Evaluation of the Anti-Alzheimer Activity of Lycium barbarum Polysaccharide in Aβ1–42-Induced Neurotoxicity in Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Agents
2.2. Experimental Animals and Surgery
2.3. Open Field Test (OFT)
2.4. Morris Water Maze (MWM)
2.5. Y-Maze
2.6. Passive Avoidance Test (PAT)
2.7. Tissue Preparation
2.8. Hematoxylin-Eosin Staining (HE)
2.9. Detection of Brain Inflammation Markers
2.10. Detection of Biomarkers for Oxidative Stress and Cholinergic System
2.11. Statistical Analysis
3. Results
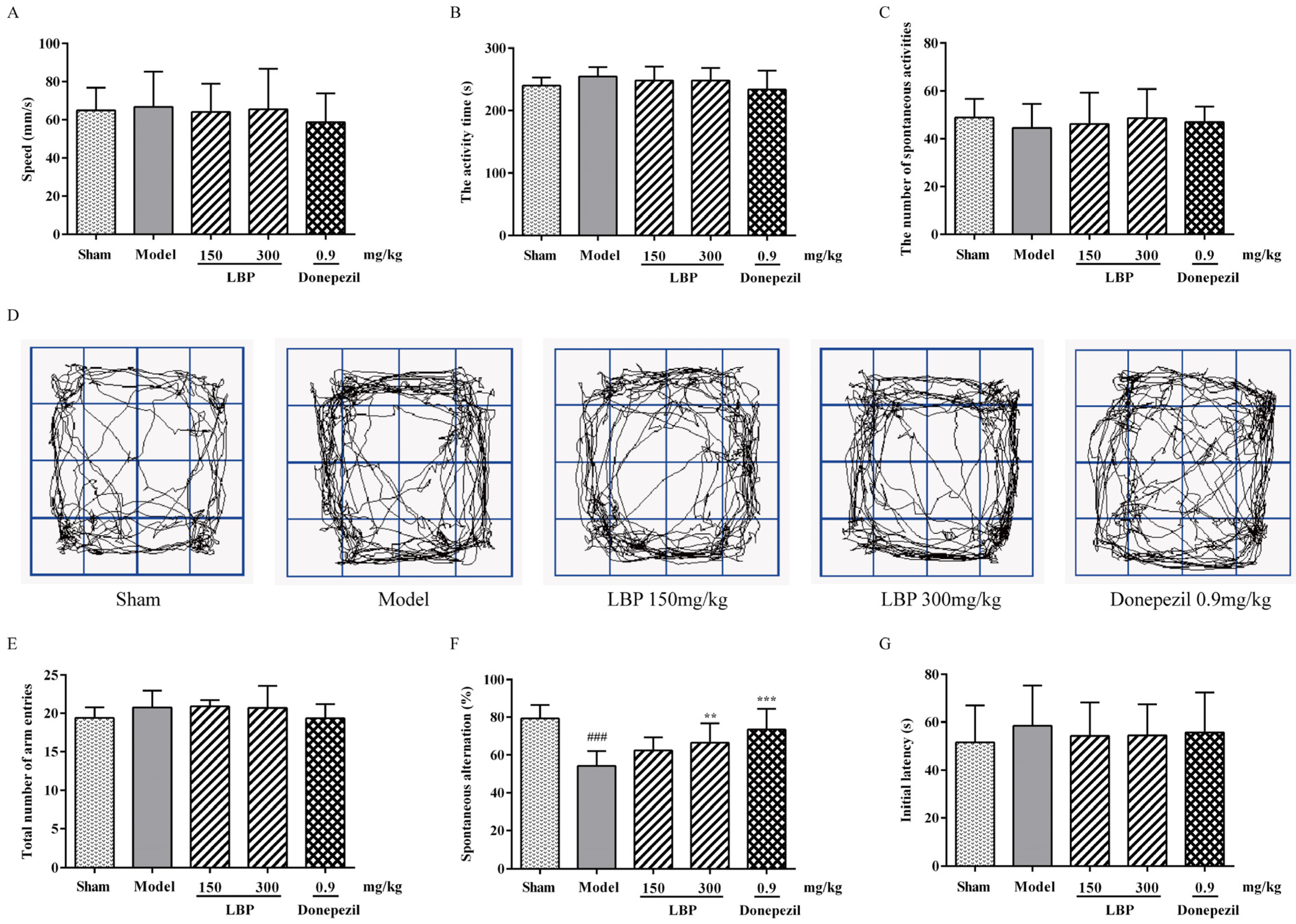
3.1. LBP Mitigates Memory Impairments Caused by Aβ1–42 Peptides
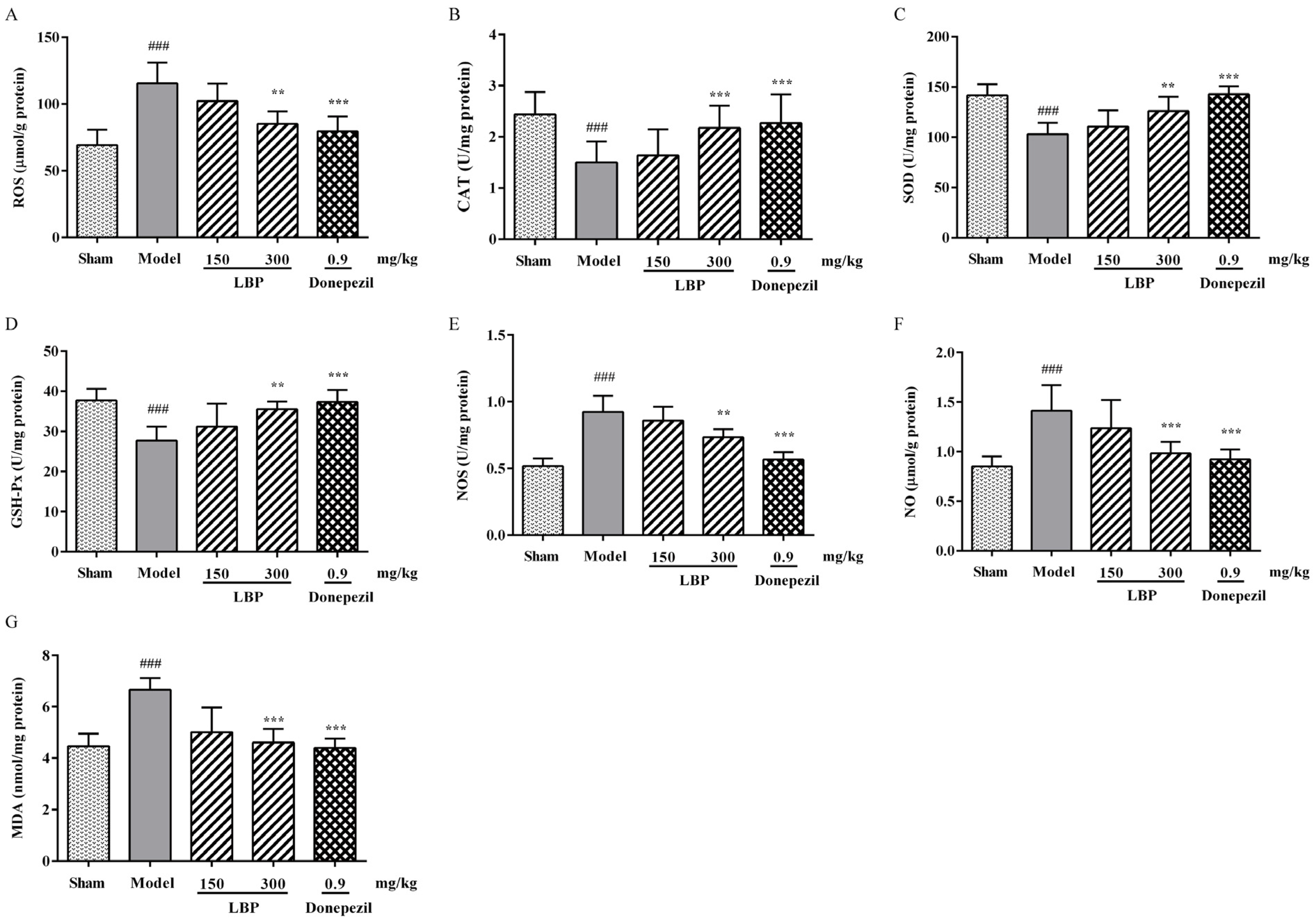
3.2. LBP Alleviates Oxidative/Nitrosative Stress Markers in the Brain of AD Rats
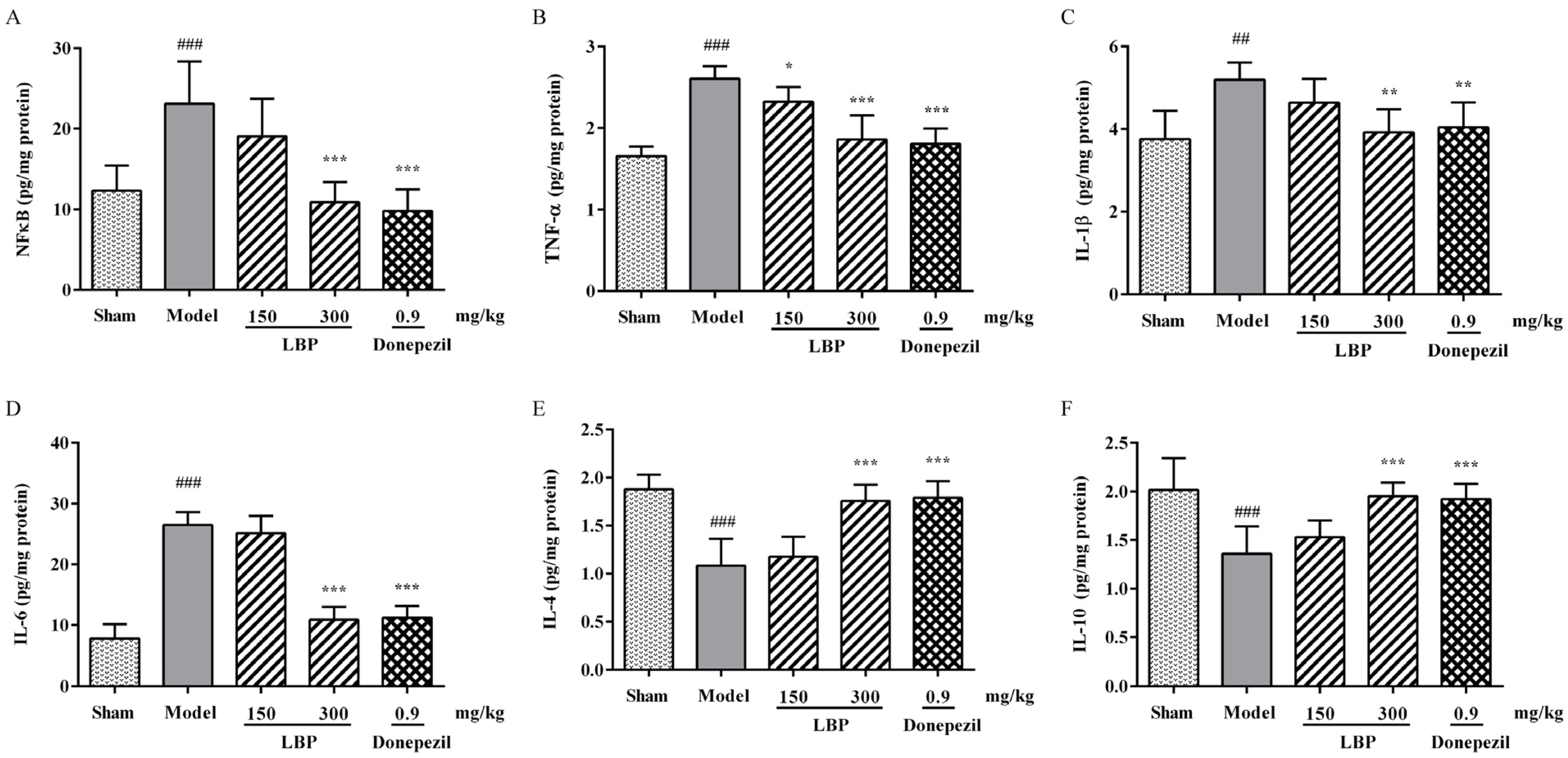
3.3. LBP Attenuates the Inflammation in Rats’ Brains Induced by Aβ1–42
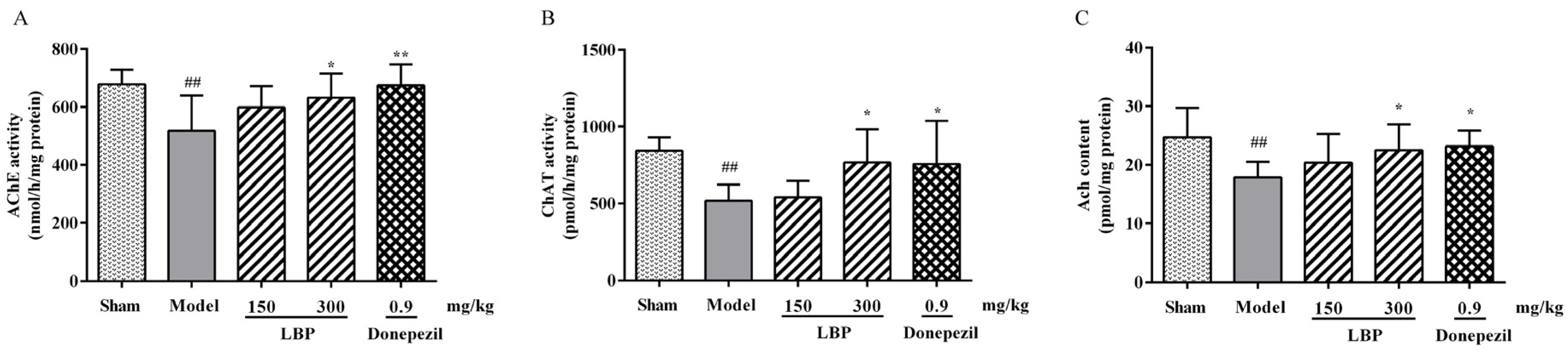
3.4. LBP Protects Cholinergic System from Aβ1–42 Peptides
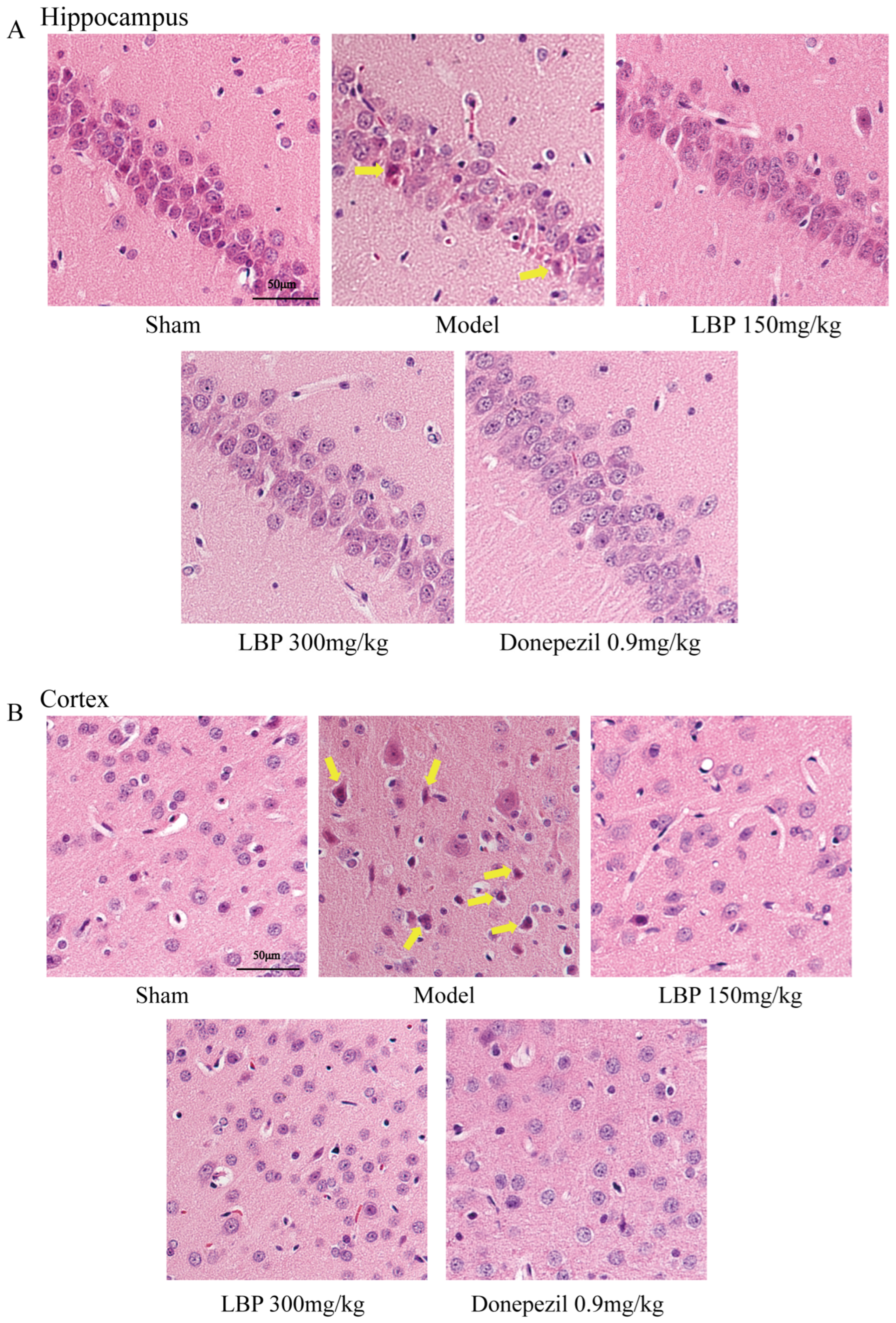
3.5. LBP Protects Against Neurological Injury in AD Model Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hamdan, A.M.E.; Alharthi, F.H.J.; Alanazi, A.H.; El-Emam, S.Z.; Zaghlool, S.S.; Metwally, K.; Albalawi, S.A.; Abdu, Y.S.; Mansour, R.E.; Salem, H.A.; et al. Neuroprotective Effects of Phytochemicals against Aluminum Chloride-Induced Alzheimer’s Disease through ApoE4/LRP1, Wnt3/β-Catenin/GSK3β, and TLR4/NLRP3 Pathways with Physical and Mental Activities in a Rat Model. Pharmaceuticals 2022, 15, 1008. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Deng, M.; Hu, G.; Li, N.; Yuan, H.; Zhou, Y. New Insights into Microglial Mechanisms of Memory Impairment in Alzheimer’s Disease. Biomolecules 2022, 12, 1722. [Google Scholar] [CrossRef] [PubMed]
