1. Introduction
Epilepsy is a common neurological disease characterized by long-term abnormal neuron discharge leading to corresponding neurological damage [
1]. Although scientists have developed approximately 20 kinds of antiseizure medications (ASMs) for clinical use, approximately 20–40% of patients with epilepsy are not sensitive to ASMs, resulting in the development of drug-resistant epilepsy (DRE) [
2]. However, the exact etiology of epilepsy and its pathogenesis are not yet fully understood.
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR) and N-methyl-D-aspartate receptor (NMDAR) are two ionotropic glutamate receptors commonly related to excitatory synaptic activity. AMPAR, a ligand-gated channel receptor, mediates the majority of fast excitatory synaptic transmission in the pathogenesis of epilepsy [
3,
4]. Given the critical role of AMPAR in epilepsy, ASMs targeting AMPAR, such as perampanide, have been used in clinical treatment [
5,
6]. AMPAR is a heterotetramer composed of four subunits, namely, GluA1-A4. The GluA2 subunit exhibits characteristically low calcium permeability, a critical property that is essential for maintaining the proper biological function of AMPAR [
7]. The trafficking of AMPAR between the cytoplasm and the postsynaptic membrane is a crucial process that regulates its distribution on the synaptic surface [
8]. AMPAR-interacting proteins play a pivotal role in AMPAR transport mechanism.
The scaffolding proteins containing PDZ domains are well-recognized as AMPAR-interacting proteins. The PDZ domain, highly conserved from yeast to humans, comprises 90 amino acids that form two α-helices and six β-folds [
9]. The structure of the PDZ domain identifies the C-terminal amino acid sequence of ligand proteins, which are widely distributed in the postsynaptic membrane of excitatory neurotransmitter receptors. It plays roles spanning cytoplasmic transport to anchoring cell membrane receptors [
10]. Recombinant glutamate receptor-interacting protein (GRIP) is a well-characterized PDZ protein involved in AMPAR transport [
11]. GRIP is a scaffolding protein that contains seven PDZ domains and a small globular protein–protein interaction domain. The PDZ domains of GRIP directly bind to the C-termini of the AMPAR GluA2 and GluA3 subunits, regulating the transport of AMPAR between the cytoplasm and the cell membrane, which plays an important role in excitatory synaptic transmission and synaptic plasticity [
11,
12].
Frmpd3 is a novel scaffold protein containing a PDZ domain and a FERM domain. While its precise distribution and biological functions in the central nervous system remain unclear, its multi-domain structure suggests a potential critical role in neuronal excitation. Hu et al. demonstrated via co-immunoprecipitation that Frmpd3 interacts with the scaffolding protein Homer1 in excitatory synapses in vitro [
13]. Spiegel et al. observed that Npas4 selectively upregulates Frmpd3 expression in inhibitory neurons [
14]. Notably, the homologous scaffold protein Frmpd2 was shown to interact with the NMDAR subunit NR2A through its PDZ domain [
15]. These findings support the hypothesis that Frmpd3 may utilize its PDZ domain to engage with neurotransmitter receptors, potentially regulating receptor trafficking and contributing to neuronal hyperexcitability and epileptogenesis.
However, the distribution and function of Frmpd3 in the central nervous system remain unclear, particularly its role in epilepsy. In this study, we reveal Frmpd3 protein expression in specific mouse brain regions and its probable functions in epilepsy. We demonstrate that Frmpd3 modulates epileptic processes through interactions with the AMPAR GluA2 subunit and the AMPAR-interacting protein GRIP.
