Selective Dual Inhibition of TNKS1 and CDK8 by TCS9725 Attenuates STAT1/β-Catenin/TGFβ1 Signaling in Renal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. High-Throughput Virtual Screening
2.2.2. Molecular Dynamic Simulation
2.2.3. ADME Predictions
2.2.4. TNKS1 Inhibition Assay
2.2.5. CDK8 Inhibition Assay
2.2.6. Cell Culture and Proliferation Assay
2.2.7. STAT1 and β-Catenin Signaling in RCC
2.2.8. Annexin V Assay
2.2.9. Tumor Cell Trans-Endothelial Cell Migration in RCC Cells
2.2.10. TGF-β-Stimulated Smad2/3 Signaling in RCC
2.2.11. Statistical Analysis
3. Results
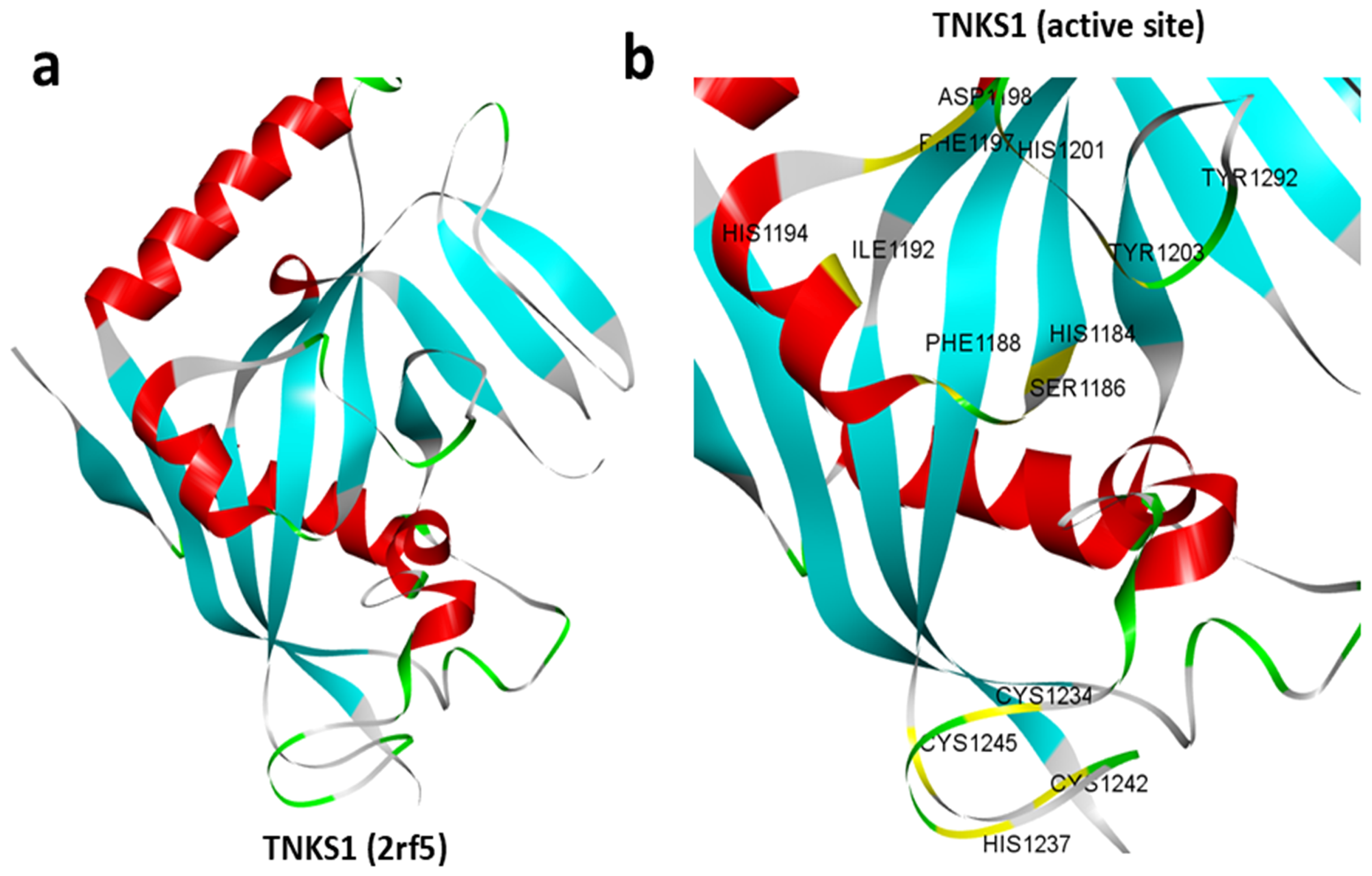
3.1. Structure of TNKS1
3.2. High-Throughput Virtual Screening of the ChemBridge Library
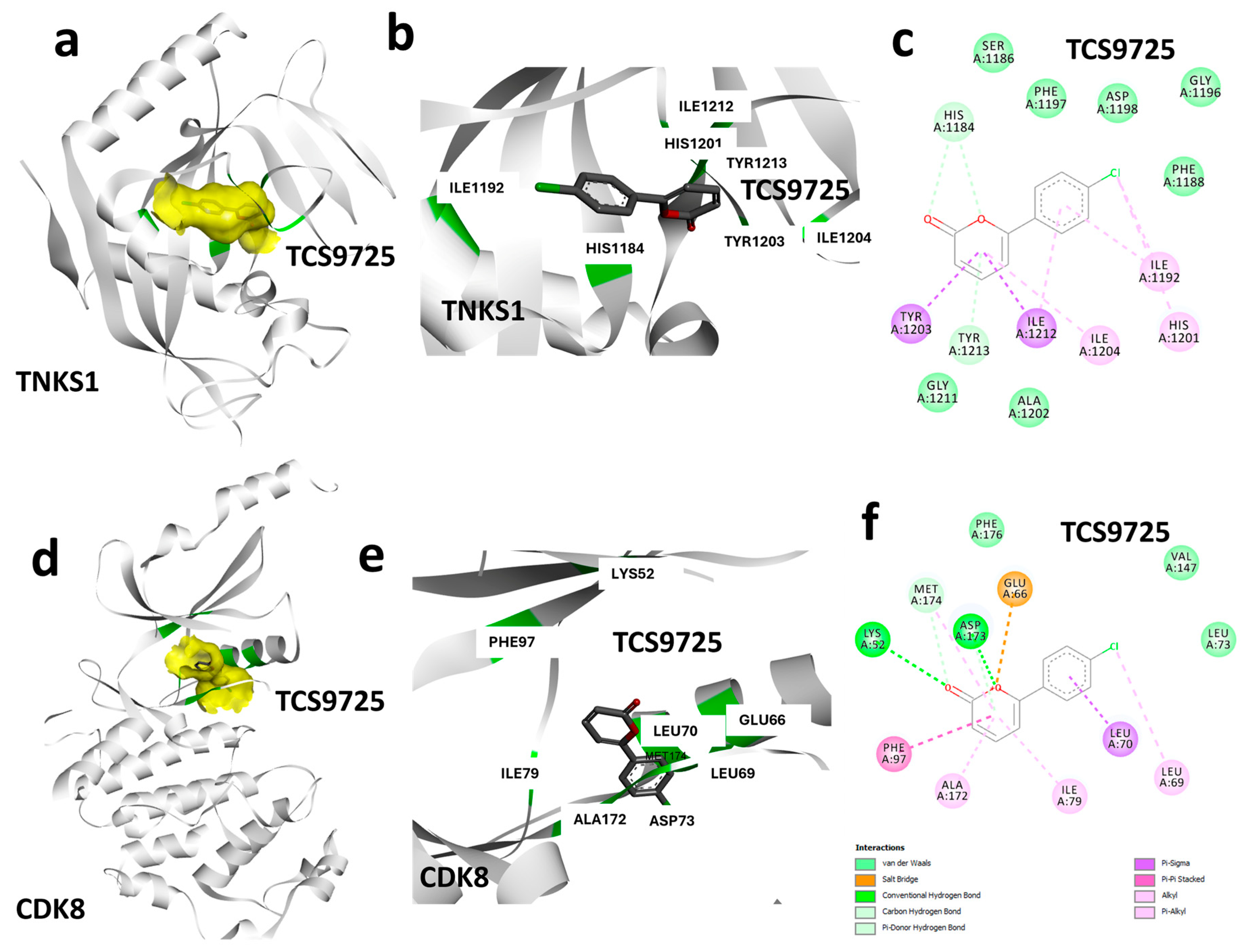
3.3. Protein–Ligand Interaction Analysis Based on Binding Characteristics of TCS9725
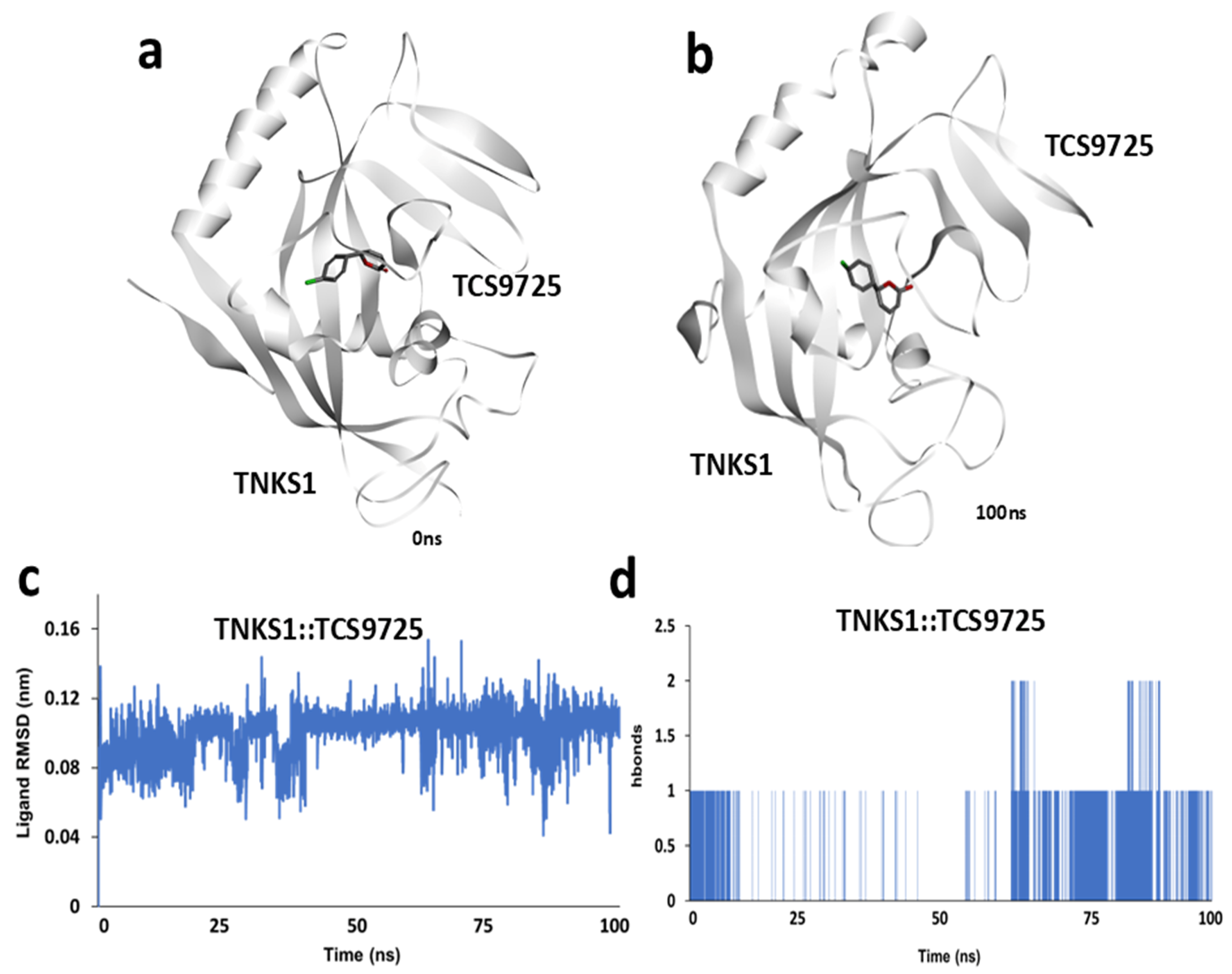
3.4. Molecular Dynamics Simulation Analysis of TNKS1::TCS9725 Complex
3.5. Molecular Dynamics Simulation of the CDK8::TCS9725 Complex
3.6. Gibbs Binding Free Energy Estimation and ADMET Property Predictions
3.7. TCS9725 Inhibited TNKS1 and CDK8 Activities and Selectively Controlled the RCC Cell Proliferation
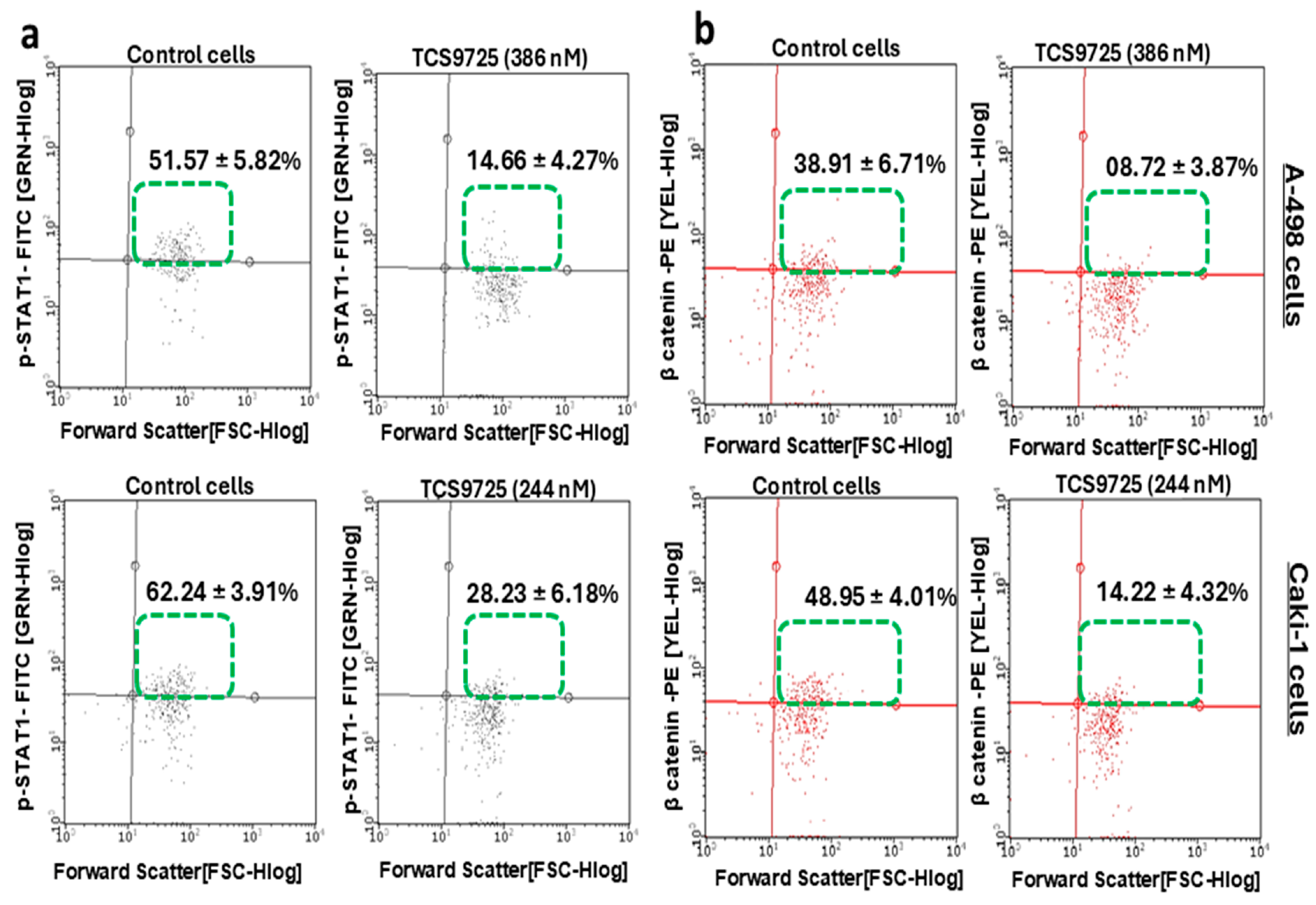
3.8. TCS9725 Reduced the Phospho-STAT-1- and β-Catenin-Positive Population in RCC Cells
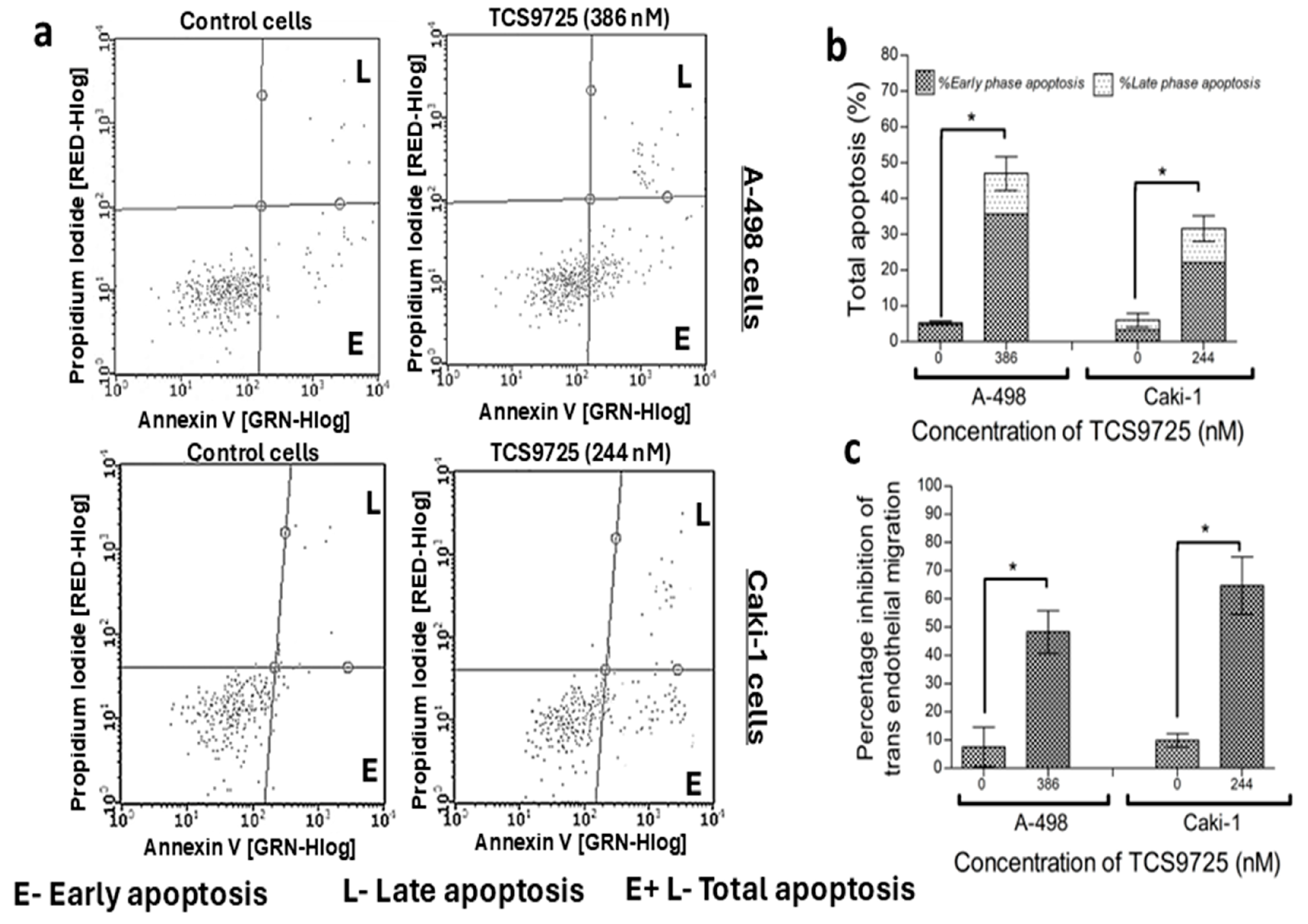
3.9. TCS9725 Induced Apoptosis and Reduced the TGFβ-Stimulated Migration in the RCC Cells
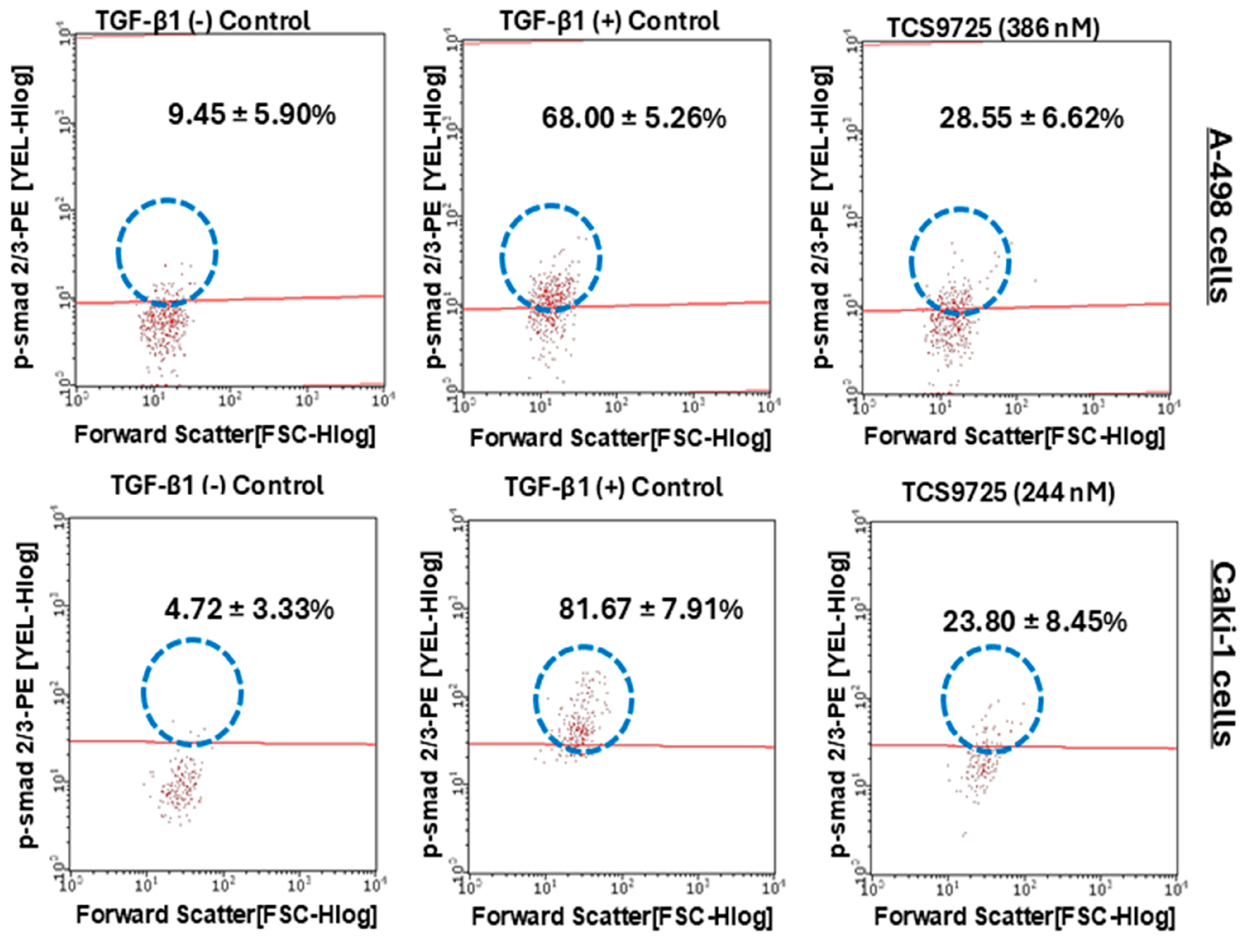
