Circulating Tumor Cells in Head and Neck Squamous-Cell Carcinoma Exhibit Distinct Properties Based on Targeted Epithelial-Related Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. CTC Detection and Gene Expression Analysis
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients with CTCs with HNSCC and Epithelial-Related Gene Expression in CTCs
3.2. Comparison of Molecular Characteristics of CTCs Based on Epithelial Marker Expression
3.3. Relationship Between the Expression of Epithelial-Related Markers in CTCs and Clinical Factors, Including Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTC | circulating tumor cell |
| EpCAM | epithelial cell adhesion molecule |
| EMT | epithelial–mesenchymal transition |
| EGFR | epidermal growth factor receptor |
| HNSCC | head and neck squamous-cell carcinoma |
| HPV | human papillomavirus |
| OS | overall survival |
| PBMC | peripheral blood mononuclear cell |
| PFS | progression-free survival |
| PD-L1 | programmed death-ligand 1 |
References
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBiomedicine 2022, 83, 104237. [Google Scholar] [CrossRef]
- Vasseur, A.; Kiavue, N.; Bidard, F.C.; Pierga, J.Y.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Shao, W.; Li, Z.; Zhao, R.; Ye, Z. Strategies for enrichment of circulating tumor cells. Transl. Cancer Res. 2020, 9, 2012–2025. [Google Scholar] [CrossRef]
- Toss, A.; Mu, Z.; Fernandez, S.; Cristofanilli, M. CTC enumeration and characterization: Moving toward personalized medicine. Ann. Transl. Med. 2014, 2, 108. [Google Scholar] [CrossRef]
- Yang, Y.P.; Giret, T.M.; Cote, R.J. Circulating tumor cells from enumeration to analysis: Current challenges and future opportunities. Cancers 2021, 13, 2723. [Google Scholar] [CrossRef]
- Eslami-S, Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. Epithelial cell adhesion molecule: An anchor to isolate clinically relevant circulating tumor cells. Cells 2020, 9, 1836. [Google Scholar] [CrossRef]
- Hyun, K.A.; Koo, G.B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.I.; Jung, H.I.; Kim, Y.S. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 2016, 7, 24677–24687. [Google Scholar] [CrossRef]
- Li, Y.M.; Xu, S.C.; Ki, J.; Han, K.O.; Pi, H.F.; Zheng, L.; Zuo, G.H.; Huang, X.B.; Li, H.Y.; Zhao, H.Z.; et al. Epithelial–mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013, 4, e831. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef]
