Evaluation of the Expression of IDO and PTEN in Human Kidney Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Kidney Tissue Samples and Cell Lines
2.2. RNA Isolation
2.3. Reverse Transcription PCR
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
2.6. Western Blotting of Human Tissue Samples and Cell Lines
2.7. Immunocytochemistry of Interferon-α2 Treated Cell Lines
3. Results
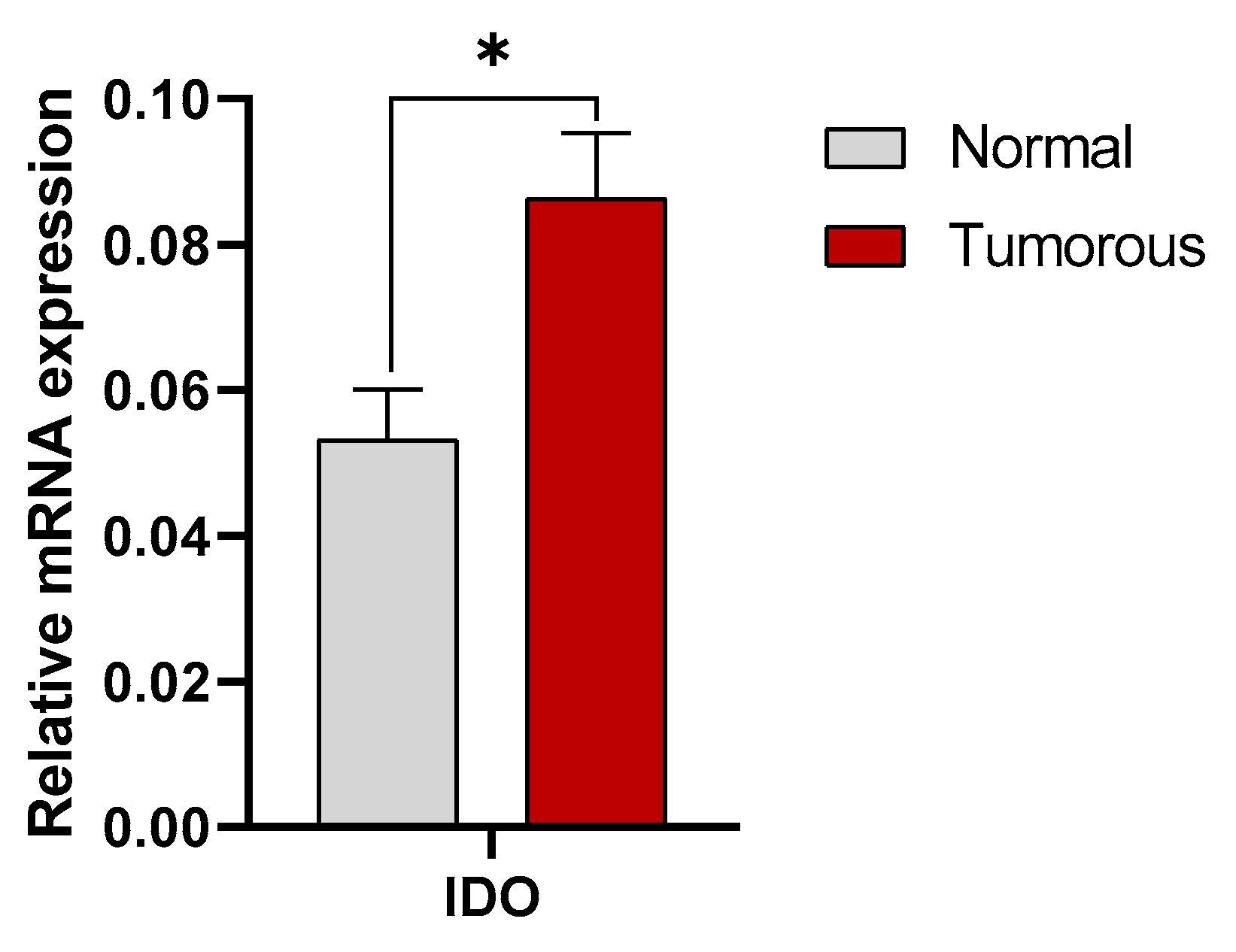
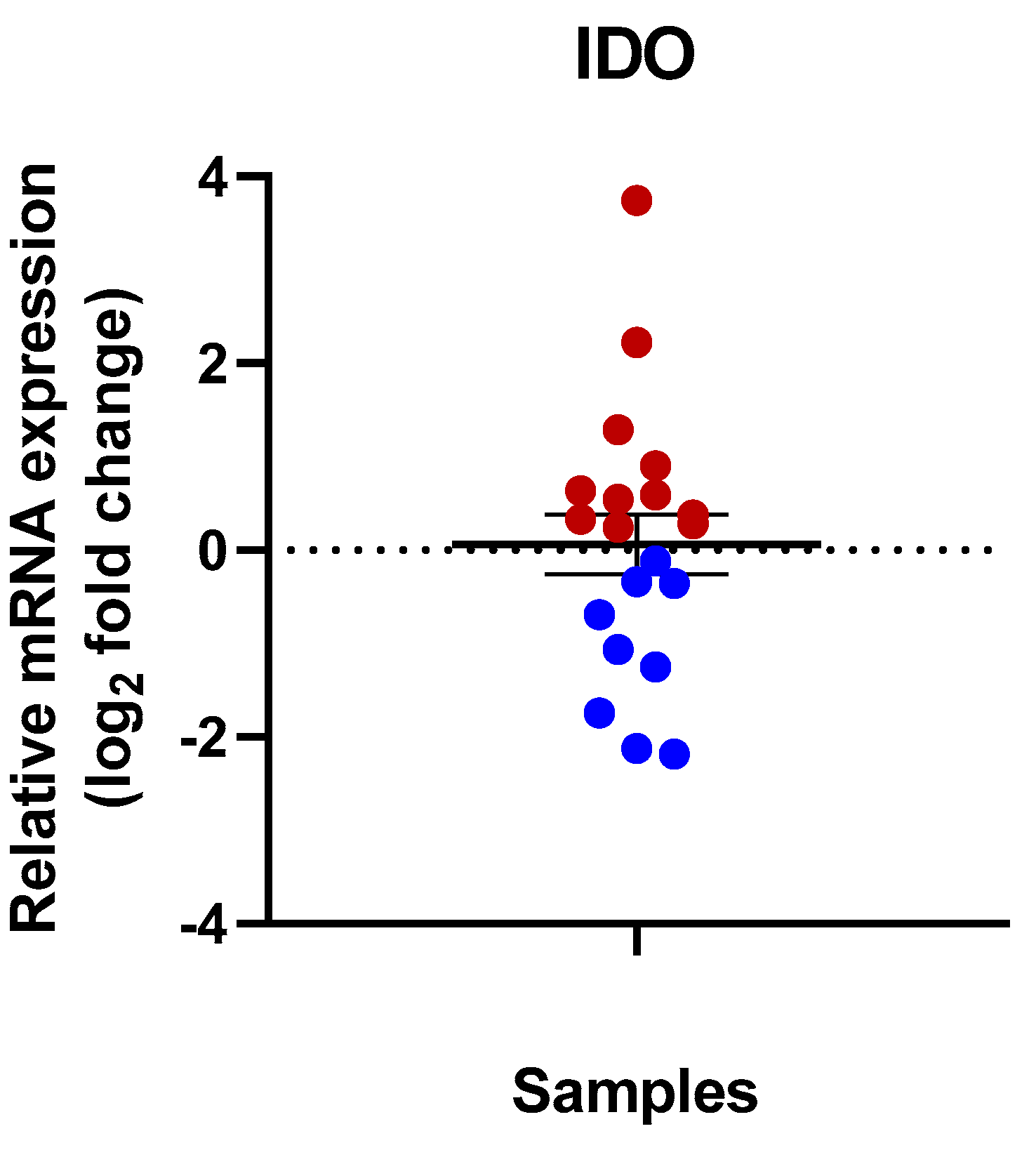
3.1. Expression of IDO in Human Kidney Tissues
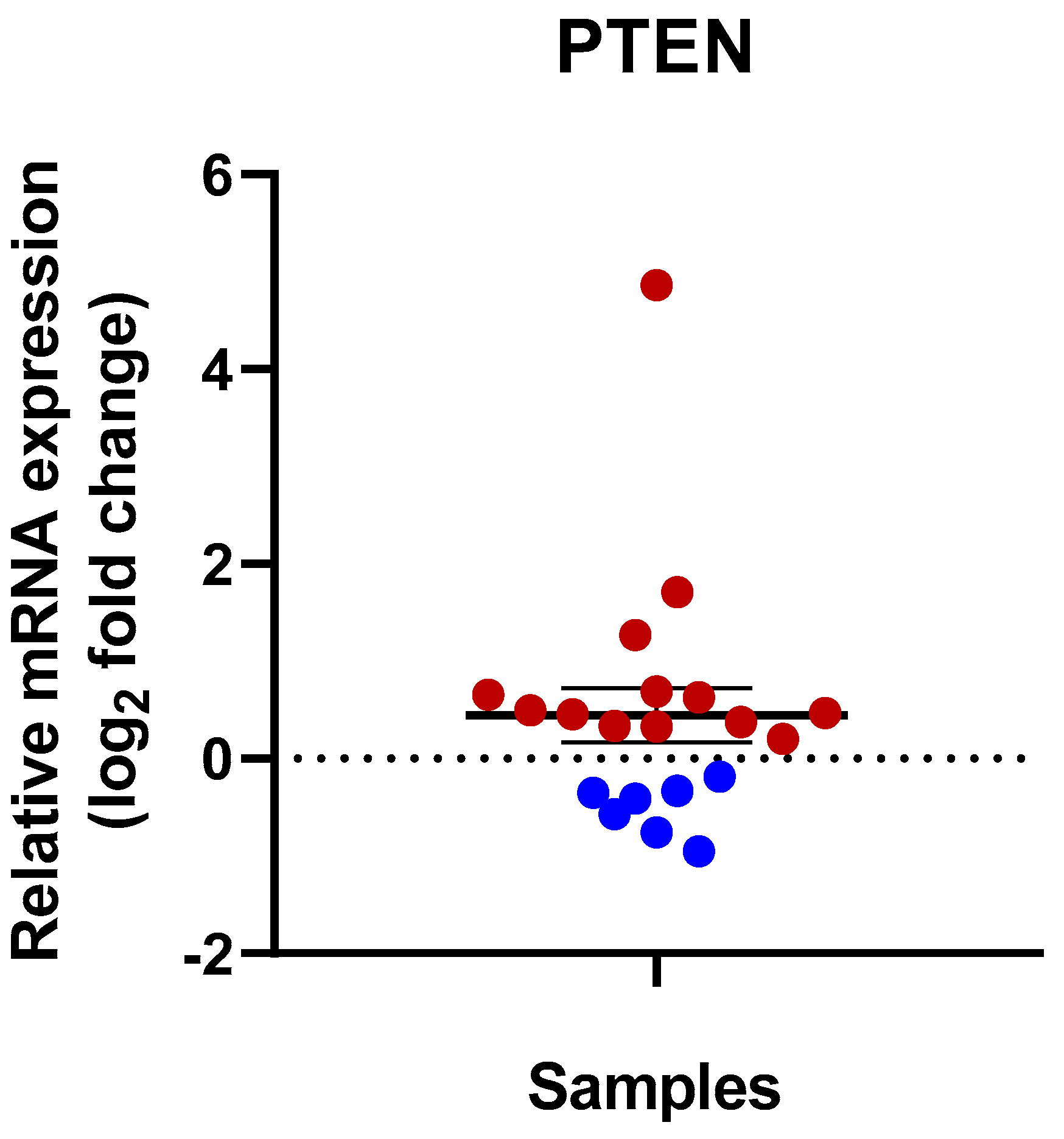
3.2. Expression of PTEN in Human Kidney Tissues
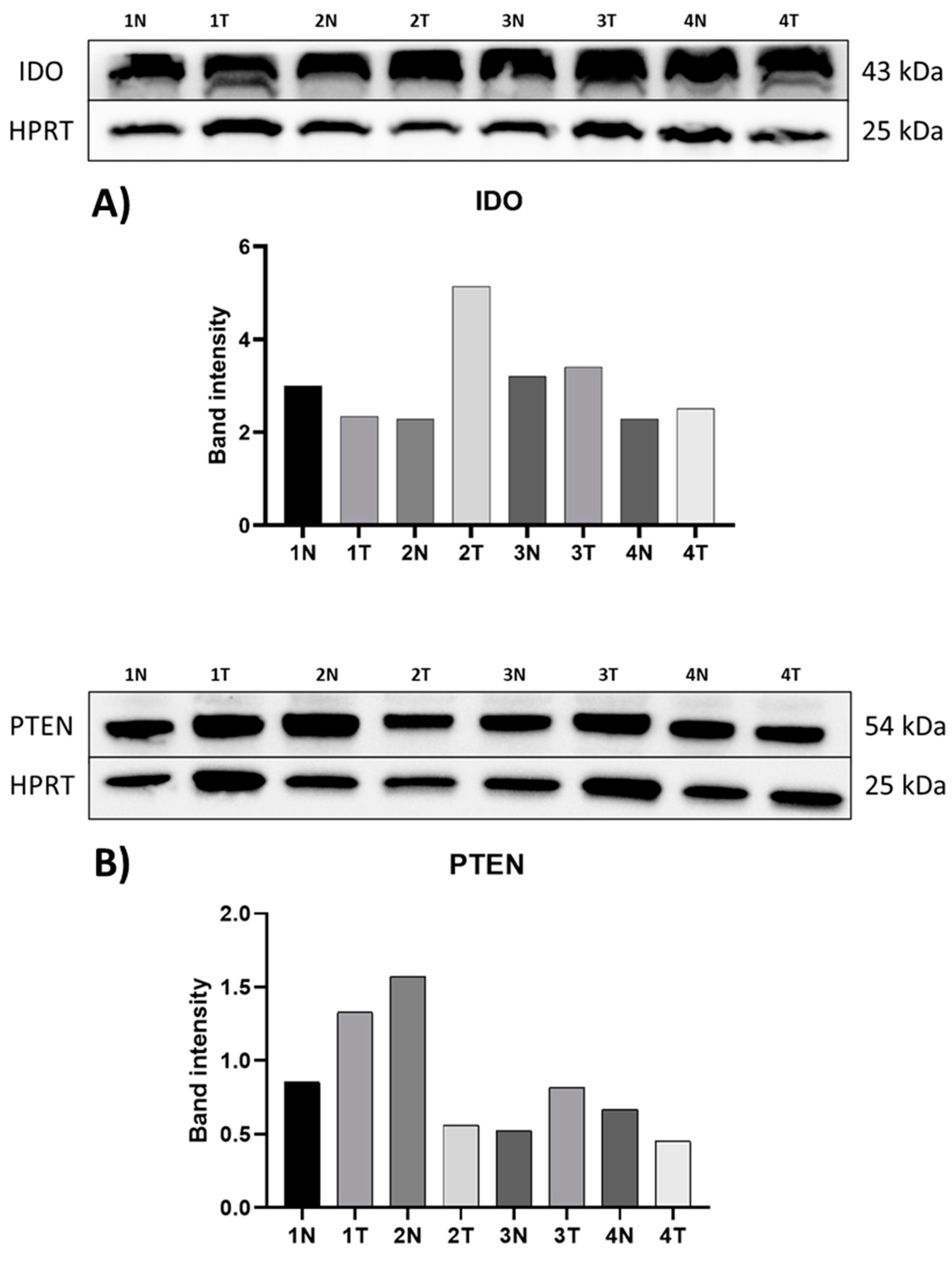
3.3. Protein Expression of IDO and PTEN
3.4. Detection of IDO in Control (Untreated) and IFN-α2-Treated CAKI-2 and A-498 Human Kidney Cancer Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IDO | Indoleamine 2,3-dioxygenase |
| PTEN | Phosphatase and tensin homolog |
| IFN-α2 | Interferon alpha-2 |
| RCC | Renal cell carcinoma |
| ccRCC | Clear-cell renal cell carcinoma |
| CA9 | Carbonic anhydrase 9 |
| MUC1 | The mucin-1 |
| miRNA | MicroRNAs (miRNA) |
| IDO2 | Indoleamine 2,3-dioxygenase-2 |
| TDO | Tryptophan-2,3-dioxygenase |
| IFN-γ | Interferon gamma |
| ATCC | American Type Culture Collection |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| BCA | Bicinchoninic Acid |
| SDA-PAGE | Dodecyl sulfate-polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene fluoride |
| HRP | Horseradish peroxidase |
| ROI | Region of Interest |
References
- Ljungberg, B.; Campbell, S.C.; Choi, H.Y.; Jacqmin, D.; Lee, J.E.; Weikert, S.; Kiemeney, L.A. The epidemiology of renal cell carcinoma. Eur. Urol. 2011, 60, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Wang, J.; Xi, J.; Zhang, H.; Li, J.; Xia, Y.; Xi, R.; Xi, Z. Somatic mutations in renal cell carcinomas from Chinese patients revealed by targeted gene panel sequencing and their associations with prognosis and PD-L1 expression. Cancer Commun. 2019, 39, 37. [Google Scholar] [CrossRef]
- Larkin, J.M.; Chowdhury, S.; Gore, M.E. Drug insight: Advances in renal cell carcinoma and the role of targeted therapies. Nat. Clin. Pract. Oncol. 2007, 4, 470–479. [Google Scholar] [CrossRef]
