Dose-Dependent Effects of TGF-β Inhibition on Osteoblast Differentiation and Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Osteoblast Differentiation
2.3. Cell Viability Assay and Dose–Response Proliferation Curve
2.4. Measurement of Apoptosis
2.5. Quantification of Alkaline Phosphatase Activity
2.6. Alkaline Phosphatase Staining
2.7. Alizarin Red S Staining for Mineralized Matrix Formation
2.8. RNA Extraction and cDNA Synthesis
2.9. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
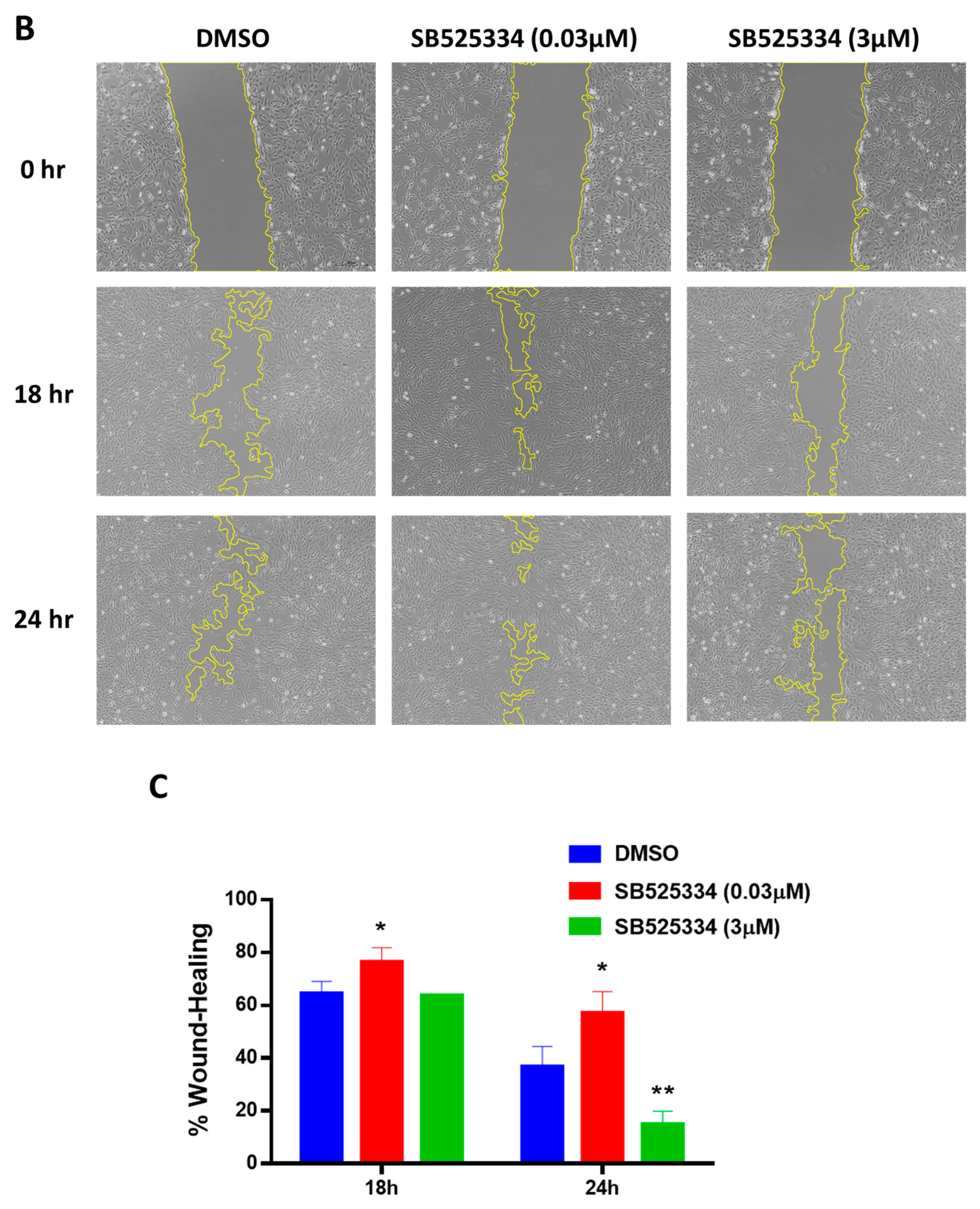
2.10. Migration/Scratch Assay
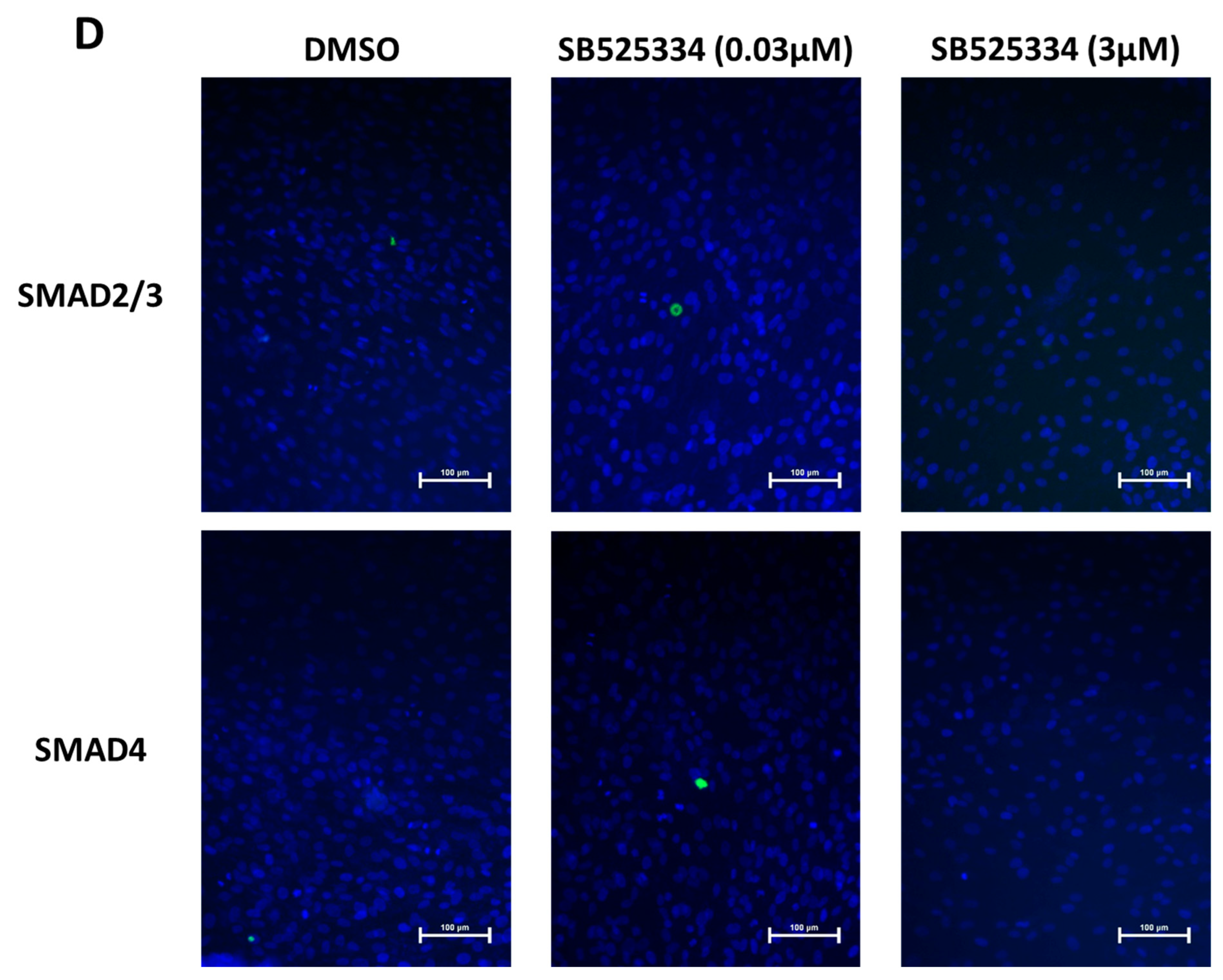
2.11. Immunocytochemistry
2.12. Statistical Analysis
3. Results
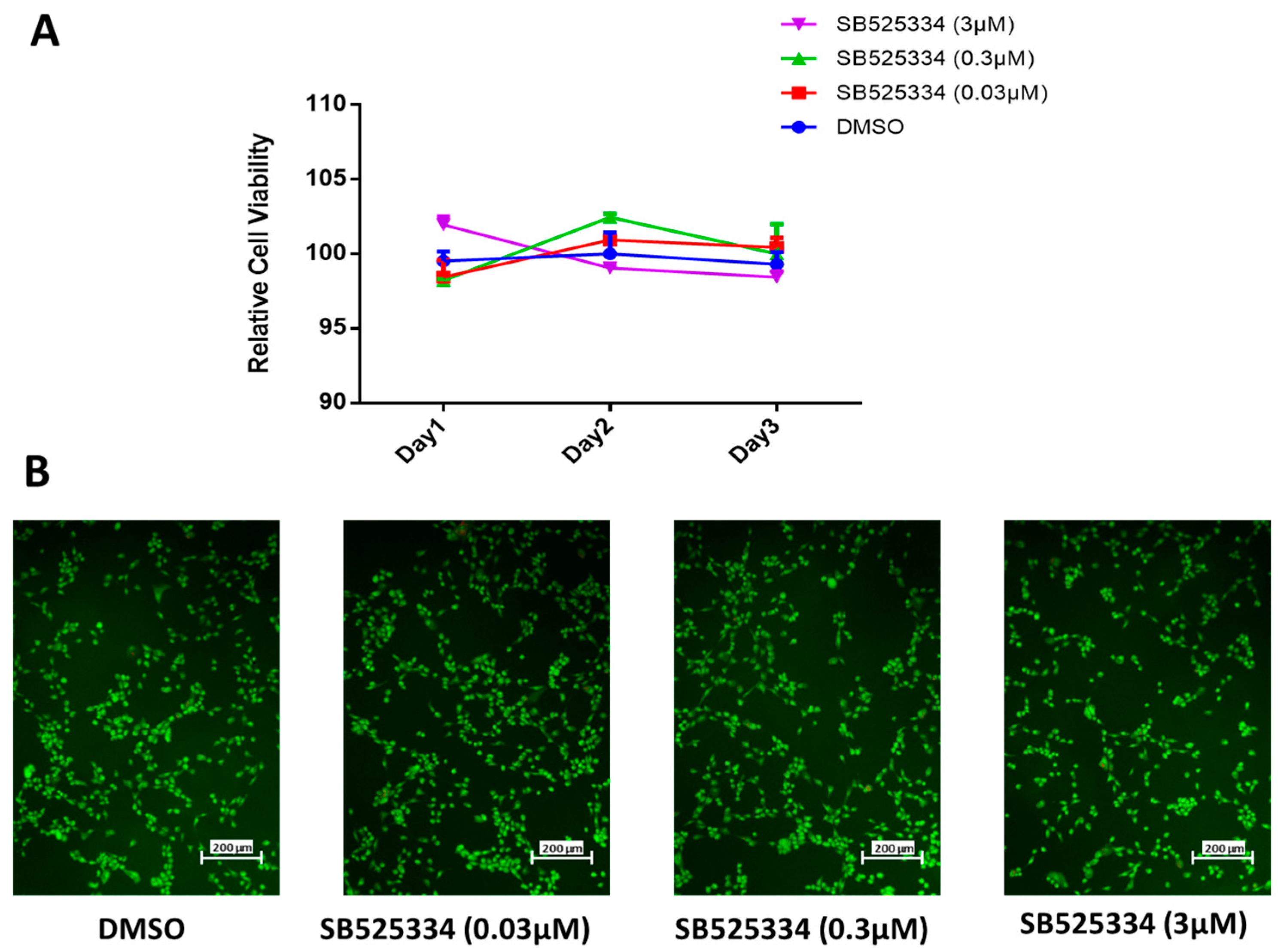
3.1. SB525334 Has no Suppressive Effect on Human MSC Proliferation
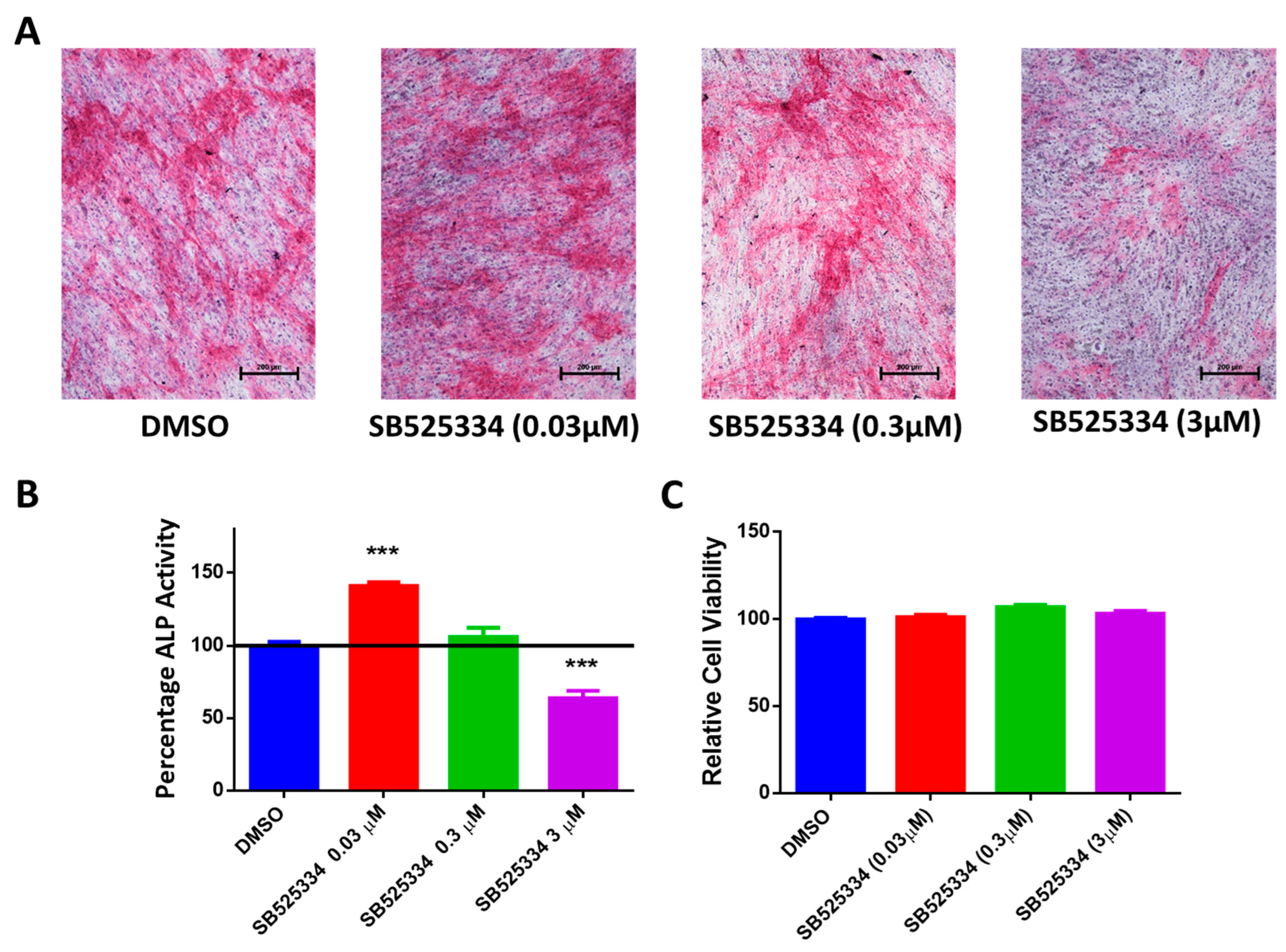
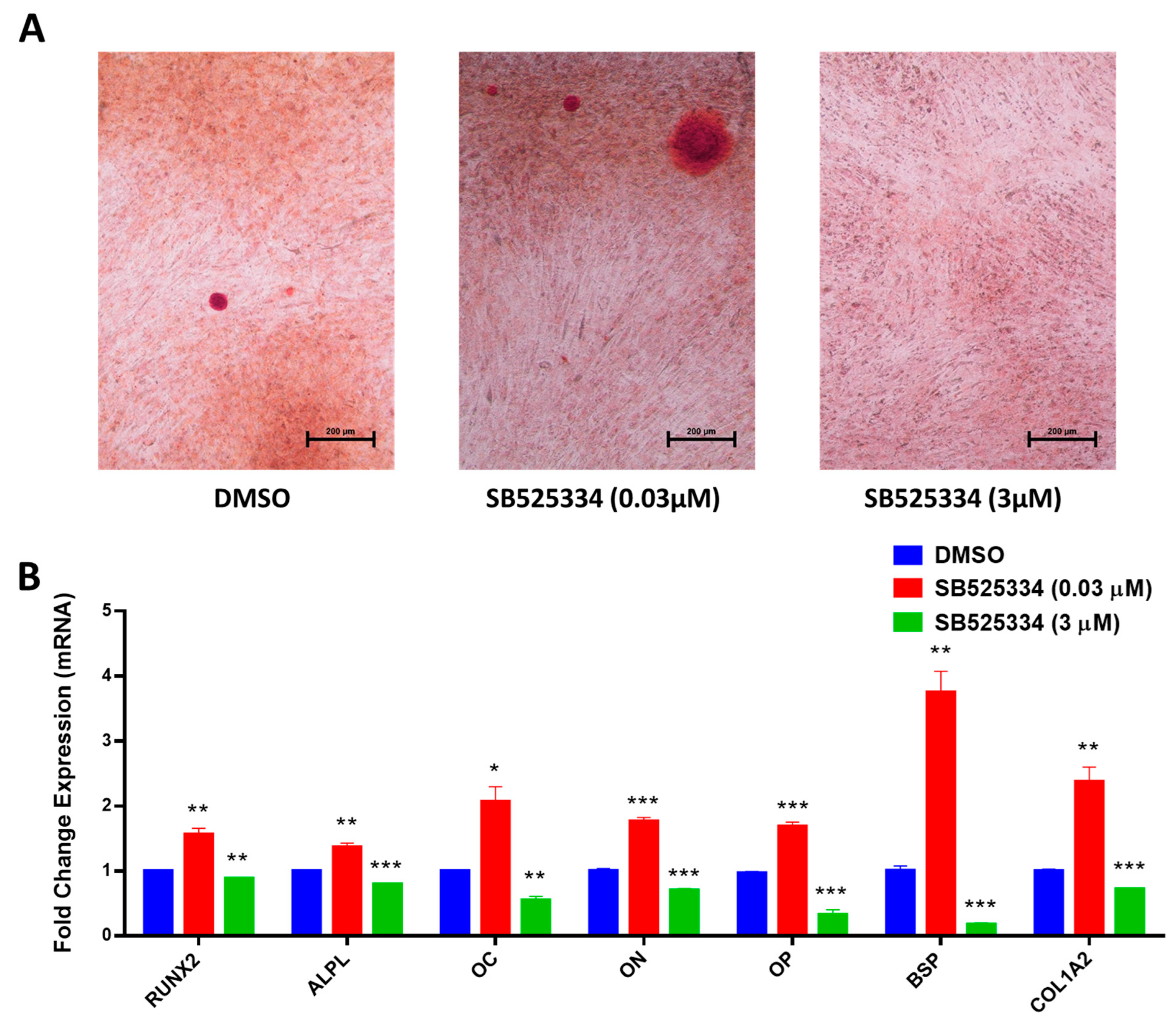
3.2. SB525334 Exhibited an Opposite Effect on the Osteoblast Differentiation of Human MSCs
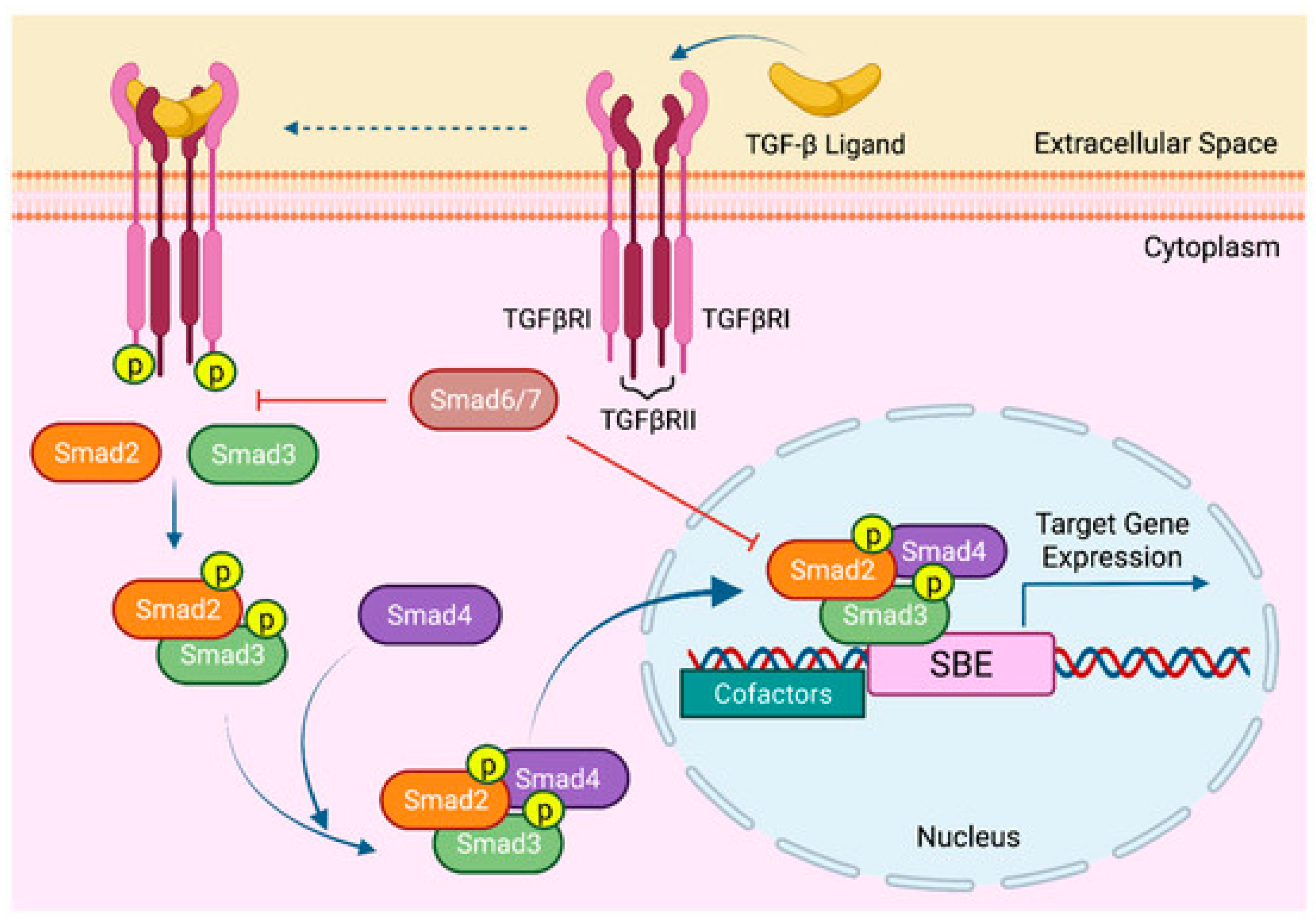
3.3. Effect of SB525334 on TGF-β Signaling Pathway
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells |
| TGF-β | Transforming growth factor β |
| TGF-βR | TGF-β receptor |
| ALK | Activin-like kinase |
| SMAD | Mothers against decapentaplegic homolog |
| ALPL | Alkaline phosphatase |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | Dimethyl sulfoxide |
| hTERT | Human telomerase reverse transcriptase |
| PBS | Phosphate-buffered saline |
| qRT-PCR | Quantitative reverse transcriptase polymerase chain reaction |
| BMP | Bone morphogenetic proteins |
| Wnt | Wingless/int1 |
| TNF | Tumor necrosis factor |
| Runx2 | Runt-related transcription factor 2 |
| OC | Osteocalcin |
| ON | Osteonectin |
| OP | Osteopontin |
| BSP | Bone sialoprotein |