- Wirths, O.; Walter, S.; Kraus, I.; Klafki, H.W.; Stazi, M.; Oberstein, T.J.; Ghiso, J.; Wiltfang, J.; Bayer, T.A.; Weggen, S. N-truncated Aβ(4-x) peptides in sporadic Alzheimer’s disease cases and transgenic Alzheimer mouse models. Alzheimer’s Res. Ther. 2017, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cao, X.; Zhou, Y.; Shi, F.; Xin, S.; He, S.; An, Y.; Gao, L.; Yang, Y.; Yu, B.; et al. An analog derived from phenylpropanoids ameliorates Alzheimer’s disease-like pathology and protects mitochondrial function. Neurobiol. Aging 2019, 80, 187–195. [Google Scholar] [CrossRef]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76 Pt A, 27–50. [Google Scholar] [CrossRef]
- Eskandari, S.; Rezayof, A.; Asghari, S.M.; Hashemizadeh, S. Neurobiochemical characteristics of arginine-rich peptides explain their potential therapeutic efficacy in neurodegenerative diseases. Neuropeptides 2023, 101, 102356. [Google Scholar] [CrossRef]
- Ojetunde, A. The Neuroprotective and Therapeutic Effects of Medicinal Plants and Natural Products against Aluminium Chloride-Induced Alzheimer’s Disease: Recent Update. Biol. Med. Nat. Prod. Chem. 2024, 13, 7–33. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimer’s Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Elshamy, S.; Abdel Motaal, A.; Abdel-Halim, M.; Medhat, D.; Handoussa, H. Potential neuroprotective activity of Mentha longifolia L. in aluminum chloride-induced rat model of Alzheimer’s disease. J. Food Biochem. 2021, 45, 1770. [Google Scholar] [CrossRef]
- Prema, A.; Justin Thenmozhi, A.; Manivasagam, T.; Mohamed Essa, M.; Guillemin, G.J. Fenugreek Seed Powder Attenuated Aluminum Chloride-Induced Tau Pathology, Oxidative Stress, and Inflammation in a Rat Model of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2017, 60, S209–S220. [Google Scholar] [CrossRef] [PubMed]
- Pengsheng, W.; Xue, L.; Haotian, G.; Jiaqi, C.; Wenxuan, L.; Liwei, Z.; Decheng, W.; Miaoqi, C.; Qiwen, Z.; Ge, J. Olfactory stimulation from edible and medicinal homologous essential oils improves Aβ-induced cognitive impairment by regulating oxidative stress and synaptic function. J. Funct. Foods 2023, 105, 105573. [Google Scholar]