- Ida, S.; Takahashi, H.; Tada, H.; Mito, I.; Matsuyama, T.; Chikamatsu, K. Dynamic changes of the EMT spectrum between circulating tumor cells and the tumor microenvironment in human papillomavirus-positive head and neck squamous cell carcinoma. Oral. Oncol. 2023, 137, 106296. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Takahashi, H.; Kawabata-Iwakawa, R.; Nagata, Y.; Uchida, M.; Shino, M.; Ida, S.; Mito, I.; Matsuyama, T.; Chikamatsu, K. Molecular phenotypes of circulating tumor cells and efficacy of nivolumab treatment in patients with head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 21573. [Google Scholar] [CrossRef]
- Chikamatsu, K.; Tada, H.; Takahashi, H.; Kuwabara-Yokobori, Y.; Ishii, H.; Ida, S.; Shino, M. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral. Oncol. 2019, 89, 34–39. [Google Scholar] [CrossRef]
- Sieuwerts, A.M.; Kraan, J.; Bolt-de Vries, J.; van der Spoel, P.; Mostert, B.; Martens, J.W.; Gratama, J.W.; Sleijfer, S.; Foekens, J.A. Molecular characterization of circulating tumor cells in large quantities of contaminating leukocytes by a multiplex real-time PCR. Breast Cancer Res. Treat. 2009, 118, 455–468. [Google Scholar] [CrossRef]
- Tinhofer, I.; Hristozova, T.; Stromberger, C.; Keilhoiz, U.; Budach, V. Monitoring of circulating tumor cells and their expression of EGFR/phospho-EGFR during combined radiotherapy regimens in locally advanced squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e685–e690. [Google Scholar] [CrossRef]
- Ilie, M.; Szafer-Glusman, E.; Hofman, V.; Long-Mira, E.; Suttmann, R.; Darbonne, W.; Butori, C.; Lalvée, S.; Fayada, J.; Selva, E.; et al. Expression of MET in circulating tumor cells correlates with expression in tumor tissue from advanced-stage lung cancer patients. Oncotarget 2017, 8, 26112–26121. [Google Scholar] [CrossRef]
- Park, J.; Chang, E.S.; Kim, J.Y.; Chelakkot, C.; Sung, M.; Song, J.Y.; Jung, K.; Lee, J.H.; Choi, J.Y.; Kim, N.Y.; et al. c-MET-positive circulating tumor cells and cell-free DNA as independent prognostic factors in hormone receptor-positive/HER2-negative metastatic breast cancer. Breast Cancer Res. 2024, 26, 13. [Google Scholar] [CrossRef]
- Keller, L.; Werner, S.; Pantel, K. Biology and clinical relevance of EpCAM. Cell Stress. 2019, 3, 165–180. [Google Scholar] [CrossRef]
