Liquid Chromatography‒Tandem Mass Spectrometry Analysis of Primary Metabolites and Phenolic Acids Across Five Citrus Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction Process for Metabolite Analysis
2.3. LC–MS/MS Conditions
2.4. Analysis of Metabolites
3. Results
3.1. The Analysis of the Metabolites Among Five Citrus Varieties by LC–MS/MS
3.2. Differential Metabolites in Different Citrus Varieties
3.3. Analysis of Differential Metabolic Pathways in Different Citrus Varieties
3.4. Metabolic Pathway and Relative Abundance of Identified Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tundis, R.; Loizzo, M.R.; Menichini, F. An overview on chemical aspects and potential health benefits of limonoids and their derivatives. Crit. Rev. Food Sci. Nutr. 2014, 54, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Stinco, C.M.; Escudero-Gilete, M.L.; Heredia, F.J.; Vicario, I.M.; Melendez-Martinez, A.J. Multivariate analyses of a wide selection of orange varieties based on carotenoid contents, color and in vitro antioxidant capacity. Food Res. Int. 2016, 90, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 566–583. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, G. An insight into the role of citrus bioactives in modulation of colon cancer. J. Funct. Foods 2015, 13, 239–261. [Google Scholar] [CrossRef]
- Spreen, T.H.; Gao, Z.; Fernandes, W., Jr.; Zansler, M.L. Global economics and marketing of citrus products. In The Genus Citrus; Elsevier: Amsterdam, The Netherlands, 2020; pp. 471–493. [Google Scholar]
- Xiong, B.; Ye, S.; Qiu, X.; Liao, L.; Sun, G.; Luo, J.; Dai, L.; Rong, Y.; Wang, Z. Transcriptome analyses of two citrus cultivars (Shiranuhi and Huangguogan) in seedling etiolation. Sci. Rep. 2017, 7, 46245. [Google Scholar] [CrossRef] [PubMed]
- Turtoi, E.; Jeudy, J.; Henry, S.; Dadi, I.; Valette, G.; Enjalbal, C.; Turtoi, A. Analysis of polar primary metabolites in biological samples using targeted metabolomics and LC‒MS. STAR Protoc. 2023, 4, 102400. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Y.; Wu, W.; Zhu, C.; Zhang, R.; Chen, J.; Zeng, J. Metabolomics analysis of the peels of different colored citrus fruits (Citrus reticulata cv.‘Shatangju’) during the maturation period based on UHPLC-QQQ-MS. Molecules 2020, 25, 396. [Google Scholar] [CrossRef]
- Zhao, X.J.; Xing, T.T.; Li, Y.F.; Jiao, B.N. Analysis of phytochemical contributors to antioxidant capacity of the peel of Chinese mandarin and orange varieties. Int. J. Food Sci. Nutr. 2019, 70, 825–833. [Google Scholar] [CrossRef]
- Francini, A.; Romeo, S.; Cifelli, M.; Gori, D.; Domenici, V.; Sebastiani, L. 1H NMR and PCA-based analysis revealed variety dependent changes in phenolic contents of apple fruit after drying. Food Chem. 2017, 221, 1206–1213. [Google Scholar] [CrossRef]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar]
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and metabolic diversity of flavonoids in citrus species. Sci. Rep. 2017, 7, 10549. [Google Scholar] [CrossRef]
- Peng, Y.; Li, M.; Song, F.; Liu, S.; Qin, Y.; Hu, B.; Cui, X. Identification of Primary Metabolite Profiles Reveals Quality Characteristics of Citrus maxima ‘Shatian Yu’from Different Origins. Curr. Issues Mol. Biol. 2024, 46, 12830–12846. [Google Scholar] [CrossRef] [PubMed]
- Goh, R.M.V.; Pua, A.; Liu, S.Q.; Lassabliere, B.; Leong, K.-C.; Sun, J.; Lau, H.; Tan, L.P.; Zhang, W.L.; Yu, B. Characterisation of volatile and non-volatile compounds in pomelo by gas chromatography-olfactometry, gas chromatography and liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Essent. Oil Res. 2020, 32, 132–143. [Google Scholar]