- Ghaderi, S.; Gholipour, P.; Komaki, A.; Salehi, I.; Rashidi, K.; Esmaeil Khoshnam, S.; Rashno, M. p-Coumaric acid ameliorates cognitive and non-cognitive disturbances in a rat model of Alzheimer’s disease: The role of oxidative stress and inflammation. Int. Immunopharmacol. 2022, 112, 109295. [Google Scholar] [CrossRef]
- Moneim, A.E. Oxidant/Antioxidant imbalance and the risk of Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 335–349. [Google Scholar] [CrossRef]
- Lecca, D.; Jung, Y.J.; Scerba, M.T.; Hwang, I.; Kim, Y.K.; Kim, S.; Modrow, S.; Tweedie, D.; Hsueh, S.C.; Liu, D.; et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 2327–2340. [Google Scholar] [CrossRef]
- Fan, M.; Liu, S.; Sun, H.M.; Ma, M.D.; Gao, Y.J.; Qi, C.C.; Xia, Q.R.; Ge, J.F. Bilateral intracerebroventricular injection of streptozotocin induces AD-like behavioral impairments and neuropathological features in mice: Involved with the fundamental role of neuroinflammation. Biomed. Pharmacother. 2022, 153, 113375. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Ahmed, M.; Surapaneni, K.M.; Veeraraghavan, V.P.; Arulselvan, P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J. Biol. Sci. 2021, 28, 4232–4239. [Google Scholar] [CrossRef]
- Di, S.; Miao, Y.; Huibo, G.; Yanyan, Z. Neuroprotective effect of Betalain against AlCl3-induced Alzheimer’s disease in Sprague Dawley Rats via putative modulation of oxidative stress and nuclear factor kappa B (NF-κB) signaling pathway. Biomed. Pharmacother. 2021, 137, 111369. [Google Scholar]
- ChunSing, L.; Lim, T.G.; KwokFai, S.; ManLung, F. Neuroprotective mechanism of Lycium barbarum polysaccharides against hippocampal-dependent spatial memory deficits in a rat model of obstructive sleep apnea. PLoS ONE 2015, 10, e0117990. [Google Scholar]