- Szegedi, K.; Szabó, Z.; Kállai, J.; Király, J.; Szabó, E.; Bereczky, Z.; Juhász, É.; Dezső, B.; Szász, C.; Zsebik, B.; et al. Potential Role of VHL, PTEN, and BAP1 Mutations in Renal Tumors. J. Clin. Med. 2023, 12, 4538. [Google Scholar] [CrossRef]
- Napolitano, L.; Manfredi, C.; Cirillo, L.; Fusco, G.M.; Passaro, F.; Abate, M.; La Rocca, R.; Mastrangelo, F.; Spirito, L.; Pandolfo, S.D.; et al. Cytoreductive Nephrectomy and Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives. Medicina 2023, 59, 767. [Google Scholar] [CrossRef]
- Yagoda, A.; Abi-Rached, B.; Petrylak, D. Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin. Oncol. 1995, 22, 42–60. [Google Scholar]
- Dutcher, J.P.; Flippot, R.; Fallah, J.; Escudier, B. On the Shoulders of Giants: The Evolution of Renal Cell Carcinoma Treatment-Cytokines, Targeted Therapy, and Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 418–435. [Google Scholar] [CrossRef]
- Anker, J.; Miller, J.; Taylor, N.; Kyprianou, N.; Tsao, C.K. From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma. Cells 2021, 10, 3231. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Regan, M.M.; Jegede, O.A.; Mantia, C.M.; Powles, T.; Werner, L.; Motzer, R.J.; Tannir, N.M.; Lee, C.H.; Tomita, Y.; Voss, M.H.; et al. Treatment-free Survival after Immune Checkpoint Inhibitor Therapy versus Targeted Therapy for Advanced Renal Cell Carcinoma: 42-Month Results of the CheckMate 214 Trial. Clin. Cancer Res. 2021, 27, 6687–6695. [Google Scholar] [CrossRef]
- Li, F.; Aljahdali, I.A.M.; Zhang, R.; Nastiuk, K.L.; Krolewski, J.J.; Ling, X. Kidney cancer biomarkers and targets for therapeutics: Survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 254. [Google Scholar] [CrossRef]
- Braun, D.A.; Bakouny, Z.; Hirsch, L.; Flippot, R.; Van Allen, E.M.; Wu, C.J.; Choueiri, T.K. Beyond conventional immune-checkpoint inhibition—Novel immunotherapies for renal cell carcinoma. Nat. Rev. Clin. Oncol. 2021, 18, 199–214. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Hu, X.; Chen, R.; Wei, Q.; Xu, X. The Landscape of Alpha Fetoprotein In Hepatocellular Carcinoma: Where Are We? Int. J. Biol. Sci. 2022, 18, 536–551. [Google Scholar] [CrossRef]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef]