2. Materials and Methods
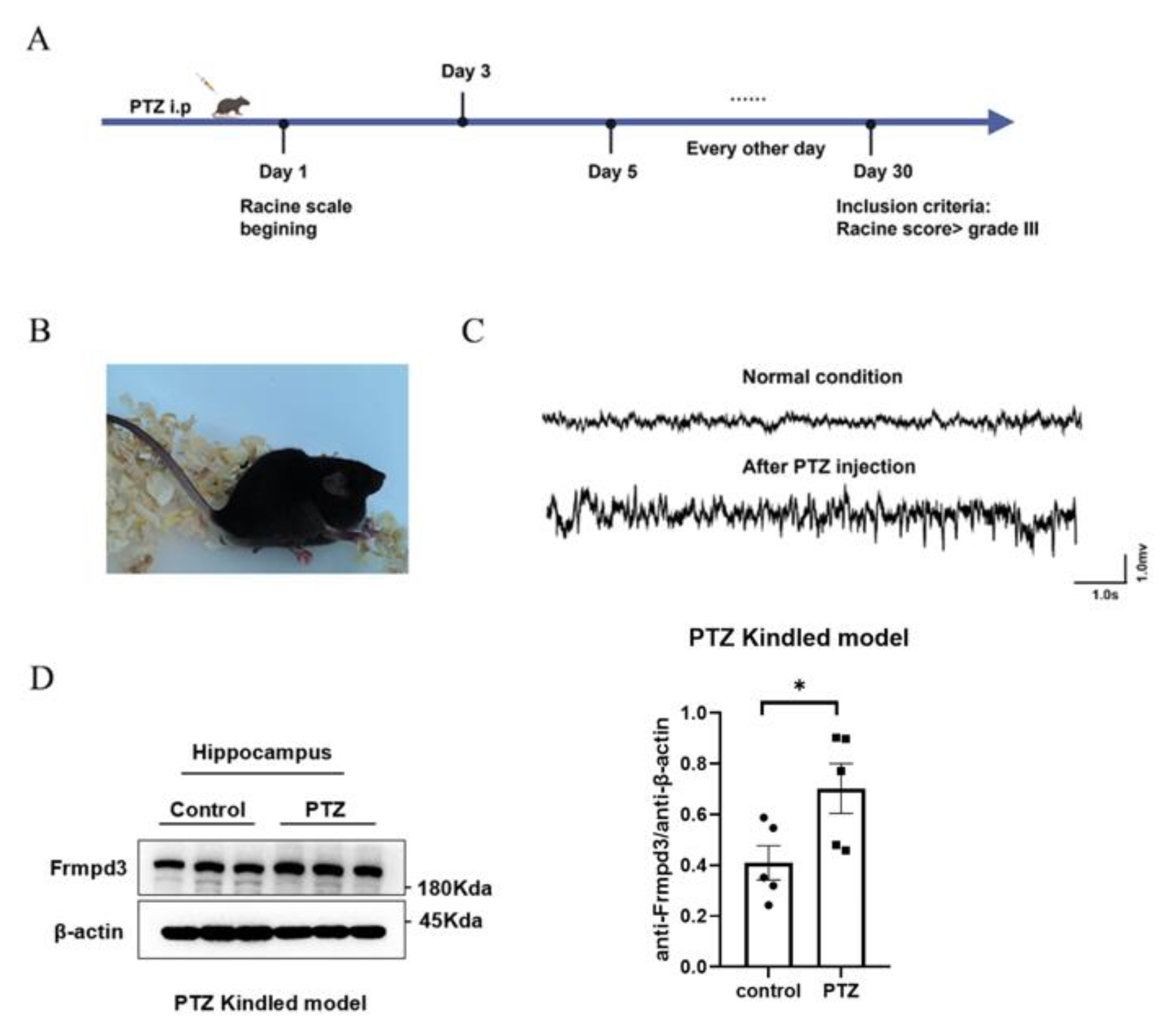
2.1. Pentylenetetrazol-Kindled Chronic Epilepsy Mouse Models
Adult wild-type C57B/L6 mice were intraperitoneally injected with pentylenetetrazole (PTZ, 35 mg/kg) every other day for one month. After injection, the samples were observed with a video camera for at least one hour [
16]. Behavioral seizure scores were evaluated according to the Racine standard scale: grade 0, arrest and normal behavior; grade 1, facial twitches (nose, lips, and eyes); grade 2, chewing and head nodding; grade 3, forelimb clonus; grade 4, rearing and falling on the forelimbs and hind limb clonus; and grade 5, rearing and falling on the side or back. Only grades 4 and 5 seizures were considered fully kindled seizures and were included in the number of seizures. The days until full kindling were recorded as the latency period.