- Zhou, Y.; Duan, Y.; Huang, S.; Zhou, X.; Zhou, L.; Hu, T.; Yang, Y.; Lu, J.; Ding, K.; Guo, D.; et al. Polysaccharides from Lycium barbarum ameliorate amyloid pathology and cognitive functions in APP/PS1 transgenic mice. Int. J. Biol. Macromol. 2020, 144, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. CR 2020, 39, 210. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Ren, H.; Li, H.; Li, X.; Dong, T.; Xu, S.; Yan, Y.; Sun, B.; Bai, J.; Li, Y. Lycium barbarum polysaccharide reduces hyperoxic acute lung injury in mice through Nrf2 pathway. Biomed. Pharmacother. 2019, 111, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Deng, Q.; Zhou, W.; Zhang, Y. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol. Ther. 2022, 229, 107921. [Google Scholar] [CrossRef]
- Li, X.; Mo, X.; Liu, T.; Shao, R.; Teopiz, K.; McIntyre, R.S.; So, K.F.; Lin, K. Efficacy of Lycium barbarum polysaccharide in adolescents with subthreshold depression: Interim analysis of a randomized controlled study. Neural Regen. Res. 2022, 17, 1582–1587. [Google Scholar] [CrossRef]
- Hu, X.; Qu, Y.; Chu, Q.; Li, W.; He, J. Investigation of the neuroprotective effects of Lycium barbarum water extract in apoptotic cells and Alzheimer’s disease mice. Mol. Med. Rep. 2018, 17, 3599–3606. [Google Scholar] [CrossRef]
- Ho, Y.S.; Yu, M.S.; Yik, S.Y.; So, K.F.; Yuen, W.H.; Chang, R.C. Polysaccharides from wolfberry antagonizes glutamate excitotoxicity in rat cortical neurons. Cell. Mol. Neurobiol. 2009, 29, 1233–1244. [Google Scholar] [CrossRef]
- Bo, R.; Sun, Y.; Zhou, S.; Ou, N.; Gu, P.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Simple nanoliposomes encapsulating Lycium barbarum polysaccharides as adjuvants improve humoral and cellular immunity in mice. Int. J. Nanomed. 2017, 12, 6289–6301. [Google Scholar] [CrossRef]
- Xing, X.; Liu, F.; Xiao, J.; So, K.F. Neuro-protective Mechanisms of Lycium barbarum. Neuromolecular Med. 2016, 18, 253–263. [Google Scholar] [CrossRef]
- Gao, L.-L.; Ma, J.-M.; Fan, Y.-N.; Zhang, Y.-N.; Ge, R.; Tao, X.-J.; Zhang, M.-W.; Gao, Q.-H.; Yang, J.-J. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int. J. Biol. Macromol. 2021, 183, 1379–1392. [Google Scholar] [CrossRef]
