Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry and Network Pharmacology Reveal the Mechanisms of Rhodiola crenulata in Improving Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Extracts and Medicinal Serum
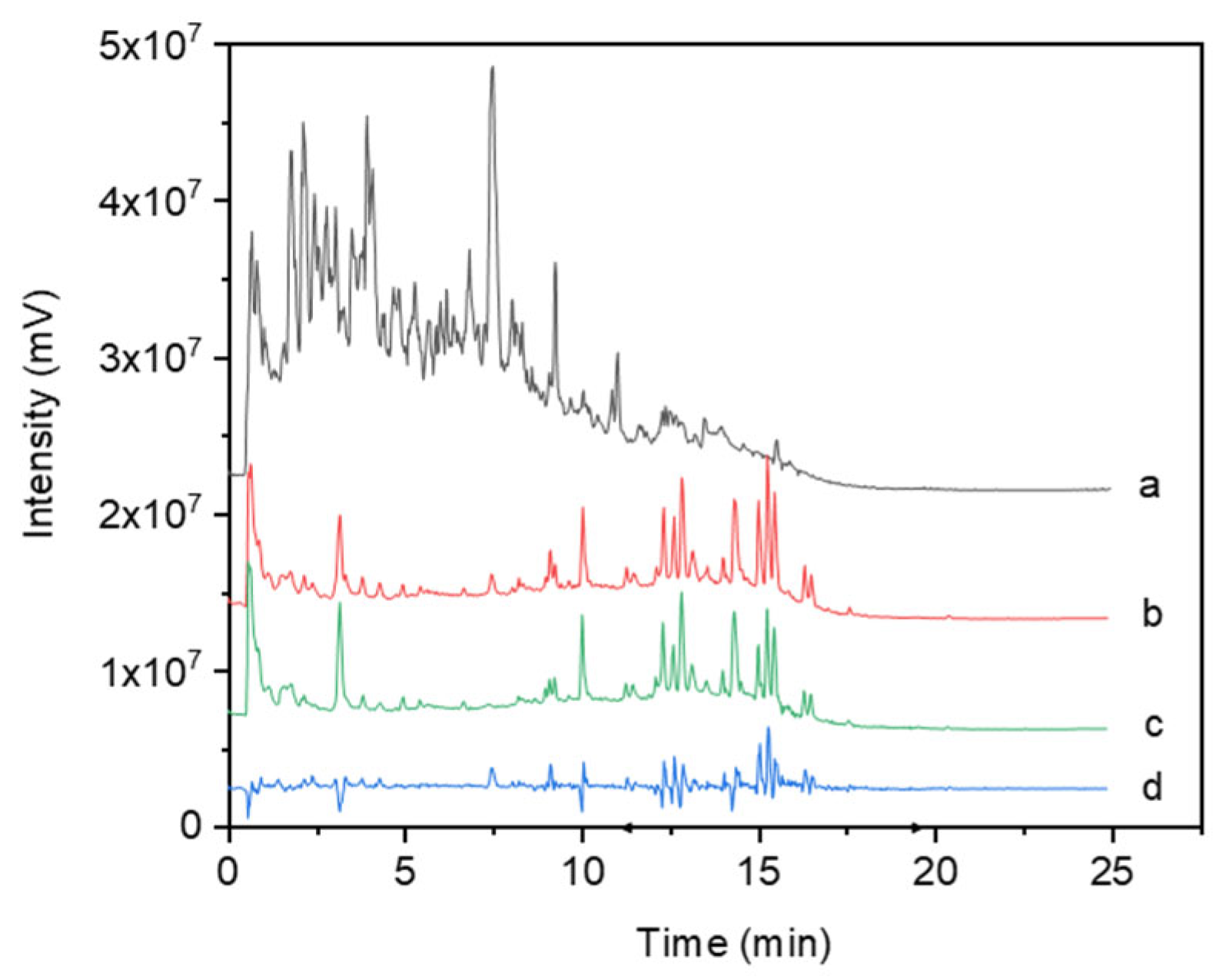
2.3. UHPLC-MS/MS Analysis
2.4. Network Pharmacology Analysis of Blood-Absorbed Components of Rhodiola crenulata
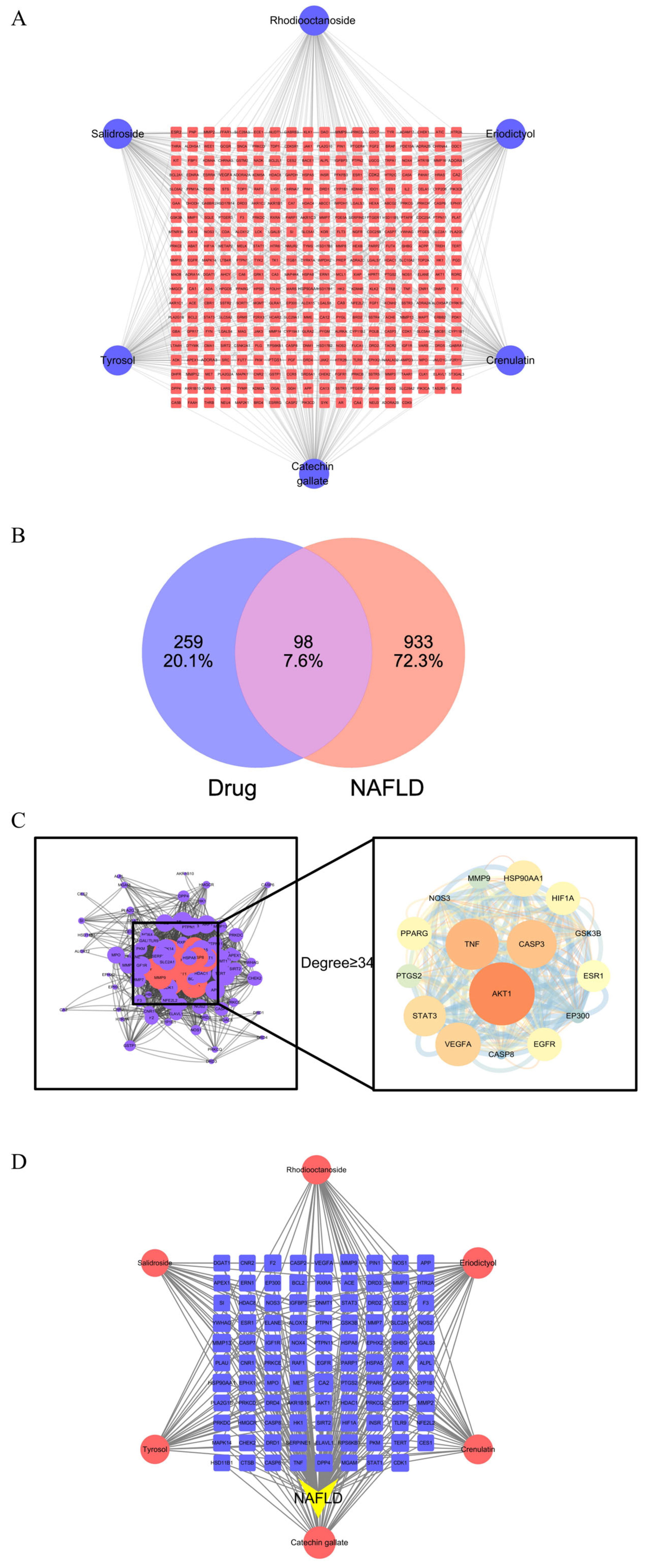
2.4.1. Identification of Active Components and Target Proteins: Construction of the “Drug-Target” Network
2.4.2. Intersection of Drug Targets and Disease Targets
2.4.3. Protein–Protein Interaction (PPI) Network
2.4.4. Construction of the “Drug–Disease–Target” Network
2.4.5. GO Biological Enrichment and KEGG Pathway Enrichment Analysis
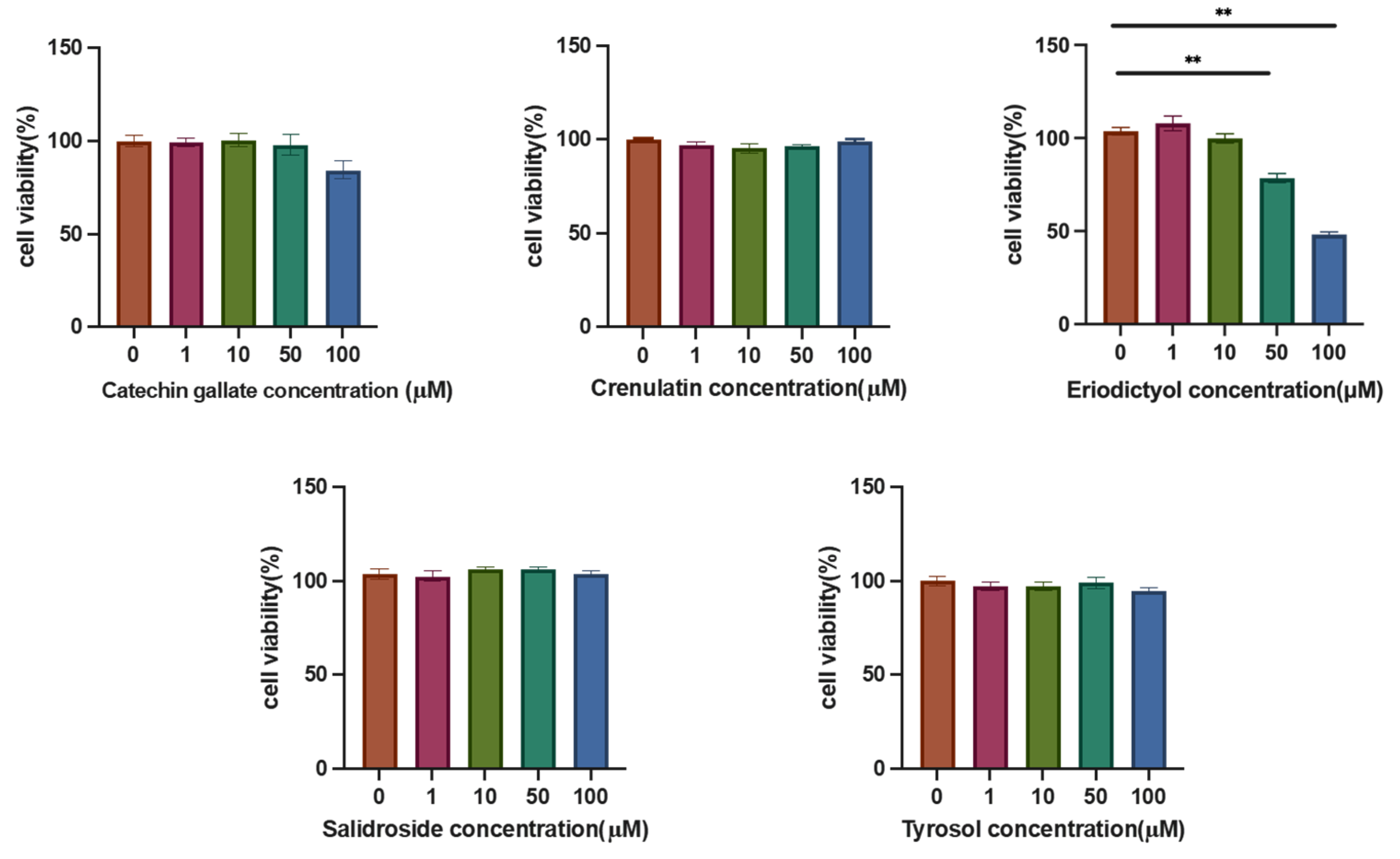
2.5. Cell Culture and Cell Viability
2.6. TG Content Measurement
2.7. Oil Red O Staining
2.8. Molecular Docking of Potential Targets
2.9. Molecular Dynamics (MD) Simulation
2.10. Data Analysis
3. Results
3.1. Analysis of the Effects of Rhodiola Crenulata Extract on Blood Components (In Vivo)
3.2. Target Network Analysis
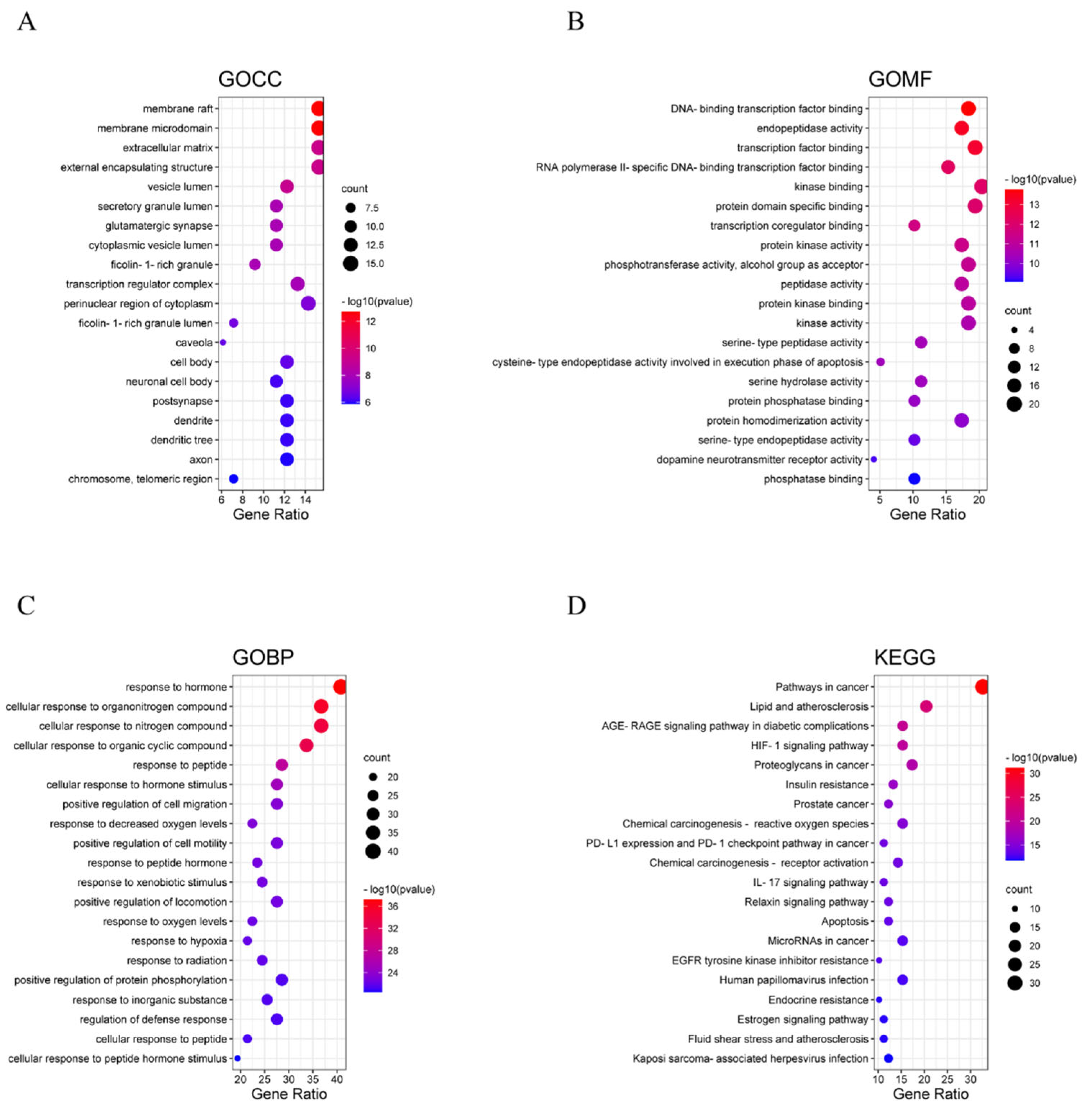
3.3. Pathway Enrichment Analysis
3.3.1. GO Biological Enrichment Analysis
3.3.2. KEGG Pathway Enrichment Analysis
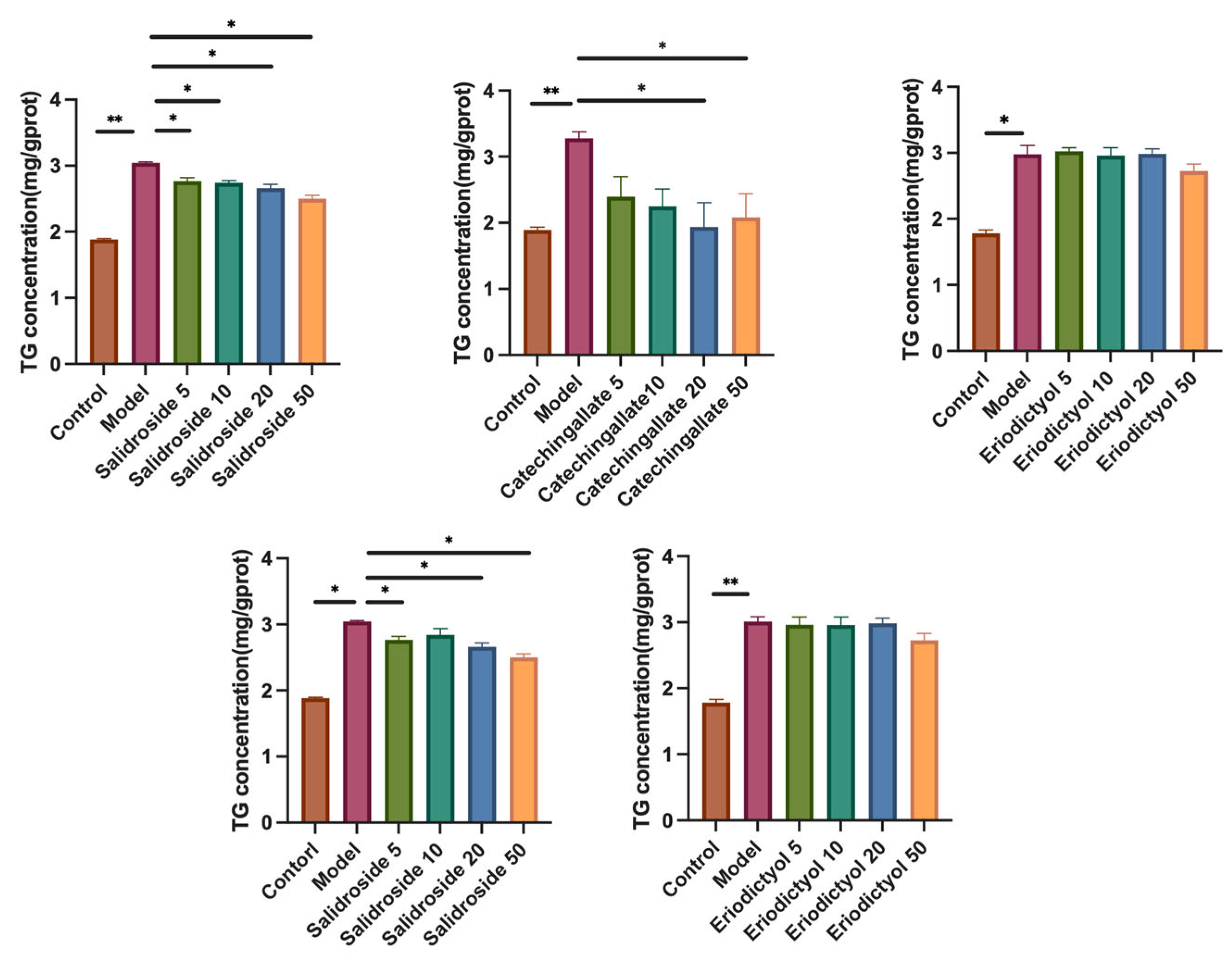
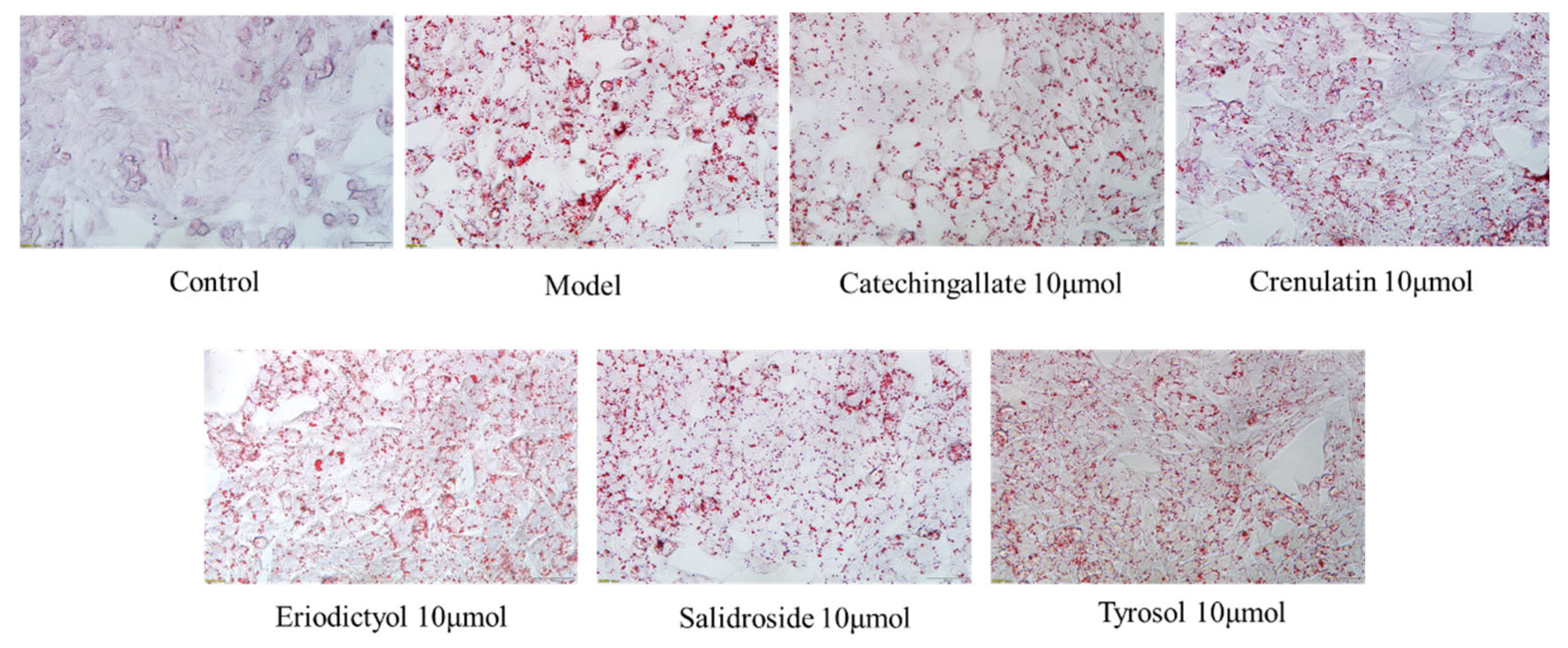
3.4. Effects of Various Components on Hepatic Lipid Accumulation
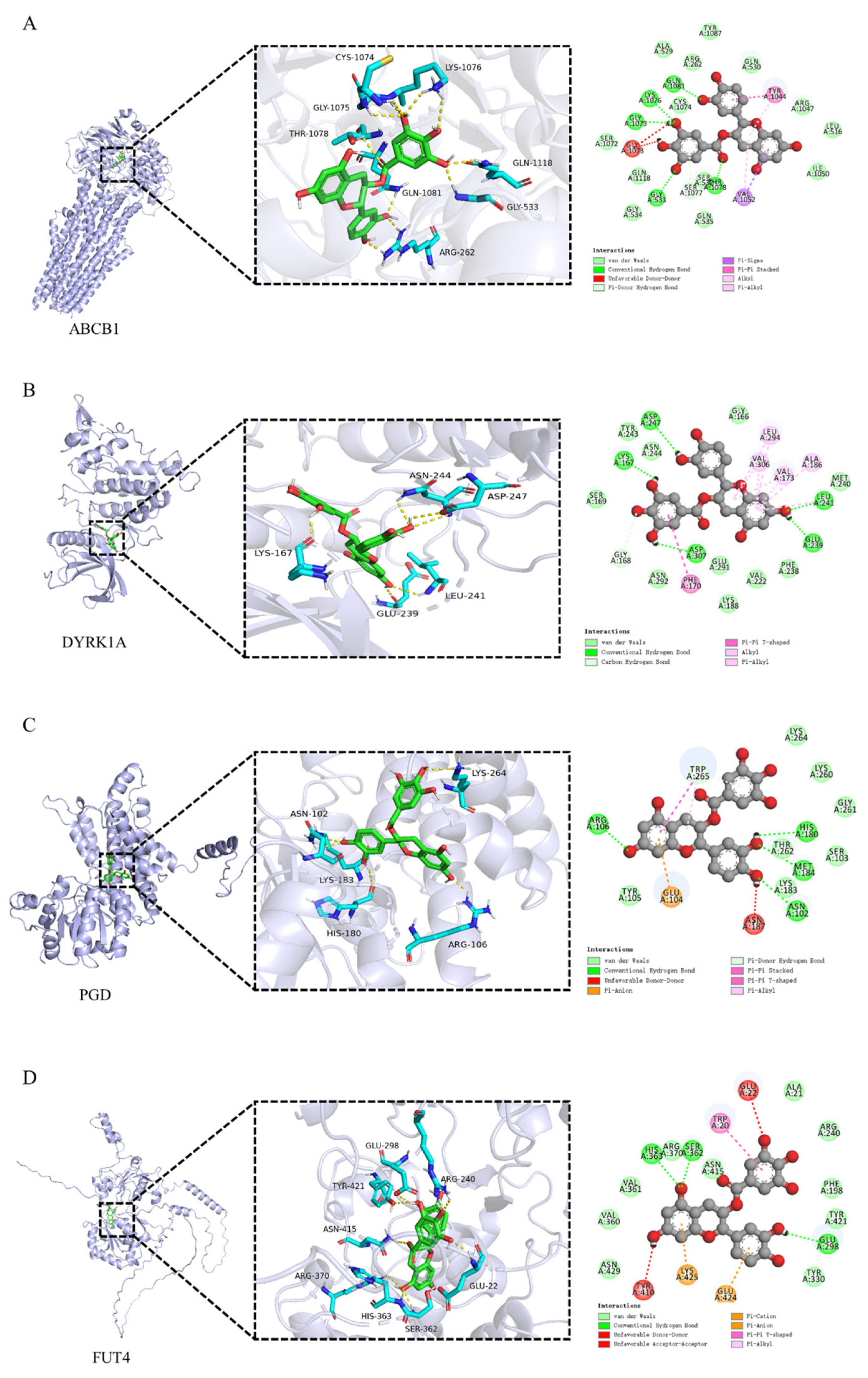
3.5. Molecular Docking of Catechin Gallate with Core Targets
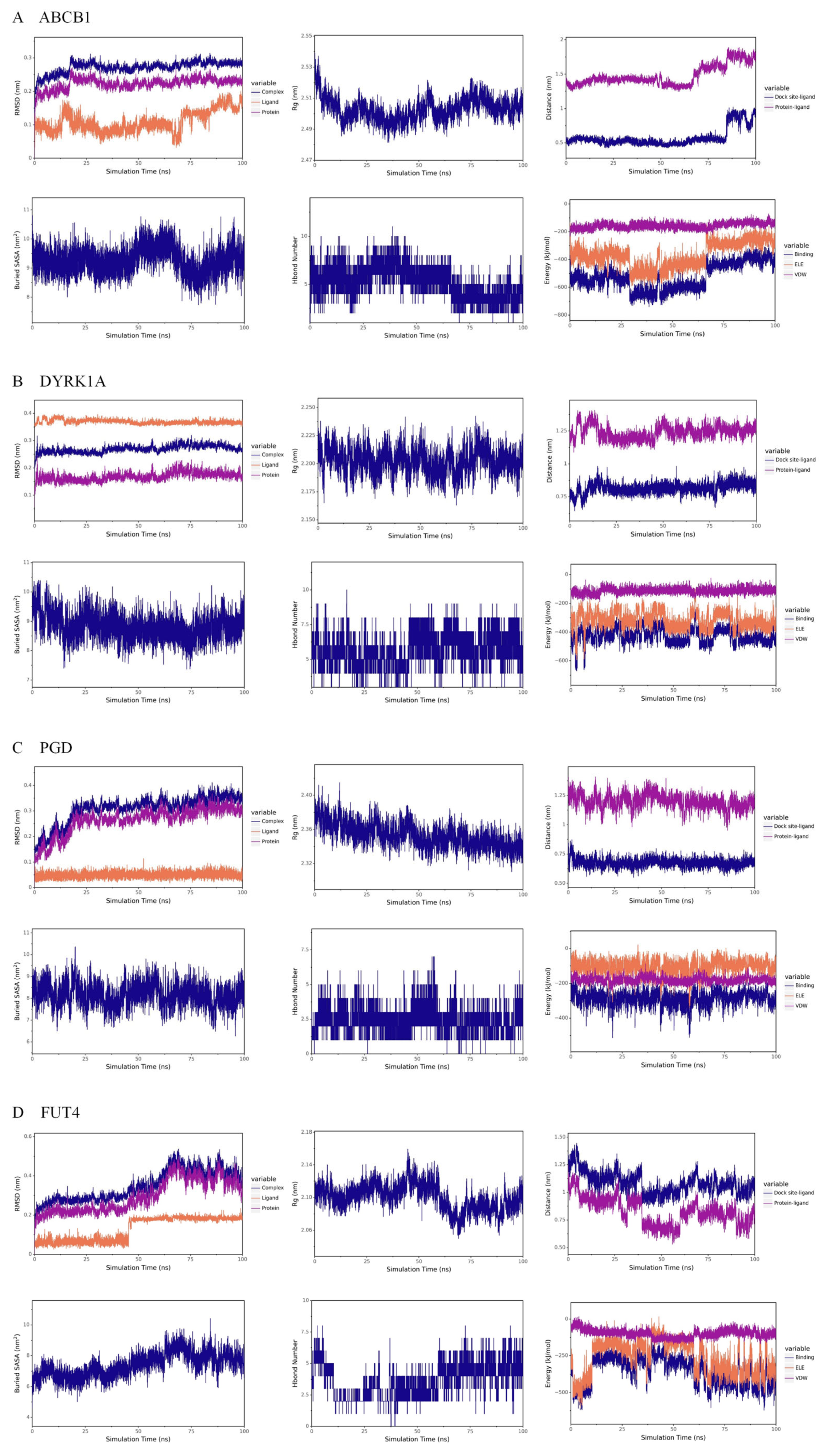
3.6. Molecular Dynamics Simulation Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, L.; Zhong, F.W.; Chen, Z.L.; Li, G.X.; Zhu, Q. Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet. Food Sci. Hum. Well. 2022, 11, 1045–1052. [Google Scholar] [CrossRef]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, R.; Wang, M.; Chen, Y.; Chen, Z.; Ke, X.; Zuo, L.; Wang, J. Baicalein prevents fructose-induced hepatic steatosis in rats: In the regulation of fatty acid de novo synthesis, fatty acid elongation and fatty acid oxidation. Front. Pharmacol. 2022, 13, 917329. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Keam, S.J. Resmetirom: First Approval. Drugs 2024, 84, 729–735. [Google Scholar] [CrossRef]