- Yanamoto, S.; Kawasaki, G.; Yoshitomi, I.; Iwamoto, T.; Hirata, K.; Mizuno, A. Clinicopathologic significance of EpCAM expression in squamous cell carcinoma of the tongue and its possibility as a potential target for tongue cancer gene therapy. Oral. Oncol. 2007, 43, 869–877. [Google Scholar] [CrossRef]
- Murakami, N.; Mori, T.; Nakamura, S.; Yoshimoto, S.; Honma, Y.; Ueno, T.; Kobayashi, K.; Kashihara, T.; Takahashi, K.; Inaba, K.; et al. Prognostic value of the expression of epithelial cell adhesion molecules in head and neck squamous cell carcinoma treated by definitive radiotherapy. J. Radiat. Res. 2019, 60, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Schinke, H.; Heider, T.; Herkommer, T.; Simon, F.; Blancke Soares, A.; Kranz, G.; Samaga, D.; Dajka, L.; Feuchtinger, A.; Walch, A.; et al. Digital scoring of EpCAM and slug expression as prognostic markers in head and neck squamous cell carcinomas. Mol. Oncol. 2021, 15, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, P.; Hollmann, A.; Kitz, J.; Afthonidou, A.; Simon, F.; Shakhtour, J.; Mack, B.; Kranz, G.; Libl, D.; Leu, M.; et al. High expression of EpCAM and Sox2 is a positive prognosticator of clinical outcome for head and neck carcinoma. Sci. Rep. 2018, 8, 14582. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, J.M.; Song, Y.S.; Cho, N.Y.; Lee, H.S.; Kang, G.H. Clinicopathologic, molecular, and prognostic implications of the loss of EPCAM expression in colorectal carcinoma. Oncotarget 2016, 7, 13372–13387. [Google Scholar] [CrossRef]
- Pal, A.; Barrett, T.F.; Paolini, R.; Parikh, A.; Puram, S.V. Partial EMT in head and neck cancer biology: A spectrum instead of a switch. Oncogene 2021, 40, 5049–5065. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; Aguilar-Gallardo, C.; Calabuig-Fariñas, S.; Sirera, R.; Jantus-Lewintre, E.; Camps, C. EpCAM duality becomes this molecule in a new Dr. Jekyll and Mr. Hyde tale. Crit. Rev. Oncol. Hematol. 2018, 126, 52–63. [Google Scholar] [CrossRef]
- Grandis, J.R.; Tweardy, D.J. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993, 53, 3579–3584. [Google Scholar]
- Brand, T.M.; Iida, M.; Li, C.; Wheeler, D.L. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov. Med. 2011, 12, 419–432. [Google Scholar]