- Gao, J.M.; Zhang, X.; Shu, G.T.; Chen, N.N.; Zhang, J.Y.; Xu, F.; Li, F.; Liu, Y.G.; Wei, Y.; He, Y.Q.; et al. Trilobatin rescues cognitive impairment of Alzheimer’s disease by targeting HMGB1 through mediating SIRT3/SOD2 signaling pathway. Acta Pharmacol. Sin. 2022, 43, 2482–2494. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, M.; Sun, Q.; Yang, T.; Lu, X.; Feng, X.; Song, M.; Cui, L.; Fan, H. Lycopene ameliorates chronic stress-induced hippocampal injury and subsequent learning and memory dysfunction through inhibiting ROS/JNK signaling pathway in rats. Food Chem. Toxicol. 2020, 145, 111688. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, J.; Pang, C.; Liu, K.; Wang, R.; Chen, Y.; Yuan, X.; Zhang, M.; Ni, J.; Dong, P.; et al. The study of therapeutic efficacy and mechanisms of Schisandra chinensis and Evodia rutaecarpa combined treatment in a rat model of Alzheimer’s disease. Heliyon 2023, 9, e21942. [Google Scholar] [CrossRef]
- Polanco, J.C.; Li, C.; Bodea, L.G.; Martinez-Marmol, R.; Meunier, F.A.; Götz, J. Amyloid-β and tau complexity—Towards improved biomarkers and targeted therapies. Nat. Rev. Neurol. 2018, 14, 22–39. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Z.; Wang, W.; Wei, M.; Si, X.; Shang, Y.; Yang, Z.; Li, T.; Guo, H.; Li, S. Piperine regulates glycogen synthase kinase-3β-related signaling and attenuates cognitive decline in D-galactose-induced aging mouse model. J. Nutr. Biochem. 2020, 75, 108261. [Google Scholar] [CrossRef]
- Niu, Q.; Li, D.; Zhang, J.; Piao, Z.; Xu, B.; Xi, Y.; Mohamed Kamal, N.; Lim, V.; Li, P.; Yin, Y. The new perspective of Alzheimer’s Disease Research: Mechanism and therapeutic strategy of neuronal senescence. Ageing Res. Rev. 2024, 102, 102593. [Google Scholar] [CrossRef]
