for mitigation of microbiologically influenced corrosion. Bioelectrochemistry 2022, 145, 108050. [Google Scholar] [CrossRef]
- Jing, Z.; Yinhang, W.; Jian, C.; Zhanbo, Q.; Xinyue, W.; Shuwen, H. Interaction between gut microbiota and T cell immunity in colorectal cancer. Autoimmun. Rev. 2025, 24, 103807. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, X.; Zhang, Y.; Bao, W.; Lu, Z.; Zhao, W.; Rukeya, Y.; He, P.; Qi, J.; Liu, S.; et al. Enterococcus faecalis hijacks FABP2 to activate quorum-sensing signals and aggravate Crohn’s disease by inducing gut dysbiosis. Gut 2025, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Cho, J.Y.; Chen, L.; Liu, Y.; Ji, F.; Salgado, K.; Ge, S.; Yang, D.; Yu, H.; Shao, J.; et al. Enriched pathways in gut microbiome predict response to immune checkpoint inhibitor treatment across demographic regions and various cancer types. iScience 2025, 28, 112162. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pessione, E. The Neuro-Endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, M. Diversity of structure and function of GABAB receptors: A complexity of GABAB-mediated signaling. Proc. Jpn. Acad. Ser. B 2018, 94, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Tynes, V.V.; Landsberg, G.M. Nutritional Management of Behavior and Brain Disorders in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 711–727. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, H.; Suzuki, K.; Ma, S.; Liu, C. Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression. Antioxidants 2019, 8, 376. [Google Scholar] [CrossRef]
- Banderet, L.E.; Lieberman, H.R. Treatment with tyrosine, a neurotransmitter precursor, reduces environmental stress in humans. Brain Res. Bull. 1989, 22, 759–762. [Google Scholar] [CrossRef]
- Durgan, D.J.; Lee, J.; McCullough, L.D.; Bryan, R.M. Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke 2019, 50, 2270–2277. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, K.E.; Demas, G.E. A gut feeling: Microbiome-brain-immune interactions modulate social and affective behaviors. Horm. Behav. 2018, 99, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut–Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-Chain Fatty Acids Regulate the Enteric Neurons and Control Gastrointestinal Motility in Rats. Gastroenterology 2010, 138, 1772–1782.e4. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Front. Neurosci. 2020, 13, 1361. [Google Scholar] [CrossRef]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Hong, T.; Van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine Derived from Probiotic Lactobacillus reuteri Suppresses TNF via Modulation of PKA and ERK Signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef]
- LaGreca, M.; Skehan, L.; Hutchinson, D. The Microbiome and Neurotransmitter Activity. JoSaM. 2022. Available online: https://www.josam.org/josam/article/view/90 (accessed on 19 June 2025).
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef]
- Thornton, T.; Mills, D.; Bliss, E. The impact of lipopolysaccharide on cerebrovascular function and cognition resulting from obesity-induced gut dysbiosis. Life Sci. 2024, 336, 122337. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.K.; Kalyan, M.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Pendyala, G.; Yelamanchili, S.V.; Yang, J.; Chidambaram, S.B.; Sakharkar, M.K.; et al. Role of the Gut Bacteria-Derived Metabolite Phenylacetylglutamine in Health and Diseases. ACS Omega 2024, 9, 3037–4137. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Ahmed, S.; Busetti, A.; Fotiadou, P.; Vincy Jose, N.; Reid, S.; Georgieva, M.; Brown, S.; Dunbar, H.; Beurket-Ascencio, G.; Delday, M.I.; et al. In vitro Characterization of Gut Microbiota-Derived Bacterial Strains With Neuroprotective Properties. Front. Cell. Neurosci. 2019, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, L.; Chang, C.