Nephroprotective Effects of Tanacetum balsamita Extract on Metabolic-Induced Renal Injury (MIRI) in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Plant Material, Sample Extraction, and Phytochemical Analyses
2.3. Experimental Design
- Group 1—control group animals, with free access to fresh water and normal pelleted food;
- Group 2—rats orally treated with low dose (150 mg/kg/p.o.) of extract of T. balsamita (ETBld) for 8 weeks;
- Group 3—rats given high dose (300 mg/kg/p.o.) of extract of T. balsamita (ETBhd) for 8 weeks;
- Group 4—rats with induced metabolic syndrome and subsequent MIRI;
- Group 5—MIRI group treated orally once a day with enalapril (enlp, 5 mg/kg/day) [18] (from 5th to 8th week) as the positive control for hypertension;
- Group 6—MIRI rats treated orally once a day with acarbose (5 mg/kg) [19] (from 5th to 8th week) as the positive control for diabetes type 2 (DT2);
- Group 7—MIRI rats treated with ETBld for the whole period (from 1st week to the end of the 8th week);
- Group 8—MIRI rats treated with ETBhd for the whole period;
2.4. Assessment of Serum Biochemical Parameters
2.5. Urinalysis
2.6. Assessment of the Oxidative Stress Biomarkers
2.7. Histopathological Examination
2.8. Statistical Analysis
3. Results
3.1. Assessment of Blood Glucose Level and Systolic Blood Pressure
3.2. Oxidative Stress Biomarkers
3.3. Assessment of Serum Biochemical Parameters and Urinalysis
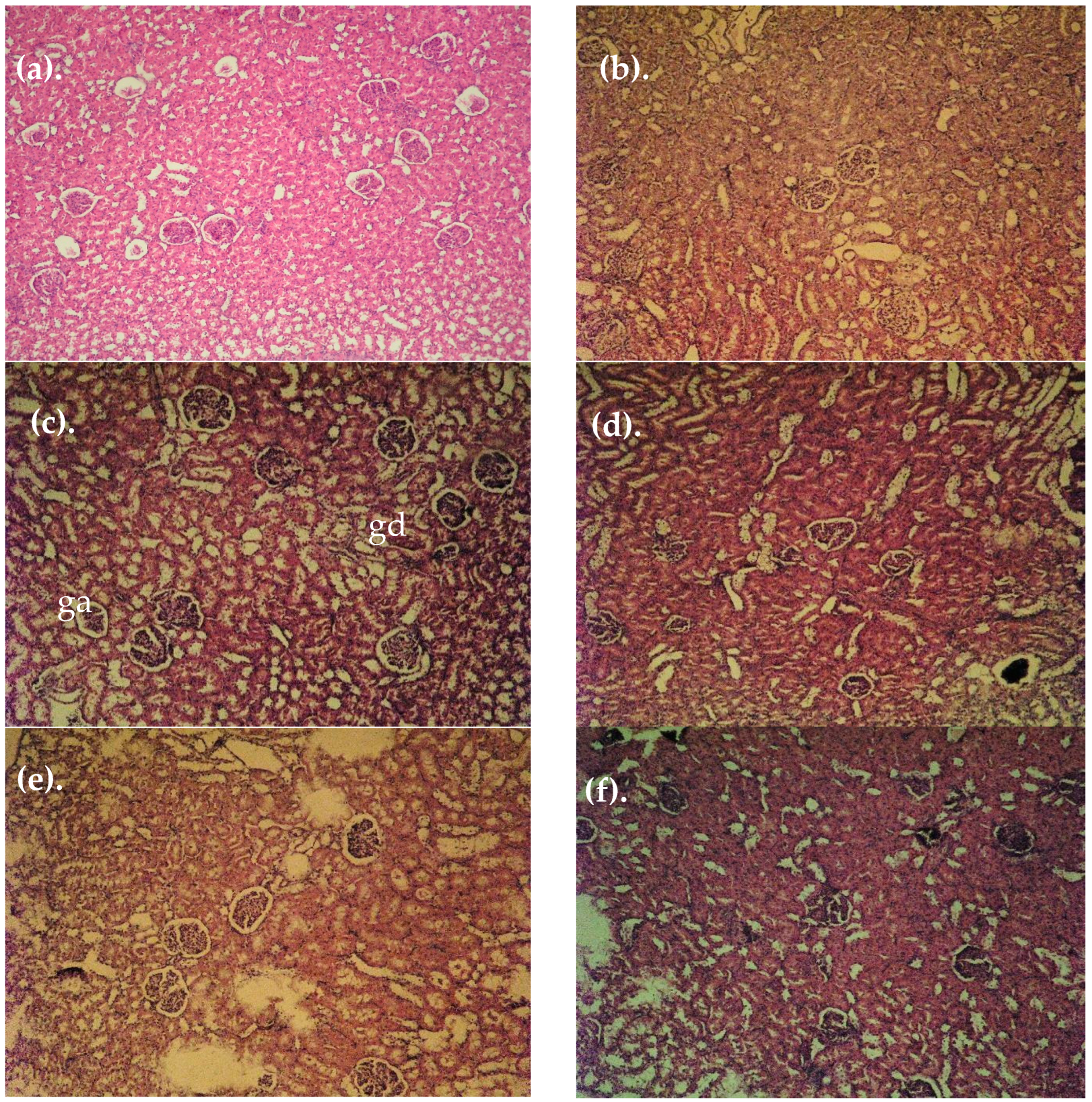
3.4. Histopathological Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIRI | Metabolic-induced renal injury |
| MAFLD | Metabolic-associated fatty liver disease |
| ETB | Tanacetum balsamita leaves extract |
| DCQA | Dicaffeoylquinic acids |
| ESRD | End-stage renal disease |
| DN | Diabetic nephropathy |
| MDA | Malondialdehyde |
| GR | Glutathione reductase |
| GPx | Glutathione peroxidase |
| GST | Glutathione-S-transferase |
| SOD | Superoxide dismutase |
| RAS | Renin-angiotensin system |
| GLP-1 | Glucagon-like peptide-1 |
| nsMRAs | Nonsteroidal mineralocorticoid receptor antagonists |
| (UHPLC-DAD) | Ultra-high-performance liquid chromatography–Diode Array Detection |
| GAE | Gallic acid equivalent |
| QE | Quercetin equivalent |
| CGA | Chlorogenic acid |
| DE | Dry extract |
| ROS | Reactive oxygen species |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| HFD | High-fat diet |
| GLUT4 | Glucose transporter 4 |
| TGF | Transforming growth factor |
| TNF | Tumor necrosis factor |
References
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. [Google Scholar]
- Han, S.; Kim, S. A New Era in Diabetic Kidney Disease Treatment: The Four Pillars and Strategies to Build Beyond. Electrolyte Blood Press. 2024, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Buławska, D.; Czarnik, W.; Hajdys, J.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Novel Insights into Diabetic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 10222. [Google Scholar] [CrossRef]
