Effects of Aerobic Exercise on Irisin and Skeletal Muscle Autophagy in ApoE−/− Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care, Grouping, and Treatment
2.2. Exercise Protocol
2.3. Skeletal Muscle Tissue Sampling
2.4. Measurement of Lipid Levels
2.5. Detection of Irisin in Skeletal Muscle and Serum
2.6. Hematoxylin-Eosin (HE) Staining
2.7. Immunoblots
2.8. Statistical Analysis
3. Results
3.1. Exercise Attenuates Dyslipidemia in ApoE−/− Mice
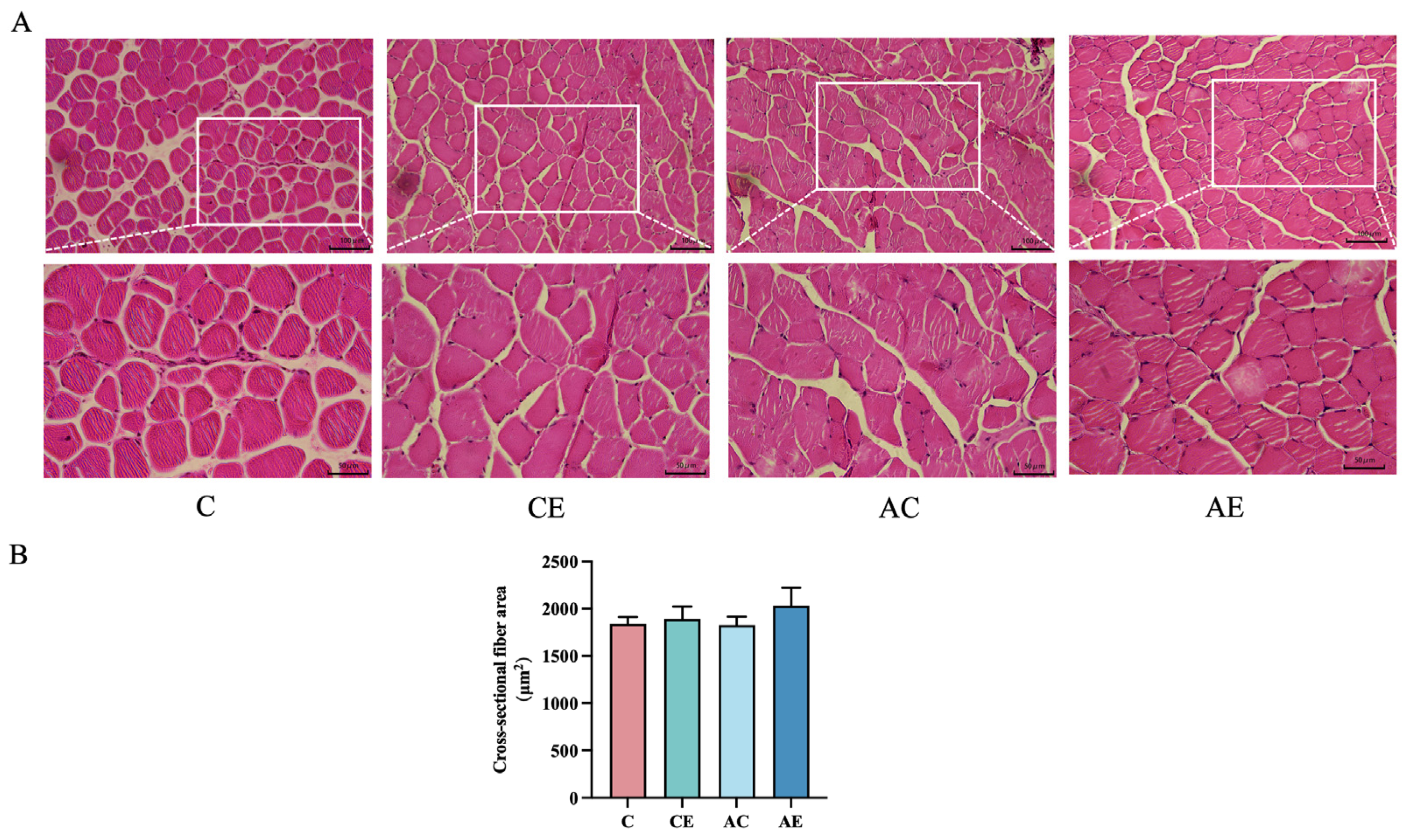
3.2. Exercise Exerted No Significant Influence on the Cross-Sectional Fiber Area Fibers in ApoE−/− Mice
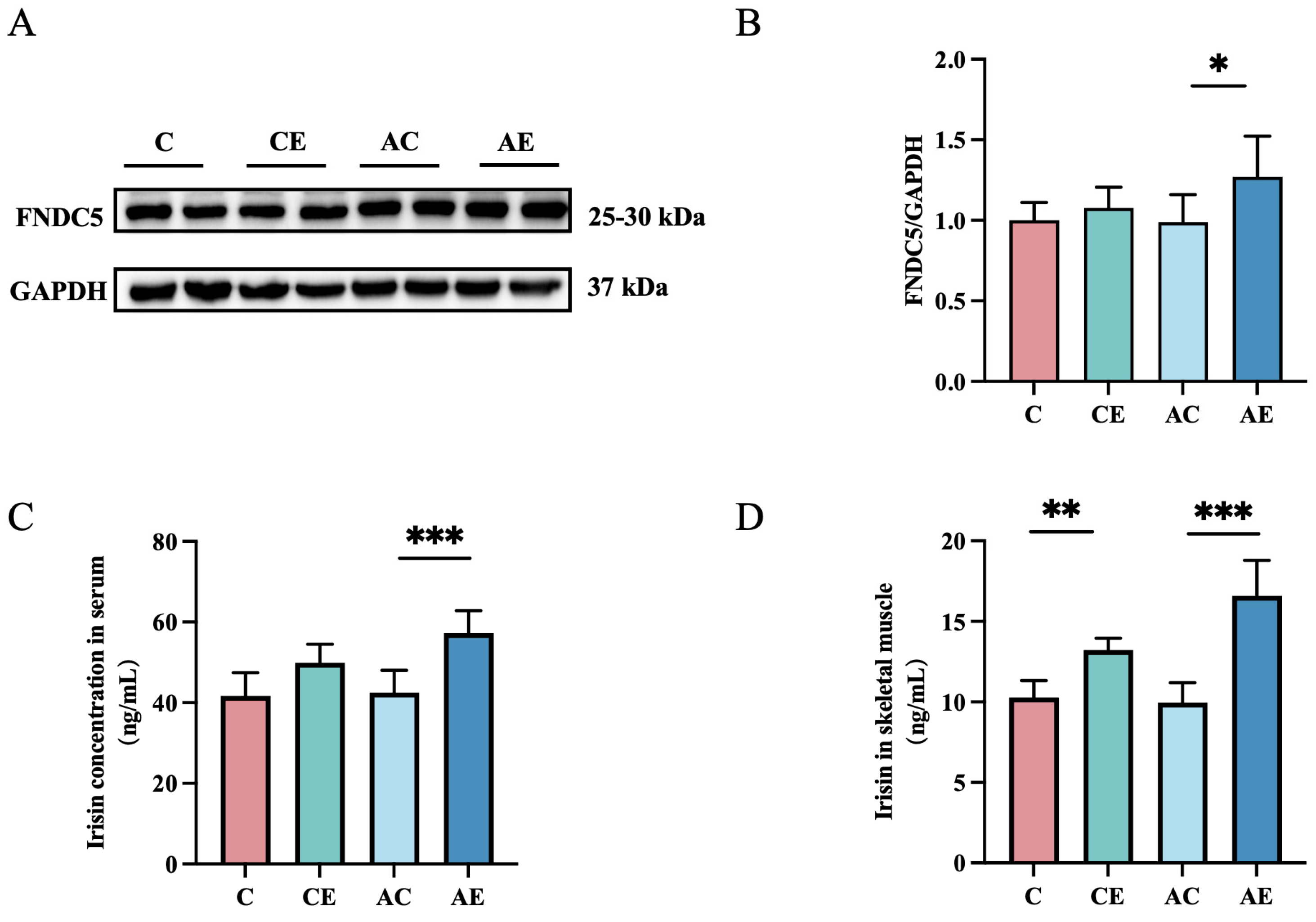
3.3. Exercise Increased Irisin Levels in ApoE−/− Mice
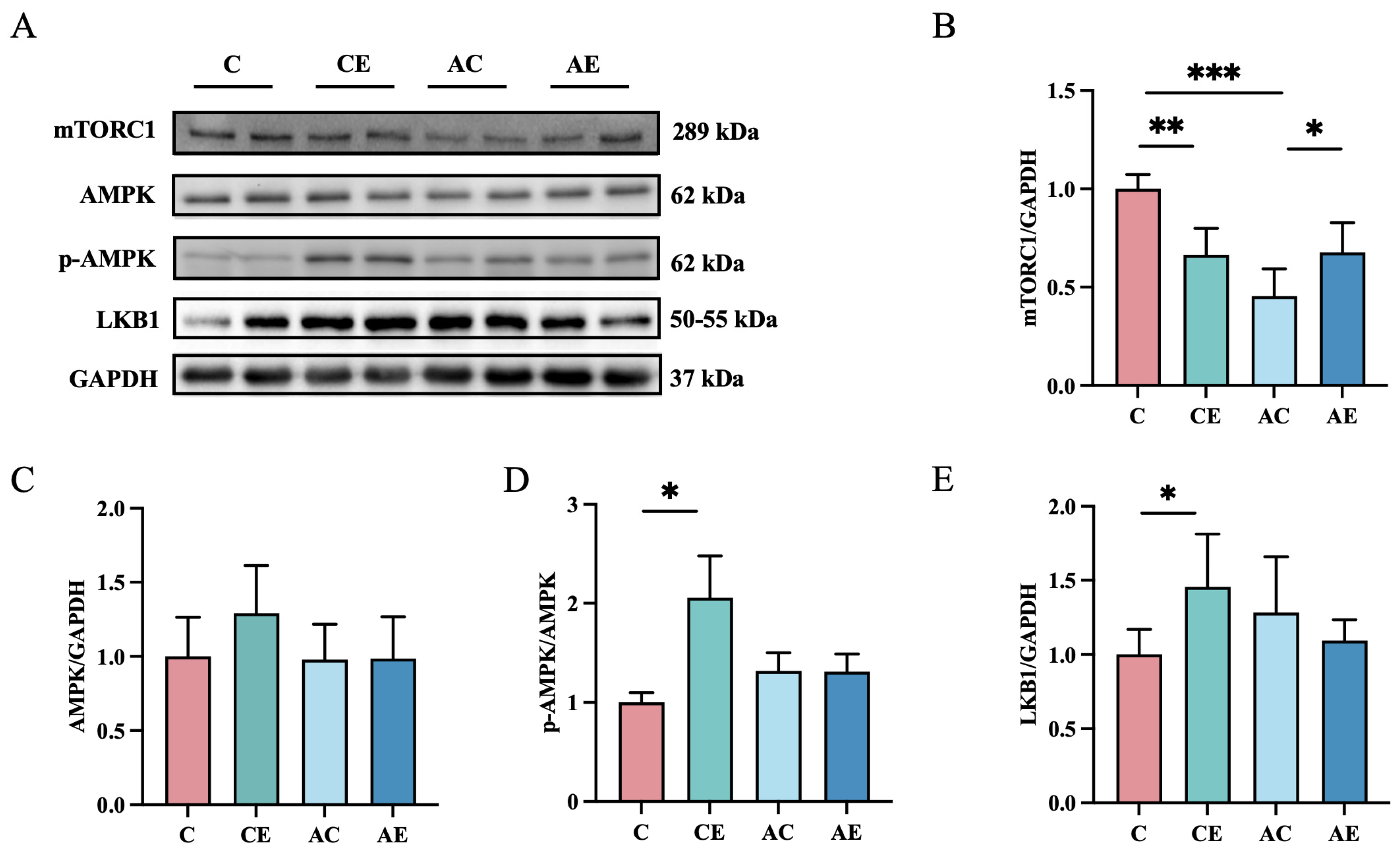
3.4. Exercise Upregulated Protein Expression of mTORC1, but Had No Effect on the APMK in ApoE−/− Mice
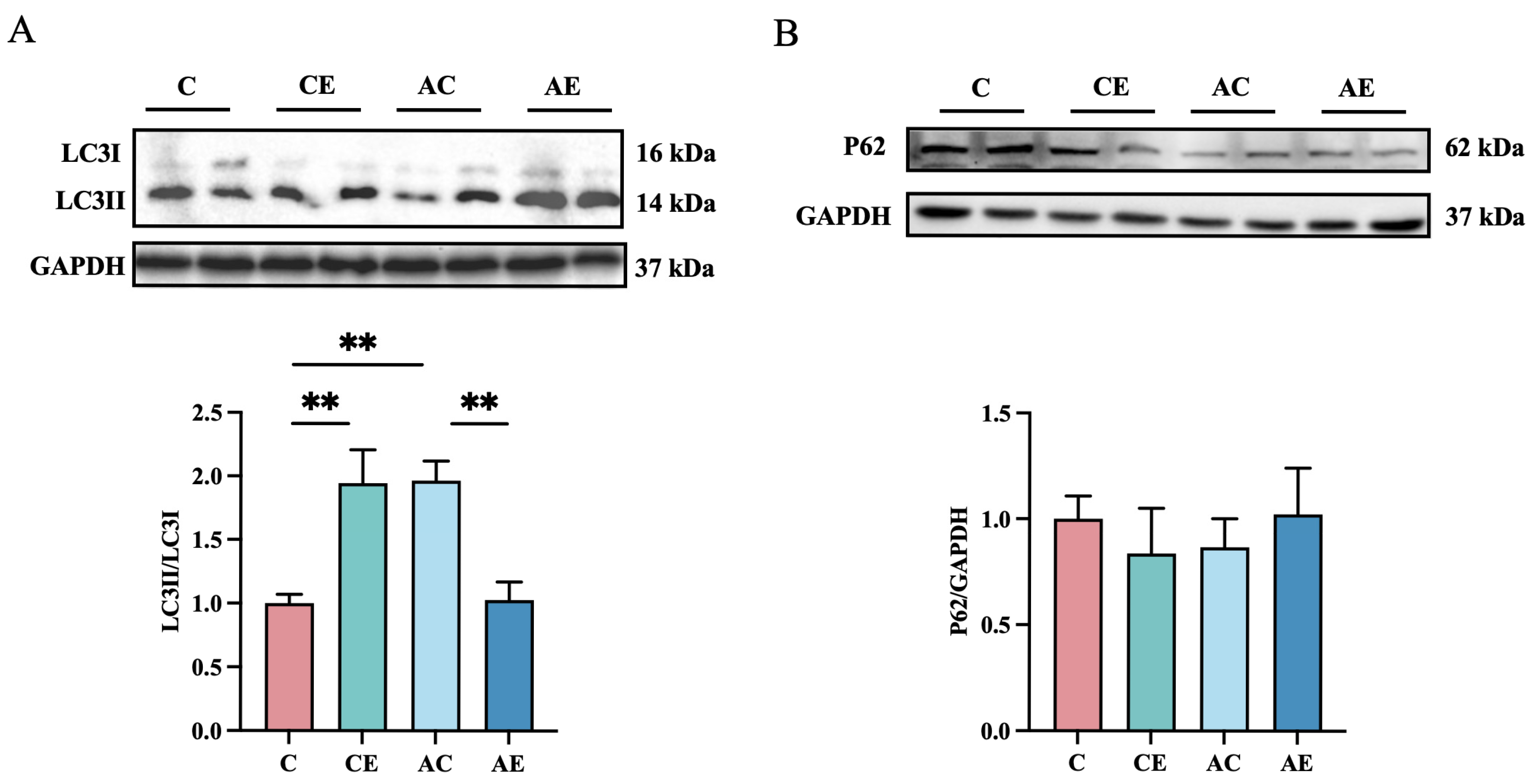
3.5. Exercise Inhibits the Expression of Autophagy-Related Proteins in Skeletal Muscle of ApoE−/− Mice
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full Term |
| FNDC5 | Fibronectin type III domain containing protein 5 |
| AMPK | Adenosine 5′-monophosphate (AMP)-activated protein kinase |
| p-AMPK | phosphorylated AMP-activated protein kinase |
| mTOR | mechanistic Target of Rapamycin |
| mTORC1 | mammalian target of rapamycin complex 1 |
| LKB1 | Serine-Threonine Kinase 11 |
| LC3 | Low Complexity Communication Codec |
| LC3 I | Microtubule-associated protein 1 light chain 3B I |
| LC3 II | Microtubule-associated protein 1 light chain 3B II |