- Kalyankrishna, S.; Grandis, J.R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, L.; Wang, Y.; Zhao, S.; Cui, M. Clinicopathology of EpCAM and EGFR in human epithelial ovarian carcinoma. Open Med. (Wars) 2017, 12, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Schinke, H.; Luxenburger, E.; Kranz, G.; Shakhtour, J.; Libl, D.; Huang, Y.; Gaber, A.; Pavšič, M.; Lenarčič, B.; et al. EpCAM ectodomain EpEX is a ligand of EGFR that counteracts EGF-mediated epithelial-mesenchymal transition through modulation of phospho-ERK1/2 in head and neck cancers. PLoS Biol. 2018, 16, e2006624. [Google Scholar] [CrossRef] [PubMed]
- Domènech, M.; Muñoz Marmol, A.M.; Mate, J.L.; Estival, A.; Moran, T.; Cucurull, M.; Saigi, M.; Hernandez, A.; Sanz, C.; Hernandez-Gallego, A.; et al. Correlation between PD-L1 expression and MET gene amplification in patients with advanced non-small cell lung cancer and no other actionable oncogenic driver. Oncotarget 2021, 12, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Kim, S.; Kwon, D.; Koh, J.; Kim, Y.A.; Kim, K.; Chung, D.H.; Jeon, Y.K. MET receptor tyrosine kinase regulates the expression of co-stimulatory and co-inhibitory molecules in tumor cells and contributes to PD-L1-mediated suppression of immune cell function. Int. J. Mol. Sci. 2019, 20, 4287. [Google Scholar] [CrossRef]
- Li, E.; Huang, X.; Zhang, G.; Liang, T. Combinational blockade of MET and PD-L1 improves pancreatic cancer immunotherapeutic efficacy. J. Exp. Clin. Cancer Res. 2021, 40, 279. [Google Scholar] [CrossRef]
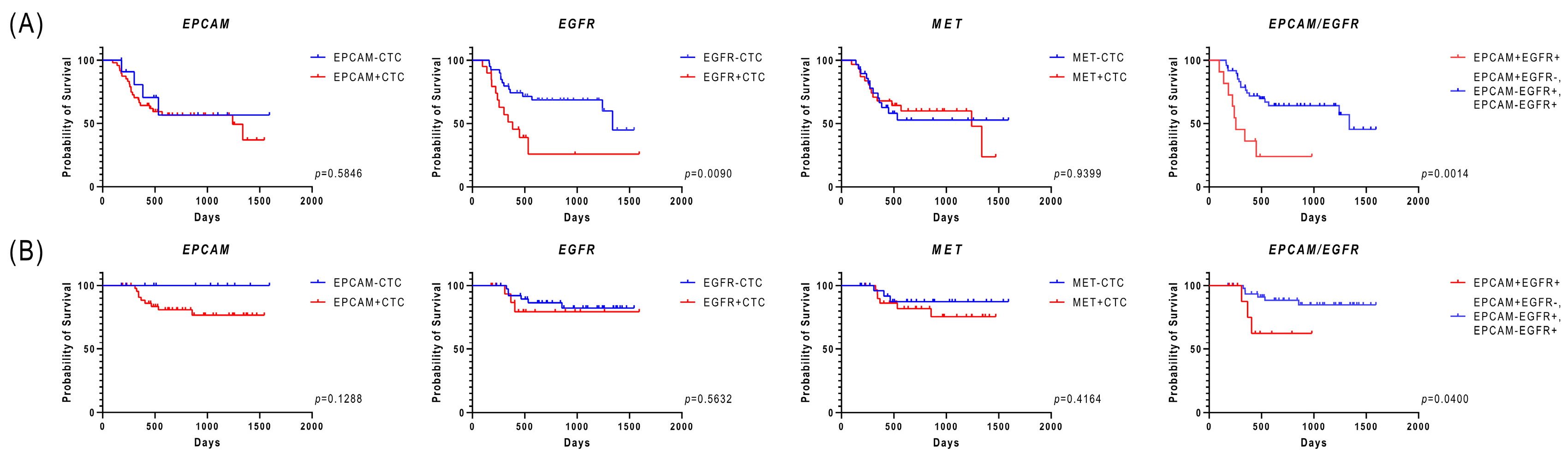
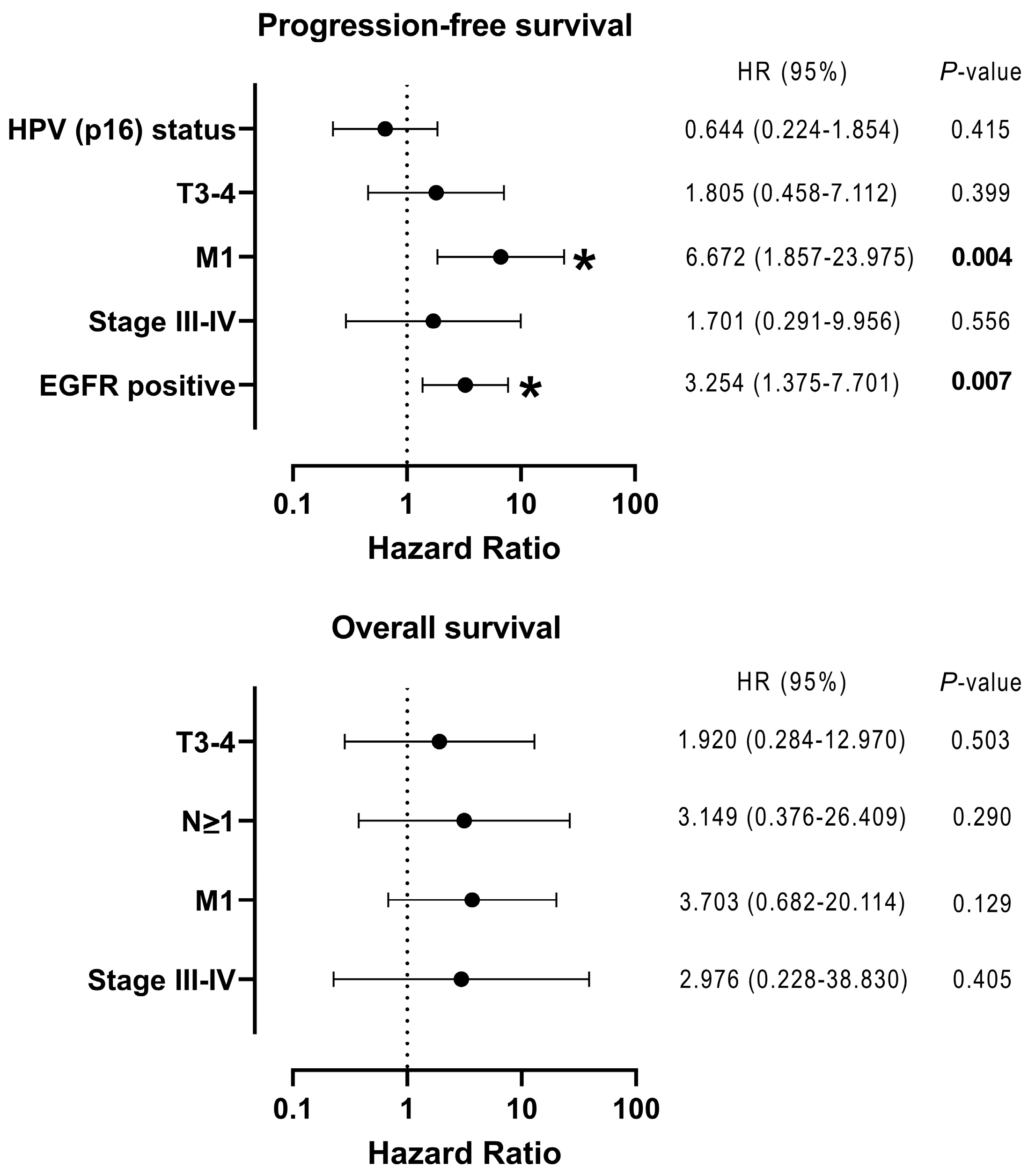
| Clinical Variable | Number of Patients (%) | |
|---|---|---|
| Age: median 69 years old | ≤69 | 31 (51.7) |
| >69 | 29 (48.3) | |
| Sex | Male | 55 (91.7) |
| Female | 5 (8.3) | |
| Primary sites | Oral cavity | 2 (3.3) |
| Larynx | 14 (23.3) | |
| Oropharynx | 28 (46.7) | |
| Hypopharynx | 16 (26.7) | |
| HPV (p16) status | Negative | 36 (60.0) |
| Positive | 24 (40.0) | |
| T classification | 1–2 | 27 (45.0) |
| 3–4 | 33 (55.0) | |
| N classification | 0 | 20 (33.3) |
| ≥1 | 40 (66.7) | |
| M classification | 0 | 56 (93.3) |
| 1 | 4 (6.7) | |
| Stage | I–II | 24 (40.0) |
| III–IV | 36 (60.0) | |
| EPCAM | Negative | 12 (20.0) |
| Positive | 48 (80.0) | |
| EGFR | Negative | 40 (66.7) |
| Positive | 20 (33.3) | |
| MET | Negative | 29 (48.3) |
| Positive | 31 (51.7) |
| EGFR | p-Value | |||
| Negative (n = 40) | Positive (n = 20) | |||
| EPCAM | Negative (n = 12) | 3 | 9 | 0.0013 |