- El-Askary, H.; Salem, H.H.; Abdel Motaal, A. Potential Mechanisms Involved in the Protective Effect of Dicaffeoylquinic Acids from Artemisia annua L. Leaves against Diabetes and Its Complications. Molecules 2022, 27, 857. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Li, Y.; Famurewa, A.C.; Olatunji, O.J. Antidiabetic and Nephroprotective Effects of Polysaccharide Extract from the Seaweed Caulerpa Racemosa in High Fructose-Streptozotocin Induced Diabetic Nephropathy. DMSO 2021, 14, 2121–2131. [Google Scholar] [CrossRef]
- Mihaylova, R.; Gevrenova, R.; Petrova, A.; Savov, Y.; Zheleva-Dimitrova, D.; Balabanova, V.; Momekov, G.; Simeonova, R. Mitigating Effects of Tanacetum balsamita L. on Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Plants 2024, 13, 2086. [Google Scholar] [CrossRef]
- Sharif, M.; Najafizadeh, P.; Asgarpanah, J.; Mousavi, Z. In Vivo Analgesic and Anti-Inflammatory Effects of the Essential Oil from Tanacetum balsamita L. Braz. J. Pharm. Sci. 2020, 56, e18357. [Google Scholar] [CrossRef]
- Gevrenova, R.; Balabanova, V.; Zheleva-Dimitrova, D.; Momekov, G. The Most Promising Southeastern European Tanacetum Species: A Review of Chemical Composition and Biological Studies. PHAR 2023, 70, 1067–1081. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Zheleva-Dimitrova, D.; Balabanova, V.; Kolmayer, M.; Voynikov, Y.; Joubert, O. An In-Depth Study of Metabolite Profile and Biological Potential of Tanacetum balsamita L. (Costmary). Plants 2022, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, R.; Zheleva-Dimitrova, D.; Elincheva, V.; Gevrenova, R.; Momekov, G.; Simeonova, R. Prenanthes Purpurea and 3,5-DiCQA Alleviate Cellular Stress in H2O2-Induced Neurotoxicity: An In Vitro Comparative Study. Int. J. Mol. Sci. 2024, 25, 9805. [Google Scholar] [CrossRef]
- Lechkova, B.; Shishmanova-Doseva, M.; Benbassat, N.; Gevrenova, R.; Atanassova, P.; Penkova, N.; Peychev, L.; Hrischev, P.; Peychev, Z.; Ivanova, S. Evaluation of the Influence of Tanacetum Vulgare Extract on Cognitive Functions and Hippocampal BDNF Expression. Molecules 2024, 29, 5723. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Simeonova, R.; Kondeva-Burdina, M.; Savov, Y.; Balabanova, V.; Zengin, G.; Petrova, A.; Gevrenova, R. Antioxidant and Hepatoprotective Potential of Echinops ritro L. Extracts on Induced Oxidative Stress In Vitro/In Vivo. Int. J. Mol. Sci. 2023, 24, 9999. [Google Scholar] [CrossRef] [PubMed]