| P62 | Sequestosome-1 (SQSTM1) |
| TC | Total Cholesterol |
| TAG | Triacylglycerides |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| HDL-C | High-Density Lipoprotein Cholesterol |
| AMP | Adenosine Monophosphate |
| ATP | Adenosine Triphosphate |
| ULK1 | Unc-51 like autophagy activating kinase 1 |
| SPF | Specific Pathogen Free |
| PBS | Phosphate Buffered Saline |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| PVDF | Polyvinylidene Fluoride |
| TBST | Tris Buffered Saline with Tween-20 |
| CSA | Cross-sectional area |
References
- Sfyri, P.; Matsakas, A. Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: Emphasis on apolipoprotein E deficiency and peripheral arterial disease. J. Biomed. Sci. 2017, 24, 42. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Loomba, R.S.; Arora, R. Preventive aspects in peripheral artery disease. Ther. Adv. Cardiovasc. Dis. 2012, 6, 53–70. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Q.; Zhou, Z.; Song, H.; Zhang, Y.; Wu, F.; Jiang, M.; Wang, F.; Zhang, W.; Li, L.; et al. Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS ONE 2016, 11, e0158038. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, K.; Han, Y.; Zhu, H.; Zhou, X.; Tan, T.; Zeng, J.; Zhang, J.; Liu, Y.; Li, Y.; et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J. Cardiovasc. Pharmacol. 2018, 72, 259–269. [Google Scholar] [CrossRef]
- Li, R.-L.; Wu, S.-S.; Wu, Y.; Wang, X.-X.; Chen, H.-Y.; Xin, J.-J.; Li, H.; Lan, J.; Xue, K.-Y.; Li, X.; et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J. Mol. Cell. Cardiol. 2018, 121, 242–255. [Google Scholar] [CrossRef]
