Recent Advances in Bone Tissue Engineering: Enhancing the Potential of Mesenchymal Stem Cells for Regenerative Therapies
Abstract
1. Introduction
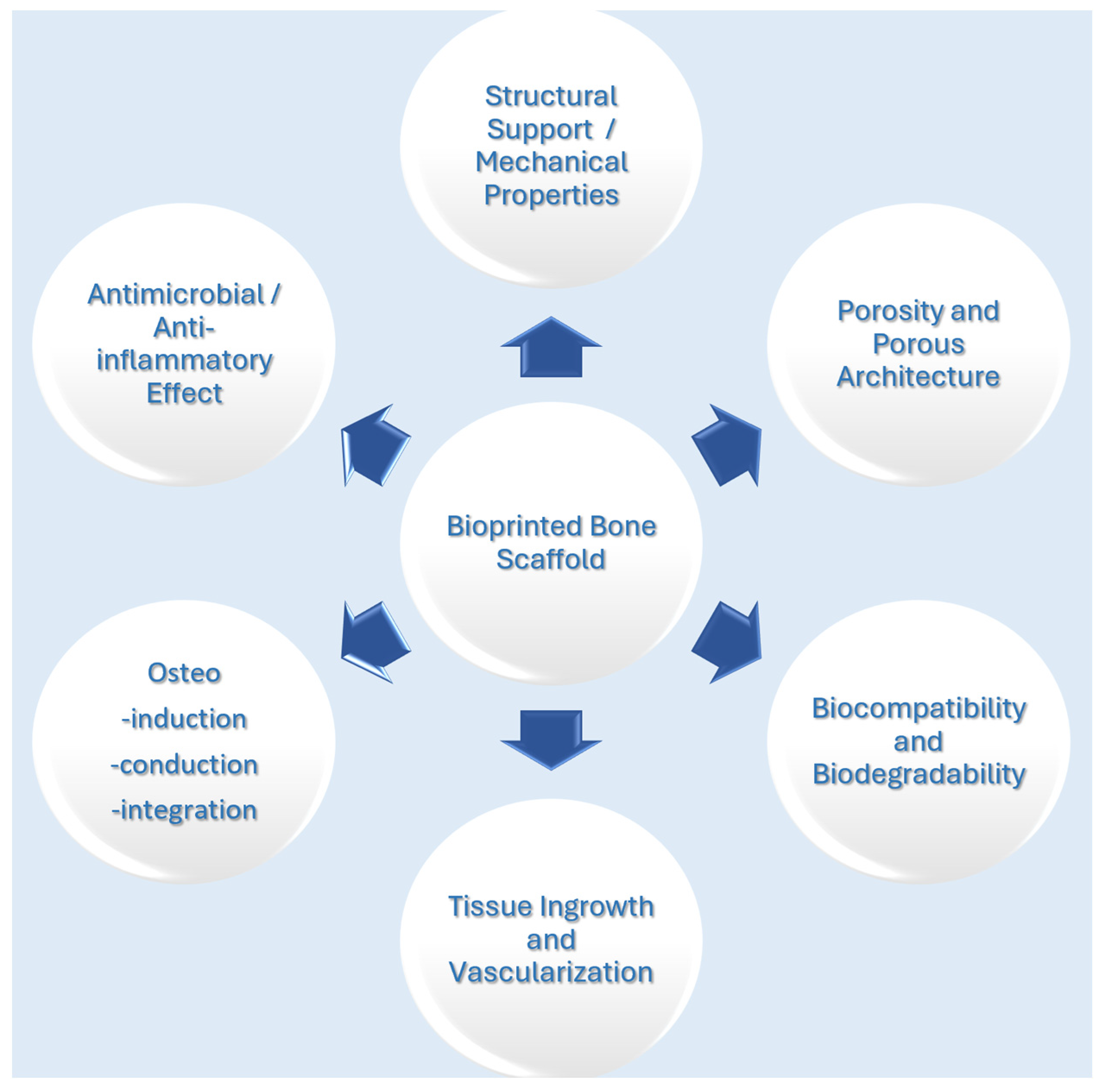
2. Bone Tissue Engineering
3. Bioprinting
4. Bioink
5. MSCs as Building Blocks for BTE
6. Osteoinductive Factors and Their Application in BTE
7. Clinical Challenges for Bioprinted MSC Applications
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Buyuksungur, S.; Hasirci, V.; Hasirci, N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J. Biomed. Mater. Res. A 2021, 109, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Meena, L.K.; Rather, H.; Kedaria, D.; Vasita, R. Polymeric microgels for bone tissue engineering applications—A review. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 381–397. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), S131–S139. [Google Scholar] [CrossRef]
- Zha, K.; Tian, Y.; Panayi, A.C.; Mi, B.; Liu, G. Recent Advances in Enhancement Strategies for Osteogenic Differentiation of Mesenchymal Stem Cells in Bone Tissue Engineering. Front. Cell Dev. Biol. 2022, 10, 824812. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef]
- Cowan, C.M.; Shi, Y.-Y.; O Aalami, O.; Chou, Y.-F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Dargah, M.S.; Azar, M.H.; Alizadeh, R.; Mahdavi, F.S.; Sayedain, S.S.; Kaviani, A.; Asadollahi, M.; Azami, M.; Beheshtizadeh, N. Enhanced bone tissue regeneration using a 3D-printed poly(lactic acid)/Ti6Al4V composite scaffold with plasma treatment modification. Sci. Rep. 2023, 13, 3139. [Google Scholar] [CrossRef]
- Hoveidaei, A.H.; Sadat-Shojai, M.; Nabavizadeh, S.S.; Niakan, R.; Shirinezhad, A.; MosalamiAghili, S.; Tabaie, S. Clinical challenges in bone tissue engineering—A narrative review. Bone 2024, 192, 117363. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Al-Nawas, B. Bone reconstruction of extensive maxillomandibular defects in adults. Periodontology 2000 2023, 93, 340–357. [Google Scholar] [CrossRef]
- Tarantino, U.; Cerocchi, I.; Scialdoni, A.; Saturnino, L.; Feola, M.; Celi, M.; Liuni, F.M.; Iolascon, G.; Gasbarra, E. Bone healing and osteoporosis. Aging Clin Exp Res 2011, 23 (Suppl. 2), 62–64. [Google Scholar] [PubMed]
- Neves, L.S.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Current approaches and future perspectives on strategies for the development of personalized tissue engineering therapies. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2012, 9, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Paetzold, R.; Coulter, F.; Singh, G.; Kelly, D.; O’Cearbhaill, E. Fused filament fabrication of polycaprolactone bioscaffolds: Influence of fabrication parameters and thermal environment on geometric fidelity and mechanical properties. Bioprinting 2022, 27, e00206. [Google Scholar] [CrossRef]
- Silva, J.C.; Carvalho, M.S.; Udangawa, R.N.; Moura, C.S.; Cabral, J.M.S.; da Silva, C.L.; Ferreira, F.C.; Vashishth, D.; Linhardt, R.J. Extracellular matrix decorated polycaprolactone scaffolds for improved mesenchymal stem/stromal cell osteogenesis towards a patient-tailored bone tissue engineering approach. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2153–2166. [Google Scholar] [CrossRef]
- Iqbal, N.; Pant, T.; Rohra, N.; Goyal, A.; Lawrence, M.; Dey, A.; Ganguly, P. Nanobiotechnology in Bone Tissue Engineering Applications: Recent Advances and Future Perspectives. Appl. Biosci. 2023, 2, 617–638. [Google Scholar] [CrossRef]
- Romagnoli, M.; Casali, M.; Zaffagnini, M.; Cucurnia, I.; Raggi, F.; Reale, D.; Grassi, A.; Zaffagnini, S. Tricalcium Phosphate as a Bone Substitute to Treat Massive Acetabular Bone Defects in Hip Revision Surgery: A Systematic Review and Initial Clinical Experience with 11 Cases. J. Clin. Med. 2023, 12, 1820. [Google Scholar] [CrossRef]
- Scheer, J.H.; Adolfsson, L.E. Tricalcium phosphate bone substitute in corrective osteotomy of the distal radius. Injury 2009, 40, 262–267. [Google Scholar] [CrossRef]
- Whitehouse, M.R.; Blom, A.W. The Use of Ceramics as Bone Substitutes in Revision Hip Arthroplasty. Materials 2009, 2, 1895–1907. [Google Scholar] [CrossRef]
- Lindfors, N.; Hyvönen, P.; Nyyssönen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed]
- López-Noriega, A.; Quinlan, E.; Celikkin, N.; O’brien, F.J. Incorporation of polymeric microparticles into collagen-hydroxyapatite scaffolds for the delivery of a pro-osteogenic peptide for bone tissue engineering. APL Mater. 2014, 3, 014910. [Google Scholar] [CrossRef]
- Villa, M.M.; Wang, L.; Huang, J.; Rowe, D.W.; Wei, M. Bone tissue engineering with a collagen–hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 103, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Baheiraei, N.; Nourani, M.R.; Mortazavi, S.M.J.; Movahedin, M.; Eyni, H.; Bagheri, F.; Norahan, M.H. Development of a bioactive porous collagen/β-tricalcium phosphate bone graft assisting rapid vascularization for bone tissue engineering applications. J. Biomed. Mater. Res. A 2017, 106, 73–85. [Google Scholar] [CrossRef]
- Fang, X.; Lei, L.; Jiang, T.; Chen, Y.; Kang, Y. Injectable thermosensitive alginate/β-tricalcium phosphate/aspirin hydrogels for bone augmentation. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 106, 1739–1751. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-ε-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. A 2017, 23, 503–514. [Google Scholar] [CrossRef]