- Zheleva-Dimitrova, D.; Petrova, A.; Zengin, G.; Sinan, K.I.; Balabanova, V.; Joubert, O.; Zidorn, C.; Voynikov, Y.; Simeonova, R.; Gevrenova, R. Metabolite Profiling and Bioactivity of Cicerbita alpina (L.) Wallr. (Asteraceae, Cichorieae). Plants 2023, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Putra, I.M.W.A.; Fakhrudin, N.; Nurrochmad, A.; Wahyuono, S. A Review of Medicinal Plants with Renoprotective Activity in Diabetic Nephropathy Animal Models. Life 2023, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Petrova, A.; Simeonova, R.; Voycheva, C.; Savov, Y.; Marinov, L.; Balabanova, V.; Gevrenova, R.; Zheleva-Dimitrova, D. Metabolic Syndrome: Comparison of Three Diet-Induced Experimental Models. Pharmacia 2023, 70, 1539–1548. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, Z. Atorvastatin Induces Hepatotoxicity in Diabetic Rats via Oxidative Stress, Inflammation, and Anti-Apoptotic Pathway. Med. Sci. Monit. 2019, 25, 6165–6173. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, Y.; Li, W.; Nie, X. The Multiple Roles of Fibroblast Growth Factor in Diabetic Nephropathy. J. Inflamm. Res. 2021, 14, 5273–5290. [Google Scholar] [CrossRef]
- Goyal, R.K.; Satia, M.C.; Bangaru, R.A.; Gandhi, T.P. Effect of Long-Term Treatment with Enalapril in Streptozotocin Diabetic and DOCA Hypertensive Rats. J. Cardiovasc. Pharmacol. 1998, 32, 317–322. [Google Scholar] [CrossRef]
- Kurt, H. Comparative Therapeutic Potentials of Acarbose and a Formulated Herbal Extract on Type 2 Diabetic Rats. Afr. J. Pharm. Pharmacol. 2012, 6, 2194–2204. [Google Scholar] [CrossRef]
- Héctor Polizio, A.; Peña, C. Effects of Angiotensin II Type 1 Receptor Blockade on the Oxidative Stress in Spontaneously Hypertensive Rat Tissues. Regul. Pept. 2005, 128, 1–5. [Google Scholar] [CrossRef]
- Bump, E.A.; Taylor, Y.C.; Brown, J.M. Role of Glutathione in the Hypoxic Cell Cytotoxicity of Misonidazole. Cancer Res. 1983, 43, 997–1002. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Tappel, A.L. [53] Glutathione Peroxidase and Hydroperoxides. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 506–513. ISBN 978-0-12-181952-1. [Google Scholar]
- Pinto, M.C.; Mata, A.M.; Lopez-barea, J. Reversible Inactivation of Saccharomyces Cerevisiae Glutathione Reductase under Reducing Conditions. Arch. Biochem. Biophys. 1984, 228, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. (Eds.) Theory and Practice of Histological Techniques, 6th ed.; Expertconsult; Churchill Livingstone/Elsevier: Philadelphia, PA, USA, 2008; ISBN 978-0-443-10279-0. [Google Scholar]
- Kumar, M.; Dev, S.; Khalid, M.U.; Siddenthi, S.M.; Noman, M.; John, C.; Akubuiro, C.; Haider, A.; Rani, R.; Kashif, M.; et al. The Bidirectional Link Between Diabetes and Kidney Disease: Mechanisms and Management. Cureus 2023, 15, e45615. [Google Scholar] [CrossRef]
- Shi, G.-J.; Shi, G.-R.; Zhou, J.; Zhang, W.; Gao, C.; Jiang, Y.; Zi, Z.-G.; Zhao, H.; Yang, Y.; Yu, J.-Q. Involvement of Growth Factors in Diabetes Mellitus and Its Complications: A General Review. Biomed. Pharmacother. 2018, 101, 510–527. [Google Scholar] [CrossRef]