-W.; Li, S.-J.; Liang, Y.-W.; Lo, Y.-C.; Chen, Y.-Y. Interplay of Neuroinflammation and Gut Microbiota Dysbiosis in Alzheimer’s Disease Using Diffusion Kurtosis Imaging Biomarker in 3 × Tg-AD Mouse Models. ACS Chem. Neurosci. 2025, 16, 1511–1528. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Song, Z.; Fan, X.; Shao, H.; Schönke, M.; Boon, M.R.; Rensen, P.C.N.; Wang, Y. Choline and butyrate beneficially modulate the gut microbiome without affecting atherosclerosis in APOE*3-Leiden.CETP mice. Atherosclerosis 2022, 362, 47–55. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Tough, I.R.; Forbes, S.; Cox, H.M. Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate. Neurogastroenterol. Motil. 2018, 30, e13454. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, H.; Ju, Y.; Liu, J.; Wang, M.; Liu, B.; Zhang, Y. Gut microbiota-derived short-chain fatty acids and depression: Deep insight into biological mechanisms and potential applications. Gen. Psychiatry 2024, 37, e101374. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut–Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef]
- Larraufie, P.; Haroun, K.; Fleury, C.; Andriamihaja, M.; Blachier, F. Regulation of enteroendocrine cell respiration by the microbial metabolite hydrogen sulfide. Front. Endocrinol. 2023, 14, 1123364. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Fragas, M.G.; Oliveira, D.M.D.; Hiyane, M.I.; Braga, T.T.; Camara, N.O.S. The dual effect of acetate on microglial TNF-α production. Clinics 2022, 77, 100062. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, N.; Agis-Balboa, R.C.; Walter, J.; Sananbenesi, F.; Fischer, A. Sodium Butyrate Improves Memory Function in an Alzheimer’s Disease Mouse Model When Administered at an Advanced Stage of Disease Progression. J. Alzheimers Dis. 2011, 26, 187–197. [Google Scholar] [CrossRef]
- Deutschmann, K.; Reich, M.; Klindt, C.; Dröge, C.; Spomer, L.; Häussinger, D.; Keitel, V. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2018, 1864, 1319–1325. [Google Scholar] [CrossRef]
- Ward, J.B.J.; Mroz, M.S.; Keely, S.J. The bile acid receptor, TGR 5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol. Motil. 2013, 25, 708–711. [Google Scholar] [CrossRef]
- Joyce, S.A.; O’Malley, D. Bile acids, bioactive signalling molecules in interoceptive gut-to-brain communication. J. Physiol. 2022, 600, 2565–2578. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, J.-Y.; Lee, A.; Lu, Y.-X.; Zhou, S.-Y.; Owyang, C. Satiety induced by bile acids is mediated via vagal afferent pathways. JCI Insight 2020, 5, e132400. [Google Scholar] [CrossRef] [PubMed]
- Parry, G.J.; Rodrigues, C.M.P.; Aranha, M.M.; Hilbert, S.J.; Davey, C.; Kelkar, P.; Low, W.C.; Steer, C.J. Safety, Tolerability, and Cerebrospinal Fluid Penetration of Ursodeoxycholic Acid in Patients With Amyotrophic Lateral Sclerosis. Clin. Neuropharmacol. 2010, 33, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, J.; Hu, W.; Wang, C.; Lu, X.; Tong, L.; Wu, F.; Zhang, W. Identification of functional farnesoid X receptors in brain neurons. FEBS Lett. 2016, 590, 3233–3242. [Google Scholar] [CrossRef]
- Poland, J.C.; Flynn, C.R. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology 2021, 36, 235–245. [Google Scholar] [CrossRef]
- Jaglin, M.; Rhimi, M.; Philippe, C.; Pons, N.; Bruneau, A.; Goustard, B.; Daugé, V.; Maguin, E.; Naudon, L.; Rabot, S. Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Front. Neurosci. 2018, 12, 216. [Google Scholar] [CrossRef]
- Panaro, B.L.; Yusta, B.; Matthews, D.; Koehler, J.A.; Song, Y.; Sandoval, D.A.; Drucker, D.J. Intestine-selective reduction of Gcg expression reveals the importance of the distal gut for GLP-1 secretion. Mol. Metab. 2020, 37, 100990. [Google Scholar] [CrossRef]