- Rai, B.; Lin, J.L.; Lim, Z.X.; Guldberg, R.E.; Hutmacher, D.W.; Cool, S.M. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL–TCP scaffolds. Biomaterials 2010, 31, 7960–7970. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, S.H.; Kim, W.D. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess Biosyst. Eng. 2010, 34, 505–513. [Google Scholar] [CrossRef]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.A.; Elisseeff, J.H.; Dorafshar, A.H.; Grayson, W.L. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef]
- Detsch, R.; Schaefer, S.; Deisinger, U.; Ziegler, G.; Seitz, H.; Leukers, B. In Vitro—Osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. J. Biomater. Appl. 2010, 26, 359–380. [Google Scholar] [CrossRef]
- Wang, L.; Fan, H.; Zhang, Z.-Y.; Lou, A.-J.; Pei, G.-X.; Jiang, S.; Mu, T.-W.; Qin, J.-J.; Chen, S.-Y.; Jin, D. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 2010, 31, 9452–9461. [Google Scholar] [CrossRef] [PubMed]
- Polini, A.; Pisignano, D.; Parodi, M.; Quarto, R.; Scaglione, S. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE 2011, 6, e26211. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Zhou, Y.; Liu, R.; Sun, R.; Li, Y.; Tian, Y.; Fan, B. Advances in growth factor-containing 3D printed scaffolds in orthopedics. Biomed. Eng. Online 2025, 24, 14. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Lange, C.; Reichert, J.; Berner, A.; Chen, F.; Fratzl, P.; Schantz, J.-T.; Hutmacher, D.W. Bone tissue engineering: From bench to bedside. Mater. Today 2012, 15, 430–435. [Google Scholar] [CrossRef]
- Kwarcinski, J.; Boughton, P.; van Gelder, J.; Damodaran, O.; Doolan, A.; Ruys, A. Clinical evaluation of rapid 3D print-formed implants for surgical reconstruction of large cranial defects. ANZ J. Surg. 2020, 91, 1226–1232. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kim, Y.-C.; Min, J.-C.; Park, H.-J.; Lee, E.-J.; Shim, J.-H.; Choi, J.-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740. [Google Scholar] [CrossRef]
- Fang, S.; Wang, Y.; Xu, P.; Zhu, J.; Liu, J.; Li, H.; Sun, X. Three-dimensional-printed titanium implants for severe acetabular bone defects in revision hip arthroplasty: Short- and mid-term results. Int. Orthop. 2022, 46, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Meng, C.; Zhang, Z.; Zhu, Q. 3D printing of polycaprolactone/bioactive glass composite scaffolds for in situ bone repair. Ceram. Int. 2022, 48, 7491–7499. [Google Scholar] [CrossRef]
- Soares, C.S.; Barros, L.C.; Saraiva, V.; Gomez-Florit, M.; Babo, P.S.; Dias, I.R.; Reis, R.L.; Carvalho, P.P.; E Gomes, M. Bioengineered surgical repair of a chronic oronasal fistula in a cat using autologous platelet-rich fibrin and bone marrow with a tailored 3D printed implant. J. Feline Med. Surg. 2018, 20, 835–843. [Google Scholar] [CrossRef]
- Shen, G.; Gao, B.; Guo, J.; Xu, W.; Chen, G.; Huang, S.; Zeng, Z.; Zhao, X. Dynamic culturing of large cell-loaded PCL/gelatin methacryloyl scaffolds for bone critical size defect repair. Int. J. Biol. Macromol. 2025, 298, 139906. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.; Lagunas, A.; Mills, C.; Rodríguez-Seguí, S.; Estévez, M.; Oberhansl, S.; Comelles, J.; Samitier, J. Stem Cell Differentiation by Functionalized Micro- and Nanostructured Surfaces. Nanomedicine 2008, 4, 65–82. [Google Scholar] [CrossRef]
- Pedroza-González, S.C.; Rodriguez-Salvador, M.; Pérez-Benítez, B.E.; Alvarez, M.M.; Santiago, G.T.-D. Bioinks for 3D Bioprinting: A Scientometric Analysis of Two Decades of Progress. Int. J. Bioprinting 2021, 7, 68–91. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.E.; Gou, M.; Xu, Y.; et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, D.; Li, R.; Sheng, S.; Li, G.; Sun, Y.; Wang, P.; Mo, Y.; Liu, H.; Chen, X.; et al. Protocol for engineering bone organoids from mesenchymal stem cells. Bioact. Mater. 2025, 45, 388–400. [Google Scholar] [CrossRef]
- Vikram Singh, A.; Hasan Dad Ansari, M.; Wang, S.; Laux, P.; Luch, A.; Kumar, A.; Patil, R.; Nussberger, S. The Adoption of Three-Dimensional Additive Manufacturing from Biomedical Material Design to 3D Organ Printing. Appl. Sci. 2019, 9, 811. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef]
- Byambaa, B.; Annabi, N.; Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Jia, W.; Kazemzadeh-Narbat, M.; Shin, S.R.; Tamayol, A.; Khademhosseini, A. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv. Healthc. Mater. 2017, 6, 1700015. [Google Scholar] [CrossRef] [PubMed]
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.-J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef]
- Haring, A.P.; Thompson, E.G.; Tong, Y.; Laheri, S.; Cesewski, E.; Sontheimer, H.; Johnson, B.N. Process- and bio-inspired hydrogels for 3D bioprinting of soft free-standing neural and glial tissues. Biofabrication 2019, 11, 025009. [Google Scholar] [CrossRef]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Ravnic, D.J.; Leberfinger, A.N.; Koduru, S.V.; Hospodiuk, M.; Moncal, K.K.; Datta, P.; Dey, M.; Rizk, E.; Ozbolat, I.T. Transplantation of Bioprinted Tissues and Organs: Technical and Clinical Challenges and Future Perspectives. Ann. Surg. 2017, 266, 48–58. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. Design aspects and characterization of hydrogel-based bioinks for extrusion-based bioprinting. Bioprinting 2023, 32, e00274. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2012, 101A, 1255–1264. [Google Scholar] [CrossRef]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Santos, J.D.; Atayde, L.; Alves, N.; Maurício, A.C. Three-Dimensional Printing/Bioprinting and Cellular Therapies for Regenerative Medicine: Current Advances. J. Funct. Biomater. 2025, 16, 28. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, X.-B.; Gao, X.-D.; Hao, D.-J.; Li, T.; Xu, Z.-W. Bioprinting for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1036375. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Min, K.; Jeon, H.; Kim, S. Physically Transient Distributed Feedback Laser Using Optically Activated Silk Bio-Ink. Adv. Opt. Mater. 2016, 4, 1738–1743. [Google Scholar] [CrossRef]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D bioprinting in tissue engineering for medical applications: The classic and the hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Kérourédan, O.; Washio, A.; Handschin, C.; Devillard, R.; Kokabu, S.; Kitamura, C.; Tabata, Y. Bioactive gelatin-sheets as novel biopapers to support prevascularization organized by laser-assisted bioprinting for bone tissue engineering. Biomed. Mater. 2024, 19, 025038. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Zandrini, T.; Florczak, S.; Levato, R.; Ovsianikov, A. Breaking the resolution limits of 3D bioprinting: Future opportunities and present challenges. Trends Biotechnol. 2022, 41, 604–614. [Google Scholar] [CrossRef]
- Guida, L.; Cavallaro, M.; Levi, M. Advancements in high-resolution 3D bioprinting: Exploring technological trends, bioinks and achieved resolutions. Bioprinting 2024, 44, e00376. [Google Scholar] [CrossRef]
- Kalaiselvan, E.; Maiti, S.K.; Shivaramu, S.; Banu, S.A.; Sharun, K.; Mohan, D.; Palakkara, S.; Bag, S.; Sahoo, M.; Ramalingam, S.; et al. Bone Marrow-Derived Mesenchymal Stem Cell-Laden Nanocomposite Scaffolds Enhance Bone Regeneration in Rabbit Critical-Size Segmental Bone Defect Model. J. Funct. Biomater. 2024, 15, 66. [Google Scholar] [CrossRef]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef]
- England, S.; Rajaram, A.; Schreyer, D.J.; Chen, X. Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Fast Setting Silk Fibroin Bioink for Bioprinting of Patient-Specific Memory-Shape Implants. Adv. Healthc. Mater. 2017, 6, 1701021. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Kinsella, J.M. Directing the Self-assembly of Tumour Spheroids by Bioprinting Cellular Heterogeneous Models within Alginate/Gelatin Hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, Z.; Yang, X.; Zhang, Y.; Liang, Y.; Chen, X.; Qiu, X.; Chen, X. Advancements in GelMA bioactive hydrogels: Strategies for infection control and bone tissue regeneration. Theranostics 2025, 15, 460–493. [Google Scholar] [CrossRef]
- E Bertassoni, L.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; A Araujo, W.; Zorlutuna, P.; E Vrana, N.; Ghaemmaghami, A.M.; Dokmeci, M.R.; et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef]
- Lei, M.; Wang, X. Biodegradable Polymers and Stem Cells for Bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef]
- Di Bella, C.; Fosang, A.; Donati, D.M.; Wallace, G.G.; Choong, P.F.M. 3D Bioprinting of Cartilage for Orthopedic Surgeons: Reading between the Lines. Front. Surg. 2015, 2, 39. [Google Scholar] [CrossRef]
- Thomas, B.H.; Fryman, J.C.; Liu, K.; Mason, J. Hydrophilic–hydrophobic hydrogels for cartilage replacement. J. Mech. Behav. Biomed. Mater. 2009, 2, 588–595. [Google Scholar] [CrossRef]
- Rasouli, R.; Sweeney, C.; Frampton, J.P. Heterogeneous and Composite Bioinks for 3D-Bioprinting of Complex Tissue. Biomed. Mater. Devices 2024, 3, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Wenz, A.; Borchers, K.; Tovar, G.E.M.; Kluger, P.J. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication 2017, 9, 044103. [Google Scholar] [CrossRef]
- Dávila, J.L.; Freitas, M.S.; Neto, P.I.; Silveira, Z.C.; Silva, J.V.L.; D’ávila, M.A. Fabrication of PCL/β-TCP scaffolds by 3D mini-screw extrusion printing. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Lee, S.; Esworthy, T.; Stake, S.; Miao, S.; Zuo, Y.Y.; Harris, B.T.; Zhang, L.G. Advances in 3D Bioprinting for Neural Tissue Engineering. Adv. Biosyst. 2018, 2, 1700213. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Mishra, A.; Lin, P.T.P.; Ng, S.H.; Yeong, W.Y.; Kim, Y.; Yoon, Y. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2016, 17, 1600250. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; De Leon-Oliva, D.; Boaru, D.L.; Fraile-Martinez, O.; García-Montero, C.; Casanova, C.; García-Honduvilla, N.; Bujan, J.; A Saez, M.; Álvarez-Mon, M.; et al. Advances in 3D bioprinting to enhance translational applications in bone tissue engineering and regenerative medicine. Histol. Histopathol. 2024, 40, 147–156. [Google Scholar] [CrossRef]
- Kim, J. Characterization of Biocompatibility of Functional Bioinks for 3D Bioprinting. Bioengineering 2023, 10, 457. [Google Scholar] [CrossRef]
- Lemarié, L.; Anandan, A.; Petiot, E.; Marquette, C.; Courtial, E.-J. Rheology, simulation and data analysis toward bioprinting cell viability awareness. Bioprinting 2020, 21, e00119. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Snyder, J.; Son, A.R.; Hamid, Q.; Wang, C.; Lui, Y.; Sun, W. Mesenchymal stem cell printing and process regulated cell properties. Biofabrication 2015, 7, 044106. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Sticker, D.; Charwat, V.; Kasper, C.; Lepperdinger, G. Lab-on-a-chip technologies for stem cell analysis. Trends Biotechnol. 2014, 32, 245–253. [Google Scholar] [CrossRef]
