The 50-nm Free Vesicles Visible in Saccharomyces cerevisiae Are Not COPII-Dependent
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Plasmids
2.2. Electron Microscopy
2.3. Quantitation
2.4. Sampling and Statistical Analysis
3. Results
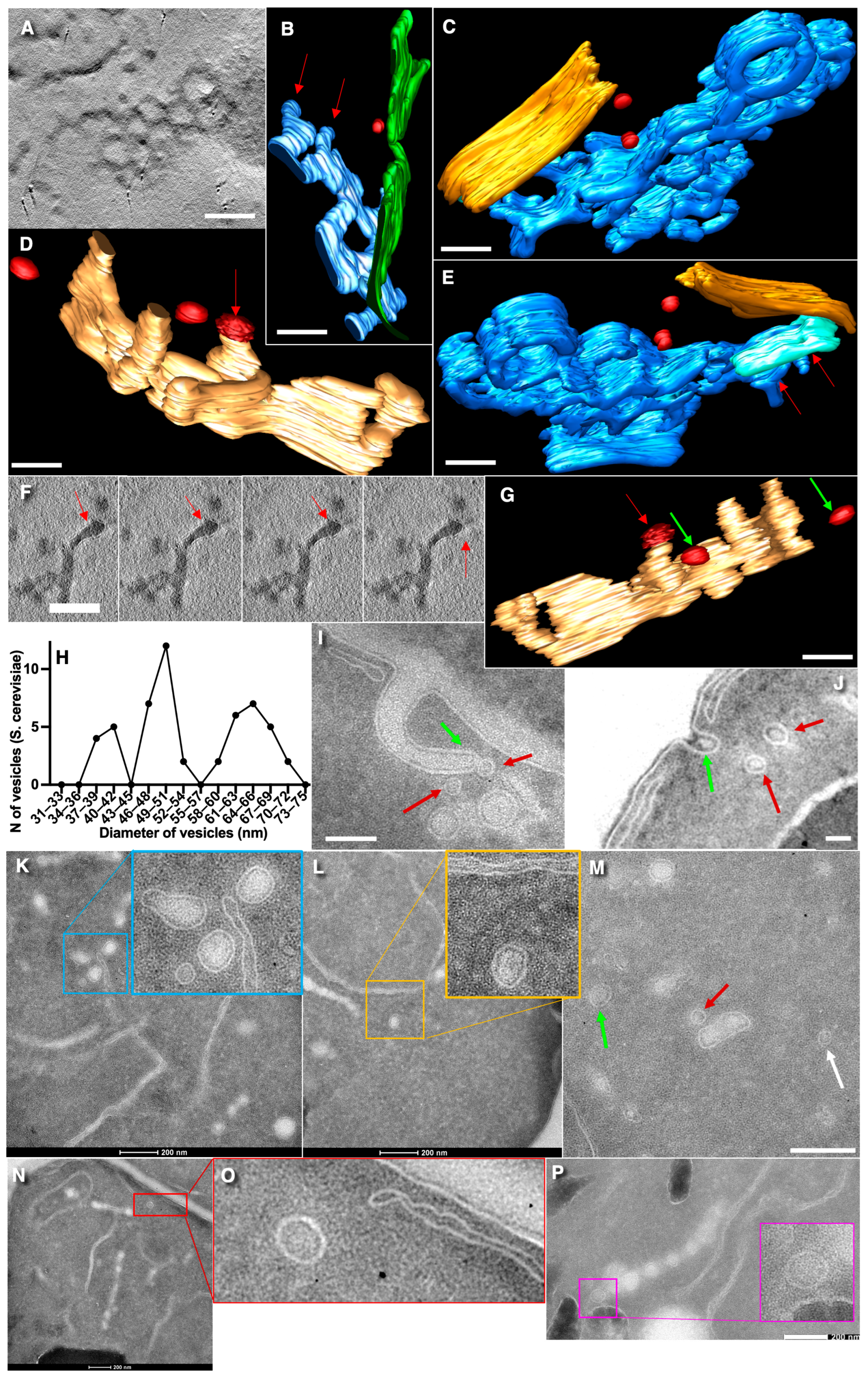
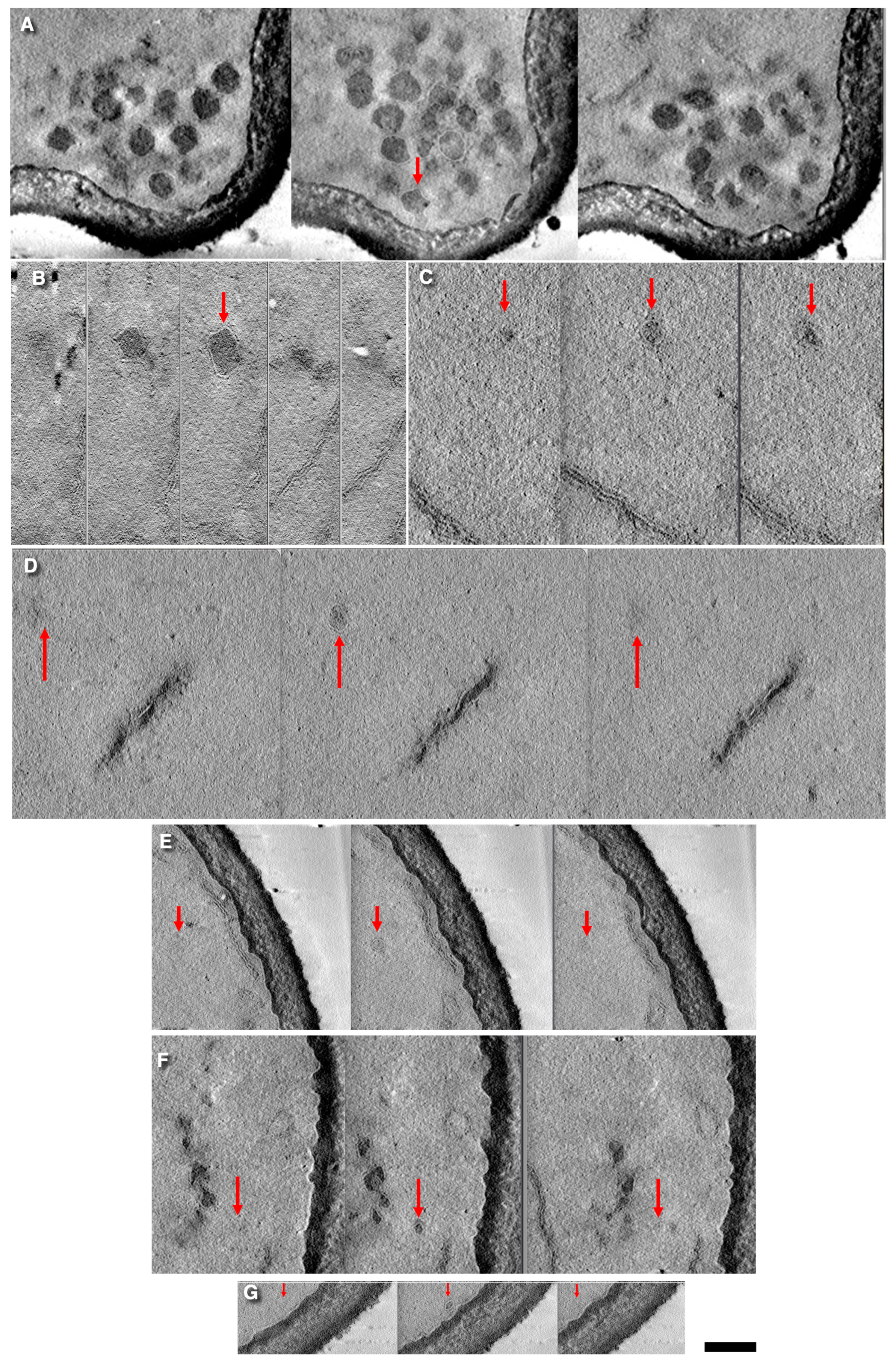
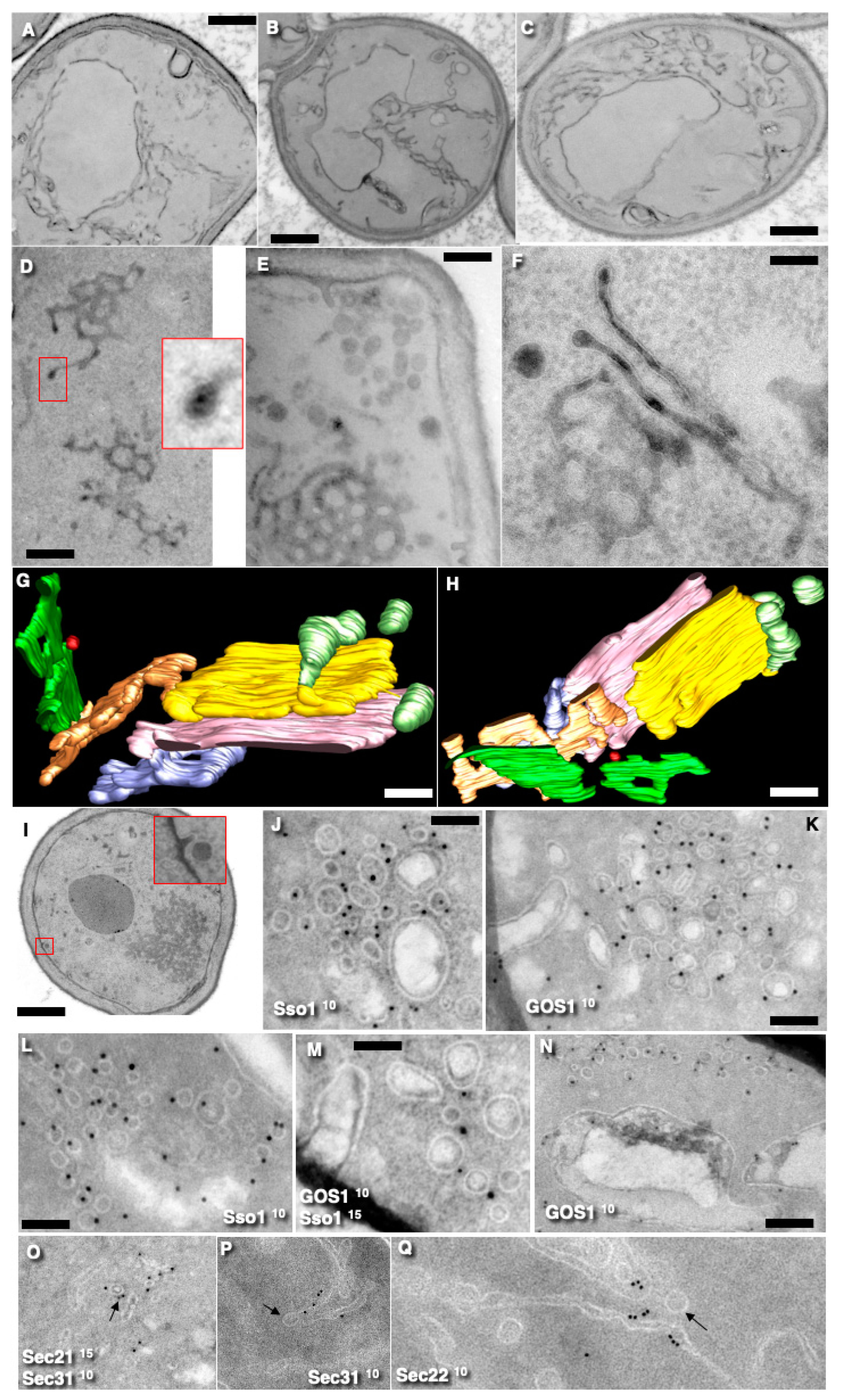
3.1. Vesicles in S. cerevisiae
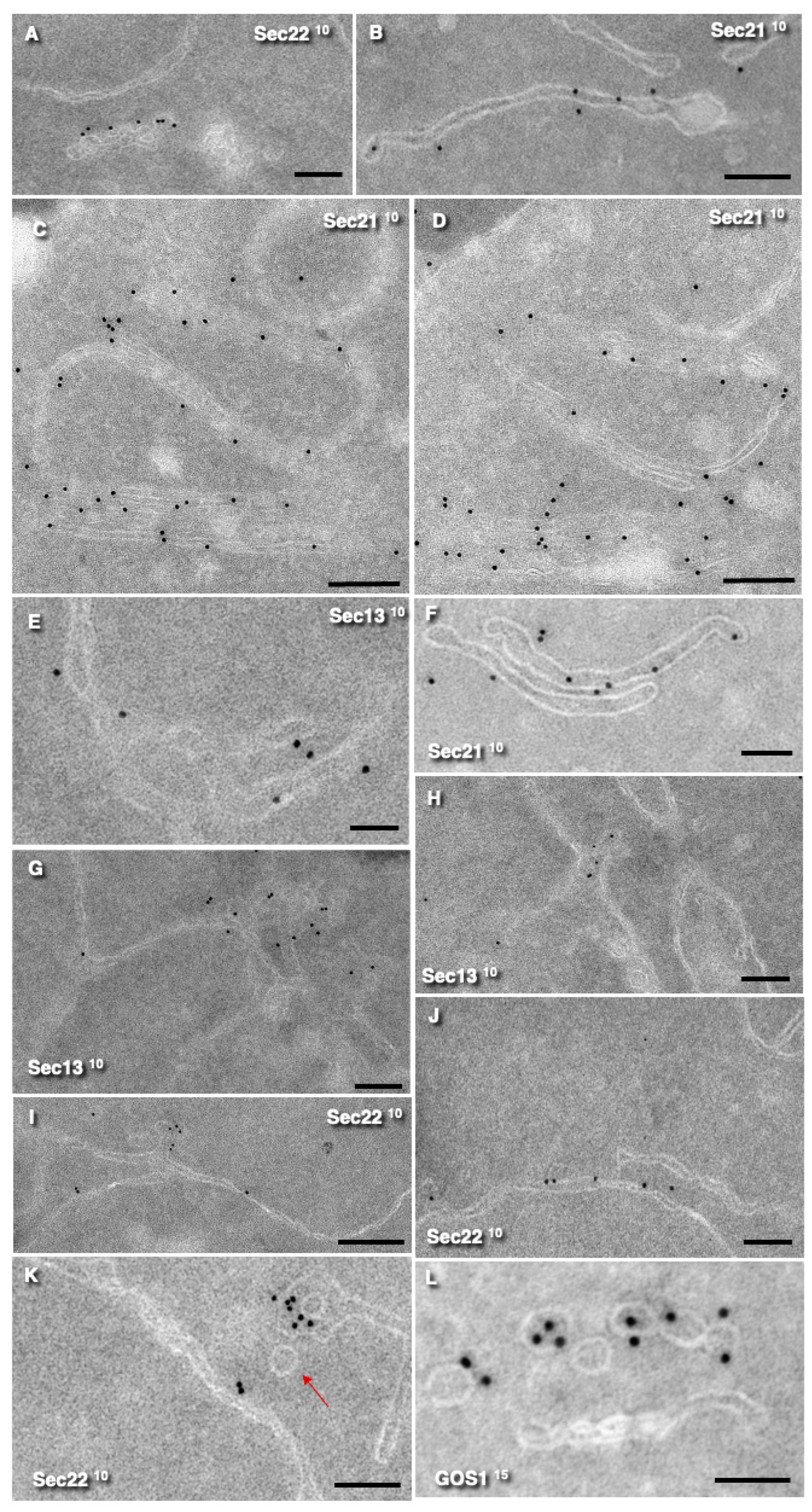
3.2. Localization of COPII and COPI Markers on Endomembranes
3.3. Inhibition of Sec23 and Its Effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mironov, A.A.; Beznoussenko, G.V. The kiss-and-run model of intra-Golgi transport. Int. J. Mol. Sci. 2012, 13, 6800–6819. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.A.; Sesorova, I.V.; Beznoussenko, G.V. Golgi’s way: A long path toward the new paradigm of the intra-Golgi transport. Histochem. Cell Biol. 2013, 140, 383–393. [Google Scholar] [CrossRef]
- Mironov, A.A.; Beznoussenko, G.V. Models of intracellular transport: Pros and cons. Front. Cell Dev. Biol. 2019, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Beznoussenko, G.V.; Kweon, H.S.; Sesorova, I.S.; Mironov, A.A. Comparison of the Cisterna Maturation-Progression Model with the Kiss-and-Run Model of Intra-Golgi Transport: Role of Cisternal Pores and Cargo Domains. Int. J. Mol. Sci. 2022, 23, 3590. [Google Scholar] [CrossRef] [PubMed]
- Beznoussenko, G.V.; Bejan, A.I.; Parashuraman, S.; Luini, A.; Kweon, H.S.; Mironov, A.A. The Diffusion Model of Intra-Golgi Transport Has Limited Power. Int. J. Mol. Sci. 2023, 24, 1375. [Google Scholar] [CrossRef]
- Kaiser, C.A.; Schekman, R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 1990, 61, 723–733. [Google Scholar] [CrossRef]
- Barlowe, C.; Orci, L.; Yeung, T.; Hosobuchi, M.; Hamamoto, S.; Salama, N.; Rexach, M.F.; Ravazzola, M.; Amherdt, M.; Schekman, R. COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 1994, 77, 895–907. [Google Scholar] [CrossRef]
- Mossessova, E.; Bickford, L.C.; Goldberg, J. SNARE selectivity of the COPII coat. Cell 2003, 114, 483–495. [Google Scholar] [CrossRef]
- Rexach, M.F.; Latterich, M.; Schekman, R.W. Characteristics of endoplasmic reticulum-derived transport vesicles. J. Cell Biol. 1994, 126, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.; Glick, B.S.; Rothman, J.E. A new type of coated vesicular carrier that appears not to contain clathrin: Its possible role in protein transport within the Golgi stack. Cell 1986, 46, 171–184. [Google Scholar] [CrossRef]