- Peters, I.; Dubrowinskaja, N.; Abbas, M.; Seidel, C.; Kogosov, M.; Scherer, R.; Gebauer, K.; Merseburger, A.S.; Kuczyk, M.A.; Grünwald, V.; et al. DNA methylation biomarkers predict progression-free and overall survival of metastatic renal cell cancer (mRCC) treated with antiangiogenic therapies. PLoS ONE 2014, 9, e91440. [Google Scholar] [CrossRef] [PubMed]
- Milella, M.; Rutigliano, M.; Lasorsa, F.; Ferro, M.; Bianchi, R.; Fallara, G.; Crocetto, F.; Pandolfo, S.D.; Barone, B.; d’Amati, A.; et al. The Role of MUC1 in Renal Cell Carcinoma. Biomolecules 2024, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.T.; Arbitrio, M.; Caracciolo, D.; Cordua, A.; Cuomo, O.; Grillone, K.; Riillo, C.; Caridà, G.; Scionti, F.; Labanca, C.; et al. miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: A systematic review. Mol. Ther. Nucleic Acids 2022, 27, 1191–1224. [Google Scholar] [CrossRef] [PubMed]
- Meireson, A.; Devos, M.; Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Kim, R.H.; Wang, X.; Evans, A.J.; Campbell, S.C.; Nguyen, J.K.; Farncombe, K.M.; Eng, C. Early-onset renal cell carcinoma in PTEN harmatoma tumour syndrome. npj Genom. Med. 2020, 5, 40. [Google Scholar] [CrossRef]
- Belladonna, M.L.; Orabona, C.; Grohmann, U.; Puccetti, P. TGF-beta and kynurenines as the key to infectious tolerance. Trends Mol. Med. 2009, 15, 41–49. [Google Scholar] [CrossRef]
- Ozaki, Y.; Edelstein, M.P.; Duch, D.S. Induction of indoleamine 2,3-dioxygenase: A mechanism of the antitumor activity of interferon gamma. Proc. Natl. Acad. Sci. USA 1988, 85, 1242–1246. [Google Scholar] [CrossRef]
- Dobos, N.; de Vries, E.F.; Kema, I.P.; Patas, K.; Prins, M.; Nijholt, I.M.; Dierckx, R.A.; Korf, J.; den Boer, J.A.; Luiten, P.G.; et al. The role of indoleamine 2,3-dioxygenase in a mouse model of neuroinflammation-induced depression. J. Alzheimer’s Dis. 2012, 28, 905–915. [Google Scholar] [CrossRef]
- Szántó, S.; Koreny, T.; Mikecz, K.; Glant, T.T.; Szekanecz, Z.; Varga, J. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res. Ther. 2007, 9, R50. [Google Scholar] [CrossRef]