2.2. Immunofluorescence Staining
Fresh animal brain tissues were postfixed for 24 h at 4 °C. Floating slices (35 μm thick) were permeabilized with 2% Triton X-100 for 30 min at room temperature and then incubated and blocked in an immunofluorescence blocking solution. The slices were incubated with primary antibodies against Frmpd3 (rabbit, Thermo Fisher, Waltham, MA, USA), and NeuN (mouse, Abcam, Waltham, MA, USA), Frmpd3 (rabbit, Thermo Fisher, Waltham, MA, USA), and GFAP (mouse, Cell Signaling Technology, Waltham, MA, USA), Frmpd3 (rabbit, Thermo Fisher, Waltham, MA, USA) and PSD95 (mouse, Cell Signaling Technology, Waltham, MA, USA), and Frmpd3 (rabbit, Thermo Fisher, Waltham, MA, USA), and GAD67 (mouse, Abcam, Waltham, MA, USA) overnight at 4 °C, followed by incubation with secondary fluorescent-conjugated antibodies for 4 h at room temperature. 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. Images were captured under a confocal microscope (Leica; Wetzlar, Germany).
2.3. Western Blot
The hippocampus of PTZ-kindled mice and control mice were collected and extracted for total protein analysis via a RIPA protein extraction kit (Beyotime Biotechnology, Shanghai, China). The protein mixture was then boiled at 95 °C for 5 min, after which the protein concentrations were calculated via an enhanced bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). The proteins were separated on 8% SDS-PAGE gels, and the separated target proteins were immunoblotted with primary antibody Frmpd3 (rabbit, Thermo Fisher, Waltham, MA, USA), β-actin (mouse, Proteintech, Wuhan, China), and α-Tubulin (mouse, Proteintech, Wuhan, China), anti-GluA2 (rabbit, Proteintech, Wuhan, China), anti-GRIP1 (rabbit, Proteintech, Wuhan, China), anti-GluN2B (rabbit, Proteintech, Wuhan, China), anti-ATP1A1 (mouse, Proteintech, Wuhan, China) overnight at 4 °C. The blots were then incubated with HRP-conjugated secondary antibodies. The blots were scanned and quantified via a fusion imaging system.
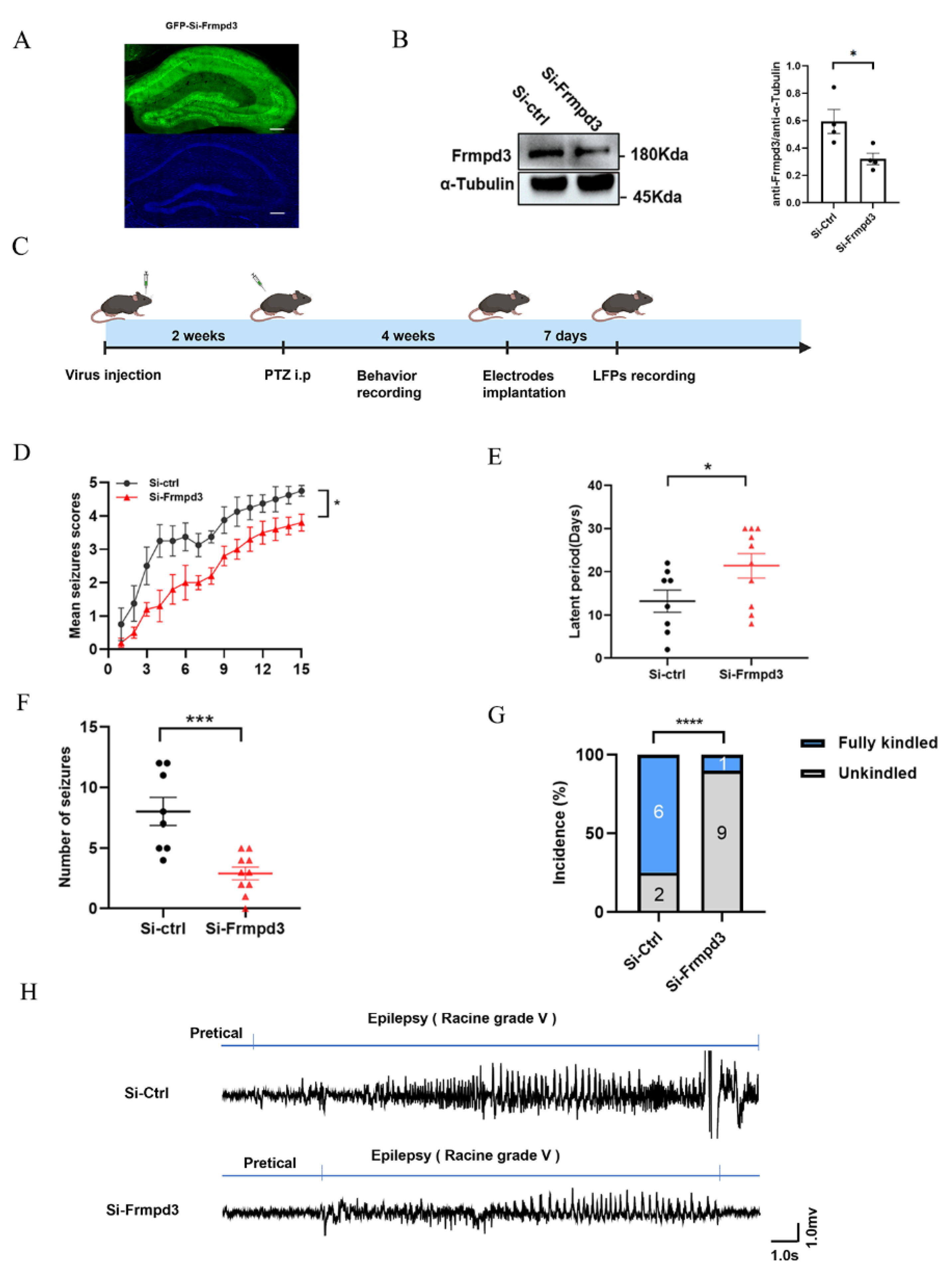