- Bannykh, S.I.; Rowe, T.; Balch, W.E. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 1996, 135, 19–35. [Google Scholar] [CrossRef]
- Bacia, K.; Futai, E.; Prinz, S.; Meister, A.; Daum, S.; Glatte, D.; Briggs, J.A.G.; Schekman, R. Multibudded tubules formed by COPII on artificial liposomes. Sci. Rep. 2011, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Bykov, Y.S.; Schaffer, M.; Dodonova, S.O.; Albert, S.; Plitzko, J.M.; Baumeister, W.; Engel, B.D.; Briggs, J.A. The structure of the COPI coat determined within the cell. Elife 2017, 6, e32493. [Google Scholar] [CrossRef] [PubMed]
- Pyle, E.; Miller, E.A.; Zanetti, G. Cryo-electron tomography reveals how COPII assembles on cargo-containing membranes. Nat. Struct. Mol. Biol. 2025, 32, 513–519. [Google Scholar] [CrossRef]
- Beznoussenko, G.V.; Ragnini-Wilson, A.; Wilson, C.; Mironov, A.A. Three-dimensional and immune electron microscopic analysis of the secretory pathway in Saccharomyces cerevisiae. Histochem. Cell Biol. 2016, 146, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Fatal, N.; Karhinen, L.; Jokitalo, E.; Makarow, M. Active and specific recruitment of a soluble cargo protein for endoplasmic reticulum exit in the absence of functional COPII component Sec24p. J. Cell Sci. 2004, 117, 1665–1673. [Google Scholar] [CrossRef]
- Fatal, N.; Suntio, T.; Makarow, M. Selective protein exit from yeast endoplasmic reticulum in absence of functional COPII coat component Sec13p. Mol. Biol. Cell 2002, 13, 4130–4140. [Google Scholar] [CrossRef]
- Gomez-Navarro, N.; Melero, A.; Li, X.H.; Boulanger, J.; Kukulski, W.; Miller, E.A. Cargo crowding contributes to sorting stringency in COPII vesicles. J. Cell Biol. 2020, 219, e201806038. [Google Scholar] [CrossRef]
- Melero, A.; Boulanger, J.; Kukulski, W.; Miller, E.A. Ultrastructure of COPII vesicle formation in yeast characterized by correlative light and electron microscopy. Mol. Biol. Cell 2022, 33, ar122. [Google Scholar] [CrossRef]
- Nair, A.; Nair, A.; Stock, P.; Jain, A.; Somerville, E.; Sanyal, A.; Kirchhausen, T. Close-Up of vesicular ER Exit Sites by Volume Electron Imaging using FIB-SEM. bioRxiv 2025. [Google Scholar] [CrossRef]
