Differential Profiles of Gut Microbiota-Derived Metabolites of Bile Acids and Propionate as Potential Predictors of Depressive Disorder in Women with Morbid Obesity at High Risk of Metabolic Dysfunction-Associated Steatotic Liver Disease—A Pilot Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Hepatopathological Diagnosis
2.3. Anthropometric Evaluation and Biochemical Analysis
2.4. Plasma Measurements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
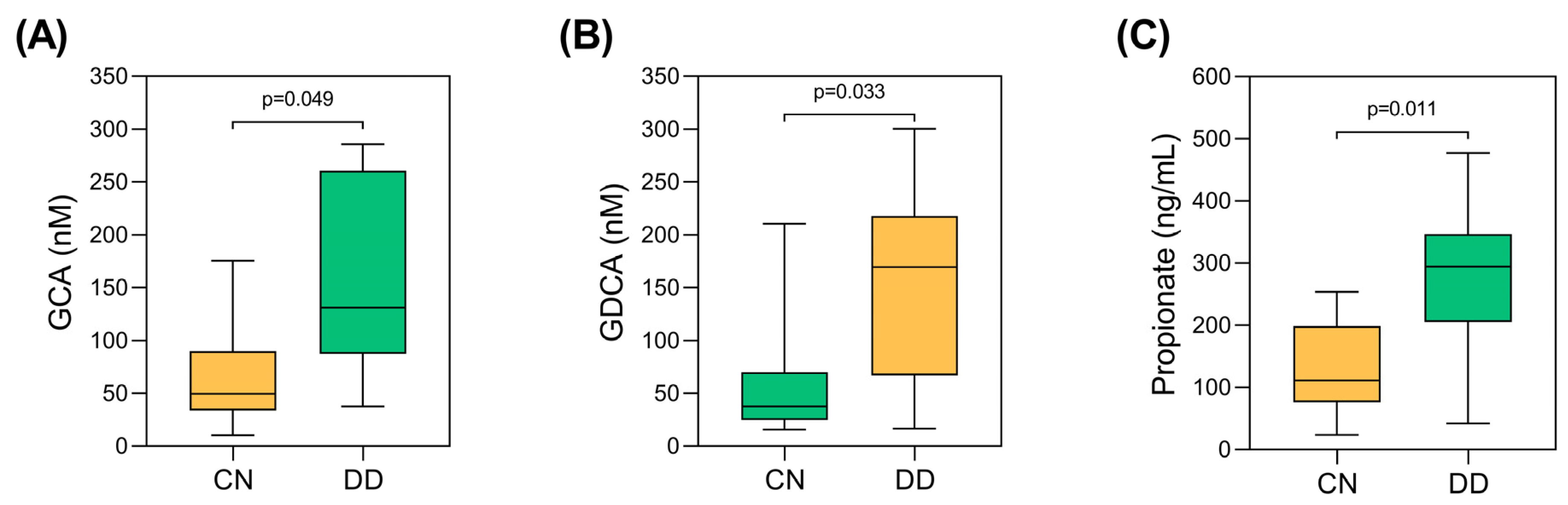
3.2. Evaluation of Microbiota-Derived Metabolites Between the Study Groups
3.3. Evaluation of Metabolic and Inflammatory Biomarkers in the Cohort
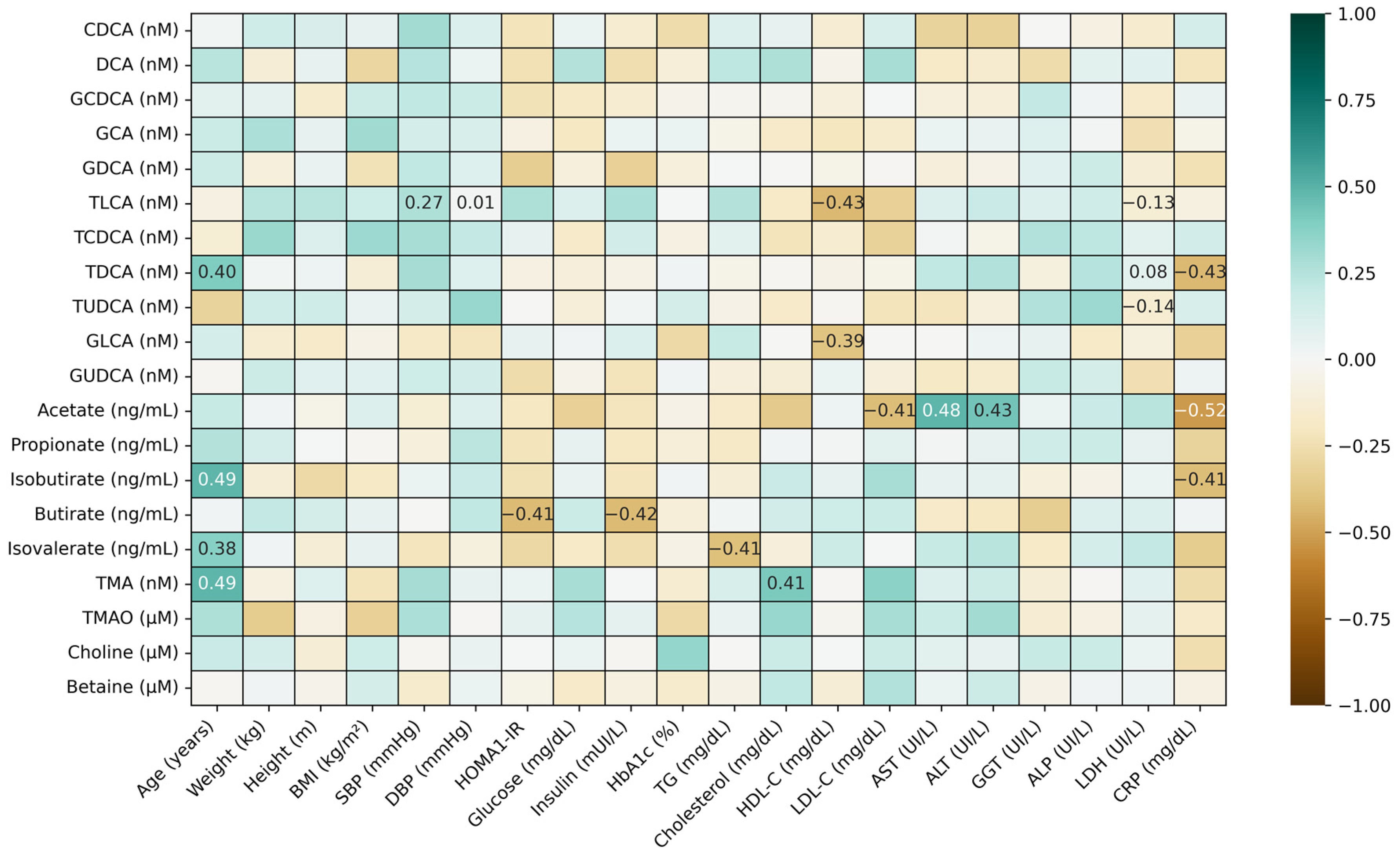
3.4. Correlations Between Microbiota-Derived Metabolites and Measured Biomarkers
3.4.1. Correlations Between BAs and SCFAs and Other Microbial Bioactives
3.4.2. Correlations Between Microbiota Metabolites and Clinical Characteristics
3.4.3. Correlations Between Microbial Metabolites and Immuno-Metabolic Biomarkers
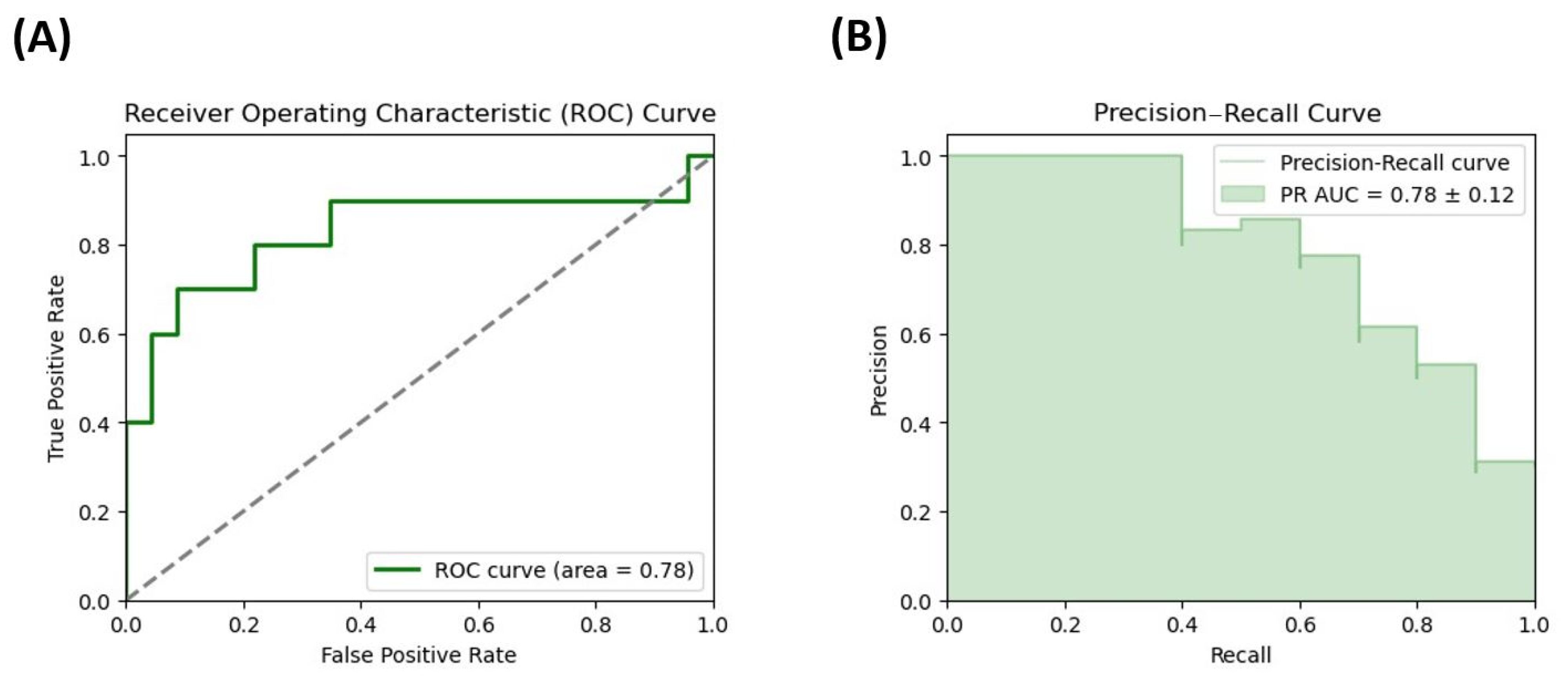
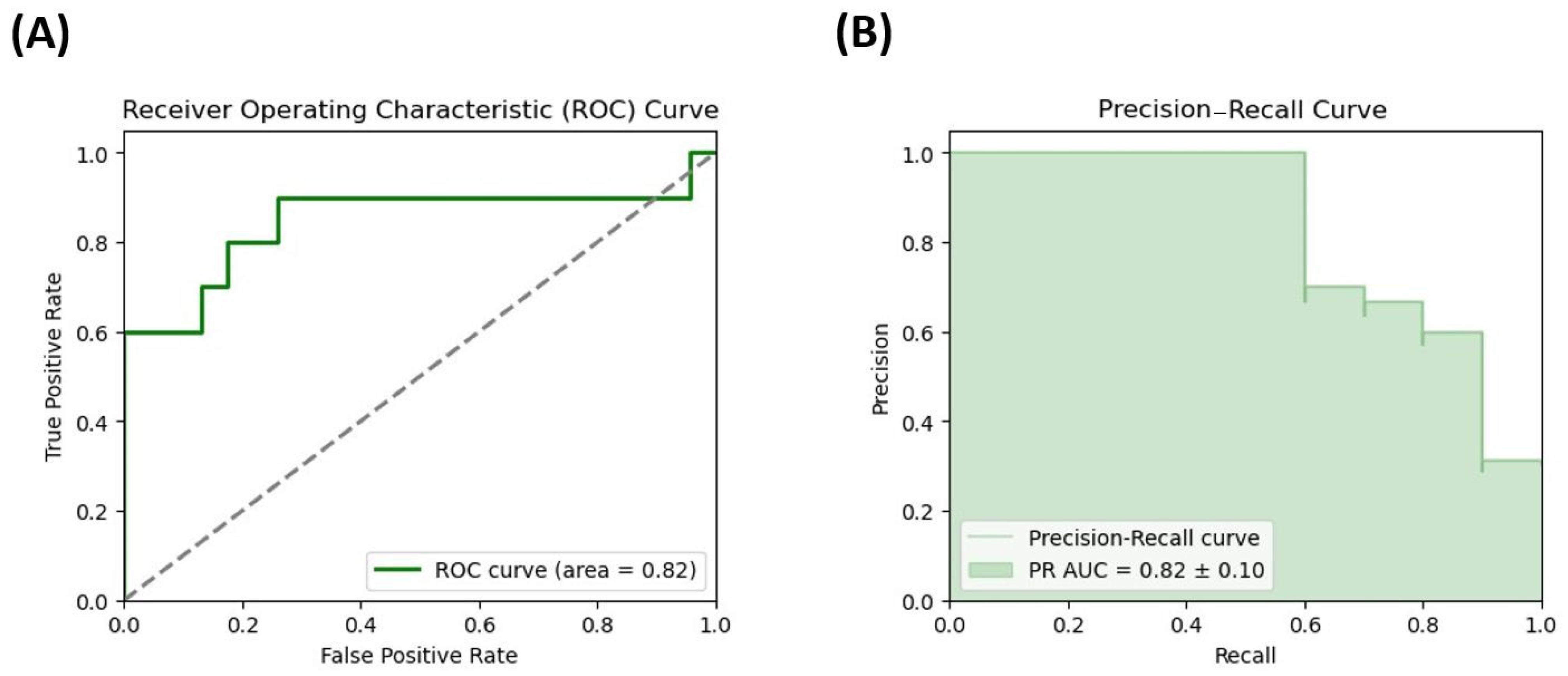
3.5. Evaluation of the Potential of Microbiota Metabolites as Potential Biomarkers of DD Risk in Female Patients with Severe Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Gut–microbiota–brain axis by bile acids in depression. Psychiatry Clin. Neurosci. 2022, 76, 281. [Google Scholar] [CrossRef]
- Youssef, N.A.; Abdelmalek, M.F.; Binks, M.; Guy, C.D.; Omenetti, A.; Smith, A.D.; Diehl, A.M.E.; Suzuki, A. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013, 33, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lim, L.K.E.; Ng, C.H.; Tan, D.J.H.; Lim, W.H.; Ho, C.S.H.; Tan, E.X.X.; Sanyal, A.J.; Muthiah, M.D. Is Fatty Liver Associated With Depression? A Meta-Analysis and Systematic Review on the Prevalence, Risk Factors, and Outcomes of Depression and Non-alcoholic Fatty Liver Disease. Front. Med. 2021, 8, 691696. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, C.; Chen, Y.; Han, B.; Chen, Y.; Cheng, J.; Wang, N.; Wang, B.; Lu, Y. High prevalence of metabolic diseases, liver steatosis and fibrosis among Chinese psychiatric patients. BMC Psychiatry 2023, 23, 206. [Google Scholar] [CrossRef]