3.10. TCS9725 Downregulated TGF-β-Stimulated Smad2(pS465/pS467)/Smad3 (pS423/pS425) Signaling in RCC Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Banumathy, G.; Cairns, P. Signaling pathways in renal cell carcinoma. Cancer Biol. Ther. 2010, 10, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Krause, M.; Samoylenko, A.; Vainio, S. Wnt Signaling in Renal Cell Carcinoma. Cancers 2016, 8, 57. [Google Scholar] [CrossRef]
- Yip, H.Y.K.; Papa, A. Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef]
- Matuszczak, M.; Kiljańczyk, A.; Salagierski, M. Surgical Approach in Metastatic Renal Cell Carcinoma: A Literature Review. Cancers 2023, 15, 1804. [Google Scholar] [CrossRef]
- Martino-Echarri, E.; Brocardo, M.G.; Mills, K.M.; Henderson, B.R. Tankyrase Inhibitors Stimulate the Ability of Tankyrases to Bind Axin and Drive Assembly of β-Catenin Degradation-Competent Axin Puncta. PLoS ONE 2016, 11, e0150484. [Google Scholar] [CrossRef] [PubMed]
- Haikarainen, T.; Krauss, S.; Lehtio, L. Tankyrases: Structure, function and therapeutic implications in cancer. Curr. Pharm. Des. 2014, 20, 6472–6488. [Google Scholar] [CrossRef]
- Fujita, M.; Demizu, Y. Advances in the development of Wnt/β-catenin signaling inhibitors. RSC Med. Chem. 2024, 16, 984–999. [Google Scholar] [CrossRef]
- Menck, K.; Heinrichs, S.; Baden, C.; Bleckmann, A. The WNT/ROR Pathway in Cancer: From Signaling to Therapeutic Intervention. Cells 2021, 10, 142. [Google Scholar] [CrossRef]
- Friedson, B.; Willis, S.D.; Shcherbik, N.; Campbell, A.N.; Cooper, K.F. The CDK8 kinase module: A novel player in the transcription of translation initiation and ribosomal genes. Mol. Biol. Cell 2025, 36, ar2. [Google Scholar] [CrossRef]