- Meireson, A.; Chevolet, I.; Hulstaert, E.; Ferdinande, L.; Ost, P.; Geboes, K.; De Man, M.; Van de Putte, D.; Verset, L.; Kruse, V.; et al. Peritumoral endothelial indoleamine 2, 3-dioxygenase expression is an early independent marker of disease relapse in colorectal cancer and is influenced by DNA mismatch repair profile. Oncotarget 2018, 9, 25216–25224. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, P.H.; Hu, C.C.; Nien, H.C.; Wang, J.T.; Sheu, J.C.; Chow, L.P. Aberrant Upregulation of Indoleamine 2,3-Dioxygenase 1 Promotes Proliferation and Metastasis of Hepatocellular Carcinoma Cells via Coordinated Activation of AhR and β-Catenin Signaling. Int. J. Mol. Sci. 2021, 22, 11661. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, R.; Weiler, C.; Spring, O.; Eder, M.; Buchner, A.; Popp, T.; Castro, M.; Kammerer, R.; Takikawa, O.; Hatz, R.A.; et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin. Cancer Res. 2007, 13, 6993–7002. [Google Scholar] [CrossRef] [PubMed]
- Seeber, A.; Klinglmair, G.; Fritz, J.; Steinkohl, F.; Zimmer, K.C.; Aigner, F.; Horninger, W.; Gastl, G.; Zelger, B.; Brunner, A.; et al. High IDO-1 expression in tumor endothelial cells is associated with response to immunotherapy in metastatic renal cell carcinoma. Cancer Sci. 2018, 109, 1583–1591. [Google Scholar] [CrossRef]
- Pallotta, M.T.; Rossini, S.; Suvieri, C.; Coletti, A.; Orabona, C.; Macchiarulo, A.; Volpi, C.; Grohmann, U. Indoleamine 2,3-dioxygenase 1 (IDO1): An up-to-date overview of an eclectic immunoregulatory enzyme. FEBS J. 2022, 289, 6099–6118. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, X. PTEN: A default gate-keeping tumor suppressor with a versatile tail. Cell Res. 2008, 18, 807–816. [Google Scholar] [CrossRef]
- Bononi, A.; Pinton, P. Study of PTEN subcellular localization. Methods 2015, 77–78, 92–103. [Google Scholar] [CrossRef]
- Ortega-Molina, A.; Serrano, M. PTEN in cancer, metabolism, and aging. Trends Endocrinol. Metab. 2013, 24, 184–189. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Gao, Y.; Chen, L.; Gu, L.; Chen, J.; Lyu, X.; Zhang, Y.; Zhang, X. Phosphatase and tensin homolog (PTEN) expression on oncologic outcome in renal cell carcinoma: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0179437. [Google Scholar] [CrossRef]