| COL1A2 | Collagen type I alpha 2 |
| JUN | Jun proto-oncogene |
| RAF1 | Raf-1 proto-oncogene |
| ATF3 | Activating transcription factor 3 |
References
- Almasoud, N.; Binhamdan, S.; Younis, G.; Alaskar, H.; Alotaibi, A.; Manikandan, M.; Alfayez, M.; Kassem, M.; AlMuraikhi, N. Author Correction: Tankyrase inhibitor XAV-939 enhances osteoblastogenesis and mineralization of human skeletal (mesenchymal) stem cells. Sci. Rep. 2021, 11, 4559. [Google Scholar] [CrossRef] [PubMed]
- AlMuraikhi, N.; Ali, D.; Alshanwani, A.; Vishnubalaji, R.; Manikandan, M.; Atteya, M.; Siyal, A.; Alfayez, M.; Aldahmash, A.; Kassem, M.; et al. Stem cell library screen identified ruxolitinib as regulator of osteoblastic differentiation of human skeletal stem cells. Stem Cell Res. Ther. 2018, 9, 319. [Google Scholar] [CrossRef]
- AlMuraikhi, N.; Almasoud, N.; Binhamdan, S.; Younis, G.; Ali, D.; Manikandan, M.; Vishnubalaji, R.; Atteya, M.; Siyal, A.; Alfayez, M.; et al. Hedgehog Signaling Inhibition by Smoothened Antagonist BMS-833923 Reduces Osteoblast Differentiation and Ectopic Bone Formation of Human Skeletal (Mesenchymal) Stem Cells. Stem Cells Int. 2019, 2019, 3435901. [Google Scholar] [CrossRef]
- AlMuraikhi, N.; Ali, D.; Vishnubalaji, R.; Manikandan, M.; Atteya, M.; Siyal, A.; Alfayez, M.; Aldahmash, A.; Kassem, M.; Alajez, N.M. Notch Signaling Inhibition by LY411575 Attenuates Osteoblast Differentiation and Decreased Ectopic Bone Formation Capacity of Human Skeletal (Mesenchymal) Stem Cells. Stem Cells Int. 2019, 2019, 3041262. [Google Scholar] [CrossRef]
- AlMuraikhi, N.; Alaskar, H.; Binhamdan, S.; Alotaibi, A.; Kassem, M.; Alfayez, M. JAK2 Inhibition by Fedratinib Reduces Osteoblast Differentiation and Mineralisation of Human Mesenchymal Stem Cells. Molecules 2021, 26, 606. [Google Scholar] [CrossRef]