- Lin, S.; Teng, J.; Li, J.; Sun, F.; Yuan, D.; Chang, J. Association of Chemerin and Vascular Endothelial Growth Factor (VEGF) with Diabetic Nephropathy. Med. Sci. Monit. 2016, 22, 3209–3214. [Google Scholar] [CrossRef]
- Sinha, S.K.; Nicholas, S.B. Pathomechanisms of Diabetic Kidney Disease. J. Clin. Med. 2023, 12, 7349. [Google Scholar] [CrossRef]
- Qiao, Y.-C.; Chen, Y.-L.; Pan, Y.-H.; Ling, W.; Tian, F.; Zhang, X.-X.; Zhao, H.-L. Changes of Transforming Growth Factor Beta 1 in Patients with Type 2 Diabetes and Diabetic Nephropathy: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2017, 96, e6583. [Google Scholar] [CrossRef]
- Czaya, B.; Faul, C. FGF23 and Inflammation—A Vicious Coalition in CKD. Kidney Int. 2019, 96, 813–815. [Google Scholar] [CrossRef]
- Zanchi, C.; Locatelli, M.; Benigni, A.; Corna, D.; Tomasoni, S.; Rottoli, D.; Gaspari, F.; Remuzzi, G.; Zoja, C. Renal Expression of FGF23 in Progressive Renal Disease of Diabetes and the Effect of Ace Inhibitor. PLoS ONE 2013, 8, e70775. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, E.S.; Choi, R.; Nawaboot, J.; Lee, M.Y.; Lee, E.Y.; Kim, H.S.; Chung, C.H. Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy. Yonsei Med. J. 2016, 57, 664. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, S.J.; Lee, J.-H.; Kim, J.-H.; Son, S.S.; Cha, S.-K.; Lee, E.S.; Chung, C.H.; Lee, E.Y. Angiotensin II-Mediated MYH9 Downregulation Causes Structural and Functional Podocyte Injury in Diabetic Kidney Disease. Sci. Rep. 2019, 9, 7679. [Google Scholar] [CrossRef]
- Chandran, G.; Sirajudeen, K.N.S.; Nik Yusoff, N.S.; Swamy, M.; Samarendra, M.S. Effect of the Antihypertensive Drug Enalapril on Oxidative Stress Markers and Antioxidant Enzymes in Kidney of Spontaneously Hypertensive Rat. Oxidative Med. Cell. Longev. 2014, 2014, 608512. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ali, T.M.; Abdel Gaid, M.A.; Elberry, A.A. Effects of Enalapril and Paricalcitol Treatment on Diabetic Nephropathy and Renal Expressions of TNF-α, P53, Caspase-3 and Bcl-2 in STZ-Induced Diabetic Rats. PLoS ONE 2019, 14, e0214349. [Google Scholar] [CrossRef] [PubMed]
- Kamarauskaite, J.; Baniene, R.; Raudone, L.; Vilkickyte, G.; Vainoriene, R.; Motiekaityte, V.; Trumbeckaite, S. Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.). Antioxidants 2021, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Qian, J.; Rao, L.; Lin, S.; Wang, C.; Xu, L.; Yuan, B.; Yuan, J.; Wan, Y.; Fu, G. 3,5-Dicaffeoylquinic Acid Promotes Intestinal Urate Excretion via the MAPK Signaling Pathway Based on Caco-2 Cell Model. Food Biosci. 2025, 63, 105559. [Google Scholar] [CrossRef]
- Acıkara, Ö.B.; Ataman, U.; Zidorn, C.; Cvacka, J.; Kurtul, E.; Buděšínský, M.; Bednarova, L.; Sarıaltın, S.Y. Undescribed and Known Phenolic Acid Derivatives with Significant Antioxidant Activity from the Scorzonera Parviflora Jacq. Roots. Fitoterapia 2025, 180, 106328. [Google Scholar] [CrossRef]