- Kim, H.L.; Seligson, D.; Liu, X.; Janzen, N.; Bui, M.H.; Yu, H.; Shi, T.; Figlin, R.A.; Horvath, S.; Belldegrun, A.S. Using protein expressions to predict survival in clear cell renal carcinoma. Clin. Cancer Res. 2004, 10, 5464–5471. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Morgan, M.; Gunaratne, P.H.; Wheeler, D.A.; Gibbs, R.A.; Gordon Robertson, A.; Chu, A.; Beroukhim, R.; Cibulskis, K.; Signoretti, S.; et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar]
- Zhu, C.; Wei, J.; Tian, X.; Li, Y.; Li, X. Prognostic role of PPAR-γ and PTEN in the renal cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 12668–12677. [Google Scholar] [PubMed]
- Sekino, Y.; Hagura, T.; Han, X.; Babasaki, T.; Goto, K.; Inoue, S.; Hayashi, T.; Teishima, J.; Shigeta, M.; Taniyama, D.; et al. PTEN Is Involved in Sunitinib and Sorafenib Resistance in Renal Cell Carcinoma. Anticancer Res. 2020, 40, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bedke, J.; Albiges, L.; Volpe, A.; Giles, R.H.; Hora, M.; Marconi, L.; Klatte, T.; Abu-Ghanem, Y.; Dabestani, S.; et al. A Renewal of the TNM Staging System for Patients with Renal Cancer To Comply with Current Decision-making: Proposal from the European Association of Urology Guidelines Panel. Eur. Urol. 2023, 83, 3–5. [Google Scholar] [CrossRef]
- Trott, J.F.; Kim, J.; Abu Aboud, O.; Wettersten, H.; Stewart, B.; Berryhill, G.; Uzal, F.; Hovey, R.C.; Chen, C.H.; Anderson, K.; et al. Inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget 2016, 7, 66540–66557. [Google Scholar] [CrossRef] [PubMed]
- Brandacher, G.; Perathoner, A.; Ladurner, R.; Schneeberger, S.; Obrist, P.; Winkler, C.; Werner, E.R.; Werner-Felmayer, G.; Weiss, H.G.; Göbel, G.; et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: Effect on tumor-infiltrating T cells. Clin. Cancer Res. 2006, 12, 1144–1151. [Google Scholar] [CrossRef]