2.4. Intrahippocampal Injection of AAV
Healthy adult C57/BL6 male mice weighing 25 ± 2 g were obtained from Henan Skobes Biological Co., Ltd., Zhengzhou, Henan, China. Intrahippocampal injections of adeno-associated virus (AAV) were administered via a stereotaxic apparatus (RWD Co., Ltd., Shenzhen, China). AAV carrying shRNA targeting mouse Frmpd3 (NM_001320946.1) was from OBiO Technology (Shanghai) Corp., Ltd., Shanghai, China. Mouse shRNA sequence was 5′-GCAGCCTCATTGAGACCTT-3′. Briefly, the mice were anesthetized with pentobarbital (80 μg/g, intraperitoneally) and then placed in a stereotaxic apparatus. AAV9 vectors (siCtrl, siFrmpd3) were injected into the CA1 hippocampal region of the mice (Paxinos Mouse Brain Atlas; coordinates from bregma; anteroposterior (AP): −1.7 mm; mediolateral (ML): ±1.3 mm; dorsoventral (DV): −1.7 mm) via a microliter syringe controlled by an injection pump. All AAV deliveries were performed bilaterally with a volume of 1 μL each at a flow rate of 0.1 μL/min. The needle was kept in place for 15 min after each injection. The injected mice were allowed to fully recover for 2 weeks to prevent infection, and recovery was followed by PTZ injection for behavioral observation.
2.5. LFP Recording
Surgery was performed 1 week prior to the recordings. Briefly, to record the hippocampal LFP (Local field potential, LFP), the mice were anesthetized and placed in a stereotaxic apparatus, and two stainless steel screws implanted in the anterior cranium served as the ground electrode. For LFP recording, the recording electrode was implanted into the prefrontal lobe (anteroposterior (AP): +1.7 mm; mediolateral (ML): −0.4 mm), and the reference electrode was implanted into the cerebellum. A typical seizure-like event is characterized by the disappearance of the electrophysiological rhythm connected to the A-M system 1800 acquisition system and the occurrence of a cluster of spontaneous paroxysmal discharges with amplitudes greater than 2 times the baseline and durations greater than 5 s.