- Pichette, J.; Fynn-Sackey, N.; Gagnon, J. Hydrogen Sulfide and Sulfate Prebiotic Stimulates the Secretion of GLP-1 and Improves Glycemia in Male Mice. Endocrinology 2017, 158, 3416–3425. [Google Scholar] [CrossRef]
- Bala, V.; Rajagopal, S.; Kumar, D.P.; Nalli, A.D.; Mahavadi, S.; Sanyal, A.J.; Grider, J.R.; Murthy, K.S. Release of GLP-1 and PYY in Response to the Activation of G Protein-Coupled Bile Acid Receptor TGR5 is Mediated by Epac/PLC-ε Pathway and Modulated by Endogenous H2S. Front Physiol. 2014, 5, 420. [Google Scholar] [CrossRef]
- Casado-Bedmar, M.; Viennois, E. MicroRNA and Gut Microbiota: Tiny but Mighty—Novel Insights into Their Cross-talk in Inflammatory Bowel Disease Pathogenesis and Therapeutics. J. Crohns Colitis 2022, 16, 992–1005. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- Moloney, G.M.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Microbial regulation of microRNA expression in the brain–gut axis. Curr. Opin. Pharmacol. 2019, 48, 120–126. [Google Scholar] [CrossRef]
- Haramati, S.; Navon, I.; Issler, O.; Ezra-Nevo, G.; Gil, S.; Zwang, R.; Hornstein, E.; Chen, A. microRNA as Repressors of Stress-Induced Anxiety: The Case of Amygdalar miR-34. J. Neurosci. 2011, 31, 14191–14203. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Gao, Z.; Zhang, Y.; Gu, J. Gut microbiota-induced microRNA-206-3p increases anxiety-like behaviors by inhibiting expression of Cited2 and STK39. Microb. Pathog. 2023, 176, 106008. [Google Scholar] [CrossRef]
- Griggs, E.M.; Young, E.J.; Rumbaugh, G.; Miller, C.A. MicroRNA-182 Regulates Amygdala-Dependent Memory Formation. J. Neurosci. 2013, 33, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Moloney, G.M.; Ryan, F.J.; Hoban, A.E.; Bastiaanssen, T.F.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Social interaction-induced activation of RNA splicing in the amygdala of microbiome-deficient mice. eLife 2018, 7, e33070. [Google Scholar] [CrossRef]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, W.-D.; Wang, Y.-D. The roles of the gut microbiota–miRNA interaction in the host pathophysiology. Mol. Med. 2020, 26, 101. [Google Scholar] [CrossRef]
- Qu, S.; Gao, Y.; Ma, J.; Yan, Q. Microbiota-derived short-chain fatty acids functions in the biology of B lymphocytes: From differentiation to antibody formation. Biomed. Pharmacother. 2023, 168, 115773. [Google Scholar] [CrossRef]
- Delgado-Ocaña, S.; Cuesta, S. From microbes to mind: Germ-free models in neuropsychiatric research. mBio 2024, 15, e02075-24. [Google Scholar] [CrossRef]
- Chen, L.L.; Abbaspour, A.; Mkoma, G.F.; Bulik, C.M.; Rück, C.; Djurfeldt, D. Gut Microbiota in Psychiatric Disorders: A Systematic Review. Psychosom. Med. 2021, 83, 679–692. [Google Scholar] [CrossRef]
- Superdock, D.K.; Zhang, W.; Poole, A.C. Processing and storage methods affect oral and gut microbiome composition. Front. Microbiol. 2023, 14, 1253570. [Google Scholar] [CrossRef]
- Forry, S.P.; Servetas, S.L.; Dootz, J.N.; Hunter, M.E.; Kralj, J.G.; Filliben, J.J.; Jackson, S.A. A sensitivity analysis of methodological variables associated with microbiome measurements. Microbiol. Spectr. 2025, 13, e00696-24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ganz, A.B.; Rayner, A.; Cheng, T.Y.; Oba, H.; Rolnik, B.; Lancaster, S.; Lu, X.; Li, Y.; Johnson, J.S.; et al. Dynamic Human Gut Microbiome and Immune Shifts During an Immersive Psychosocial Therapeutic Program. bioRXiv 2024. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Shen, M.; Li, R.; Liu, Y.; Zeng, Z.; Zhou, J.; Niu, D.; Zhang, Q.; Wang, R.; Yao, J.; et al. Elucidating the specific mechanisms of the gut-brain axis: The short-chain fatty acids-microglia pathway. J. Neuroinflamm. 2025, 22, 133. [Google Scholar] [CrossRef]
- Zhan, Y.; Al-Nusaif, M.; Ding, C.; Zhao, L.; Dong, C. The potential of the gut microbiome for identifying Alzheimer’s disease diagnostic biomarkers and future therapies. Front. Neurosci. 2023, 17, 1130730. [Google Scholar] [CrossRef]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: A systematic review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef]