- Odunsi, K.; Qian, F.; Lugade, A.A.; Yu, H.; Geller, M.A.; Fling, S.P.; Kaiser, J.C.; Lacroix, A.M.; D’Amico, L.; Ramchurren, N.; et al. Metabolic adaptation of ovarian tumors in patients treated with an IDO1 inhibitor constrains antitumor immune responses. Sci. Transl. Med. 2022, 14, eabg8402. [Google Scholar] [CrossRef]
- Hornyák, L.; Dobos, N.; Koncz, G.; Karányi, Z.; Páll, D.; Szabó, Z.; Halmos, G.; Székvölgyi, L. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front. Immunol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Shin Lee, J.; Seok Kim, H.; Bok Kim, Y.; Cheol Lee, M.; Soo Park, C. Expression of PTEN in renal cell carcinoma and its relation to tumor behavior and growth. J. Surg. Oncol. 2003, 84, 166–172. [Google Scholar] [CrossRef]
- Antonelli, G.; Scagnolari, C.; Moschella, F.; Proietti, E. Twenty-five years of type I interferon-based treatment: A critical analysis of its therapeutic use. Cytokine Growth Factor Rev. 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Flanigan, R.C.; Salmon, S.E.; Blumenstein, B.A.; Bearman, S.I.; Roy, V.; McGrath, P.C.; Caton, J.R., Jr.; Munshi, N.; Crawford, E.D. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 2001, 345, 1655–1659. [Google Scholar] [CrossRef]
- Dai, X.; Zhu, B.T. Indoleamine 2,3-dioxygenase tissue distribution and cellular localization in mice: Implications for its biological functions. J. Histochem. Cytochem. 2010, 58, 17–28. [Google Scholar] [CrossRef]
- Recht, M.; Borden, E.C.; Knight, E., Jr. A human 15-kDa IFN-induced protein induces the secretion of IFN-gamma. J. Immunol. 1991, 147, 2617–2623. [Google Scholar] [CrossRef]
- Yuan, F.; Liu, Y.; Fu, X.; Chen, J. Indoleamine-pyrrole 2,3-dioxygenase might be a prognostic biomarker for patients with renal cell carcinoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2012, 37, 649–655. [Google Scholar] [PubMed]
- Vidotto, T.; Saggioro, F.P.; Jamaspishvili, T.; Chesca, D.L.; Picanço de Albuquerque, C.G.; Reis, R.B.; Graham, C.H.; Berman, D.M.; Siemens, D.R.; Squire, J.A.; et al. PTEN-deficient prostate cancer is associated with an immunosuppressive tumor microenvironment mediated by increased expression of IDO1 and infiltrating FoxP3+ T regulatory cells. Prostate 2019, 79, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Sim, W.; Kim, J.H. Differential Dependency of Human Pancreatic Cancer Cells on Targeting PTEN via PLK 1 Expression. Cancers 2020, 12, 277. [Google Scholar] [CrossRef]