- Matsuoka, K.; Orci, L.; Amherdt, M.; Bednarek, S.Y.; Hamamoto, S.; Schekman, R.; Yeung, T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 1998, 93, 263–275. [Google Scholar] [CrossRef]
- Morin-Ganet, M.N.; Rambourg, A.; Clermont, Y.; Képès, F. Role of endoplasmic reticulum-derived vesicles in the formation of Golgi elements in sec23 and sec18 Saccharomyces cerevisiae mutants. Anat. Rec. 1998, 251, 256–264. [Google Scholar] [CrossRef]
- Morin-Ganet, M.N.; Rambourg, A.; Deitz, S.B.; Franzusoff, A.; Képès, F. Morphogenesis and dynamics of the yeast Golgi apparatus. Traffic 2000, 1, 56–68. [Google Scholar] [CrossRef]
- Movafeghi, A.; Happel, N.; Pimpl, P.; Tai, G.H.; Robinson, D.G. Arabidopsis Sec21p and Sec23p homologs. Probable coat proteins of plant COP-coated vesicles. Plant Physiol. 1999, 119, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Pimpl, P.; Movafeghi, A.; Coughlan, S.; Denecke, J.; Hillmer, S.; Robinson, D.G. In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 2000, 12, 2219–2236. [Google Scholar] [CrossRef]
- Ossig, R.; Dascher, C.; Trepte, H.H.; Schmitt, H.D.; Gallwitz, D. The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol. Cell Biol. 1991, 11, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
- Dascher, C.; Ossig, R.; Gallwitz, D.; Schmitt, H.D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell Biol. 1991, 11, 872–885. [Google Scholar] [CrossRef]
- Ballensiefen, W.; Ossipov, D.; Schmitt, H.D. Recycling of the yeast v-SNARE Sec22p involves COPI-proteins and the ER transmembrane proteins Ufe1p and Sec20p. J. Cell Sci. 1998, 111 Pt 11, 1507–1520. [Google Scholar] [CrossRef]
- Katz, L.; Hanson, P.I.; Heuser, J.E.; Brennwald, P. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO J. 1998, 17, 6200–6209. [Google Scholar] [CrossRef]
- Jäntti, J.; Hildén, P.; Rönk, ä.H.; Mäkiranta, V.; Keränen, S.; Kuismanen, E. Immunocytochemical analysis of Uukunieli virus budding compartments: Role of the intermediate compartment and the Golgi stack in virus maturation. J. Virol. 1997, 71, 1162–1172. [Google Scholar] [CrossRef]
- Petkovic, M.; Jemaiel, A.; Daste, F.; Specht, C.G.; Izeddin, I.; Vorkel, D.; Verbavatz, J.M.; Darzacq, X.; Triller, A.; Pfenninger, K.H.; et al. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat. Cell Biol. 2014, 16, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Calvert, C.M.; Sanders, D. Inositol trisphosphate-dependent and -independent Ca2+ mobilization pathways at the vacuolar membrane of Candida albicans. J. Biol. Chem. 1995, 270, 7272–7280. [Google Scholar] [CrossRef]