- Ray, K. Resmetirom proves positive for NASH with liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 218. [Google Scholar] [CrossRef]
- Kokkorakis, M.; Boutari, C.; Hill, M.A.; Kotsis, V.; Loomba, R.; Sanyal, A.J.; Mantzoros, C.S. Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: Trials, opportunities, and challenges. Metab. Clin. Exp. 2024, 154, 155835. [Google Scholar] [CrossRef]
- Ma, C.; Wang, C.; Zhang, Y.; Zhou, H.; Li, Y. Potential natural compounds for the prevention and treatment of nonalcoholic fatty liver disease: A review on molecular mechanisms. Curr. Mol. Pharmacol. 2022, 15, 846–861. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, S.; Peng, Z.; Wang, M.; Zhao, K.; Xu, B.B.; Yin, X.; Ibrahim, M.M.; Mersal, G.A.M.; El-Bahy, Z.M.; et al. Rapid screening and sensing of stearoyl-CoA desaturase 1 (SCD1) inhibitors from ginger and their efficacy in ameliorating non-alcoholic fatty liver disease. J. Food Meas. Charact. 2024, 18, 6843–6857. [Google Scholar] [CrossRef]
- Khanna, K.; Mishra, K.P.; Ganju, L.; Singh, S.B. Golden root: A wholesome treat of immunity. Biomed. Pharmacother. 2017, 87, 496–502. [Google Scholar] [CrossRef]
- Recio, M.C.; Giner, R.M.; Manez, S. Immunmodulatory and antiproliferative properties of Rhodiola species. Planta Med. 2016, 82, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wang, L.; Jin, Y.; Gu, L.; Yin, G.; Wang, J.; Yu, X.A.; Huang, H.; Zhang, Z.; Wang, B.; et al. Chemical characteristics of Rhodiola crenulata and its mechanism in acute mountain sickness using UHPLC-Q-TOF-MS/MS combined with network pharmacology analysis. J. Ethnopharmacol. 2022, 294, 115345. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Jia, N.; Pei, C.; Shen, Z.; Zhao, S.; Wang, Y.; Wu, Y.; Shi, S.; Li, S.; Wang, Z. Rosavidin protects against PM2.5-induced lung toxicity via inhibition of NLRP3 inflammasome-mediated pyroptosis by activating the PI3K/AKT pathway. Biochem. Pharmacol. 2023, 213, 115623. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Wu, L.; Li, J.X.; Cailang, B.; Zhao, X.H.; Yue, H.L. Rhodiosin from Rhodiola crenulata effectively alleviate postprandial hyperglycemia by inhibiting both the activity and production of α- glucosidase. Phytomedicine 2024, 132, 155836. [Google Scholar] [CrossRef]