- Yin, X.-L.; Xu, B.-Q.; Zhang, Y.-Q. Gynura Divaricata Rich in 3, 5−/4, 5-Dicaffeoylquinic Acid and Chlorogenic Acid Reduces Islet Cell Apoptosis and Improves Pancreatic Function in Type 2 Diabetic Mice. Nutr. Metab. 2018, 15, 73. [Google Scholar] [CrossRef]
- Bao, L.; Gong, Y.; Xu, W.; Dao, J.; Rao, J.; Yang, H. Chlorogenic Acid Inhibits NLRP3 Inflammasome Activation through Nrf2 Activation in Diabetic Nephropathy. PLoS ONE 2025, 20, e0316615. [Google Scholar] [CrossRef]
- Poloni, J.A.T.; Rotta, L.N. Diabetic Kidney Disease: Pathophysiological Changes and Urinalysis Contribution to Diagnosis—A Narrative Review. J. Lab. Precis. Med. 2022, 7, 3. [Google Scholar] [CrossRef]
- Alain Prudence, I.; Tharcisse, G.; Donatien, M.; Jean Paul, N.; Bernard, I.; Phillipe, H.; Lauben, N. Assessment of Glucosuria and Ketonuria in Diabetic Patients Attending Gitwe District Hospital. J. Drug Deliv. Ther. 2025, 15, 125–129. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Farooq, U.; Saleem, A.; Saleem, M.; Rahman, M.H.; Ashraf, G.M. Ameliorating Effect of Malva Neglecta Wallr on Obesity and Diabetes in Wistar Rats: A Mechanistic Study. BioMed Res. Int. 2022, 2022, 2614599. [Google Scholar] [CrossRef]
- Fauzi, A.; Titisari, N.; Noor, M.H.M.; Azlan, A.; Hamzah, H. Therapeutic Effect of Morus Alba Leaf Extract and Chlorogenic Acid on Inhibiting the Progression of Kidney Disease. Cogent Food Agric. 2024, 10, 2301841. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Rademaekers, A.; Weisert, A.; Häberlein, H.; Franken, S. Pharmacological Profile of Dicaffeoylquinic Acids and Their Role in the Treatment of Respiratory Diseases. Front. Pharmacol. 2024, 15, 1371613. [Google Scholar] [CrossRef]
- Alsawaf, S.; Alnuaimi, F.; Afzal, S.; Thomas, R.M.; Chelakkot, A.L.; Ramadan, W.S.; Hodeify, R.; Matar, R.; Merheb, M.; Siddiqui, S.S.; et al. Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation. Biology 2022, 11, 1717. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Romecín, P.; García-Guillén, A.I.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Flavonoids in Kidney Health and Disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Hu, Q.; Qu, C.; Xiao, X.; Zhang, W.; Jiang, Y.; Wu, Z.; Song, D.; Peng, X.; Ma, X.; Zhao, Y. Flavonoids on Diabetic Nephropathy: Advances and Therapeutic Opportunities. Chin. Med. 2021, 16, 74. [Google Scholar] [CrossRef]
- Matboli, M.; Ibrahim, D.; Hasanin, A.H.; Hassan, M.K.; Habib, E.K.; Bekhet, M.M.; Afifi, A.M.; Eissa, S. Epigenetic Modulation of Autophagy Genes Linked to Diabetic Nephropathy by Administration of Isorhamnetin in Type 2 Diabetes Mellitus Rats. Epigenomics 2021, 13, 187–202. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and Its Metabolite Isorhamnetin Promote Glucose Uptake through Different Signalling Pathways in Myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed]