- Alonso, M.; Burgos, H.I.; Pannunzio, V.; Monti Hughes, A.; Mattoon, J.R.; Stella, C.A. Brefeldin A decreases the activity of the general amino acid permease (GAP1) and the more specific systems for L-leucine uptake in Saccharomyces cerevisiae. Cell Mol. Biol. Lett. 2006, 11, 256–263. [Google Scholar] [CrossRef]
- Kolpakov, V.; Polishchuk, R.; Bannykh, S.; Rekhter, M.; Solovjev, P.; Romanov, Y.E.; Tararak, E.; Antonov, A.; Mironov, A. Atherosclerosis prone branch regions in human aorta: Microarchitecture and cell composition of intima. Atherosclerosis 1996, 122, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Vanhecke, D.; Studer, D.; Ochs, M. Stereology meets electron tomography: Towards quantitative 3D electron microscopy. J. Struct. Biol. 2007, 159, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Marini, G.; Pigino, G. Chapter 6—Yeast membraneless compartments revealed by correlative light microscopy and electron tomography. Methods Cell Biol. 2019, 152, 103–117. [Google Scholar] [PubMed]
- Mironov, A.A., Jr.; Mironov, A.A. Estimation of subcellular organelle volume from ultrathin sections through centrioles with a discretized version of vertical rotator. J. Microsc. 1998, 192, 29–36. [Google Scholar] [CrossRef]
- Kukulski, W.; Schorb, M.; Welsch, S.; Picco, A.; Kaksonen, M.; Briggs, J.A. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J. Cell Biol. 2011, 192, 111–119. [Google Scholar] [CrossRef]
- Kukulski, W.; Schorb, M.; Welsch, S.; Picco, A.; Kaksonen, M.; Briggs, J.A. Precise, correlated fluorescence microscopy and electron tomography of lowicryl sections using fluorescent fiducial markers. Methods Cell Biol. 2012, 111, 235–257. [Google Scholar] [CrossRef] [PubMed]
- Rambourg, A.; Clermont, Y.; Jackson, C.L.; Képès, F. Ultrastructural modifications of vesicular and Golgi elements in the Saccharomyces cerevisiae sec21 mutant at permissive and non-permissive temperatures. Anat. Rec. 1994, 240, 32–41. [Google Scholar] [CrossRef]