- Liang, T.S.; Zhou, J.X.; Jing, P.; He, Z.J.; Jiao, S.S.; Zhao, W.J.; Tong, Q.; Jia, G.F. Anti-senescence effects of Rhodiola crenulate extracts on LO2 cells and bioactive compounds. J. Ethnopharmacol. 2023, 306, 116179. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, F.; Wang, X.; Cheng, Y.; Guo, Y.; Qian, H.; Liu, Y. Reshaped gut microbial composition and functions associated with the antifatigue effect of salidroside in exercise mice. Mol. Nutr. Food Res. 2023, 67, e2300015. [Google Scholar] [CrossRef]
- Xie, N.; Fan, F.F.; Jiang, S.N.; Hou, Y.; Zhang, Y.; Cairang, N.J.; Wang, X.B.; Meng, X.L. Rhodiola crenulate alleviates hypobaric hypoxia-induced brain injury via adjusting NF-κB/NLRP3-mediated inflammation. Phytomedicine 2022, 103, 154240. [Google Scholar] [CrossRef]
- Lin, K.T.; Hsu, S.W.; Lai, F.Y.; Chang, T.C.; Shi, L.S.; Lee, S.Y. Rhodiola crenulata extract regulates hepatic glycogen and lipid metabolism via activation of the AMPK pathway. BMC Complement. Altern. Med. 2016, 16, 127. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lai, F.Y.; Shi, L.S.; Chou, Y.C.; Yen, I.C.; Chang, T.C. Rhodiola crenulata extract suppresses hepatic gluconeogenesis via activation of the AMPK pathway. Phytomedicine 2015, 22, 477–486. [Google Scholar] [CrossRef]
- Yi, Q.; Sun, P.; Li, J.; Kong, S.; Tian, J.; Li, X.; Yang, Y.; Zhang, P.; Liu, Y.; Han, J.; et al. Rho, a fraction from Rhodiola crenulate, ameliorates hepatic steatosis in mice models. Front. Physiol. 2018, 9, 222. [Google Scholar] [CrossRef]
- Wang, J.; Rong, X.; Li, W.; Yang, Y.; Yamahara, J.; Li, Y. Rhodiola crenulata root ameliorates derangements of glucose and lipid metabolism in a rat model of the metabolic syndrome and type 2 diabetes. J. Ethnopharmacol. 2012, 142, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jin, Y.; Yao, L.; Liu, L.; Li, J.; Li, H.; Lai, Y.; Chen, Z.; Pan, Z.; Han, T.; et al. Rhodiola crenulata root extract ameliorates fructose-induced hepatic steatosis in rats: Association with activating autophagy. Biomed. Pharmacother. 2020, 125, 109836. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Ding, M.; Ma, Z.; Li, S.; Qu, J.; Li, X. Chuanxiong Rhizoma extracts prevent liver fibrosis via targeting CTCF-c-MYC-H19 pathway. Chin. Herb. Med. 2024, 16, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Zhu, Y.; Zhang, X.W.; Yang, K.P.; Yang, X.; An, M.Y.; Tian, C.L.; Li, J. Based on network pharmacology and experiments to explore the underlying mechanism of Mahonia bealei (Fortune) Carri’ere for treating alcoholic hepatocellular carcinoma. J. Ethnopharmacol. 2024, 318, 116919. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Zhang, J.; Zhao, Y.; Hua, Y.; Zhang, C.; Zheng, G.; Yang, G.; Guan, J.; Li, H.; et al. Exploring Ganweikang Tablet as a Candidate Drug for NAFLD Through Network Pharmacology Analysis and Experimental Validation. Front. Pharmacol. 2022, 13, 893336. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Z.; Zhang, L.; Xue, C.; Feng, X.; Chai, X.; Wang, Y. Identification of prototype compounds and their metabolites in rats’ serum from Xuefu Zhuyu Decoction by UPLC-Q-TOF/MS. Chin. Herb. Med. 2023, 15, 139–150. [Google Scholar] [CrossRef]
- Wang, L.; Pu, X.L.; Nie, X.; Wang, D.; Jiang, H.J.; Chen, Y.; Pang, L.; Wang, S.J.; Wang, X.; Xu, Z.Y.; et al. Integrated serum pharmacochemistry and network pharmacological analysis used to explore possible anti-rheumatoid arthritis mechanisms of the Shentong-Zhuyu decoction. J. Ethnopharmacol. 2021, 273, 113988. [Google Scholar] [CrossRef]
- Xiang, W.; Xia, Z.N.; Xu, L. UPLC-MS/MS profiling, antioxidant, α-glucosidase inhibitory, cholinesterase inhibitory, and cardiovascular protection potentials of Jialing 20 (Morus multicaulis Perr.) Mulberry Branch Extract. Foods 2021, 10, 2659. [Google Scholar] [CrossRef]
- Shi, X.W.; Xu, L.; Zhang, J.Q.; Mo, J.F.; Zhuang, P.; Zheng, L. Oxyresveratrol from mulberry branch extract protects HUVECs against oxidized Low-density Lipoprotein-induced oxidative injury via activation of the Nrf-2/HO-1 pathway. J. Funct. Foods 2023, 100, 105371. [Google Scholar] [CrossRef]
- Xiang, W.; Xu, L.; Zheng, L.; Zhang, Q.A.; Shi, X.W. Purification, structural characterization, and immunoregulatory activity of a polysaccharide from mulberry branch. Chem. Biol. Technol. Agric. 2024, 11, 39. [Google Scholar] [CrossRef]