- Liang, J.-X.; Ampuero, J.; Niu, H.; Imajo, K.; Noureddin, M.; Behari, J.; Lee, D.H.; Ehman, R.L.; Rorsman, F.; Vessby, J.; et al. An individual patient data meta-analysis to determine cut-offs for and confounders of NAFLD-fibrosis staging with magnetic resonance elastography. J. Hepatol. 2023, 79, 592–604. [Google Scholar] [CrossRef]
- Custodio, R.J.P.; Hobloss, Z.; Myllys, M.; Hassan, R.; González, D.; Reinders, J.; Bornhorst, J.; Weishaupt, A.-K.; Seddek, A.-L.; Abbas, T.; et al. Cognitive Functions, Neurotransmitter Alterations, and Hippocampal Microstructural Changes in Mice Caused by Feeding on Western Diet. Cells 2023, 12, 2331. [Google Scholar] [CrossRef]
- Lai, J.; Luo, L.; Zhou, T.; Feng, X.; Ye, J.; Zhong, B. Alterations in Circulating Bile Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Systematic Review and Meta-Analysis. Biomolecules 2023, 13, 1356. [Google Scholar] [CrossRef]
- Li, T.; Hasan, M.N.; Gu, L. Bile acids regulation of cellular stress responses in liver physiology and diseases. Egastroenterology 2024, 2, e100074. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhou, X.; Chen, W.; Zhou, H. Dysregulated bile acid homeostasis: Unveiling its role in metabolic diseases. Med. Rev. 2024, 4, 262–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. eBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- MahmoudianDehkordi, S.; Bhattacharyya, S.; Brydges, C.R.; Jia, W.; Fiehn, O.; Rush, A.J.; Dunlop, B.W.; Kaddurah-Daouk, R. Gut Microbiome-Linked Metabolites in the Pathobiology of Major Depression With or Without Anxiety—A Role for Bile Acids. Front. Neurosci. 2022, 16, 937906. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, S4–S14. [Google Scholar] [CrossRef]