- Heydari, S.; Hedayati Ch, M.; Saadat, F.; Abedinzade, M.; Nikokar, I.; Aboutaleb, E.; Khafri, A.; Mokarram, A.R. Diphtheria toxoid nanoparticles improve learning and memory impairment in animal model of Alzheimer’s disease. Pharmacol. Rep. PR 2020, 72, 814–826. [Google Scholar] [CrossRef]
- Dai, S.J.; Zhang, J.Y.; Bao, Y.T.; Zhou, X.J.; Lin, L.N.; Fu, Y.B.; Zhang, Y.J.; Li, C.Y.; Yang, Y.X. Intracerebroventricular injection of Aβ(1-42) combined with two-vessel occlusion accelerate Alzheimer’s disease development in rats. Pathol. Res. Pract. 2018, 214, 1583–1595. [Google Scholar] [CrossRef]
- Meng, X.; Fu, M.; Wang, S.; Chen, W.; Wang, J.; Zhang, N. Naringin ameliorates memory deficits and exerts neuroprotective effects in a mouse model of Alzheimer’s disease by regulating multiple metabolic pathways. Mol. Med. Rep. 2021, 23, 332. [Google Scholar] [CrossRef]
- Hwang, E.S.; Kim, H.B.; Lee, S.; Kim, M.J.; Lee, S.O.; Han, S.M.; Maeng, S.; Park, J.H. Loganin enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Physiol. Behav. 2017, 171, 243–248. [Google Scholar] [CrossRef]
- Liu, M.; Guo, H.; Li, C.; Wang, D.; Wu, J.; Wang, C.; Xu, J.; Qin, R.A. Cognitive improvement of compound danshen in an Aβ25-35 peptide-induced rat model of Alzheimer’s disease. BMC Complement. Altern. Med. 2015, 15, 382. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Xu, P.; Song, S.; Liu, P.; Chi, T.; Ji, X.; Jin, G.; Qiu, S.; Hou, Y.; et al. Xanthoceras sorbifolia extracts ameliorate dendritic spine deficiency and cognitive decline via upregulation of BDNF expression in a rat model of Alzheimer’s disease. Neurosci. Lett. 2016, 629, 208–214. [Google Scholar] [CrossRef]
- Zhihui, Q. Modulating nitric oxide signaling in the CNS for Alzheimer’s disease therapy. Future Med. Chem. 2013, 5, 1451–1468. [Google Scholar] [CrossRef]
- Asiimwe, N.; Yeo, S.G.; Kim, M.S.; Jung, J.; Jeong, N.Y. Nitric Oxide: Exploring the Contextual Link with Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2016, 2016, 7205747. [Google Scholar] [CrossRef]