| Positive (n = 48) | 37 | 11 | ||
| MET | p-Value | |||
| Negative (n = 29) | Positive (n = 31) | |||
| EPCAM | Negative (n = 12) | 7 | 5 | 0.5269 |
| Positive (n = 48) | 22 | 26 | ||
| MET | p-Value | |||
| Negative (n = 29) | Positive (n = 31) | |||
| EGFR | Negative (n = 40) | 16 | 24 | 0.1004 |
| Positive (n = 20) | 13 | 7 | ||
| VIM | p-Value | CDH1 | p-Value | CDH2 | p-Value | SNAI1 | p-Value | ZEB1 | p-Value | ZEB2 | p-Value | TWIST1 | p-Value | CD274 | p-Value | PDCD1LG2 | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPCAM | Negative | 35.97 (34.39–36.85) | 0.877 | 26.11 (25.92–27.73) | 0.025 | 21.10 (18.19–22.79) | 0.017 | 25.49 (24.33–27.05) | 0.877 | 28.14 (26.98–29.00) | 0.448 | 32.97 (31.99–33.11) | 0.891 | 24.07 (23.37–25.13) | 0.002 | 25.11 (24.98–26.74) | 0.143 | 23.93 (23.16–25.42) | 0.222 |
| Positive | 36.01 (35.03–36.48) | 25.18 (24.95–26.07) | 23.00 (22.05–24.01) | 25.50 (24.02–26.07) | 28.51 (27.98–29.04) | 32.99 (32.00–33.05) | 23.09 (22.08–24.04) | 26.02 (25.11–26.27) | 24.34 (23.98–25.18) | ||||||||||
| EGFR | Negative | 36.02 (35.05–36.82) | 0.171 | 25.13 (24.96–26.02) | 0.010 | 23.03 (22.19–24.05) | 0.001 | 25.90 (24.03–26.73) | 0.658 | 28.98 (28.00–29.06) | 0.012 | 32.99 (32.01–33.07) | 0.182 | 23.08 (21.96–24.02) | 0.013 | 26.02 (25.14–26.96) | 0.054 | 24.64 (23.98–25.28) | 0.127 |
| Positive | 35.97 (34.32–36.04) | 26.15 (25.24–27.02) | 22.03 (19.19–22.99) | 25.02 (24.33–26.06) | 28.04 (26.98–28.77) | 32.95 (31.98–33.01) | 23.97 (23.09–25.04) | 25.38 (25.03–26.05) | 24.10 (23.12–25.07) | ||||||||||
| MET | Negative | 35.04 (34.51–36.32) | 0.002 | 25.06 (24.89–26.10) | 0.121 | 22.84 (20.10–23.07) | 0.058 | 25.94 (23.65–26.05) | 0.436 | 28.02 (26.98–29.02) | 0.038 | 32.97 (32.00–33.04) | 0.354 | 23.14 (22.47–25.04) | 0.803 | 25.22 (25.03–26.08) | 0.023 | 24.06 (23.18–24.64) | <0.001 |
| Positive | 36.04 (36.00–36.89) | 25.98 (25.02–27.00) | 23.03 (22.05–24.05) | 25.14 (24.32–27.02) | 28.95 (28.02–29.04) | 33.00 (32.01–33.07) | 23.17 (22.90–24.05) | 26.04 (25.53–26.97) | 25.08 (24.16–26.03) |
| Number | EPCAM | EGFR | MET | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | p-Value | Negative | Positive | p-Value | Negative | Positive | p-Value | |||
| (n = 12) | (n = 48) | (n = 40) | (n = 20) | (n = 29) | (n = 31) | ||||||
| HPV (p16) status | Negative | 36 | 6 | 30 | 0.4292 | 26 | 10 | 0.2636 | 16 | 20 | 0.4603 |