- Müller, S.J.; Fabry, B.; Gekle, S. Predicting Cell Stress and Strain during Extrusion Bioprinting. Phys. Rev. Appl. 2023, 10, 64061. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Rodríguez-Rego, J.M.; Macías-García, A.; Mendoza-Cerezo, L.; Díaz-Parralejo, A. Relationship between shear-thinning rheological properties of bioinks and bioprinting parameters. Int. J. Bioprinting 2023, 9, 422–431. [Google Scholar] [CrossRef]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009, 4, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, A.; Chen, X. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef]
- Rossi, A.; Pescara, T.; Gambelli, A.M.; Gaggia, F.; Asthana, A.; Perrier, Q.; Basta, G.; Moretti, M.; Senin, N.; Rossi, F.; et al. Biomaterials for extrusion-based bioprinting and biomedical applications. Front. Bioeng. Biotechnol. 2024, 12, 1393641. [Google Scholar] [CrossRef]
- Czyżewski, P.; Marciniak, D.; Nowinka, B.; Borowiak, M.; Bieliński, M. Influence of Extruder’s Nozzle Diameter on the Improvement of Functional Properties of 3D-Printed PLA Products. Polymers 2022, 14, 356. [Google Scholar] [CrossRef]
- Ravanbakhsh, H.; Karamzadeh, V.; Bao, G.; Mongeau, L.; Juncker, D.; Zhang, Y.S. Emerging Technologies in Multi-Material Bioprinting. Adv. Mater. 2021, 33, 2104730. [Google Scholar] [CrossRef]
- Krishna, D.V.; Sankar, M.R. Persuasive factors on the bioink printability and cell viability in the extrusion-based 3D bioprinting for tissue regeneration applications. Eng. Regen. 2023, 4, 396–410. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Boretti, G.; Baldursson, H.E.; Buonarrivo, L.; Simonsson, S.; Brynjólfsson, S.; Gargiulo, P.; Sigurjónsson, Ó.E. Mechanical and Biological Characterization of Ionic and Photo-Crosslinking Effects on Gelatin-Based Hydrogel for Cartilage Tissue Engineering Applications. Polymers 2024, 16, 2741. [Google Scholar] [CrossRef] [PubMed]
- Rühle, A.; Thomsen, A.; Saffrich, R.; Voglstätter, M.; Bieber, B.; Sprave, T.; Wuchter, P.; Vaupel, P.; Huber, P.E.; Grosu, A.-L.; et al. Multipotent mesenchymal stromal cells are sensitive to thermic stress—Potential implications for therapeutic hyperthermia. Int. J. Hyperth. 2020, 37, 430–441. [Google Scholar] [CrossRef]
- Fischer, L.; Nosratlo, M.; Hast, K.; Karakaya, E.; Ströhlein, N.; Esser, T.U.; Gerum, R.; Richter, S.; Engel, F.B.; Detsch, R.; et al. Calcium supplementation of bioinks reduces shear stress-induced cell damage during bioprinting. Biofabrication 2022, 14, 045005. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.-J. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf. Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Presen, D.M.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal Stromal Cell-Based Bone Regeneration Therapies: From Cell Transplantation and Tissue Engineering to Therapeutic Secretomes and Extracellular Vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352. [Google Scholar] [CrossRef]
- Choe, G.; Lee, M.; Oh, S.; Seok, J.M.; Kim, J.; Im, S.; A Park, S.; Lee, J.Y. Three-dimensional bioprinting of mesenchymal stem cells using an osteoinductive bioink containing alginate and BMP-2-loaded PLGA nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2022, 136, 212789. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Hutton, D.L.; Grayson, W.L. Stem cell-based approaches to engineering vascularized bone. Curr. Opin. Chem. Eng. 2014, 3, 75–82. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Rehman, A.; Nigam, A.; Laino, L.; Russo, D.; Todisco, C.; Esposito, G.; Svolacchia, F.; Giuzio, F.; Desiderio, V.; Ferraro, G. Mesenchymal Stem Cells in Soft Tissue Regenerative Medicine: A Comprehensive Review. Medicina 2023, 59, 1449. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, Y.; Kim, M.; Zhao, G.; Wang, Y.; Collins, C.P.; Mei, O.; Zhang, Y.; Duan, C.; Zhong, J.; et al. Effective Bone Tissue Fabrication Using 3D-Printed Citrate-Based Nanocomposite Scaffolds Laden with BMP9-Stimulated Human Urine Stem Cells. ACS Appl. Mater. Interfaces 2024, 17, 197–210. [Google Scholar] [CrossRef]
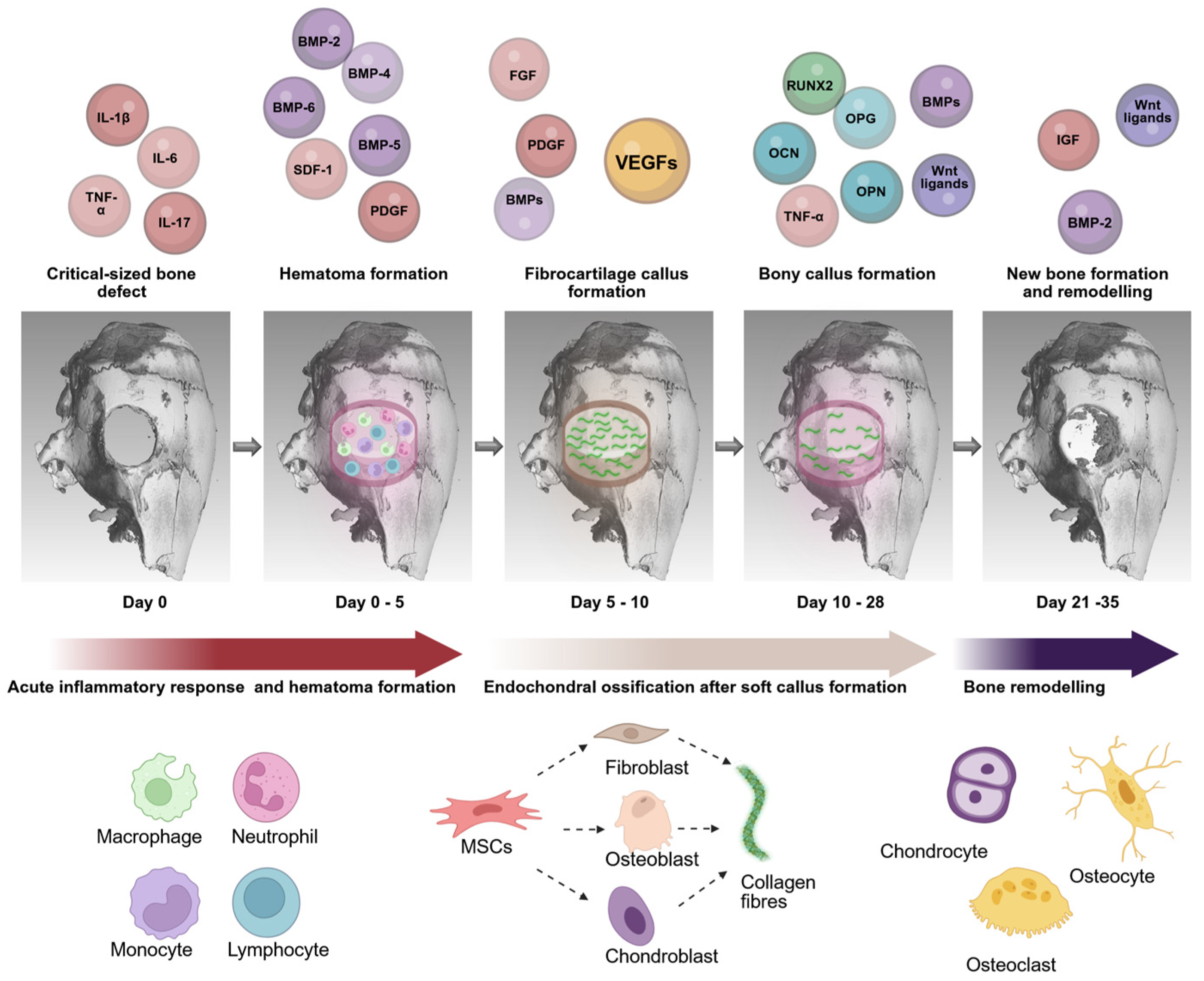
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Thompson, Z.; Miclau, T.; Hu, D.; Helms, J.A. A model for intramembranous ossification during fracture healing. J. Orthop. Res. 2002, 20, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Mazur, M.M.; Buck, A.C.; E Wandtke, M.; Liu, J.; A Ebraheim, N. Prospective Review of Mesenchymal Stem Cells Differentiation into Osteoblasts. Orthop. Surg. 2017, 9, 13–19. [Google Scholar] [CrossRef]
- Romano, I.R.; D’angeli, F.; Vicario, N.; Russo, C.; Genovese, C.; Furno, D.L.; Mannino, G.; Tamburino, S.; Parenti, R.; Giuffrida, R. Adipose-Derived Mesenchymal Stromal Cells: A Tool for Bone and Cartilage Repair. Biomedicines 2023, 11, 1781. [Google Scholar] [CrossRef]
- Chen, D.; Liu, S.; Chu, X.; Reiter, J.; Gao, H.; McGuire, P.; Yu, J.; Xuei, X.; Liu, Y. Osteogenic Differentiation Potential of Mesenchymal Stem. Genes 2023, 14, 1871. [Google Scholar] [CrossRef]
- Simunovic, F.; Finkenzeller, G. Vascularization strategies in bone tissue engineering. Cells 2021, 10, 1749. [Google Scholar] [CrossRef] [PubMed]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Baron, M.; Drohat, P.; Crawford, B.; Hornicek, F.J.; Best, T.M.; Kouroupis, D. Mesenchymal Stem/Stromal Cells: Immunomodulatory and Bone Regeneration Potential after Tumor Excision in Osteosarcoma Patients. Bioengineering 2023, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef] [PubMed]