- Ntona, S.; Papaefthymiou, A.; Kountouras, J.; Gialamprinou, D.; Kotronis, G.; Boziki, M.; Polyzos, S.A.; Tzitiridou, M.; Chatzopoulos, D.; Thavayogarajah, T.; et al. Impact of nonalcoholic fatty liver disease-related metabolic state on depression. Neurochem. Int. 2023, 163, 105484. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, J.; Wang, J.; Liu, Z.; Wang, X.; Kang, P.; Yang, C.; Liu, P.; Zhang, K. Abnormal gut microbiota and bile acids in patients with first-episode major depressive disorder and correlation analysis. Psychiatry Clin. Neurosci. 2022, 76, 321–328. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Yu, J.; Chen, H.; Zhang, D.; Zhang, T.; Ma, Y.; Zou, C.; Zhang, Z.; Ma, L.; et al. Gut microbiome determines therapeutic effects of OCA on NAFLD by modulating bile acid metabolism. NPJ Biofilms Microbiomes 2023, 9, 29. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Chen, S.; Shao, Q.; Chen, J.; Lv, X.; Ji, J.; Liu, Y.; Song, Y. Bile acid signalling and its role in anxiety disorders. Front. Endocrinol. 2023, 14, 1268865. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.-H.; Chen, Y.-C.; Lin, Y.-T.; Huang, S.-Y. N-3 PUFA Ameliorates the Gut Microbiota, Bile Acid Profiles, and Neuropsychiatric Behaviours in a Rat Model of Geriatric Depression. Biomedicines 2022, 10, 1594. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Chen, Y.; Haque, Z.; De Veer, M.; Egan, G.; Wang, B. Sialylated milk oligosaccharides alter neurotransmitters and brain metabolites in piglets: An In vivo magnetic resonance spectroscopic (MRS) study. Nutr. Neurosci. 2021, 24, 885–895. [Google Scholar] [CrossRef]
- Tomkin, G.H.; Owens, D. Obesity diabetes and the role of bile acids in metabolism. J. Transl. Intern. Med. 2016, 4, 73–80. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Chiang, J.Y.L. Understanding Bile Acid Signaling in Diabetes: From Pathophysiology to Therapeutic Targets. Diabetes Metab. J. 2019, 43, 257. [Google Scholar] [CrossRef]
- Iwaki, M.; Kessoku, T.; Tanaka, K.; Ozaki, A.; Kasai, Y.; Kobayashi, T.; Nogami, A.; Honda, Y.; Ogawa, Y.; Imajo, K.; et al. Combined, elobixibat, and colestyramine reduced cholesterol toxicity in a mouse model of metabolic dysfunction-associated steatotic liver disease. Hepatol. Commun. 2023, 7, e0285. [Google Scholar] [CrossRef]