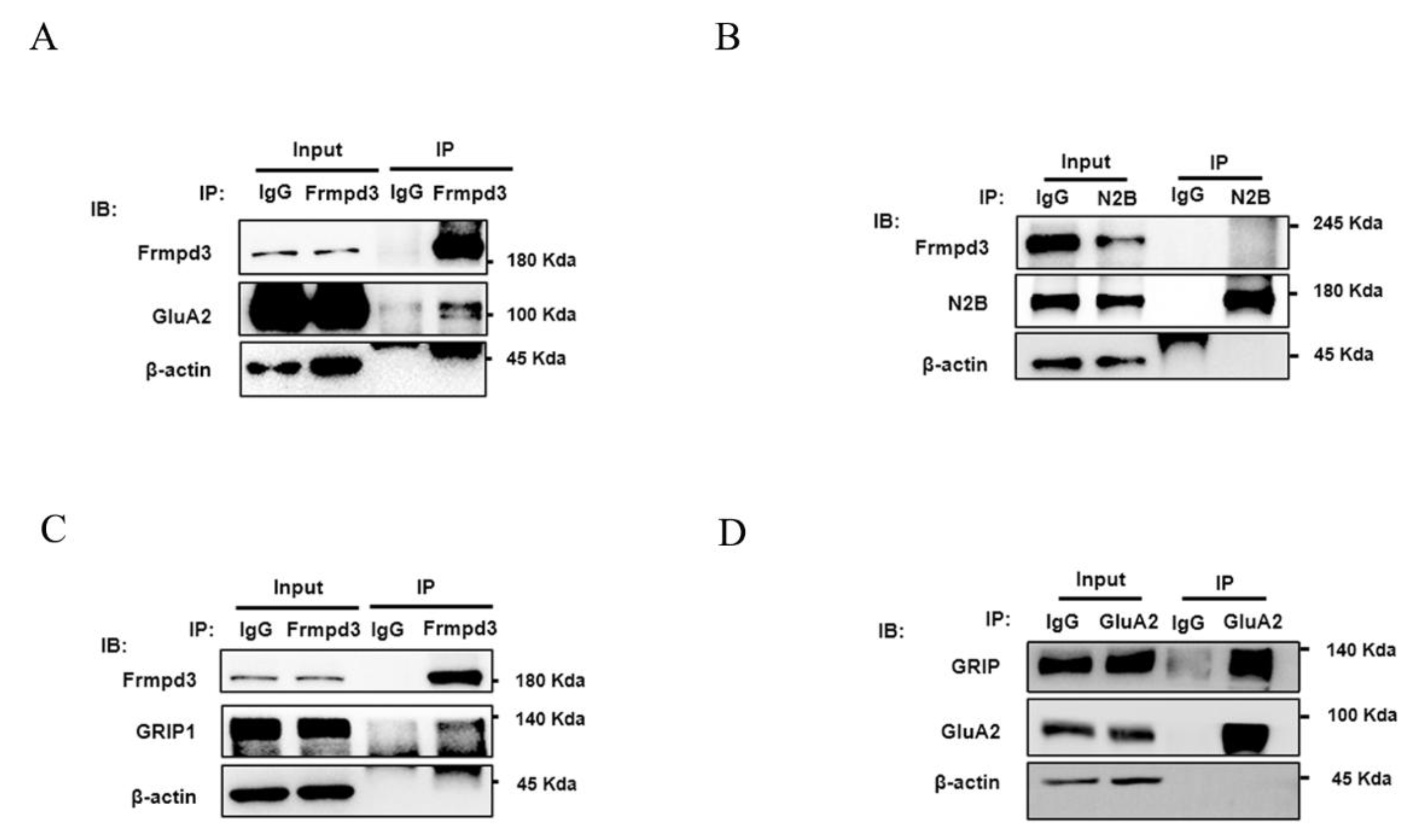
2.6. Immunoprecipitation
Hippocampal tissue samples were lysed with RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) and supplemented with a protease inhibitor cocktail (Thermo Fisher, Waltham, MA, USA) at 4 °C for 30 min. Lysates containing equal amounts of protein were incubated with protein A/G magnetic beads (Beyotime Biotechnology, Shanghai, China) bound to anti-Frmpd3, anti-GluA2 (rabbit, Proteintech, Wuhan, China), anti-GRIP1 (rabbit, Proteintech, Wuhan, China), or anti-GluN2B (rabbit, Proteintech, Wuhan, China) antibodies at 4 °C overnight, according to the manufacturer’s instructions. After washing, the proteins were harvested by magnetic separation, and the magnetic beads were eluted by adding 100 μL of 1× SDS–PAGE loading buffer. Proteins were subsequently analyzed via Western Blotting.
2.7. Statistical Analysis
GraphPad Prism 9.0 software (San Diego, CA, USA) was used for statistical analysis. Experimental data are presented as either medians with interquartile ranges or means ± standard deviations (SDs). p < 0.05 was considered statistically significant. The normality of the data was analyzed via the Shapiro–Wilk test. When the variance of the dataset was significantly different, we used nonparametric statistical analysis. Two-tailed Student’s t-tests were used to compare two independent groups.