- Adams, V.; Reich, B.; Uhlemann, M.; Niebauer, J. Molecular effects of exercise training in patients with cardiovascular disease: Focus on skeletal muscle, endothelium, and myocardium. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H72–H88. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Jiang, Y.; Liu, H. Aerobic exercise inhibits inflammatory response in atherosclerosis via Sestrin1 protein. Exp. Gerontol. 2021, 155, 111581. [Google Scholar] [CrossRef]
- Lesniewski, L.A.; Durrant, J.R.; Connell, M.L.; Henson, G.D.; Black, A.D.; Donato, A.J.; Seals, D.R. Aerobic exercise reverses arterial inflammation with aging in mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1025–H1032. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, D.B.; Shaw, R.J. The LKB1–AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Chen, Y.; Wang, Y.; Wang, W.; Long, S.; Yang, H.-Y.; Wu, J.; Li, M.; Tian, X.; Wei, X.; et al. Lithocholic acid binds TULP3 to activate sirtuins and AMPK to slow down ageing. Nature 2024. [Google Scholar] [CrossRef]
- Rubio-Tomás, T.; Sotiriou, A.; Tavernarakis, N. The interplay between selective types of (macro)autophagy: Mitophagy and xenophagy. Int. Rev. Cell Mol. Biol. 2023, 374, 129–157. [Google Scholar]