| Positive | 24 | 6 | 18 | 14 | 10 | 13 | 11 | ||||
| T classification | 1–2 | 27 | 8 | 19 | 0.1139 | 19 | 8 | 0.7836 | 14 | 13 | 0.7955 |
| 3–4 | 33 | 4 | 29 | 21 | 12 | 15 | 18 | ||||
| N classification | 0 | 20 | 6 | 14 | 0.1893 | 15 | 5 | 0.3950 | 8 | 12 | 0.4194 |
| >1 | 40 | 6 | 34 | 25 | 15 | 21 | 19 | ||||
| M classification | 0 | 56 | 12 | 44 | 0.5744 | 37 | 19 | >0.9999 | 27 | 29 | >0.9999 |
| 1 | 4 | 0 | 4 | 3 | 1 | 2 | 2 | ||||
| Stage | I–II | 24 | 8 | 16 | 0.0498 | 16 | 8 | >0.9999 | 11 | 13 | 0.7969 |
| III–IV | 36 | 4 | 32 | 24 | 12 | 18 | 18 | ||||
| Relapse | Negative | 34 | 8 | 26 | 0.5258 | 26 | 8 | 0.0975 | 17 | 17 | 0.7997 |
| Positive | 26 | 4 | 22 | 14 | 12 | 12 | 14 | ||||
| Locoregional relapse | Negative | 43 | 9 | 34 | >0.9999 | 33 | 10 | 0.0143 | 21 | 22 | >0.9999 |
| Positive | 17 | 3 | 14 | 7 | 10 | 8 | 9 | ||||
| Distant metastasis | Negative | 50 | 11 | 39 | 0.6697 | 32 | 18 | 0.4714 | 25 | 25 | 0.7324 |
| Positive | 10 | 1 | 9 | 8 | 2 | 4 | 6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikamatsu, K.; Takahashi, H.; Tada, H.; Uchida, M.; Ida, S.; Tomidokoro, Y.; Motegi, M. Circulating Tumor Cells in Head and Neck Squamous-Cell Carcinoma Exhibit Distinct Properties Based on Targeted Epithelial-Related Markers. Curr. Issues Mol. Biol. 2025, 47, 240. https://doi.org/10.3390/cimb47040240
Chikamatsu K, Takahashi H, Tada H, Uchida M, Ida S, Tomidokoro Y, Motegi M. Circulating Tumor Cells in Head and Neck Squamous-Cell Carcinoma Exhibit Distinct Properties Based on Targeted Epithelial-Related Markers. Current Issues in Molecular Biology. 2025; 47(4):240. https://doi.org/10.3390/cimb47040240
Chicago/Turabian StyleChikamatsu, Kazuaki, Hideyuki Takahashi, Hiroe Tada, Miho Uchida, Shota Ida, Yuichi Tomidokoro, and Masaomi Motegi. 2025. "Circulating Tumor Cells in Head and Neck Squamous-Cell Carcinoma Exhibit Distinct Properties Based on Targeted Epithelial-Related Markers" Current Issues in Molecular Biology 47, no. 4: 240. https://doi.org/10.3390/cimb47040240
APA StyleChikamatsu, K., Takahashi, H., Tada, H., Uchida, M., Ida, S., Tomidokoro, Y., & Motegi, M. (2025). Circulating Tumor Cells in Head and Neck Squamous-Cell Carcinoma Exhibit Distinct Properties Based on Targeted Epithelial-Related Markers. Current Issues in Molecular Biology, 47(4), 240. https://doi.org/10.3390/cimb47040240