- Yue, H.; Wang, L.; Jiang, S.; Banma, C.; Jia, W.; Tao, Y.; Zhao, X. Hypoglycemic effects of Rhodiola crenulata (HK. f. et. Thoms) H. Ohba in vitro and in vivo and its ingredient identification by UPLC-triple-TOF/MS. Food Funct. 2022, 13, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Li, Y.; Mao, X.; Xu, R.; Yin, R. Characterization of chemical constituents in Rhodiola Crenulate by high-performance liquid chromatography coupled with Fourier-transform ion cyclotron resonance mass spectrometer (HPLC-FT-ICR MS). J. Mass Spectrom. 2016, 51, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Al-Massarani, S.M.; El Gamal, A.A.; Abd El Halim, M.F.; Al-Said, M.S.; Abdel-Kader, M.S.; Basudan, O.A.; Alqasoumi, S.I. New acyclic secondary metabolites from the biologically active fraction of Albizia lebbeck flowers. Saudi Pharm. J. 2017, 25, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.C.; Wu, X.Y.; Yi, H.; Chen, R.; Fan, G. The Therapeutic Effects and Mechanisms of Salidroside on Cardiovascular and Metabolic Diseases: An Updated Review. Chem. Biodivers. 2021, 18, e2100033. [Google Scholar] [CrossRef]
- Liu, J.X.; Cai, J.P.; Zhang, N.S.; Tai, J.D.; Fan, P.; Dong, X.; Cao, Y.G. Salidroside protects mice from high-fat diet-induced obesity by modulating the gut microbiota. Int. Immunopharmacol. 2023, 120, 110278. [Google Scholar] [CrossRef]
- Hanau, S.; Helliwell, J.R. 6-Phosphogluconate dehydrogenase and its crystal structures. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2022, 78, 96–112. [Google Scholar] [CrossRef]
- Asemani, M.; Sepahdari, A.; Hafezieh, M.; Dadgar, S. Effect of different carbohydrate-to-lipid ratios elicits growth, feed utilization, lipid deposition and lipogenic enzyme activity in striped catfish (Pangasianodon hypophthalmus, Sauvage, 1878) fingerlings. Iran. J. Fish. Sci. 2020, 19, 1818–1827. [Google Scholar] [CrossRef]
- Chen, Q.L.; Luo, Z.; Shi, X.; Wu, K.; Zhuo, M.Q.; Song, Y.F.; Hu, W. Dietary methimazole-induced hypothyroidism reduces hepatic lipid deposition by down-regulating lipogenesis and up-regulating lipolysis in Pelteobagrus fulvidraco. Gen. Comp. Endocrinol. 2015, 217, 28–36. [Google Scholar] [CrossRef]
- Margier, M.; Collet, X.; le May, C.; Desmarchelier, C.; André, F.; Lebrun, C.; Defoort, C.; Bluteau, A.; Borel, P.; Lespine, A.; et al. ABCB1 (P-glycoprotein) regulates vitamin D absorption and contributes to its transintestinal efflux. FASEB J. 2019, 33, 2084–2094. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.X.; Wang, J.X.; Ren, C.Y.; Chen, H.; Zhang, J.F. DYRK1A inhibitors for disease therapy: Current status and perspectives. Eur. J. Med. Chem. 2022, 229, 114062. [Google Scholar] [CrossRef]