- AlMuraikhi, N.; Binhamdan, S.; Alaskar, H.; Alotaibi, A.; Tareen, S.; Muthurangan, M.; Alfayez, M. Inhibition of GSK-3β Enhances Osteoblast Differentiation of Human Mesenchymal Stem Cells through Wnt Signalling Overexpressing Runx2. Int. J. Mol. Sci. 2023, 24, 7164. [Google Scholar] [CrossRef] [PubMed]
- Almuraikhi, N. Inhibition of TGF-β type I receptor by SB505124 down-regulates osteoblast differentiation and mineralization of human mesenchymal stem cells. Cell Biochem. Funct. 2023, 41, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β receptors: In and beyond TGF-β signaling. Cell Signal 2018, 52, 112–120. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, Z.; Li, J.; Chen, X.; Ping, Y.; Liu, S.; Shi, X.; Li, L.; Wang, L.; Huang, L.; et al. Transforming growth factor-beta1 promotes the migration and invasion of sphere-forming stem-like cell subpopulations in esophageal cancer. Exp. Cell Res. 2015, 336, 141–149. [Google Scholar] [CrossRef]
- de Kroon, L.M.; Narcisi, R.; Blaney Davidson, E.N.; Cleary, M.A.; van Beuningen, H.M.; Koevoet, W.J.; van Osch, G.J.; van der Kraan, P.M. Activin Receptor-Like Kinase Receptors ALK5 and ALK1 Are Both Required for TGFβ-Induced Chondrogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0146124. [Google Scholar] [CrossRef]
- Heo, J.Y.; Do, J.Y.; Lho, Y.; Kim, A.Y.; Kim, S.W.; Kang, S.H. TGF-β1 Receptor Inhibitor SB525334 Attenuates the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells via the TGF-β1 Signaling Pathway. Biomedicines 2021, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Lian, N.; Lin, T.; Liu, W.; Wang, W.; Li, L.; Sun, S.; Nyman, J.S.; Yang, X. Transforming growth factor β suppresses osteoblast differentiation via the vimentin activating transcription factor 4 (ATF4) axis. J. Biol. Chem. 2012, 287, 35975–35984. [Google Scholar] [CrossRef] [PubMed]