- Chu, Y.; Yuan, X.; Tao, Y.; Yang, B.; Luo, J. Autophagy in muscle regeneration: Mechanisms, targets, and therapeutic perspective. Int. J. Mol. Sci. 2024, 25, 11901. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Liu, T.; Zhu, X.; Pan, X. The multifaceted role of the SASP in atherosclerosis: From mechanisms to therapeutic opportunities. Cell Biosci. 2022, 12, 74. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y. AMPK and autophagy. Adv. Exp. Med. Biol. 2019, 1206, 85–108. [Google Scholar] [CrossRef]
- Yee, E.M.; Hauser, C.T.; Petrocelli, J.J.; de Hart, N.M.M.P.; Ferrara, P.J.; Bombyck, P.; Fennel, Z.J.; van Onselen, L.; Mookerjee, S.; Funai, K.; et al. Treadmill training does not enhance skeletal muscle recovery following disuse atrophy in older male mice. Front. Physiol. 2023, 14, 1263500. [Google Scholar] [CrossRef]
- Bedford, T.G.; Tipton, C.M.; Wilson, N.C.; Oppliger, R.A.; Gisolfi, C.V. Maximum oxygen consumption of rats and its changes with various experimental procedures. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 47, 1278–1283. [Google Scholar] [CrossRef]
- Gaggini, M.; Gorini, F.; Vassalle, C. Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. Int. J. Mol. Sci. 2022, 24, 75. [Google Scholar] [CrossRef]
- Bezsonov, E.; Khotina, V.; Glanz, V.; Sobenin, I.; Orekhov, A. Lipids and lipoproteins in atherosclerosis. Biomedicines 2023, 11, 1424. [Google Scholar] [CrossRef]
- Ahmadabadi, F.; Nakhaei, H.; Mogharnasi, M.; Huang, C.-J. Aerobic interval training improves irisin and chemerin levels of both liver and visceral adipose tissues and circulating asprosin in rats with metabolic syndrome. Physiol. Int. 2021, 108, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, B.; Wang, Y.; Tian, X.; Lin, J.; Sun, X.; Sun, Y.; Zhang, X.; Xu, H.; Li, M.; et al. Exercise ameliorates dysregulated mitochondrial fission, mitochondrial respiration, and neuronal apoptosis in Parkinson’s disease mice via the irisin/AMPK/SIRT1 pathway. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Sanotra, M.R.; Huang, C.-C.; Hsu, Y.-J.; Liao, C.-C. Aerobic exercise modulates proteomic profiles in gastrocnemius muscle of db/db mice, ameliorating sarcopenia. Life 2024, 14, 412. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Harber, M.P. Skeletal muscle hypertrophy after aerobic exercise training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef]
- Ouyang, Q.; Chen, Q.; Ke, S.; Ding, L.; Yang, X.; Rong, P.; Feng, W.; Cao, Y.; Wang, Q.; Li, M.; et al. Rab8a as a mitochondrial receptor for lipid droplets in skeletal muscle. Dev. Cell 2023, 58, 289–305.e6. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/irisin: Physiology and pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef]
- Pesce, M.; Ballerini, P.; Paolucci, T.; Puca, I.; Farzaei, M.H.; Patruno, A. Irisin and autophagy: First update. Int. J. Mol. Sci. 2020, 21, 7587. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Botella, J.; Jamnick, N.A.; Granata, C.; Genders, A.J.; Perri, E.; Jabar, T.; Garnham, A.; Lazarou, M.; Bishop, D.J. Exercise and training regulation of autophagy markers in human and rat skeletal muscle. Int. J. Mol. Sci. 2022, 23, 2619. [Google Scholar] [CrossRef] [PubMed]