- Bian, J.; Dannappel, M.; Wan, C.; Firestein, R. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer. Cells 2020, 9, 2125. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, J.; Liang, J.; Thompson, Z.S.; Kathrein, K.; Broude, E.V.; Roninson, I.B. Systemic Toxicity Reported for CDK8/19 Inhibitors CCT251921 and MSC2530818 Is Not Due to Target Inhibition. Cells 2019, 8, 1413. [Google Scholar] [CrossRef]
- Arnett, A.; Moo, K.G.; Flynn, K.J.; Sundberg, T.B.; Johannessen, L.; Shamji, A.F.; Gray, N.S.; Decker, T.; Zheng, Y.; Gersuk, V.H.; et al. The Cyclin-Dependent Kinase 8 (CDK8) Inhibitor DCA Promotes a Tolerogenic Chemical Immunophenotype in CD4(+) T Cells via a Novel CDK8-GATA3-FOXP3 Pathway. Mol. Cell. Biol. 2021, 41, e0008521. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; He, Z.; Chen, K.; Ouyang, K.; Yang, C.; Li, J.; Tang, H.; Cai, M. Unveiling the impact of CDK8 on tumor progression: Mechanisms and therapeutic strategies. Front. Pharmacol. 2024, 15, 1386929. [Google Scholar] [CrossRef] [PubMed]
- Yesilkanal, A.E.; Johnson, G.L.; Ramos, A.F.; Rosner, M.R. New strategies for targeting kinase networks in cancer. J. Biol. Chem. 2021, 297, 101128. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Al Shahrani, M.; AboHassan, M.; Gahtani, R.; Alshahrani, M.Y.; Suliman, M.; Ahmad, I.; Saeed, M. High-throughput screening and in vitro evaluation of CSB-0914; a novel small molecule NF-κB inhibitor attenuating inflammatory responses through NF-κB, Nrf2 and HO-1 cross-talk. J. Biomol. Struct. Dyn. 2025, 43, 2762–2771. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Deshpande, H. Dual Targeting of MEK1 and Akt Kinase Identified SBL-027 as a Promising Lead Candidate to Control Cell Proliferations in Gastric Cancer. Biotechnol. Appl. Biochem. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Utami, P.D.; Setianingsih, H.; Sari, D.R.T. In Silico Approach Triterpene Glycoside of H. atra Targeting Orotidine 5-Monophosphate Decarboxylase Protein (PfOMPDC) in P. falciparum Infection Mechanism. BioMed Res. Int. 2024, 2024, 5924799. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.; Walther, P.; Leitz, J.; Mukherjee, S.; Wu, J.C.; Shivnaraine, R.V.; Zou, J. ADMET-AI: A machine learning ADMET platform for evaluation of large-scale chemical libraries. Bioinformatics 2024, 40, btae416. [Google Scholar] [CrossRef]
- Abohassan, M.; Alshahrani, M.; Alshahrani, M.Y.; Rajagopalan, P. Insilco and Invitro approaches identify novel dual PI3K/AKT pathway inhibitors to control acute myeloid leukemia cell proliferations. Med. Oncol. 2022, 39, 249. [Google Scholar] [CrossRef]
- Al Shahrani, M.; Abohassan, M.; Alshahrani, M.; Gahtani, R.M.; Rajagopalan, P. Identification of 8-(2-methyl phenyl)-9H-benzo[f]indeno [2,1-c]quinolin-9-one (C-5635020) as a novel and selective TGFβ RII kinase inhibitor for breast cancer therapy. Biochem. Biophys. Res. Commun. 2025, 746, 151225. [Google Scholar] [CrossRef]
- Halder, S.K.; Sultana, I.; Shuvo, M.N.; Shil, A.; Himel, M.K.; Hasan, M.A.; Shawan, M. In Silico Identification and Analysis of Potentially Bioactive Antiviral Phytochemicals against SARS-CoV-2: A Molecular Docking and Dynamics Simulation Approach. BioMed Res. Int. 2023, 2023, 5469258. [Google Scholar] [CrossRef]
- Santos, L.H.S.; Ferreira, R.S.; Caffarena, E.R. Integrating Molecular Docking and Molecular Dynamics Simulations. Methods Mol. Biol. 2019, 2053, 13–34. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Mygland, L.; Brinch, S.A.; Strand, M.F.; Olsen, P.A.; Aizenshtadt, A.; Lund, K.; Solberg, N.T.; Lycke, M.; Thorvaldsen, T.E.; Espada, S.; et al. Identification of response signatures for tankyrase inhibitor treatment in tumor cell lines. iScience 2021, 24, 102807. [Google Scholar] [CrossRef]