- Alliston, T.; Choy, L.; Ducy, P.; Karsenty, G.; Derynck, R. TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001, 20, 2254–2272. [Google Scholar] [CrossRef]
- Centrella, M.; Horowitz, M.C.; Wozney, J.M.; McCarthy, T.L. Transforming growth factor-β gene family members and bone. Endocr. Rev. 1994, 15, 27–39. [Google Scholar] [CrossRef]
- Robey, P.G.; Young, M.F.; Flanders, K.C.; Roche, N.S.; Kondaiah, P.; Reddi, A.H.; Termine, J.D.; Sporn, M.B.; Roberts, A.B. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J. Cell Biol. 1987, 105, 457–463. [Google Scholar] [CrossRef]
- Gungor, M.Z.; Uysal, M.; Senturk, S. The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications. Cancers 2022, 14, 940. [Google Scholar] [CrossRef]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Haack-Sorensen, M.; Burns, J.S.; Elsnab, B.; Jakob, F.; Hokland, P.; Kassem, M. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem. Biophys. Res. Commun. 2005, 326, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Elango, R.; Al-Toub, M.; Manikandan, M.; Al-Rikabi, A.; Harkness, L.; Ditzel, N.; Atteya, M.; Hamam, R.; Alfayez, M.; et al. Neoplastic Transformation of Human Mesenchymal Stromal Cells Mediated via LIN28B. Sci. Rep. 2019, 9, 8101. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, L.; Qin, W.H.; Zhou, Y.Y.; Chen, B.; Zhao, X.Q.; Zhao, H.L.; Mi, E.; Mi, E.; Wang, Q.M.; Ning, J.L. Transforming growth factor β plays an important role in enhancing wound healing by topical application of Povidone-iodine. Sci. Rep. 2017, 7, 991. [Google Scholar] [CrossRef]