- Kengelbach-Weigand, A.; Thielen, C.; Bäuerle, T.; Götzl, R.; Gerber, T.; Körner, C.; Beier, J.P.; Horch, R.E.; Boos, A.M. Personalized medicine for reconstruction of critical-size bone defects—A translational approach with customizable vascularized bone tissue. npj Regen. Med. 2021, 6, 49. [Google Scholar] [CrossRef]
- Schott, N.G.; Friend, N.E.; Stegemann, J.P. Coupling Osteogenesis and Vasculogenesis in Engineered Orthopedic Tissues. Tissue Eng. B Rev. 2021, 27, 199–214. [Google Scholar] [CrossRef]
- Peng, J.; Chen, L.; Peng, K.; Chen, X.; Wu, J.; He, Z.; Xiang, Z. Bone Marrow Mesenchymal Stem Cells and Endothelial Progenitor Cells Co-Culture Enhances Large Segment Bone Defect Repair. J. Biomed. Nanotechnol. 2019, 15, 742–755. [Google Scholar] [CrossRef]
- Tsiapalis, D.; O’driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef]
- Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory Effects of MSCs in Bone Healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- James, A.W. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica 2013, 2013, 684736. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Jann, J.; Gascon, S.; Roux, S.; Faucheux, N. Influence of the TGF-β Superfamily on Osteoclasts/Osteoblasts Balance in Physiological and Pathological Bone Conditions. Int. J. Mol. Sci. 2020, 21, 7597. [Google Scholar] [CrossRef]
- Santibanez, J.F.; Echeverria, C.; Millan, C.; Simon, F. Transforming growth factor-beta superfamily regulates mesenchymal stem cell osteogenic differentiation: A microRNA linking. Acta Histochem. 2023, 125, 152096. [Google Scholar] [CrossRef]
- Stains, J.P.; Civitelli, R. Genomic approaches to identifying transcriptional regulators of osteoblast differentiation. Genome Biol. 2003, 4, 222. [Google Scholar] [CrossRef][Green Version]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef]
- Frith, J.; Genever, P. Transcriptional control of mesenchymal stem cell differentiation. Transfus. Med. Hemotherapy 2008, 35, 216–227. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional Scaffolds with Improved Antimicrobial Properties and Osteogenicity Based on Piezoelectric Electrospun Fibers Decorated with Bioactive Composite Microcapsules. ACS Appl. Mater. Interfaces 2018, 10, 34849–34868. [Google Scholar] [CrossRef]
- Sluzalska, K.D.; Slawski, J.; Sochacka, M.; Lampart, A.; Otlewski, J.; Zakrzewska, M. Intracellular partners of fibroblast growth factors 1 and 2—Implications for functions. Cytokine Growth Factor Rev. 2021, 57, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in growth factor delivery for bone tissue engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhao, J.; Brochmann, E.J.; Wang, J.C.; Murray, S.S. Bone morphogenetic protein-2 and tumor growth: Diverse effects and possibilities for therapy. Cytokine Growth Factor Rev. 2017, 34, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.S.; Pashkuleva, I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 63–76. [Google Scholar] [CrossRef]
- Khan, S.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Nyberg, E.; Holmes, C.; Witham, T.; Grayson, W.L. Growth factor-eluting technologies for bone tissue engineering. Drug Deliv. Transl. Res. 2015, 6, 184–194. [Google Scholar] [CrossRef]
- Stamnitz, S.; Klimczak, A. Mesenchymal stem cells, bioactive factors, and scaffolds in bone repair: From research perspectives to clinical practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef]
- Luginbuehl, V.; Zoidis, E.; Meinel, L.; von Rechenberg, B.; Gander, B.; Merkle, H.P. Impact of IGF-I release kinetics on bone healing: A preliminary study in sheep. Eur. J. Pharm. Biopharm. 2013, 85, 99–106. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef]
- Marie, P.J.; Miraoui, H.; Sévère, N. FGF/FGFR signaling in bone formation: Progress and perspectives. Growth Factors 2012, 30, 117–123. [Google Scholar] [CrossRef]
- Kleinheinz, J.; Jung, S.; Wermker, K.; Fischer, C.; Joos, U. Release kinetics of VEGF165 from a collagen matrix and structural matrix changes in a circulation model. Head Face Med. 2010, 6, 17. [Google Scholar] [CrossRef]
- Ding, J.; Ghali, O.; Lencel, P.; Broux, O.; Chauveau, C.; Devedjian, J.; Hardouin, P.; Magne, D. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009, 84, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dai, S.; Fan, G. Glucocorticoid-induced differentiation of primary cultured bone marrow mesenchymal cells into adipocytes is antagonized by exogenous Runx2. APMIS 2010, 118, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Z.; Ge, C.; Krebsbach, P.; Franceschi, R. Healing Cranial Defects with AdRunx2-transduced Marrow Stromal Cells. J. Dent. Res. 2007, 86, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 6, 32–52. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Jt. Surg. 2003, 85, 1544–1552. [Google Scholar] [CrossRef]
- Xiao, Y.-T.; Xiang, L.-X.; Shao, J.-Z. Bone morphogenetic protein. Biochem. Biophys. Res. Commun. 2007, 362, 550–553. [Google Scholar] [CrossRef]
- Shen, B.; Bhargav, D.; Wei, A.; A Williams, L.; Tao, H.; Ma, D.D.F.; Diwan, A.D. BMP-13 Emerges as a Potential Inhibitor of Bone Formation. Int. J. Biol. Sci. 2009, 5, 192–200. [Google Scholar] [CrossRef]