- Zhong, L.; Ding, Y.; Bandyopadhyay, G.; Waaler, J.; Börgeson, E.; Smith, S.; Zhang, M.; Phillips, S.A.; Mahooti, S.; Mahata, S.K.; et al. The PARsylation activity of tankyrase in adipose tissue modulates systemic glucose metabolism in mice. Diabetologia 2016, 59, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.; Holsworth, D.D.; Waaler, J.; Wilson, S.R.; Ekblad, B.; Perdreau-Dahl, H.; Dinh, H.; Drewes, G.; Hopf, C.; Morth, J.P.; et al. Structural basis and SAR for G007-LK, a lead stage 1,2,4-triazole based specific tankyrase 1/2 inhibitor. J. Med. Chem. 2013, 56, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Weng, W.; Zhang, Q.; Wu, Y.; Ni, S.; Tan, C.; Xu, M.; Sun, H.; Liu, C.; Wei, P.; et al. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J. Hematol. Oncol. 2018, 11, 113. [Google Scholar] [CrossRef]
- Cao, M.Q.; You, A.B.; Zhu, X.D.; Zhang, W.; Zhang, Y.Y.; Zhang, S.Z.; Zhang, K.W.; Cai, H.; Shi, W.K.; Li, X.L.; et al. miR-182-5p promotes hepatocellular carcinoma progression by repressing FOXO3a. J. Hematol. Oncol. 2018, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Si, J.; Gan, L.; Guo, M.; Yan, J.; Chen, Y.; Wang, F.; Xie, Y.; Wang, Y.; Zhang, H. Inhibition of Wnt signalling pathway by XAV939 enhances radiosensitivity in human cervical cancer HeLa cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 479–487. [Google Scholar] [CrossRef]
- Stratford, E.W.; Daffinrud, J.; Munthe, E.; Castro, R.; Waaler, J.; Krauss, S.; Myklebost, O. The tankyrase-specific inhibitor JW74 affects cell cycle progression and induces apoptosis and differentiation in osteosarcoma cell lines. Cancer Med. 2014, 3, 36–46. [Google Scholar] [CrossRef]