- Popov, J.; Despot, T.; Rodriguez, D.A.; Khan, I.; Mech, E.; Khan, M.; Bojadzija, M.; Pai, N. Implications of Microbiota and Immune System in Development and Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients 2024, 16, 1668. [Google Scholar] [CrossRef]
- Auguet, T.; Berlanga, A.; Guiu-Jurado, E.; Martinez, S.; Porras, J.A.; Aragonès, G.; Sabench, F.; Hernandez, M.; Aguilar, C.; Sirvent, J.J.; et al. Altered Fatty Acid Metabolism-Related Gene Expression in Liver from Morbidly Obese Women with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 22173–22187. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Auguet, T.; Lopez-Dupla, M.; Ramos, J.; Bertran, L.; Riesco, D.; Aguilar, C.; Ardévol, A.; Pinent, M.; Sabench, F.; Del Castillo, D.; et al. Lipocalin, Resistin and Gut Microbiota-Derived Propionate Could Be Used to Predict Metabolic Bariatric Surgery Selected Outcomes. Processes 2022, 10, 143. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Sternby, H.; Hartman, H.; Thorlacius, H.; Regnér, S. The Initial Course of IL1β, IL-6, IL-8, IL-10, IL-12, IFN-γ and TNF-α with Regard to Severity Grade in Acute Pancreatitis. Biomolecules 2021, 11, 591. [Google Scholar] [CrossRef]
- Faraj, S.S.; Jalal, P.J. IL1β, IL-6, and TNF-α cytokines cooperate to modulate a complicated medical condition among COVID-19 patients: Case-control study. Ann. Med. Surg. 2023, 85, 2291–2297. [Google Scholar] [CrossRef]
- Rasouli, N.; Kern, P.A. Adipocytokines and the Metabolic Complications of Obesity. J. Clin. Endocrinol. Metab. 2008, 93, s64–s73. [Google Scholar] [CrossRef]
- Park, S.; Shimokawa, I. Influence of Adipokines on Metabolic Dysfunction and Aging. Biomedicines 2024, 12, 873. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Markowitz, S.M.; Gonzalez, J.S.; Wilkinson, J.L.; Safren, S.A. A Review of Treating Depression in Diabetes: Emerging Findings. Psychosomatics 2011, 52, 1–18. [Google Scholar] [CrossRef]