The workflow for fluorescence colocalization analysis includes the following: Defining regions of interest in Leica Las X to encompass colocalization signals; extracting intensity values from both channels along spatial/temporal axes and plotting synchronized curves; assessing curve synchrony via visual inspection; interpreting results where co-expression indicates synchronous curve trends, while no correlation reflects random fluctuations.
4. Discussion
In our study, we identified a novel scaffold protein, Frmpd3, and provided insights into the mechanism by which Frmpd3 is involved in epilepsy susceptibility and the regulation of pathological neuronal excitability.
Our results demonstrated that the Frmpd3 protein is expressed in hippocampus, cortex, hypothalamus, and cerebellum neurons of mice. The hippocampus, cortex [
19], hypothalamus [
20], and cerebellum [
21] play pivotal roles in the occurrence and development of epilepsy. Notably, the hippocampus, which is particularly vulnerable to damage in temporal lobe epilepsy, serves as a critical region for epileptogenesis [
22]. These findings collectively suggest that the Frmpd3 protein is anatomically associated with epilepsy-related brain regions. Furthermore, we observed a significant upregulation of Frmpd3 protein expression in the hippocampus of PTZ-induced epileptic mice. Considering that genetic and protein alterations have been implicated in the development of epileptic phenotypes, we further investigated the potential involvement of Frmpd3 in epileptogenic processes. Through hippocampal-specific knockdown of Frmpd3 expression, we demonstrated a marked suppression of seizure activity, characterized by prolonged seizure latency and reduced seizure severity. These experimental results strongly indicate that Frmpd3 may play a regulatory role in modulating epileptic susceptibility.
Previous studies have identified Frmpd3 localization in excitatory synapses in vitro [