- Siddiqui, N.; Ali, J.; Parvez, S.; Zameer, S.; Najmi, A.K.; Akhtar, M. Linagliptin, a DPP-4 inhibitor, ameliorates Aβ (1-42) peptides induced neurodegeneration and brain insulin resistance (BIR) via insulin receptor substrate-1 (IRS-1) in rat model of Alzheimer’s disease. Neuropharmacology 2021, 195, 108662. [Google Scholar] [CrossRef]
- Hua, H.; Yang, T.; Huang, L.; Chen, R.; Li, M.; Zou, Z.; Wang, N.; Yang, D.; Liu, Y. Protective Effects of Lanosterol Synthase Up-Regulation in UV-B-Induced Oxidative Stress. Front. Pharmacol. 2019, 10, 947. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 62, 1345–1367. [Google Scholar] [CrossRef]
- Yao, D.; Shi, W.; Gou, Y.; Zhou, X.; Yee Aw, T.; Zhou, Y.; Liu, Z. Fatty acid-mediated intracellular iron translocation: A synergistic mechanism of oxidative injury. Free. Radic. Biol. Med. 2005, 39, 1385–1398. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.Y. Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes. Nutrients 2019, 11, 975. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548. [Google Scholar] [CrossRef]
- Gray, S.C.; Kinghorn, K.J.; Woodling, N.S. Shifting equilibriums in Alzheimer’s disease: The complex roles of microglia in neuroinflammation, neuronal survival and neurogenesis. Neural Regen. Res. 2020, 15, 1208–1219. [Google Scholar] [CrossRef]
- Abbas, F.; Eladl, M.A.; El-Sherbiny, M.; Abozied, N.; Nabil, A.; Mahmoud, S.M.; Mokhtar, H.I.; Zaitone, S.A.; Ibrahim, D. Celastrol and thymoquinone alleviate aluminum chloride-induced neurotoxicity: Behavioral psychomotor performance, neurotransmitter level, oxidative-inflammatory markers, and BDNF expression in rat brain. Biomed. Pharmacother. 2022, 151, 113072. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Ahmed, H.I.; Zaky, H.S.; Badr, A.M. Sesame oil mitigates memory impairment, oxidative stress, and neurodegeneration in a rat model of Alzheimer’s disease. A pivotal role of NF-κB/p38MAPK/BDNF/PPAR-γ pathways. J. Ethnopharmacol. 2021, 267, 113468. [Google Scholar] [CrossRef]