- Botega, I.I.; Zamarioli, A.; Guedes, P.M.S.G.; da Silva, R.A.B.; Issa, J.P.M.; Butezloff, M.M.; Sousa, Y.T.C.S.; Ximenez, J.P.B.; Volpon, J.B. Bone callus formation is highly disrupted by dietary restriction in growing rats sustaining a femoral fracture. Acta Cir. Bras. 2019, 34, e20190010000002. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef]
- Tong, Z.; Guo, J.; Glen, R.C.; Morrell, N.W.; Li, W. A Bone Morphogenetic Protein (BMP)-derived Peptide Based on the Type I Receptor-binding Site Modifies Cell-type Dependent BMP Signalling. Sci. Rep. 2019, 9, 13446. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Willie, B.M.; Schwabe, P.; Seemann, P.; Duda, G.N. BMPs in bone regeneration: Less is more effective, a paradigm-shift. Cytokine Growth Factor Rev. 2016, 27, 141–148. [Google Scholar] [CrossRef]
- Shen, B.; Wei, A.; Whittaker, S.; Williams, L.A.; Tao, H.; Ma, D.D.; Diwan, A.D. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 2009, 109, 406–416. [Google Scholar] [CrossRef]
- Ye, G.; Li, C.; Xiang, X.; Chen, C.; Zhang, R.; Yang, X.; Yu, X.; Wang, J.; Wang, L.; Shi, Q.; et al. Bone Morphogenetic Protein-9 Induces PDLSCs Osteogenic Differentiation through the ERK and p38 Signal Pathways. Int. J. Med. Sci. 2014, 11, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Song, W.; Luo, J.; Luo, X.; Chen, J.; Sharff, K.A.; Bi, Y.; He, B.; Huang, J.; Zhu, G.; et al. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/β-catenin signalling. J. Cell. Mol. Med. 2008, 13, 2448–2464. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Ma, E.; Shen, W.; Li, H.; Zhang, Y. Synergistic effect of BMP9 and TGF-β in the proliferation and differentiation of osteoblasts. Genet. Mol. Res. 2015, 14, 7605–7615. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.; Zhao, Z.; Xu, H.H. Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials 2013, 34, 7862–7872. [Google Scholar] [CrossRef]
- Sun, X.; Ma, Z.; Zhao, X.; Jin, W.; Zhang, C.; Ma, J.; Qiang, L.; Wang, W.; Deng, Q.; Yang, H.; et al. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact. Mater. 2021, 6, 757–769. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Bononi, I.; Frontini, F.; Mazzoni, E.; Oton-Gonzalez, L.; Rotondo, J.C.; Torreggiani, E.; Tognon, M.; et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 2021, 11, 6573–6591. [Google Scholar] [CrossRef] [PubMed]
- Pelissier, P.H.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, L.; Xu, L.; Xu, Q.; Karsenty, G.; Chen, C.D. Histone demethylase JMJD3 is required for osteoblast differentiation in mice. Sci. Rep. 2015, 5, 13418. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Jadlowiec, J.A.; Campbell, P.G. Insulin-like growth factor-I induces early osteoblast gene expression in human mesenchymal stem cells. Stem Cells Dev. 2005, 14, 621–631. [Google Scholar] [CrossRef]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef]
- Shah, N.J.; Hyder, M.N.; Quadir, M.A.; Courchesne, N.-M.D.; Seeherman, H.J.; Nevins, M.; Spector, M.; Hammond, P.T. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl. Acad. Sci. USA 2014, 111, 12847–12852. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.-H.; Li, L.-Y.; Lang, J.; Yeh, S.-P.; Shi, B.; Yang, C.-C.; Yang, J.-Y.; Lin, C.-Y.; Lai, C.-C.; et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat. Cell Biol. 2010, 13, 87–94. [Google Scholar] [CrossRef]
- Ito, H. Chemokines in mesenchymal stem cell therapy for bone repair: A novel concept of recruiting mesenchymal stem cells and the possible cell sources. Mod. Rheumatol. 2010, 21, 113–121. [Google Scholar] [CrossRef]
- Hosogane, N.; Huang, Z.; Rawlins, B.A.; Liu, X.; Boachie-Adjei, O.; Boskey, A.L.; Zhu, W. Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2010, 42, 1132–1141. [Google Scholar] [CrossRef]
- Hong, J.-H.; Yaffe, M.B. TAZ: A β-Catenin-like Molecule that Regulates Mesenchymal Stem Cell Differentiation. Cell Cycle 2005, 5, 176–179. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.-J.; Park, J.-Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2–Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Chiu, Y.-S.; Chen, W.-Y.; Huang, K.-Y.; Jou, I.-M.; Wu, P.-T.; Wu, C.-H.; Chang, M.-S. Anti-IL-20 monoclonal antibody promotes bone fracture healing through regulating IL-20-mediated osteoblastogenesis. Sci. Rep. 2016, 6, 24339. [Google Scholar] [CrossRef] [PubMed]
- Dupont, K.M.; Sharma, K.; Stevens, H.Y.; Boerckel, J.D.; García, A.J.; Guldberg, R.E. Human stem cell delivery for treatment of large segmental bone defects. Proc. Natl. Acad. Sci. USA 2010, 107, 3305–3310. [Google Scholar] [CrossRef]
- Manassero, M.; Paquet, J.; Deschepper, M.; Viateau, V.; Retortillo, J.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Comparison of Survival and Osteogenic Ability of Human Mesenchymal Stem Cells in Orthotopic and Ectopic Sites in Mice. Tissue Eng. A 2016, 22, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.; Saxer, F.; Scotti, C.; Schreiner, S.; Studer, P.; Scherberich, A.; Heberer, M.; Martin, I. Perspective on the Evolution of Cell-Based Bone Tissue Engineering Strategies. Eur. Surg. Res. 2012, 49, 1–7. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Benning, L.; Gutzweiler, L.; Tröndle, K.; Riba, J.; Zengerle, R.; Koltay, P.; Zimmermann, S.; Stark, G.B.; Finkenzeller, G. Cytocompatibility testing of hydrogels toward bioprinting of mesenchymal stem cells. J. Biomed. Mater. Res. A 2017, 105, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Aghajanpour, S.; Esfandyari-Manesh, M.; Ghahri, T.; Ghahremani, M.H.; Atyabi, F.; Heydari, M.; Motasadizadeh, H.; Dinarvand, R. Impact of oxygen-calcium-generating and bone morphogenetic protein-2 nanoparticles on survival and differentiation of bone marrow-derived mesenchymal stem cells in the 3D bio-printed scaffold. Colloids Surf. B Biointerfaces 2022, 216, 112581. [Google Scholar] [CrossRef]