- Rambourg, A.; Jackson, C.L.; Clermont, Y. Three dimensional configuration of the secretory pathway and segregation of secretion granules in the yeast Saccharomyces cerevisiae. J. Cell Sci. 2001, 114 Pt 12, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Rambourg, A.; Clermont, Y.; Nicaud, J.M.; Gaillardin, C.; Kepes, F. Transformations of membrane-bound organelles in sec 14 mutants of the yeasts Saccharomyces cerevisiae and Yarrowia lipolytica. Anat. Rec. 1996, 245, 447–458. [Google Scholar] [CrossRef]
- Deitz, S.B.; Rambourg, A.; Képès, F.; Franzusoff, A. Sec7p directs the transitions required for yeast Golgi biogenesis. Traffic 2000, 1, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.S.; Beznoussenko, G.V.; Micaroni, M.; Polishchuk, R.S.; Trucco, A.; Martella, O.; Di Giandomenico, D.; Marra, P.; Fusella, A.; Di Pentima, A.; et al. Golgi enzymes are enriched in perforated zones of golgi cisternae but are depleted in COPI vesicles. Mol. Biol. Cell 2004, 15, 4710–4724. [Google Scholar] [CrossRef]
- Trucco, A.; Polishchuk, R.S.; Martella, O.; Di Pentima, A.; Fusella, A.; Di Giandomenico, D.; Pietro, E.S.; Beznoussenko, G.V.; Polishchuk, E.V.; Baldassarre, M.; et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat. Cell Biol. 2004, 6, 1071–1081. [Google Scholar] [CrossRef]
- Fusella, A.; Micaroni, M.; Di Giandomenico, D.; Mironov, A.A.; Beznoussenko, G.V. Segregation of the Qb-SNAREs GS27 and GS28 into Golgi vesicles regulates intra-Golgi transport. Traffic 2013, 14, 568–584. [Google Scholar] [CrossRef]
- Welihinda, A.A.; Beavis, A.D.; Trumbly, R.J. Mutations in LIS1 (ERG6) gene confer increased sodium and lithium uptake in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1994, 1193, 107–117. [Google Scholar] [CrossRef]
- Gaber, R.F.; Copple, D.M.; Kennedy, B.K.; Vidal, M.; Bard, M. The yeast gene erg6 is required for normal mem-brane function but is not essential for biosynthe-sis of the cell-cycle-sparking sterol. Mol. Cell Biol. 1989, 9, 3447–3456. [Google Scholar]
- Umebayashi, K.; Nakano, A. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J. Cell Biol. 2003, 161, 1117–1131. [Google Scholar] [CrossRef]
- Rambourg, A.; Clermont, Y.; Ovtracht, L.; Képès, F. Three-dimensional structure of tubular networks, presumably Golgi in nature, in various yeast strains: A comparative study. Anat. Rec. 1995, 243, 283–293. [Google Scholar] [CrossRef]
- Denisova, G.N.; Dimov, I.D.; Zaitseva, A.V.; Artiux, L.J.; Mironov, A.A.; Karelina, N.R. Overloading of differentiated Caco-2 cells during lipid transcytosis induces glycosylation mistakes in the Golgi complex. Biocell 2021, 45, 773–783. [Google Scholar] [CrossRef]