- Yang, Y.P.; Fan, X.X.; Liu, Y.J.; Ye, D.Y.; Liu, C.; Yang, H.L.; Su, Z.J.; Zhang, Y.Y.; Liu, Y.G. Function and inhibition of DYRK1A: Emerging roles of treating multiple human diseases. Biochem. Pharmacol. 2023, 212, 115521. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Xiong, X.; Liu, L.; Liang, Q.; Tong, R.S.; Feng, X.L.; Bai, L.; Shi, J.Y. Recent research and development of DYRK1A inhibitors. Chin. Chem. Lett. 2022, 33, 1841–1849. [Google Scholar] [CrossRef]
- Liu, E.C.; Qian, X.W.; He, Y.; Chen, K.L. FUT4 promotes the progression of Cholangiocarcinoma by modulating epithelial-mesenchymal transition. Cell Cycle 2024, 23, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Adhikari, E.; Lester, D.K.; Fang, B.; Johnson, J.O.; Tian, Y.J.; Mockabee-Macias, A.T.; Izumi, V.; Guzman, K.M.; White, M.G.; et al. Androgen drives melanoma invasiveness and metastatic spread by inducing tumorigenic fucosylation. Nat. Commun. 2024, 15, 1148. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.D.; Chen, Y.Y.; Liu, J.J.; Xue, J.H.; Sheng, X.M.; Qin, J.; Xue, Q.; Liu, X.C. Silencing FUT4 inhibits the progression of osteosarcoma through activation of FOXO1. Curr. Pharm. Des. 2024, 30, 440–447. [Google Scholar] [CrossRef]
- Wang, H.; Cui, X.Y.; Wang, L.Y.; Fan, N.N.; Yu, M.; Qin, H.M.; Liu, S.; Yan, Q. α1,3-fucosylation of MEST promotes invasion potential of cytotrophoblast cells by activating translation initiation. Cell Death Dis. 2023, 14, 651. [Google Scholar] [CrossRef]
- Shikha, D.; Singh, A.; Rangra, N.K.; Monga, V.; Bhatia, R. Insights to therapeutic potentials, pharmaceutical formulations, chemistry and analytical methods of catechin. Phytochem. Rev. 2024, 23, 1557–1598. [Google Scholar] [CrossRef]
- Du, Y.; Paglicawan, L.; Soomro, S.; Abunofal, O.; Baig, S.; Vanarsa, K.; Hicks, J.; Mohan, C. Epigallocatechin-3-gallate dampens non-alcoholic fatty liver by modulating liver function, Lipid profile, and Macrophage polarization. Nutrients 2021, 13, 599. [Google Scholar] [CrossRef]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef]