- Arqués, O.; Chicote, I.; Puig, I.; Tenbaum, S.P.; Argilés, G.; Dienstmann, R.; Fernández, N.; Caratù, G.; Matito, J.; Silberschmidt, D.; et al. Tankyrase Inhibition Blocks Wnt/β-Catenin Pathway and Reverts Resistance to PI3K and AKT Inhibitors in the Treatment of Colorectal Cancer. Clin. Cancer Res. 2016, 22, 644–656. [Google Scholar] [CrossRef]
- Thorne, C.A.; Hanson, A.J.; Schneider, J.; Tahinci, E.; Orton, D.; Cselenyi, C.S.; Jernigan, K.K.; Meyers, K.C.; Hang, B.I.; Waterson, A.G.; et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat. Chem. Biol. 2010, 6, 829–836. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Sung, T.-Y.; Pan, S.-L.; Huang, W.-J.; Hsu, K.-C.; Hsu, J.-Y.; Lin, T.E.; Hsu, C.-M.; Yang, C.-R. The study of a novel CDK8 inhibitor E966-0530–45418 that inhibits prostate cancer metastasis in vitro and in vivo. Biomed. Pharmacother. 2023, 162, 114667. [Google Scholar] [CrossRef]
- Brägelmann, J.; Klümper, N.; Offermann, A.; von Mässenhausen, A.; Böhm, D.; Deng, M.; Queisser, A.; Sanders, C.; Syring, I.; Merseburger, A.S.; et al. Pan-Cancer Analysis of the Mediator Complex Transcriptome Identifies CDK19 and CDK8 as Therapeutic Targets in Advanced Prostate Cancer. Clin. Cancer Res. 2017, 23, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Taatjes, D.J. The Mediator complex: A central integrator of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 155–166. [Google Scholar] [CrossRef]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of Colorectal Cancer on a TGF-β-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef]