| Authors and Year | Number of Subjects | G-B-M Intervention | Disease | Intervention | Outcomes |
|---|---|---|---|---|---|
| Boehme, M. et al. (2023) [237] | 47 patients | High doses of probiotic (Bifidobacterium longum (BL) NCC3001) and its effect on stress. | Stress | Questionnaires regarding stress and its effects on daily activities. Cortisol levels from saliva. Analysis of fecal abundance of Bifidobacterium longum (BL) NCC3001. | Significant perceived stress and improvement in sleep were reported after probiotic supplementation (p = 0.017 and p = 0.037). Acute stress response represented by the salivary cortisol levels was decreased by probiotic administration. Probiotic supplementation decreased the overall stress- and anxiety-related symptoms. |
| Önning, G. et al. (2023) [238] | 132 patients | Lactiplantibacillus plantarum HEAL9 can ameliorate cognitive functions in stress-related disorders. | Stress | Questionnaires regarding stress, mood, and quality of life. Cortisol serum levels as well as serum levels of ransforming growth factor β 1 (TGF-β1), galectin-3, fractalkine/CX3CL/CX3CL1, brain-derived neurotrophic factor (BDNF), tryptophan, L-kynurenine, and high-sensitivity C-reactive protein (hs-CRP). | Probiotic supplementation was associated with improvement in stress levels. (Cortisol levels were significantly reduced; p < 0.039). Improvement in sleep quality was observed after 12 weeks of trials. Significant improvement in short-term memory was observed (p = 0.003). No significant changes were reported among biomarker levels. |
| Casertano, M. et al. (2024) [239] | 44 patients | Probiotic supplement consisting of Levi-lactobacillus brevis P30021 and Lactiplantibacillus plantarum P30025 ant its ability of having a positive impact on mental well being. | Stress | Genomic analyzation of gut microbiota Cognitive and stress levels assessment Neurotransmitters analyzation | No effect on stress levels were observed, however depressive symptoms were ameliorated (p = 0.034). No significant changes in gut microbiota were reported, as well as no correlation of neurotransmitters weas reported. |
| Martin, F.P. et al. (2024) [240] | 36 patients | Bifidobacterium longum (BL) NCC3001 decreases emotional reactivity and ameliorates depression via modulation of gut microbiota. | IBS | PCR analysis of BLNCC3001. Brain mapping activity. | Higher levels of butyric acid were associated with improvement in clinical symptoms related to depression, as well a decrease in activation of the amygdala. The abundance of BLNCC3001 ameliorated anxiety and depressive symptoms via increasing the synthesis of butyric acid. |
| Sarkawi, M. et al. (2024) [241] | 110 patients | Effects of high doses of Lactobacillus acidophilus LA-5 and Lactobacillus paracasei L. CASEI-01 on IBS symptoms. | IBS | Questionnaires regarding IBS symptoms and quality of life. Serum levels of serotonin and cortisol. | Significant improvement in IBS-related symptoms after probiotic supplementation (p < 0.05). Significant increase (p = 0.002) in serotonin levels after probiotic treatment but no change in cortisol levels. |
| Chao, W.-C. et al. (2024) [242] | 31 patients | Yoga and probiotic supplementation | IBS | Genomic analyzation of gut microbiota. Fitness and quality of life assessment. | Significant changes in gut microbiota were observed, especially in Klebsiella and Prevotella species (p < 0.05). Improvements in fitness levels were also observed. |
| Microbial Metabolite | Produced by | Mechanism of Action | Effects on the CNS |
|---|---|---|---|