- Chai, S.; Huang, J.; Mahmut, A.; Wang, B.; Yao, Y.; Zhang, X.; Zhuang, Z.; Xie, C.; Xu, Z.; Jiang, Q. Injectable Photo-Crosslinked Bioactive BMSCs-BMP2-GelMA Scaffolds for Bone Defect Repair. Front. Bioeng. Biotechnol. 2022, 10, 875363. [Google Scholar] [CrossRef]
- Liu, B.; Wu, J.; Sun, X.; Meng, Q.; Zhang, J. Sustained delivery of osteogenic growth peptide through injectable photoinitiated composite hydrogel for osteogenesis. Front. Bioeng. Biotechnol. 2023, 11, 1228250. [Google Scholar] [CrossRef]
- Lu, K.; Wang, D.; Zou, G.; Wu, Y.; Li, F.; Song, Q.; Sun, Y. A multifunctional composite hydrogel that sequentially modulates the process of bone healing and guides the repair of bone defects. Biomed. Mater. 2024, 19, 035010. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, L.; Lin, X.; Yu, Y.; Zhao, Y. Pearl Powder Hybrid Bioactive Scaffolds from Microfluidic 3D Printing for Bone Regeneration. Adv. Sci. 2023, 10, e2304190. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Ganguly, K.; Randhawa, A.; Patil, T.V.; Patel, D.K.; Lim, K.-T. Electrically stimulated 3D bioprinting of gelatin-polypyrrole hydrogel with dynamic semi-IPN network induces osteogenesis via collective signaling and immunopolarization. Biomaterials 2023, 294, 121999. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Li, M.; Song, P.; Lei, H.; Gui, X.; Zhou, C.; Liu, L. Biomimetic Methacrylated Gelatin Hydrogel Loaded With Bone Marrow Mesenchymal Stem Cells for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770049. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.D.; Bao, X.; Kaneda, G.; Avalos, P.; Behrens, P.; Salehi, K.; Da, X.; Chen, A.; Castaneda, C.; Nakielski, P.; et al. iPSC-neural crest derived cells embedded in 3D printable bio-ink promote cranial bone defect repair. Sci. Rep. 2022, 12, 18701. [Google Scholar] [CrossRef]
- Freeman, F.E.; Burdis, R.; Kelly, D.J. Printing New Bones: From Print-and-Implant Devices to Bioprinted Bone Organ Precursors. Trends Mol. Med. 2021, 27, 700–711. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef]
- Shetty, S.S.; Shetty, S.; Venkatesh, S.B. Tissue Engineering in Oral and Maxillofacial Rehabilitation—Current Status and Future Prospects. Curr. Oral Health Rep. 2024, 11, 191–197. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Rasoolzade, B.; Namanloo, R.A.; Azarpira, N.; Dortaj, H. Stem cells and common biomaterials in dentistry: A review study. J. Mater. Sci. Mater. Med. 2022, 33, 55. [Google Scholar] [CrossRef]
- Hung, M.; Sadri, M.; Katz, M.; Schwartz, C.; Mohajeri, A. A Systematic Review of Stem Cell Applications in Maxillofacial Regeneration. Dent. J. 2024, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Yao, Y.; Giannobile, W.V.; Wang, H.-L. Current and future trends in periodontal tissue engineering and bone regeneration. Plast. Aesthetic Res. 2021, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Tel, A.; Miotti, G.; Ius, T.; de Marco, L.; Robiony, M.; Parodi, P.C.; Panciani, P.P.; Zeppieri, M. Stem Cells in Facial Regenerative Surgery: Current Clinical Applications. A Multidisciplinary, Systematic Review. Front. Biosci. 2023, 28, 123. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Fauzi, M.B.; Abu Kasim, N.H. In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. Int. J. Mol. Sci. 2023, 24, 12881. [Google Scholar] [CrossRef]
- Koçak, E.; Kemaloğlu, C.A.; Gönen, Z.B.; Gökdemir, N.S.; Soylu, E.; Bolat, D.; Yay, A. Mesenchymal Stem Cells-Derived Exosomes Combined With Bone Grafts Ameliorate Bone Regeneration in Mandibular Defects. J. Plast. Reconstr. Aesthetic Surg. 2023, 87, S11. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; Malandain, N.; Ferreira-Gonçalves, T.; Ardao, I.; Reis, C.P.; Laromaine, A.; Roig, A.; García-González, C.A. Cellulose-in-cellulose 3D-printed bioaerogels for bone tissue engineering. Cellulose 2023, 31, 515–534. [Google Scholar] [CrossRef]
| Resolution | Speed | Mechanical Properties | Porosity | Cost | |
|---|---|---|---|---|---|
| Extrusion bioprinting | + | ++ | ++ | + | ++ |
| Injected bioprinting | ++ | ++++ | + | ++ | + |
| Stereolithography | +++ | + | ++++ | +++ | +++ |
| LAB | ++++ | +++ | +++ | ++++ | ++++ |
| Cells | Bioink | Bioprinting Technique | Growth Factor | Study Type (In Vitro/In Vivo) | Bone Defect | Summary of Findings | REF |
|---|---|---|---|---|---|---|---|
| Rat BM-MSCs | GelMA/gelatine/PEG/0.4% MSNs composite hydrogels | Extrusion-based 3D bioprinting; thermo-crosslinking; photo-crosslinking of GelMA | 1 μg/mL−1 BMP-4 added to 1 mg mL−1 MSNs- Mesoporous silica nanoparticles | Implanted subcutaneously on the back of C57BL/6 mouse | Calvaria defect in diabetes mellitus rats | GelMA/gelatine/PEG/MSNs composite bioinks showed satisfactory printability, mechanical stability, and biocompatibility. The sustained release of BMP-4 from MSNs induced M2-type macrophage polarization and thereby inhibited inflammatory reactions. Loading of BMP-4 and secretion of BMP-2 by M2 type macrophages promoted the osteogenic differentiation of BM-MSCs and further accelerated bone repair in DM bone defects. | [170] |