- Bonilla-Jaime, H.; Sánchez-Salcedo, J.A.; Estevez-Cabrera, M.M.; Molina-Jiménez, T.; Cortes-Altamirano, J.L.; Alfaro-Rodríguez, A. Depression and Pain: Use of Antidepressants. Curr. Neuropharmacol. 2022, 20, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Kessing, L.V.; Rytgaard, H.C.; Ekstrøm, C.T.; Knop, F.K.; Berk, M.; Gerds, T.A. Antidiabetes Agents and Incident Depression: A Nationwide Population-Based Study. Diabetes Care 2020, 43, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- De Groot, M. Diabetes and Depression: Strategies to Address a Common Comorbidity Within the Primary Care Context. Am. J. Med. Open 2023, 9, 100039. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, R.; Zhao, C.; Wang, Y.; Tu, X.; Duan, W. Associations of depression score with metabolic dysfunction-associated fatty liver disease and liver fibrosis. J. Affect Disord. 2023, 334, 332–336. [Google Scholar] [CrossRef]
- Åström, H.; Shang, Y.; Hagström, H.; Wester, A. Persons with metabolic dysfunction-associated steatotic liver disease are at increased risk of severe depression. Liver Int. 2024, 44, 2551–2563. [Google Scholar] [CrossRef]
- Luo, M.; Xin, R.-J.; Hu, F.-R.; Yao, L.; Hu, S.-J.; Bai, F.-H. Role of gut microbiota in the pathogenesis and therapeutics of minimal hepatic encephalopathy via the gut-liver-brain axis. World J. Gastroenterol. 2023, 29, 144–156. [Google Scholar] [CrossRef]
- Ha, S.; Wong, V.W.-S.; Zhang, X.; Yu, J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut 2024, 74, 141–152. [Google Scholar] [CrossRef]
- Szczesniak, O.; Hestad, K.A.; Hanssen, J.F.; Rudi, K. Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 2016, 19, 279–283. [Google Scholar] [CrossRef]
- Zhou, J.; Tripathi, M.; Sinha, R.A.; Singh, B.K.; Yen, P.M. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. 2021, 2021, 11. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L.; Cai, M.-X.; Wang, Z.-L.; Zhuang, M.; Tan, C.-Y.; Xie, T.-H.; Yao, Y.; Wei, T.-T. TGR5 supresses cGAS/STING pathway by inhibiting GRP75-mediated endoplasmic reticulum-mitochondrial coupling in diabetic retinopathy. Cell Death Dis. 2023, 14, 583. [Google Scholar] [CrossRef]