- Arai, E.; Sakamoto, H.; Ichikawa, H.; Totsuka, H.; Chiku, S.; Gotoh, M.; Mori, T.; Nakatani, T.; Ohnami, S.; Nakagawa, T.; et al. Multilayer-omics analysis of renal cell carcinoma, including the whole exome, methylome and transcriptome. Int. J. Cancer 2014, 135, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Bancerek, J.; Poss, Z.C.; Steinparzer, I.; Sedlyarov, V.; Pfaffenwimmer, T.; Mikulic, I.; Dölken, L.; Strobl, B.; Müller, M.; Taatjes, D.J.; et al. CDK8 Kinase Phosphorylates Transcription Factor STAT1 to Selectively Regulate the Interferon Response. Immunity 2013, 38, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Liu, S.; Wang, Y.; Liang, Y.; Jablons, D.M. MK256 is a novel CDK8 inhibitor with potent antitumor activity in AML through downregulation of the STAT pathway. Oncotarget 2022, 13, 1217–1236. [Google Scholar] [CrossRef]
- Spear, J.M.; Lu, Z.; Russu, W.A. Pharmacological Inhibition of CDK8 in Triple-Negative Breast Cancer Cell Line MDA-MB-468 Increases E2F1 Protein, Induces Phosphorylation of STAT3 and Apoptosis. Molecules 2020, 25, 5728. [Google Scholar] [CrossRef]
- Solum, E.; Hansen, T.V.; Aesoy, R.; Herfindal, L. New CDK8 inhibitors as potential anti-leukemic agents—Design, synthesis and biological evaluation. Bioorganic Med. Chem. 2020, 28, 115461. [Google Scholar] [CrossRef]
- Boström, A.-K.; Lindgren, D.; Johansson, M.E.; Axelson, H. Effects of TGF-β signaling in clear cell renal cell carcinoma cells. Biochem. Biophys. Res. Commun. 2013, 435, 126–133. [Google Scholar] [CrossRef]
- Menzl, I.; Witalisz-Siepracka, A.; Sexl, V. CDK8-Novel Therapeutic Opportunities. Pharmaceuticals 2019, 12, 92. [Google Scholar] [CrossRef]