| Short-chain fatty acids [214] | Firmicutes (Lactobacillaceae, Ruminococcaceae, Lachnospiraceae) Bifidobacteriaceae | Influence microglial function Influence gene expression via HDAC inhibition | Anti-inflammatory role Enhance blood–brain barrier integrity Influence mood and cognition |
| Tryptophan metabolites [263] | LactobacillusBifidobacterium | Influence serotonin synthesis | Regulate mood, anxiety, and cognitive function |
| Gamma-aminobutyric acid (GABA) [264] | LactobacillusBifidobacterium | Modulates neuronal excitability | Regulates anxiety and may have antidepressant properties |
| Dopamine and precursors [265] | Bacillus spp. Escherichia spp. | Influence host dopamine pathways | May affect motivation, reward, and motor control |
| Histamine [266,267] | Lactobacillus reuteri | Modulates immune responses Acts via H1 and H2 receptors in CNS | Can influence wakefulness, appetite, and cognitive processes |
| Lipopolysaccharide [268] | Gram-negative bacteria | Activates systemic inflammation via TLR4 signaling | Neuroinflammation Role in depression and cognitive decline |
| Peptidoglycans [269] | Gram-positive bacteria | Activate innate immune responses | May have a role in neuroimmune interactions |
| Phenylacetylglutamine [270] | Christensenellaceae, Ruminococcaceae, Lachnospiraceae, Bacteroidetes, Firmicutes, Proteobacteria, some Gram-negative bacteria | Found in CNS but mechanism not clear | Linked to cardiovascular and possibly cognitive functions |
| SCFA | Produced by | Mechanism of Action | Effect on CNS |
| Acetate [271] | Bacteroides Prevotella Firmicutes | Activates hypothalamic neurons and modulates glial activity | Influences appetite and energy balance Possible neuroprotective effects |
| Propionate [271] | Bacteroidetes Firmicutes | Influences neurotransmission Interacts with G-protein-coupled receptors (GPCRs) | Anti-inflammatory effects May improve memory Potential anxiolytic effects |
| Butyrate [271] | Clostridium spp., Eubacterium, Roseburia, and Blautia Faecalibacterium prausnitzii | Histone deacetylase (HDAC) inhibitor (role in gene expression) Modulates microglia and enhances blood–brain barrier | Anti-inflammatory effects Improves neuroplasticity Reduces anxiety and depression |
| Isobutyrate [272] | Protein fermentation bacteria: Clostridium spp., Desulforhabdus amnigenus | Less studied, but may influence signaling through GPCR pathways | Not clear |
| Valerate [273] | Fermentation of amino acids: Clostridium spp. | HDAC inhibitor | May have anti-inflammatory role May have neuroprotective properties |
| Isovalerate [274] | Protein and amino acid fermentation: Bacteroides and Clostridium spp. | May affect microglial activation | May have a role in neuroinflammation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savulescu-Fiedler, I.; Benea, S.-N.; Căruntu, C.; Nancoff, A.-S.; Homentcovschi, C.; Bucurica, S. Rewiring the Brain Through the Gut: Insights into Microbiota–Nervous System Interactions. Curr. Issues Mol. Biol. 2025, 47, 489. https://doi.org/10.3390/cimb47070489
Savulescu-Fiedler I, Benea S-N, Căruntu C, Nancoff A-S, Homentcovschi C, Bucurica S. Rewiring the Brain Through the Gut: Insights into Microbiota–Nervous System Interactions. Current Issues in Molecular Biology. 2025; 47(7):489. https://doi.org/10.3390/cimb47070489
Chicago/Turabian StyleSavulescu-Fiedler, Ilinca, Serban-Nicolae Benea, Constantin Căruntu, Andreea-Simona Nancoff, Corina Homentcovschi, and Sandica Bucurica. 2025. "Rewiring the Brain Through the Gut: Insights into Microbiota–Nervous System Interactions" Current Issues in Molecular Biology 47, no. 7: 489. https://doi.org/10.3390/cimb47070489
APA StyleSavulescu-Fiedler, I., Benea, S.-N., Căruntu, C., Nancoff, A.-S., Homentcovschi, C., & Bucurica, S. (2025). Rewiring the Brain Through the Gut: Insights into Microbiota–Nervous System Interactions. Current Issues in Molecular Biology, 47(7), 489. https://doi.org/10.3390/cimb47070489