- Velazquez-Villegas, L.A.; Perino, A.; Lemos, V.; Zietak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Hirano, K.; Tsujimoto, G. The SCFA Receptor GPR43 and Energy Metabolism. Front. Endocrinol. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Shao, J.-H.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.-J.; Liu, Z.-Z.; He, D.-D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Wittenberg, G.M.; Bullmore, E.T.; Manji, H.K. Immune targets for therapeutic development in depression: Towards precision medicine. Nat. Rev. Drug Discov. 2022, 21, 224–244. [Google Scholar] [CrossRef]
- Ortiz-López, N.; Fuenzalida, C.; Dufeu, M.S.; Pinto-León, A.; Escobar, A.; Poniachik, J.; Roblero, J.P.; Valenzuela-Pérez, L.; Beltrán, C.J. The immune response as a therapeutic target in non-alcoholic fatty liver disease. Front. Immunol. 2022, 13, 954869. [Google Scholar] [CrossRef]
- Lirong, W.; Mingliang, Z.; Mengci, L.; Qihao, G.; Zhenxing, R.; Xiaojiao, Z.; Tianlu, C. The clinical and mechanistic roles of bile acids in depression, Alzheimer’s disease, and stroke. Proteomics 2022, 22, 2100324. [Google Scholar] [CrossRef]
| Variables | Total Cohort (n = 33) | Depression Disorder (DD) Group (n = 10) | Control (CN) Group (n = 23) | p Value |
|---|---|---|---|---|
| Age (years) | 49.39 (41.39–57.53) | 51.48 (42.43–56.98) | 48.54 (37.12–54.40) | 0.232 |
| Weight (kg) | 116.00 (110.10–130.50) | 118.00 (110.75–128.50) | 116.00 (111.10–132.00) | 0.953 |
| Height (m) | 1.60 (1.58–1.67) | 1.58 (1.56–1.60) | 1.62 (1.58–1.68) | 0.146 |
| BMI (kg/m2) | 44.06 (42.35–48.73) | 44.10 (43.74–50.23) | 43.93 (42.36–47.86) | 0.518 |
| SBP (mmHg) | 132.00 (107.50–138.50) | 130.50 (107.25–138.00) | 132.00 (109.75–138.00) | 0.951 |
| DBP (mmHg) | 63.00 (57.75–74.75) | 61.50 (59.25–73.50) | 65.00 (57.75–72.50) | 0.976 |
| HOMA1-IR | 3.60 (1.77–7.34) | 6.82 (3.32–17.40) | 2.84 (1.58–4.71) | 0.060 |
| Glucose (mg/dL) | 107.00 (87.00–148.00) | 138.00 (110.75–157.50) | 100.00 (86.50–112.50) | 0.088 |
| Insulin (mUI/L) | 13.64 (6.92–31.19) | 22.60 (14.18–48.76) | 12.18 (6.35–17.95) | 0.150 |
| HbA1c (%) | 5.75 (5.38–7.83) | 7.55 (5.68–8.12) | 5.60 (5.30–6.15) | 0.078 |
| TG (mg/dL) | 132.00 (105.00–166.00) | 134.00 (121.50–152.50) | 132.00 (103.00–187.00) | 0.949 |
| Cholesterol (mg/dL) | 169.00 (153.00–188.00) | 165.00 (133.57–189.25) | 170.00 (154.00–186.20) | 0.597 |