- Zeuschner, D.; Geerts, W.J.; van Donselaar, E.; Humbel, B.M.; Slot, J.W.; Koster, A.J.; Klumperman, J. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat. Cell Biol. 2006, 8, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Menárguez, J.A.; Prekeris, R.; Oorschot, V.M.; Scheller, R.; Slot, J.W.; Geuze, H.J.; Klumperman, J. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J. Cell Biol. 2001, 155, 1213–1224. [Google Scholar] [CrossRef]
- Sesorova, I.S.; Beznoussenko, G.V.; Kazakova, T.E.; Sesorov, V.V.; Dimov, I.D.; Mironov, A.A. New opportunities of light microscopy in cytology and histology. Tsitologia 2018, 60, 319–329. [Google Scholar] [CrossRef]
- Ryan, K.J.; Wente, S.R. Isolation and characterization of new Saccharomyces cerevisiae mutants perturbed in nuclear pore complex assembly. BMC Genet. 2002, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Enninga, J.; Levay, A.; Fontoura, B.M. Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol. Cell Biol. 2003, 23, 7271–7284. [Google Scholar] [CrossRef]
- Dokudovskaya, S.; Waharte, F.; Schlessinger, A.; Pieper, U.; Devos, D.P.; Cristea, I.M.; Williams, R.; Salamero, J.; Chait, B.T.; Sali, A.; et al. A conserved coatomer-related complex containing Sec13 and Seh1 dynamically associates with the vacuole in Saccharomyces cerevisiae. Mol. Cell Proteom. 2011, 10, M110.006478. [Google Scholar] [CrossRef]
- West, M.; Zurek, N.; Hoenger, A.; Voeltz, G.K. A 3D analysis of yeast ER structure reveals how ER domains are organized by membrane curvature. J. Cell Biol. 2011, 193, 333–346. [Google Scholar] [CrossRef]
- Papanikou, E.; Glick, B.S. The yeast Golgi apparatus: Insights and mysteries. FEBS Lett. 2009, 583, 3746–3751. [Google Scholar] [CrossRef]
- Mueller, S.C.; Branton, D. Identification of coated vesicles in Saccharomyces cerevisiae. J. Cell Biol. 1984, 98, 341–346. [Google Scholar] [CrossRef]
- Huang, K.M.; D’Hondt, K.; Riezman, H.; Lemmon, S.K. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 1999, 18, 3897–3908. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Salminen, A.; Rice, L.M.; Brünger, A.T.; Brennwald, P. Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J. Biol. Chem. 1997, 272, 16610–16617. [Google Scholar] [CrossRef]