13]. Our results also showed that Frmpd3 is distributed within the postsynaptic density (PSD) of excitatory synapses in the hippocampal CA1 region of mice. It is suggested that Frmpd3 may contribute to epileptogenesis through the modulation of excitatory synaptic transmission. The process of synaptic transmission initiates with the release of readily releasable neurotransmitters from presynaptic terminals, followed by their diffusion across the synaptic cleft and subsequent binding to postsynaptic receptors. Importantly, disruptions in any aspect of neurotransmitter receptor biology—including expression, clustering, trafficking, localization, recycling, and degradation—have been established as fundamental pathological mechanisms underlying various neurological disorders, such as dementia, schizophrenia, and epilepsy.
PDZ scaffold proteins, which bind to neurotransmitter receptors and participate in receptor trafficking and anchoring, have been recognized as crucial regulators of synaptic plasticity, learning, memory, cognition, and seizure activity [
10]. Through their PDZ domains, these scaffold proteins exhibit the capacity to interact with various neurotransmitter receptors and their distinct subunits. The homologous scaffold protein Frmpd2 has been shown to specifically interact with the C-terminus of the NR2A [
15]. Similarly, the scaffold protein GRIP, containing seven PDZ domains, demonstrates specific binding properties: its fourth and fifth PDZ domains directly interact with the C-termini of AMPAR subunits GluA2 and GluA3, respectively [
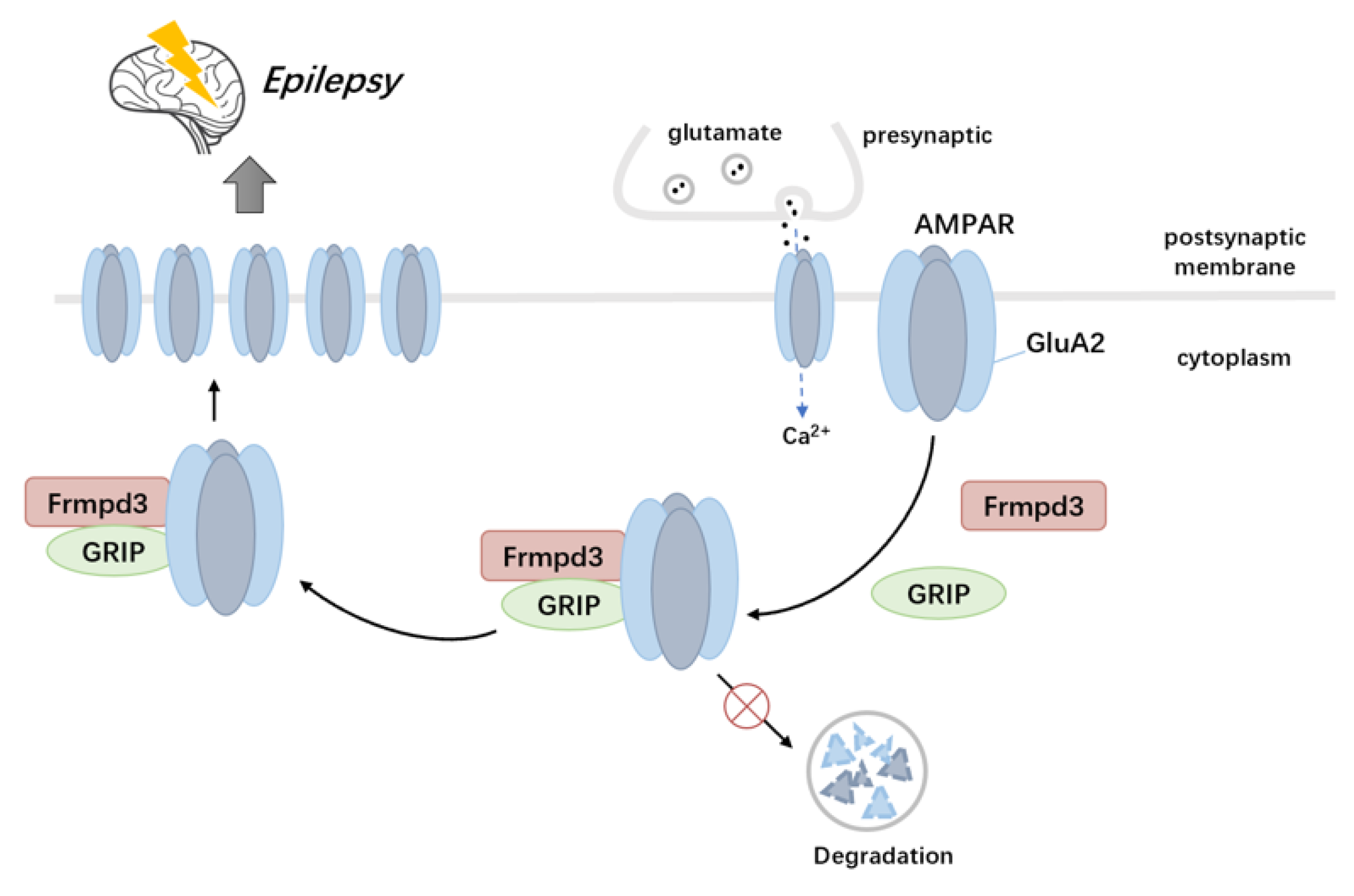
8]. In our current study, we have identified that the scaffold protein Frmpd3 interacts with both the AMPAR subunit GluA2 and GRIP through in vivo immunoprecipitation experiments. These findings suggest that the PDZ domain of Frmpd3 may serve as a critical structural determinant mediating the association between Frmpd3, GluA2, and GRIP.
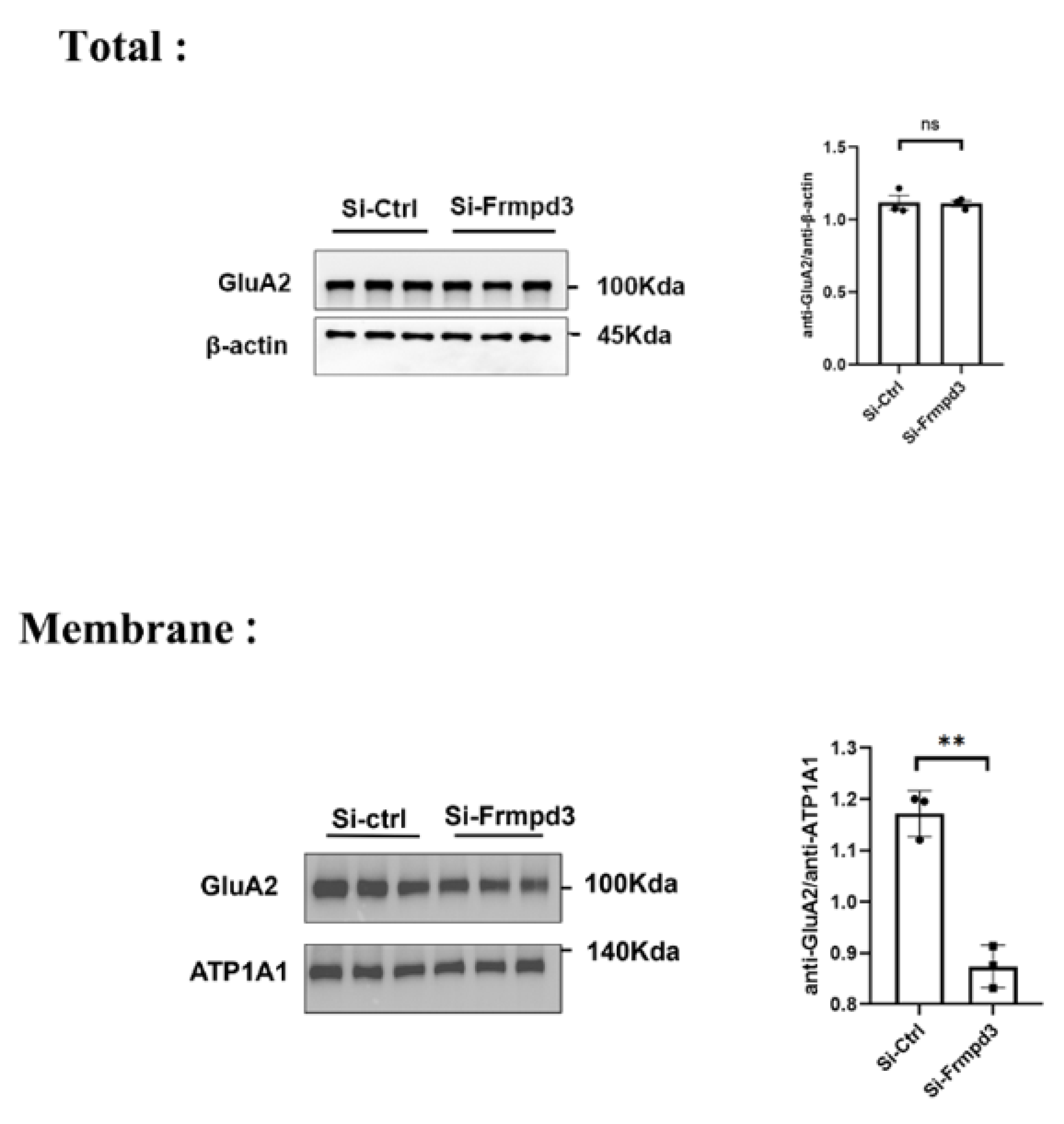