- Xu, X.; Tang, Y.Y.; Liang, X.; Luo, W.; Jiang, D.M.; Chen, J. PTEN suppresses renal cell carcinoma proliferation and migration via inhibition of the PI3K/AKT pathway. World J. Surg. Oncol. 2025, 23, 42. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, F.; Chen, X.; Liu, Z.; Ouyang, Y.; Zhao, W.; Yu, D. Downregulation of miR-221/222 by a microRNA sponge promotes apoptosis in oral squamous cell carcinoma cells through upregulation of PTEN. Oncol. Lett. 2016, 12, 4419–4426. [Google Scholar] [CrossRef]
- Alves, Â.; Medeiros, R.; Teixeira, A.L.; Dias, F. Decoding PTEN regulation in clear cell renal cell carcinoma: Pathway for biomarker discovery and therapeutic insights. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189165. [Google Scholar] [CrossRef]
- Ji, Y.; Zheng, M.; Ye, S.; Chen, J.; Chen, Y. PTEN and Ki67 expression is associated with clinicopathologic features of non-small cell lung cancer. J. Biomed. Res. 2014, 28, 462–467. [Google Scholar]



| Number | Gender | Age | Histology | Grade | TNM |
|---|---|---|---|---|---|
| 1 | Female | 52 | cc. RCC | 3 | pT1b |
| 2 | Female | 79 | cc. RCC | 2 | pT3a pN0 |
| 3 | Female | 59 | cc. RCC | 3 | pT1a |
| 4 | Male | 67 | angioleiomyolipoma | 0 | 0 |
| 5 | Female | 63 | cc. RCC | 2 | pT1a |
| 6 | Male | 60 | Papillary RCC | 2 | pT1a |
| 7 | Female | 83 | cc. RCC | 2 | pT1a |
| 8 | Female | 67 | cc. RCC | 1 | pT1b |
| 9 | Male | 78 | cc. RCC | 2 | pT1b |
| 10 | Female | 49 | oncocytoma | 0 | 0 |
| 11 | Female | 95 | oncocytoma | 0 | 0 |
| 12 | Male | 67 | cc. RCC | 1 | pT1b |
| 13 | Female | 58 | cc. RCC | 1 | pT1b |
| 14 | Female | 51 | cc. RCC | 3 | pT3a pN1 |
| 15 | Male | 65 | cc. RCC | 3 | pT3a pN1 |
| 16 | Male | 68 | Papillary RCC | 2 | pT1a |
| 17 | Female | 76 | cc. RCC | 2 | pT1a |
| 18 | Female | 57 | cc. RCC | 2 | pT1a |
| 19 | Female | 65 | cc. RCC | 3 | pT1b |
| 20 | Male | 83 | cc. RCC | 2 | pT1b |
| Number | Gender | Age | Relative IDO Expression (ΔΔCp) | Relative PTEN Expression (ΔΔCp) | ||
|---|---|---|---|---|---|---|