- Kukulski, W.; Picco, A.; Specht, T.; Briggs, J.A.; Kaksonen, M. Clathrin modulates vesicle scission, but not invagination shape, in yeast endocytosis. Elife 2016, 5, e16036. [Google Scholar] [CrossRef]
- Buser, C.; Drubin, D.G. Ultrastructural imaging of endocytic sites in Saccharomyces cerevisiae by transmission electron microscopy and immunolabeling. Microsc. Microanal. 2013, 19, 381–392. [Google Scholar] [CrossRef]
- Idrissi, F.Z.; Grötsch, H.; Fernández-Golbano, I.M.; Presciatto-Baschong, C.; Riezman, H.; Geli, M.I. Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. J. Cell Biol. 2008, 180, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.A.; Mironov, A.; Derganc, J.; Beznoussenko, G.V. Membrane Curvature, Trans-Membrane Area Asymmetry, Budding, Fission and Organelle Geometry. Int. J. Mol. Sci. 2020, 21, 7594. [Google Scholar] [CrossRef]
- Payne, G.S.; Schekman, R. Clathrin: A role in the intracellular retention of a Golgi membrane protein. Science 1989, 245, 1358–1365. [Google Scholar] [CrossRef]
- Payne, G.S. Genetic analysis of clathrin function in yeast. J. Membr. Biol. 1990, 116, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Okamoto, M.; Nakano, A. Contact of cis-Golgi with ER exit sites executes cargo capture and delivery from the ER. Nat. Commun. 2014, 5, 3653. [Google Scholar] [CrossRef]
- Ayscough, K.; Warren, G. Inhibition of protein synthesis disrupts the Golgi apparatus in the fission yeast, Schizosaccharomyces pombe. Yeast 1994, 10, 1–11. [Google Scholar] [CrossRef]
- Valdez-Taubas, J.; Pelham, H.R. Slow Diffusion of Proteins in the Yeast Plasma Membrane Allows Polarity to Be Maintained by Endocytic Cycling. Curr. Biol. 2003, 13, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Kuliawat, R.; Klumperman, J.; Ludwig, T.; Arvan, P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J. Cell Biol. 1997, 137, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.A.; Arvan, P. Origin of the regulated secretory pathway. In Golgi Apparatus; Mironov, A.A., Pavelka, M., Eds.; Chapter 3.11; Springer: Vienna, Austria, 2008; pp. 482–515. [Google Scholar] [CrossRef]
- Banfield, D.K.; Hong, W. SNAREs. In The Golgi Apparatus: State of the Art 110 Years After Camillo Golgi’s Discovery; Mironov, A.A., Pavelka, M., Eds.; Chapter 2.1; Springer: Vienna, Austria; New York, NY, USA, 2008; pp. 43–65. [Google Scholar]
- Malhotra, V.; Serafini, T.; Orci, L.; Shepherd, J.C.; Rothman, J.E. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 1989, 58, 329–336. [Google Scholar] [CrossRef]