| Human BM-MSCs | Alginate hydrogel | Extrusion-based 3D bioprinting; ionic crosslinking | Calcium peroxide nanoparticles (CPO NPs), BMP-2 (BMP2 NPs) | In vitro | - | The viability of encapsulated hBM-MSCs was increased and osteogenic differentiation was improved. Applying a sustained-release formulation of BMP-2 resulted in greater improvement in hBM-MSCs’ osteogenic differentiation and in the upregulation of RUNX2, OCN, and COL1A1 genes. | [188] |
| Rat BM-MSCs | GelMA hydrogel, photo-crosslinked under UV light (365 nm) for 30 s | Injected and UV-crosslinked at the site of injury | BMP-2 | SPF male SD rats | Distal femur defect, diameter 3 mm, depth of 2 mm | A photo-crosslinked BM-MSCs-BMP-2-GelMA bioactive hydrogel scaffold effectively promotes BM-MSC osteogenic differentiation and bone tissue regeneration. The active scaffold released about 70% of the BMP-2 in the first week, which continuously stimulated the adhesion and osteogenic differentiation of BM-MSCs inside and outside the scaffold. | [189] |
| Pre-osteoblast cell line MC3T3-E1 | GelMA/HAMA hydrogel loaded with OGP | Photo-crosslinking, injectable | Osteogenic growth peptide (OGP) | In vitro | - | The hydrogel promoted cell proliferation and adhesion and increased osteogenic-related gene and protein expression in vitro. | [190] |
| Rat BM-MSCs | GelMA hydrogels and porous CaCO3 microspheres (CMs) | Injected into the site of bone defect | BMP-2 | In vitro and SD rats | Skull defects | Rapid osteogenesis was induced, mainly involving MSC recruitment and differentiation in the later stage. The inflammatory response was balanced; macrophage polarization was modulated. Appropriate and timely modulation of bone healing process, such as the early inflammatory stage and the later osteogenic stage, was crucial to the healing of bone defects. | [191] |
| Rat BM-MSCs MC3T3-E1 cells HUVECs | Pearl powder (PP) hybrid fish gelatine methacrylate (GelMA) | Microfluidic-assisted 3D printing technology | VEGF | Rats | Skull defects | Controlled release of VEGF enables the scaffold to promote angiogenesis. A synergic effect of osteogenesis and angiogenesis was seen. | [192] |
| human BM-MSCs | Polypyrrole-grafted gelatin methacryloyl (GelMA-PPy) with triple crosslinking (thermo-photo-ioni-cally) | Extrusion-based 3D printing | Microcurrent stimulation (250 mV/20 min/day). | In vitro | Printed full-thickness rat bone model | Three-dimensional-bioprinted hBM-MSCs highly expressed gene hallmarks for NOTCH/mitogen-activated protein kinase (MAPK)/SMAD signaling while downregulating the Wnt/β-Catenin and epigenetic signalling pathways during osteogenic differentiation for up to 7 days. | [193] |
| BM-MSCs | Photo-crosslinked biomimetic methacrylated gelatin (Bio-GelMA) hydrogel | GelMA-BM-MSC suspension added into PDMS mold and photocured as scaffold | - | In vitro and rats | Segmental bone defect | The BM-MSC-carrying GelMA hydrogel scaffold has good mechanical properties and biological compatibility. It promotes the regeneration of bone and blood vessels, improves the mechanical strength of bone defects, and effectively promotes the repair of bone defects. | [194] |
| iPSC-derived cells via neural crest or mesoderm overexpressing BMP-6 | CellInk Bone or GelXA Bone | Cellink Bio X™ 3D bioprinter (in vitro tests); In vivo bone defect was filled with spatula with ink/cell mixture and crosslinking agent was added | BMP-6 | NOD/SCID mice | Cranial bone defect; frontal and parietal bones | The combination of bioprintable bioink and BMP-6 transfected iNCC-MPCs is capable of stimulating bone regeneration. | [195] |
| MSCs | Matrigel, fibrin, collagen, gelatine, and gelatine/alginate at various hydrogel concentrations | Drop-on-demand (DoD) printing | HA | In vitro | - | The inclusion of HA enhanced the proliferation and osteogenic differentiation of MSCs and prevented the degradation of fibrin in vitro. | [187] |
| MSCs HUVEC | Naturally derived hydrogel GelMA | Extrusion-based direct-writing bioprinting | Silicate nanoplatelets and VEGF | In vitro | Bone-like tissue constructs containing a perfusable vascular lumen | Encapsulated hMSCs formed a mature bone niche after 21 days of culture under the medium perfused condition. | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostadinova, M.; Raykovska, M.; Simeonov, R.; Lolov, S.; Mourdjeva, M. Recent Advances in Bone Tissue Engineering: Enhancing the Potential of Mesenchymal Stem Cells for Regenerative Therapies. Curr. Issues Mol. Biol. 2025, 47, 287. https://doi.org/10.3390/cimb47040287
Kostadinova M, Raykovska M, Simeonov R, Lolov S, Mourdjeva M. Recent Advances in Bone Tissue Engineering: Enhancing the Potential of Mesenchymal Stem Cells for Regenerative Therapies. Current Issues in Molecular Biology. 2025; 47(4):287. https://doi.org/10.3390/cimb47040287
Chicago/Turabian StyleKostadinova, Milena, Miryana Raykovska, Radoil Simeonov, Stephan Lolov, and Milena Mourdjeva. 2025. "Recent Advances in Bone Tissue Engineering: Enhancing the Potential of Mesenchymal Stem Cells for Regenerative Therapies" Current Issues in Molecular Biology 47, no. 4: 287. https://doi.org/10.3390/cimb47040287
APA StyleKostadinova, M., Raykovska, M., Simeonov, R., Lolov, S., & Mourdjeva, M. (2025). Recent Advances in Bone Tissue Engineering: Enhancing the Potential of Mesenchymal Stem Cells for Regenerative Therapies. Current Issues in Molecular Biology, 47(4), 287. https://doi.org/10.3390/cimb47040287