| HDL-C (mg/dL) | 37.40 (30.88–46.25) | 36.00 (32.00–48.00) | 37.80 (31.00–44.00) | 0.839 |
| LDL-C (mg/dL) | 96.25 (79.15–120.05) | 90.20 (66.10–114.20) | 100.00 (92.60–118.60) | 0.189 |
| AST (UI/L) | 28.50(19.00–45.00) | 22.00 (15.00–36.00) | 32.00 (22.00–45.00) | 0.341 |
| ALT (UI/L) | 33.00 (22.00–45.00) | 26.50 (20.25–43.00) | 34.00 (24.00–43.00) | 0.512 |
| GGT (UI/L) | 21.00 (16.50–32.50) | 29.00 (18.25–34.75) | 18.00 (15.50–26.50) | 0.098 |
| ALP (Ul/L) | 69.00 (53.50–79.25) | 72.00 (53.00–74.00) | 64.00 (56.50–77.50) | 0.941 |
| LDH (Ul/L) | 405.50 (354.75–457.25) | 330.00 (296.50–419.50) | 406.00 (374.50–457.50) | 0.185 |
| CRP (mg/dL) | 0.70 (0.45–1.60) | 0.60 (0.40–1.30) | 0.70 (0.50–1.50) | 0.686 |
| Medications/Treatment | Total Cohort (n = 33) | Depression Disorder (DD) Group (n = 10) | Control (CN) Group (n = 23) | p Value |
|---|---|---|---|---|
| Antihypertensive | 19 (57.6%) | 8 (24.2%) | 11 (33.3%) | 0.089 |
| Lipid-lowering agents—statins | 8 (24.2%) | 4 (12.1%) | 4 (12.1%) | 0.164 |
| Lipid-lowering agents—fibrates | 2 (6.1%) | 1 (3.05%) | 1 (3.05%) | 0.532 |
| Type 2 diabetes treatment—insulin * | 7 (21.2%) | 5 (15.2%) | 2 (6.1%) | 0.008 |
| Type 2 diabetes treatment—oral * | 13 (39.4%) | 7 (21.2%) | 6 (18.2%) | 0.018 |
| Analgesics | 3 (9.1%) | 2 (6.1%) | 1 (3.0%) | 0.151 |
| Opioid analgesics * | 2 (6.1%) | 2 (6.1%) | 0 (0%) | 0.027 |
| Antibiotics | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Anti-inflammatory drugs | 6 (18.2%) | 3 (9.1%) | 3 (9.1%) | 0.246 |
| Benzodiazepines | 3 (9.1%) | 2 (6.1%) | 1 (3.0%) | 0.151 |
| Cytostatic immunosuppressants | 1 (3.0%) | 1 (3.0%) | 0 (0%) | 0.124 |
| Corticosteroids | 2 (6.1%) | 1 (3.0%) | 1 (3.0%) | 0.532 |
| Comorbidity | Depression Disorder (DD) Group (n = 10) | Control (CN) Group (n = 23) | Xi2 (df) | p Value | |
|---|---|---|---|---|---|
| T2DM * | No | 3 | 17 | 5.629 (1) | 0.018 |
| Yes | 7 | 6 | |||
| Metabolic Syndrome | No | 4 | 5 | 1.172 (1) | 0.279 |
| Yes | 6 | 18 | |||
| High Blood Pressure | No | 2 | 12 | 2.954 (1) | 0.086 |
| Yes | 8 | 11 | |||
| MASLD | No | 3 | 8 | 0.072 (1) | 0.789 |