- Algazlan, A.S.; Almuraikhi, N.; Muthurangan, M.; Balto, H.; Alsalleeh, F. Silver Nanoparticles Alone or in Combination with Calcium Hydroxide Modulate the Viability, Attachment, Migration, and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2023, 24, 702. [Google Scholar] [CrossRef]
- Wang, L.; Ko, C.Y.; Meyers, E.E.; Pedroja, B.S.; Pelaez, N.; Bernstein, A.M. Concentration-dependent effects of transforming growth factor β1 on corneal wound healing. Mol. Vis. 2011, 17, 2835–2846. [Google Scholar] [PubMed]
- Shi, Y.; Massague, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel therapies emerging in oncology to target the TGF-β pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef]
- Fink, S.P.; Mikkola, D.; Willson, J.K.V.; Markowitz, S. TGF-β-induced nuclear localization of Smad2 and Smad3 in Smad4 null cancer cell lines. Oncogene 2003, 22, 1317–1323. [Google Scholar] [CrossRef]
- Massagué, J.; Wotton, D. Transcriptional control by the TGF-β/Smad signaling system. Embo J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Li, Y.C.; Luo, W.X.; Yang, W.D. Nuclear Transport and Accumulation of Smad Proteins Studied by Single-Molecule Microscopy. Biophys. J. 2018, 114, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Miyazono, K. TGFβ signalling: A complex web in cancer progression. Nat. Rev. Cancer 2010, 10, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGFβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Massague, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Massague, J. Roles of TGFβ in metastasis. Cell Res. 2009, 19, 89–102. [Google Scholar] [CrossRef]
- Bierie, B.; Moses, H.L. TGF-β and cancer. Cytokine Growth Factor. Rev. 2006, 17, 29–40. [Google Scholar] [CrossRef]