- Wu, D.D.; Liu, Z.G.; Wang, Y.Z.; Zhang, Q.Q.; Li, J.M.; Zhong, P.Y.; Xie, Z.W.; Ji, A.L.; Li, Y.Z. Epigallocatechin-3-gallate alleviates high-fat diet-induced nonalcoholic fatty liver disease via inhibition of apoptosis and promotion of autophagy through the ROS/MAPK signaling pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5599997. [Google Scholar] [CrossRef]
- James, A.; Wang, K.; Wang, Y.S. Therapeutic activity of green tea epigallocatechin-3-gallate on metabolic diseases and non-alcoholic fatty liver diseases: The current updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, X.; Wang, T.T.; Wu, S.S.; Guan, H.N.; Wang, D.X. (-)-Epigallocatechin-3-gallate reduces perfluorodecanoic acid-exacerbated adiposity and hepatic lipid accumulation in high-fat diet-fed male C57BL/6J mice. Molecules 2023, 28, 7832. [Google Scholar] [CrossRef]
| Time | Name | Molecular Formula | [M−H]− | Ms/Ms | Ref. |
|---|---|---|---|---|---|
| 1.78 | Salidroside | C14H20O7 | 299.1130 | 203.0832, 119.0515, 89.0247, 71.0148 | [12,31] |
| 1.99 | Tyrosol | C8H10O2 | 137.0260 | 93.0350 | [12] |
| 2.15 | Crenulatin | C11H20O6 | 247.1196 | 167.0762, 101.0255, 85.0293 | [12] |
| 3.90 | Catechin gallate | C22H18O10 | 441.1007 | 289.0736, 245.0763, 169.0125, 125.0227 | [31] |
| 6.43 | Eriodictyol | C15H12O6 | 287.0537 | 151.0043, 96.9618 | [32] |
| 7.46 | Rhodiooctanoside | C19H36O10 | 423.2205 | 291.1818, 161.0476, 101.0245, 89.0239, 59.0135 | [33] |
| Target | Common Name | Target Class | Probability | PDB ID | Binding Energy |
|---|---|---|---|---|---|
| 6-phosphogluconate dehydrogenase | PGD | Enzyme | 0.999640903 | 4GWG | −9.2 |
| Beta-secretase 1 | BACE1 | Protease | 0.999640903 | 1TQF | −7.5 |
| Apoptosis regulator Bcl-2 | BCL2 | Other ion channel | 0.928564807 | 1G5M | −8 |
| MAP kinase p38 alpha | MAPK14 | Kinase | 0.895379597 | 1A9U | −8.6 |
| Microtubule-associated protein tau | MAPT | Unclassified protein | 0.862200422 | 2MZ7 | −5.9 |
| DNA (cytosine-5)-methyltransferase 1 | DNMT1 | Writer | 0.862200422 | 3PTA | −8.6 |
| Dual-specificity tyrosine-phosphorylation regulated kinase 1A | DYRK1A | Kinase | 0.862200422 | 4YLK | −9.6 |
| Beta amyloid A4 protein | APP | Membrane receptor | 0.862200422 | 1AAP | −7.7 |