The presence of the AMPAR subunit GluA2 on the cell membrane plays a crucial regulatory role in controlling calcium permeation and voltage rectification properties of AMPARs. Notably, the absence of the GluA2 subunit leads to excessive calcium influx, which poses a significant risk for both synaptic plasticity impairment and the development of various neurological disorders, particularly epilepsy [
23]. Our experimental results demonstrated that Frmpd3 enhances the membrane localization of GluA2 without affecting its total protein expression level in neurons. It is suggested that Frmpd3, through its interactions with both GluA2 and GRIP proteins, facilitates the trafficking of GluA2 from the cytoplasm to the cell membrane, thereby increasing its membrane expression level. These findings highlight the cooperative nature of PDZ scaffold proteins in regulating neurotransmitter receptor trafficking. GRIP-AMPAR interactions are also reported to play a pivotal role in modulating synaptic AMPAR levels at the cell surface by controlling the dynamic trafficking of AMPARs between the cytoplasmic compartment and plasma membrane [
8]. The synapse-associated protein SAP97 in mice has been shown to specifically interact with the C-terminus of the AMPAR subunit GluA1 through its PDZ domain [
24]. Notably, overexpression of SAP97 can effectively compensate for impaired AMPAR transmission resulting from PSD-95 deficiency. Both proteins belong to the PDZ domain-containing protein family, with PSD-95 serving as another crucial scaffold protein that interacts with AMPARs and forms complexes with SAP97 [
8].
Our study has identified the novel scaffold protein Frmpd3 as a crucial regulator of both seizure activity and AMPAR trafficking through its interactions with GRIP and GluA2. The dysregulation of this scaffold protein-mediated regulatory network leads to impaired excitatory synaptic transmission (
Figure 6), which represents a fundamental pathological mechanism underlying epilepsy and related neurological disorders.