- Yang, L.; Moses, H.L. Transforming growth factor β: Tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008, 68, 9107–9111. [Google Scholar] [CrossRef]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordon-Cardo, C.; Guise, T.A.; Massague, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Grafe, I.; Yang, T.; Alexander, S.; Homan, E.P.; Lietman, C.; Jiang, M.M.; Bertin, T.; Munivez, E.; Chen, Y.; Dawson, B.; et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat. Med. 2014, 20, 670–675. [Google Scholar] [CrossRef]
- van der Kraan, P.M. The changing role of TGFβ in healthy, ageing and osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Pallero, M.A.; Lei, W.; Hong, H.; Yang, Y.; Suto, M.J.; Murphy-Ullrich, J.E. Inhibition of Transforming Growth Factor-beta Activation Diminishes Tumor Progression and Osteolytic Bone Disease in Mouse Models of Multiple Myeloma. Am. J. Pathol. 2016, 186, 678–690. [Google Scholar] [CrossRef]
- Takeuchi, K.; Abe, M.; Hiasa, M.; Oda, A.; Amou, H.; Kido, S.; Harada, T.; Tanaka, O.; Miki, H.; Nakamura, S.; et al. Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth. PLoS ONE 2010, 5, e9870. [Google Scholar] [CrossRef]
- Grygielko, E.T.; Martin, W.M.; Tweed, C.; Thornton, P.; Harling, J.; Brooks, D.P.; Laping, N.J. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-β type I receptor kinase in puromycin-induced nephritis. J. Pharmacol. Exp. Ther. 2005, 313, 943–951. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Jung, N.; Park, J.W.; Park, H.Y.; Jung, S.C. Epithelial—Mesenchymal Transition in Kidney Tubular Epithelial Cells Induced by Globotriaosylsphingosine and Globotriaosylceramide. PLoS ONE 2015, 10, e0136442. [Google Scholar] [CrossRef]