- Beznoussenko, G.V.; Pilyugin, S.S.; Geerts, W.J.; Kozlov, M.M.; Burger, K.N.; Luini, A.; Derganc, J.; Mironov, A.A. Trans-membrane area asymmetry controls the shape of cellular organelles. Int. J. Mol. Sci. 2015, 16, 5299–5333. [Google Scholar] [CrossRef] [PubMed]
| Necessary Features | Model of Transport | ||
|---|---|---|---|
| VM | CM | KARM | |
| 1. COPII vesicles | necessary | not necessary | not necessary |
| 2. Golgi resident proteins in COPI vesicles | not necessary | necessary | not necessary |
| 3. Temporal fusion of the distal compartment with the proximal one | not necessary | not necessary | necessary |
| 4. Presence of GOS1 in 50-nm vesicles | not necessary | not necessary | necessary |
| 5. Presence of Sec22 in 50-nm vesicles | necessary | not necessary | not necessary |
| Membranes (N = 6) | Markers | ||||
|---|---|---|---|---|---|
| Sec22 | Sec21 | GOS1 | Sso1 | Sec13 | |
| Vesicles 40 nm | 111 ± 20 | 95 ± 18 | 98 ± 9 | 871 ± 80 * | 125 ± 30 |
| Vesicles 50 nm | 93 ± 11 | 99 ± 10 | 101 ± 21 | 110 ± 12 | 133 ± 24 |
| ER | 344 ± 15 * | 141 ± 26 | 121 ± 36 | 95 ± 24 | 232 ± 24 * |
| ERES | 563 ± 34 * | 710 ± 68 * | 489 ± 46 * | 89 ± 27 | 583 ± 35 * |
| Golgi | 182 ± 41 * | 637 ± 53 * | 701 ± 27 * | 119 ± 17 | 154 ± 56 |
| PM | 109 ± 19 | 120 ± 24 | 133 ± 25 | 1012 ± 134 * | 126 ± 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironov, A.A.; Fusella, A.; Beznoussenko, G.V. The 50-nm Free Vesicles Visible in Saccharomyces cerevisiae Are Not COPII-Dependent. Curr. Issues Mol. Biol. 2025, 47, 336. https://doi.org/10.3390/cimb47050336
Mironov AA, Fusella A, Beznoussenko GV. The 50-nm Free Vesicles Visible in Saccharomyces cerevisiae Are Not COPII-Dependent. Current Issues in Molecular Biology. 2025; 47(5):336. https://doi.org/10.3390/cimb47050336
Chicago/Turabian StyleMironov, Alexander A., Aurora Fusella, and Galina V. Beznoussenko. 2025. "The 50-nm Free Vesicles Visible in Saccharomyces cerevisiae Are Not COPII-Dependent" Current Issues in Molecular Biology 47, no. 5: 336. https://doi.org/10.3390/cimb47050336
APA StyleMironov, A. A., Fusella, A., & Beznoussenko, G. V. (2025). The 50-nm Free Vesicles Visible in Saccharomyces cerevisiae Are Not COPII-Dependent. Current Issues in Molecular Biology, 47(5), 336. https://doi.org/10.3390/cimb47050336