| Telomerase reverse transcriptase | TERT | Enzyme | 0.862200422 | 5UGW | −7.4 |
| Matrix metalloproteinase 2 | MMP2 | Protease | 0.862200422 | 1CK7 | −8.4 |
| Hepatocyte growth factor receptor | MET | Kinase | 0.862200422 | 1R0P | −8.6 |
| Matrix metalloproteinase 14 | MMP14 | Protease | 0.862200422 | 3MA2 | −8.1 |
| P-glycoprotein 1 | ABCB1 | Primary active transporter | 0.862200422 | 6C0V | −10.5 |
| Signal transducer and activator of transcription 1-alpha/beta | STAT1 | Transcription factor | 0.862200422 | 7NUF | −8.7 |
| Squalene monooxygenase (by homology) | SQLE | Enzyme | 0.862200422 | 6C6N | −8 |
| CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase | ST3GAL3 | Transferase | 0.812371847 | α-Fold | −8.5 |
| Alpha-(1,3)-fucosyltransferase 7 | FUT7 | Transferase | 0.812371847 | α-Fold | −8.1 |
| Fucosyltransferase 4 | FUT4 | Enzyme | 0.812371847 | α-Fold | −9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Wang, J.; Xu, Q.; Deng, C.; Yi, X.; Wang, S.; Yao, L.; Xiang, W. Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry and Network Pharmacology Reveal the Mechanisms of Rhodiola crenulata in Improving Non-Alcoholic Fatty Liver Disease. Curr. Issues Mol. Biol. 2025, 47, 324. https://doi.org/10.3390/cimb47050324
Zeng X, Wang J, Xu Q, Deng C, Yi X, Wang S, Yao L, Xiang W. Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry and Network Pharmacology Reveal the Mechanisms of Rhodiola crenulata in Improving Non-Alcoholic Fatty Liver Disease. Current Issues in Molecular Biology. 2025; 47(5):324. https://doi.org/10.3390/cimb47050324
Chicago/Turabian StyleZeng, Xin, Jianwei Wang, Qinyi Xu, Chengdan Deng, Xi Yi, Shang Wang, Ling Yao, and Wei Xiang. 2025. "Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry and Network Pharmacology Reveal the Mechanisms of Rhodiola crenulata in Improving Non-Alcoholic Fatty Liver Disease" Current Issues in Molecular Biology 47, no. 5: 324. https://doi.org/10.3390/cimb47050324
APA StyleZeng, X., Wang, J., Xu, Q., Deng, C., Yi, X., Wang, S., Yao, L., & Xiang, W. (2025). Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry and Network Pharmacology Reveal the Mechanisms of Rhodiola crenulata in Improving Non-Alcoholic Fatty Liver Disease. Current Issues in Molecular Biology, 47(5), 324. https://doi.org/10.3390/cimb47050324