- Alarcón, C.; Zaromytidou, A.-I.; Xi, Q.; Gao, S.; Yu, J.; Fujisawa, S.; Barlas, A.; Miller, A.N.; Manova-Todorova, K.; Macias, M.J.; et al. Nuclear CDKs Drive Smad Transcriptional Activation and Turnover in BMP and TGF-β Pathways. Cell 2009, 139, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Firestein, R.; Bass, A.J.; Kim, S.Y.; Dunn, I.F.; Silver, S.J.; Guney, I.; Freed, E.; Ligon, A.H.; Vena, N.; Ogino, S.; et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature 2008, 455, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Cavalli, A.; Cascio, A. STAT1 and Its Crucial Role in the Control of Viral Infections. Int. J. Mol. Sci. 2022, 23, 4095. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Fayi, M.S.; Alshyarba, M. Selective Dual Inhibition of TNKS1 and CDK8 by TCS9725 Attenuates STAT1/β-Catenin/TGFβ1 Signaling in Renal Cancer. Curr. Issues Mol. Biol. 2025, 47, 463. https://doi.org/10.3390/cimb47060463
Al Fayi MS, Alshyarba M. Selective Dual Inhibition of TNKS1 and CDK8 by TCS9725 Attenuates STAT1/β-Catenin/TGFβ1 Signaling in Renal Cancer. Current Issues in Molecular Biology. 2025; 47(6):463. https://doi.org/10.3390/cimb47060463
Chicago/Turabian StyleAl Fayi, Majed Saad, and Mishari Alshyarba. 2025. "Selective Dual Inhibition of TNKS1 and CDK8 by TCS9725 Attenuates STAT1/β-Catenin/TGFβ1 Signaling in Renal Cancer" Current Issues in Molecular Biology 47, no. 6: 463. https://doi.org/10.3390/cimb47060463
APA StyleAl Fayi, M. S., & Alshyarba, M. (2025). Selective Dual Inhibition of TNKS1 and CDK8 by TCS9725 Attenuates STAT1/β-Catenin/TGFβ1 Signaling in Renal Cancer. Current Issues in Molecular Biology, 47(6), 463. https://doi.org/10.3390/cimb47060463