- Higashiyama, H.; Yoshimoto, D.; Kaise, T.; Matsubara, S.; Fujiwara, M.; Kikkawa, H.; Asano, S.; Kinoshita, M. Inhibition of activin receptor-like kinase 5 attenuates Bleomycin-induced pulmonary fibrosis. Exp. Mol. Pathol. 2007, 83, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Q.; Liao, Z.Y.; Wang, L.H.; Liao, Y.; Xu, X.D.; Liu, P.; Wang, X.; Hou, J.; Jiang, H.J.; Wu, X.W.; et al. A combination of pirfenidone and TGF-β inhibition mitigates cystic echinococcosis-associated hepatic injury. Parasitology 2021, 148, 767–778. [Google Scholar] [CrossRef]
- Wen, H.Y.; Qian, M.; He, J.; Li, M.H.; Yu, Q.; Leng, Z.W. Inhibiting of self-renewal, migration and invasion of ovarian cancer stem cells by blocking TGF-β pathway. PLoS ONE 2020, 15, e0230230. [Google Scholar] [CrossRef]
- Liu, S.L.; Cao, S.G.; Li, Y.; Sun, B.; Chen, D.; Wang, D.S.; Zhou, Y.B. Pancreatic stellate cells facilitate pancreatic cancer cell viability and invasion. Oncol. Lett. 2019, 17, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, R.Z.; Lu, D.; Zhuo, J.Y.; Zhang, X.Y.; Wang, K.; Wei, X.Y.; Wei, Q.; Wang, W.; Xie, H.Y.; Zhou, L.; et al. CR6-interacting factor 1 inhibits invasiveness by suppressing TGF-β-mediated epithelial-mesenchymal transition in hepatocellular carcinoma. Oncotarget 2017, 8, 94759–94768. [Google Scholar] [CrossRef][Green Version]
- DaCosta Byfield, S.; Major, C.; Laping, N.J.; Roberts, A.B. SB-505124 is a selective inhibitor of transforming growth factor-β type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2004, 65, 744–752. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Hough, C.D.; Cho, K.R.; Zonderman, A.B.; Schwartz, D.R.; Morin, P.J. Coordinately up-regulated genes in ovarian cancer. Cancer Res. 2001, 61, 3869–3876. [Google Scholar] [PubMed]

| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| ACTB | 5′AGCCATGTACGTTGCTA | 5′AGTCCGCCTAGAAGCA |
| ALPL | 5′GGAACTCCTGACCCTTGACC3′ | 5′TCCTGTTCAGCTCGTACTGC3′ |