- Mohapel, P.; Leanza, G.; Kokaia, M.; Lindvall, O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol. Aging 2005, 26, 939–946. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.-F.; Wan, C.-Q.; Cai, M.; Zhou, N.-K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Zhao, Q.; Jing, Y.-M.; He, M.-T.; Jing, L.; Xi, Y.-F.; Zhang, J.-Z. Lycium Barbarum polysaccharides ameliorates hyperglycemia-exacerbatedcerebral ischemia/reperfusion injury via protecting blood-brain barrier. Transpl. Immunol. 2023, 76, 101757. [Google Scholar] [CrossRef]
- Al-Wraikat, M.; Zhang, L.; Li, L.; Abubaker, M.A.; Liu, Y. Recent advances in wolfberry polysaccharides and whey protein-based biopolymers for regulating the diversity of gut microbiota and its mechanism: A review. Int. J. Biol. Macromol. 2024, 281, 136401. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Meng, Y.; Feng, H.; Di, X.; Guo, X. Evaluation of the Anti-Alzheimer Activity of Lycium barbarum Polysaccharide in Aβ1–42-Induced Neurotoxicity in Rat Model. Curr. Issues Mol. Biol. 2025, 47, 226. https://doi.org/10.3390/cimb47040226
Lu Q, Meng Y, Feng H, Di X, Guo X. Evaluation of the Anti-Alzheimer Activity of Lycium barbarum Polysaccharide in Aβ1–42-Induced Neurotoxicity in Rat Model. Current Issues in Molecular Biology. 2025; 47(4):226. https://doi.org/10.3390/cimb47040226
Chicago/Turabian StyleLu, Qingxin, Yixin Meng, Haichi Feng, Xin Di, and Xiaoli Guo. 2025. "Evaluation of the Anti-Alzheimer Activity of Lycium barbarum Polysaccharide in Aβ1–42-Induced Neurotoxicity in Rat Model" Current Issues in Molecular Biology 47, no. 4: 226. https://doi.org/10.3390/cimb47040226
APA StyleLu, Q., Meng, Y., Feng, H., Di, X., & Guo, X. (2025). Evaluation of the Anti-Alzheimer Activity of Lycium barbarum Polysaccharide in Aβ1–42-Induced Neurotoxicity in Rat Model. Current Issues in Molecular Biology, 47(4), 226. https://doi.org/10.3390/cimb47040226