| Enzyme Activity (nmol/min/mg) | Controls | ETBld | ETBhd | MIRI | MIRI + enlp | MIRI + acarb | MIRI + ETBld | MIRI + ETBhd |
|---|---|---|---|---|---|---|---|---|
| GPx | 27.35 ± 1.08 | 26.5 ± 1.29 | 28.37 ± 0.47 | 18.75 ± 1.71 * | 25.27 ± 0.57 + | 22.02 ± 1.48 | 24.37 ± 1.88 + | 27.45 ± 2.51 + |
| GR | 52.42 ± 0.61 | 50.7 ± 2.58 | 52.95 ± 2.16 | 41.27 ± 2.39 * | 47.72 ± 1.69 + | 44.7 ± 3.18 | 48.2 ± 2.17 + | 50.2 ± 0.91 + |
| GST | 13.17 ± 0.67 | 13.10 ± 0.62 | 12.32 ± 0.68 | 8.90 ± 0.68 * | 12.27 ± 0.63 + | 10.42 ± 1.22 | 11.0 ± 0.81 + | 11.77 ± 0.68 + |
| SOD | 0.32 ± 0.015 | 0.36 ± 0.021 | 0.35 ± 0.023 | 0.23 ± 0.017 | 0.41± 0.026 * | 0.38 ± 0.012 | 0.44 ± 0.022 | 0.45± 0.031 |
| Urine Parameters | Controls | ETBld | ETBhd | MIRI | MIRI + enlp | MIRI + acarb | MIRI + ETBld | MIRI + ETBhd |
|---|---|---|---|---|---|---|---|---|
| Ketone mmol/L | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 12.0 ± 4.6 * | 3.5 ± 3.3 | 4.38 ± 2.7 | 0.88 ± 0.7 + | 0.63 ± 0.6 + |
| Protein g/L | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 13.25 ± 7.2 * | 2.5 ± 1.0 + | 2.0 ± 1.1 + | 0.65 ± 0.4 + | 0.58 ± 0.5 + |
| Glucose mmol/L | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 41.25 ± 14 * | 10.0 ± 5.7 + | 8.75 ± 2.5 + | 7.5 ± 2.8 + | 2.5 ± 2.8 + |
| pH | 7.25 ± 0.2 | 7.13 ± 0.2 | 7.0 ± 0.5 | 5.25 ± 0.5 * | 5.75 ± 0.5 | 7 ± 0.5 + | 7.25 ± 0.2 + | 7.5 ± 0.5 + |
| Volume ml/24 h | 32.5 ± 4.2 | 38.6 ± 3.5 | 42.3 ± 3.8 | 66.2 ± 5.2 * | 56.2 ± 6.4 | 48.6 ± 3.8 + | 47.2 ± 3.2 + | 46.5 ± 4.1 + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeonova, R.; Gevrenova, R.; Marinov, L.; Savov, Y.; Zheleva-Dimitrova, D. Nephroprotective Effects of Tanacetum balsamita Extract on Metabolic-Induced Renal Injury (MIRI) in Rats. Curr. Issues Mol. Biol. 2025, 47, 293. https://doi.org/10.3390/cimb47040293
Simeonova R, Gevrenova R, Marinov L, Savov Y, Zheleva-Dimitrova D. Nephroprotective Effects of Tanacetum balsamita Extract on Metabolic-Induced Renal Injury (MIRI) in Rats. Current Issues in Molecular Biology. 2025; 47(4):293. https://doi.org/10.3390/cimb47040293
Chicago/Turabian StyleSimeonova, Rumyana, Reneta Gevrenova, Lyubomir Marinov, Yonko Savov, and Dimitrina Zheleva-Dimitrova. 2025. "Nephroprotective Effects of Tanacetum balsamita Extract on Metabolic-Induced Renal Injury (MIRI) in Rats" Current Issues in Molecular Biology 47, no. 4: 293. https://doi.org/10.3390/cimb47040293
APA StyleSimeonova, R., Gevrenova, R., Marinov, L., Savov, Y., & Zheleva-Dimitrova, D. (2025). Nephroprotective Effects of Tanacetum balsamita Extract on Metabolic-Induced Renal Injury (MIRI) in Rats. Current Issues in Molecular Biology, 47(4), 293. https://doi.org/10.3390/cimb47040293