| OC | GGCAGCGAGGTAGTGAAGAG | CTCACACACCTCCCTCCTG |
| ON | 5′GAGGAAACCGAAGAGGAGG3′ | 5′GGGGTGTTGTTCTCATCCAG3′ |
| RUNX2 | 5′GTAGATGGACCTCGGGAACC3′ | 5′GAGGCGGTCAGAGAACAAAC3′ |
| OP | GGTGATGTCCTCGTCTGTA | CCAAGTAAGTCCAACGAAAG |
| BSP | GCAGTAGTGACTCATCCGAAGAA | GCCTCAGAGTCTTCATCTTCATTC |
| COL1A2 | GGTCTTCCAGGCCTCTCC | ACCCTTGGCACCAGTAAGG |
| SMAD2 | TGCTCTGAAATTTGGGGACTGA | ACGACCATCAAGAGACCTGG |
| SMAD3 | TAATTTATTGCCGCCGCTCG | GGCCATCCAGGGACTCAAAC |
| SMAD4 | ATTTGCCTCACCACCAAAAC | AGCAGGATGATTGGAAATGG |
| JUN | CAGCCAGGTCGGCAGTATAG | GGGACTCTGCCACTTGTCTC |
| RAF1 | CCTGGCTCCCTCAGGTTTAAG | TGATCGTCTTCCAAGCTCCC |
| ATF3 | GTGAGTCCTCGGTGCTCG | GCATCATTTTGCTCCAGGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuraikhi, N.; Alkhamees, L.; Tareen, S.; Alshammari, H.; Muthurangan, M. Dose-Dependent Effects of TGF-β Inhibition on Osteoblast Differentiation and Wound Healing. Curr. Issues Mol. Biol. 2025, 47, 360. https://doi.org/10.3390/cimb47050360
Almuraikhi N, Alkhamees L, Tareen S, Alshammari H, Muthurangan M. Dose-Dependent Effects of TGF-β Inhibition on Osteoblast Differentiation and Wound Healing. Current Issues in Molecular Biology. 2025; 47(5):360. https://doi.org/10.3390/cimb47050360
Chicago/Turabian StyleAlmuraikhi, Nihal, Latifa Alkhamees, Sumaiya Tareen, Hessah Alshammari, and Manikandan Muthurangan. 2025. "Dose-Dependent Effects of TGF-β Inhibition on Osteoblast Differentiation and Wound Healing" Current Issues in Molecular Biology 47, no. 5: 360. https://doi.org/10.3390/cimb47050360
APA StyleAlmuraikhi, N., Alkhamees, L., Tareen, S., Alshammari, H., & Muthurangan, M. (2025). Dose-Dependent Effects of TGF-β Inhibition on Osteoblast Differentiation and Wound Healing. Current Issues in Molecular Biology, 47(5), 360. https://doi.org/10.3390/cimb47050360