| Yes | 7 | 15 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurek, J.M.; Xifré, B.; Rusu, E.C.; Clavero-Mestres, H.; Mahmoudian, R.; Aguilar, C.; Riesco, D.; Ugarte Chicote, J.; Martinez, S.; Vives, M.; et al. Differential Profiles of Gut Microbiota-Derived Metabolites of Bile Acids and Propionate as Potential Predictors of Depressive Disorder in Women with Morbid Obesity at High Risk of Metabolic Dysfunction-Associated Steatotic Liver Disease—A Pilot Study. Curr. Issues Mol. Biol. 2025, 47, 353. https://doi.org/10.3390/cimb47050353
Jurek JM, Xifré B, Rusu EC, Clavero-Mestres H, Mahmoudian R, Aguilar C, Riesco D, Ugarte Chicote J, Martinez S, Vives M, et al. Differential Profiles of Gut Microbiota-Derived Metabolites of Bile Acids and Propionate as Potential Predictors of Depressive Disorder in Women with Morbid Obesity at High Risk of Metabolic Dysfunction-Associated Steatotic Liver Disease—A Pilot Study. Current Issues in Molecular Biology. 2025; 47(5):353. https://doi.org/10.3390/cimb47050353
Chicago/Turabian StyleJurek, Joanna Michalina, Belen Xifré, Elena Cristina Rusu, Helena Clavero-Mestres, Razieh Mahmoudian, Carmen Aguilar, David Riesco, Javier Ugarte Chicote, Salomé Martinez, Marga Vives, and et al. 2025. "Differential Profiles of Gut Microbiota-Derived Metabolites of Bile Acids and Propionate as Potential Predictors of Depressive Disorder in Women with Morbid Obesity at High Risk of Metabolic Dysfunction-Associated Steatotic Liver Disease—A Pilot Study" Current Issues in Molecular Biology 47, no. 5: 353. https://doi.org/10.3390/cimb47050353
APA StyleJurek, J. M., Xifré, B., Rusu, E. C., Clavero-Mestres, H., Mahmoudian, R., Aguilar, C., Riesco, D., Ugarte Chicote, J., Martinez, S., Vives, M., Sabench, F., & Auguet, T. (2025). Differential Profiles of Gut Microbiota-Derived Metabolites of Bile Acids and Propionate as Potential Predictors of Depressive Disorder in Women with Morbid Obesity at High Risk of Metabolic Dysfunction-Associated Steatotic Liver Disease—A Pilot Study. Current Issues in Molecular Biology, 47(5), 353. https://doi.org/10.3390/cimb47050353