| 1 | Female | 52 | Up | 1.45 | Up * | 3.25 |
| 2 | Female | 79 | Down | 0.48 | Down | 0.81 |
| 3 | Female | 59 | Up | 1.18 | Up | 1.04 |
| 4 | Male | 67 | Up | 1.26 | Up | 1.32 |
| 5 | Female | 63 | Up | 1.51 | Up | 1.22 |
| 6 | Male | 60 | Up | 1.87 | Up | 1.61 |
| 7 | Female | 83 | Up * | 13.38 | Up * | 31.79 |
| 8 | Female | 67 | Down | 0.62 | Down | 0.59 |
| 9 | Male | 78 | Up | 1.56 | Up | 1.29 |
| 10 | Female | 49 | Down | 0.78 | Up | 1.41 |
| 11 | Female | 95 | Up | 1.21 | Down | 0.88 |
| 12 | Male | 67 | Down | 0.23 | Down | 0.68 |
| 13 | Female | 58 | Down | 0.42 | Down | 0.75 |
| 14 | Female | 51 | Down | 0.30 | Up | 1.25 |
| 15 | Male | 65 | Up * | 4.65 | Up * | 2.94 |
| 16 | Male | 68 | Down | 0.22 | Down | 0.68 |
| 17 | Female | 76 | Up * | 2.44 | Up | 1.12 |
| 18 | Female | 57 | Down | 0.92 | Up | 1.18 |
| 19 | Female | 65 | Down | 0.79 | Down | 0.97 |
| 20 | Male | 83 | Up | 1.30 | Up | 1.39 |
| Mean ΔΔCp | 1.83 | 2.81 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kónya, G.; Szabó, Z.; Dobos, N.; Király, J.; Szegedi, K.; Vass, A.; Steli, Á.; Szász, C.; Dezső, B.; Zsebik, B.; et al. Evaluation of the Expression of IDO and PTEN in Human Kidney Cancer. Curr. Issues Mol. Biol. 2025, 47, 359. https://doi.org/10.3390/cimb47050359
Kónya G, Szabó Z, Dobos N, Király J, Szegedi K, Vass A, Steli Á, Szász C, Dezső B, Zsebik B, et al. Evaluation of the Expression of IDO and PTEN in Human Kidney Cancer. Current Issues in Molecular Biology. 2025; 47(5):359. https://doi.org/10.3390/cimb47050359
Chicago/Turabian StyleKónya, Gábor, Zsuzsanna Szabó, Nikoletta Dobos, József Király, Krisztián Szegedi, Anna Vass, Ákos Steli, Csaba Szász, Balázs Dezső, Barbara Zsebik, and et al. 2025. "Evaluation of the Expression of IDO and PTEN in Human Kidney Cancer" Current Issues in Molecular Biology 47, no. 5: 359. https://doi.org/10.3390/cimb47050359
APA StyleKónya, G., Szabó, Z., Dobos, N., Király, J., Szegedi, K., Vass, A., Steli, Á., Szász, C., Dezső, B., Zsebik, B., & Halmos, G. (2025). Evaluation of the Expression of IDO and PTEN in Human Kidney Cancer. Current Issues in Molecular Biology, 47(5), 359. https://doi.org/10.3390/cimb47050359