- Wiese, W.; Barczuk, J.; Racinska, O.; Siwecka, N.; Rozpedek-Kaminska, W.; Slupianek, A.; Sierpinski, R.; Majsterek, I. PI3K/Akt/mTOR signaling pathway in blood malignancies—New therapeutic possibilities. Cancers 2023, 15, 5297. [Google Scholar] [CrossRef] [PubMed]
- Dossou, A.S.; Basu, A. The emerging roles of mTORC1 in macromanaging autophagy. Cancers 2019, 11, 1422. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zheng, F.; Zhou, J.; Cao, Y.; Zhang, L.; Lu, Y.; Li, Q.; Li, T.; Korivi, M.; Wang, L.; et al. Effects of Aerobic Exercise on Irisin and Skeletal Muscle Autophagy in ApoE−/− Mice. Curr. Issues Mol. Biol. 2025, 47, 371. https://doi.org/10.3390/cimb47050371
Wang W, Zheng F, Zhou J, Cao Y, Zhang L, Lu Y, Li Q, Li T, Korivi M, Wang L, et al. Effects of Aerobic Exercise on Irisin and Skeletal Muscle Autophagy in ApoE−/− Mice. Current Issues in Molecular Biology. 2025; 47(5):371. https://doi.org/10.3390/cimb47050371
Chicago/Turabian StyleWang, Wenxin, Fengting Zheng, Jiawei Zhou, Yangfan Cao, Liang Zhang, Yao Lu, Qingbo Li, Ting Li, Mallikarjuna Korivi, Lifeng Wang, and et al. 2025. "Effects of Aerobic Exercise on Irisin and Skeletal Muscle Autophagy in ApoE−/− Mice" Current Issues in Molecular Biology 47, no. 5: 371. https://doi.org/10.3390/cimb47050371
APA StyleWang, W., Zheng, F., Zhou, J., Cao, Y., Zhang, L., Lu, Y., Li, Q., Li, T., Korivi, M., Wang, L., & Li, W. (2025). Effects of Aerobic Exercise on Irisin and Skeletal Muscle Autophagy in ApoE−/− Mice. Current Issues in Molecular Biology, 47(5), 371. https://doi.org/10.3390/cimb47050371