- Xiao, L.; Shibuya, T.; Watanabe, T.; Kato, K.; Kanayama, Y. Effect of Light Quality on Metabolomic, Ionomic, and Transcriptomic Profiles in Tomato Fruit. Int. J. Mol. Sci. 2022, 23, 13288. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.; Bianconi, M.; Poletti, S.; Negri, S.; Munari, F.; Ceoldo, S.; Guzzo, F. Metabolomic profiling and antioxidant activity of fruits representing diverse apple and pear cultivars. Biology 2021, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, Y.; Shen, W.; Liao, B.; Liu, X.; Yang, Z.; Chen, J.; Zhao, C.; Liao, Z.; Cao, J. Genomic and metabolomic insights into the selection and differentiation of bioactive compounds in citrus. Mol. Plant 2024, 17, 1753–1772. [Google Scholar]
- Seminara, S.; Bennici, S.; Di Guardo, M.; Caruso, M.; Gentile, A.; La Malfa, S.; Distefano, G. Sweet Orange: Evolution, characterization, varieties, and breeding perspectives. Agriculture 2023, 13, 264. [Google Scholar] [CrossRef]
- Xiao, L.; Ye, F.; Zhou, Y.; Zhao, G. Utilization of pomelo peels to manufacture value-added products: A review. Food Chem. 2021, 351, 129247. [Google Scholar] [CrossRef]
- Rafique, S.; Hassan, S.M.; Mughal, S.S.; Hassan, S.K.; Shabbir, N.; Perveiz, S.; Mushtaq, M.; Farman, M. Biological attributes of lemon: A review. J. Addict. Med. Ther. Sci. 2020, 6, 030–034. [Google Scholar]
- Li, X.; Meenu, M.; Xu, B. Recent development in bioactive compounds and health benefits of kumquat fruits. Food Rev. Int. 2023, 39, 4312–4332. [Google Scholar] [CrossRef]
- Porat, R.; Deterre, S.; Giampaoli, P.; Plotto, A. The flavor of citrus fruit. Biotechnol. Flavor Prod. 2016, 1–31. [Google Scholar]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography−mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an optimized workflow for global metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Klebeko, J.; Krüger, O.; Dubicki, M.; Ossowicz-Rupniewska, P.; Janus, E. Isopropyl amino acid esters ionic liquids as vehicles for non-steroidal anti-Inflammatory drugs in potential topical drug delivery systems with antimicrobial activity. Int. J. Mol. Sci. 2022, 23, 13863. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Olaniyan, O.T.; Emeruwa, C.N.; Saha, S.; Saso, L.; Tucci, P. An insight into role of amino acids as antioxidants via NRF2 activation. Amino Acids 2024, 56, 23. [Google Scholar] [CrossRef]
- Wang, S.; Shen, S.; Wang, C.; Wang, X.; Yang, C.; Zhou, S.; Zhang, R.; Zhou, Q.; Yu, H.; Guo, H. A metabolomics study in citrus provides insight into bioactive phenylpropanoid metabolism. Hortic. Res. 2024, 11, uhad267. [Google Scholar] [CrossRef]
- Argamasilla, R.; Gómez-Cadenas, A.; Arbona, V. Metabolic and regulatory responses in citrus rootstocks in response to adverse environmental conditions. J. Plant Growth Regul. 2014, 33, 169–180. [Google Scholar] [CrossRef]
- Zhao, S.-Y.; Liu, Z.-L.; Shu, Y.-S.; Wang, M.-L.; He, D.; Song, Z.-Q.; Zeng, H.-L.; Ning, Z.-C.; Lu, C.; Lu, A.-P. Chemotaxonomic classification applied to the identification of two closely-related citrus TCMs using UPLC-Q-TOF-MS-based metabolomics. Molecules 2017, 22, 1721. [Google Scholar] [CrossRef]
- Kolukisaoglu, Ü. D-amino acids in plants: Sources, metabolism, and functions. Int. J. Mol. Sci. 2020, 21, 5421. [Google Scholar] [CrossRef]
- Du, S.; Wey, M.; Armstrong, D.W. d-Amino acids in biological systems. Chirality 2023, 35, 508–534. [Google Scholar]
- Tsai, G.E.; Yang, P.; Chang, Y.-C.; Chong, M.-Y. D-alanine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 2006, 59, 230–234. [Google Scholar] [PubMed]
- Marcone, G.L.; Rosini, E.; Crespi, E.; Pollegioni, L. D-amino acids in foods. Appl. Microbiol. Biotechnol. 2020, 104, 555–574. [Google Scholar]
- Hussain, S.B.; Shi, C.-Y.; Guo, L.-X.; Kamran, H.M.; Sadka, A.; Liu, Y.-Z. Recent advances in the regulation of citric acid metabolism in citrus fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Zhang, H.; Gao, Q.; Song, F.; Cui, X.; Mo, F. Genome-Wide Identification of APX Gene Family in Citrus maxima and Expression Analysis at Different Postharvest Preservation Times. Genes 2024, 15, 911. [Google Scholar] [CrossRef]
- Sadka, A.; Shlizerman, L.; Kamara, I.; Blumwald, E. Primary metabolism in citrus fruit as affected by its unique structure. Front. Plant Sci. 2019, 10, 1167. [Google Scholar]
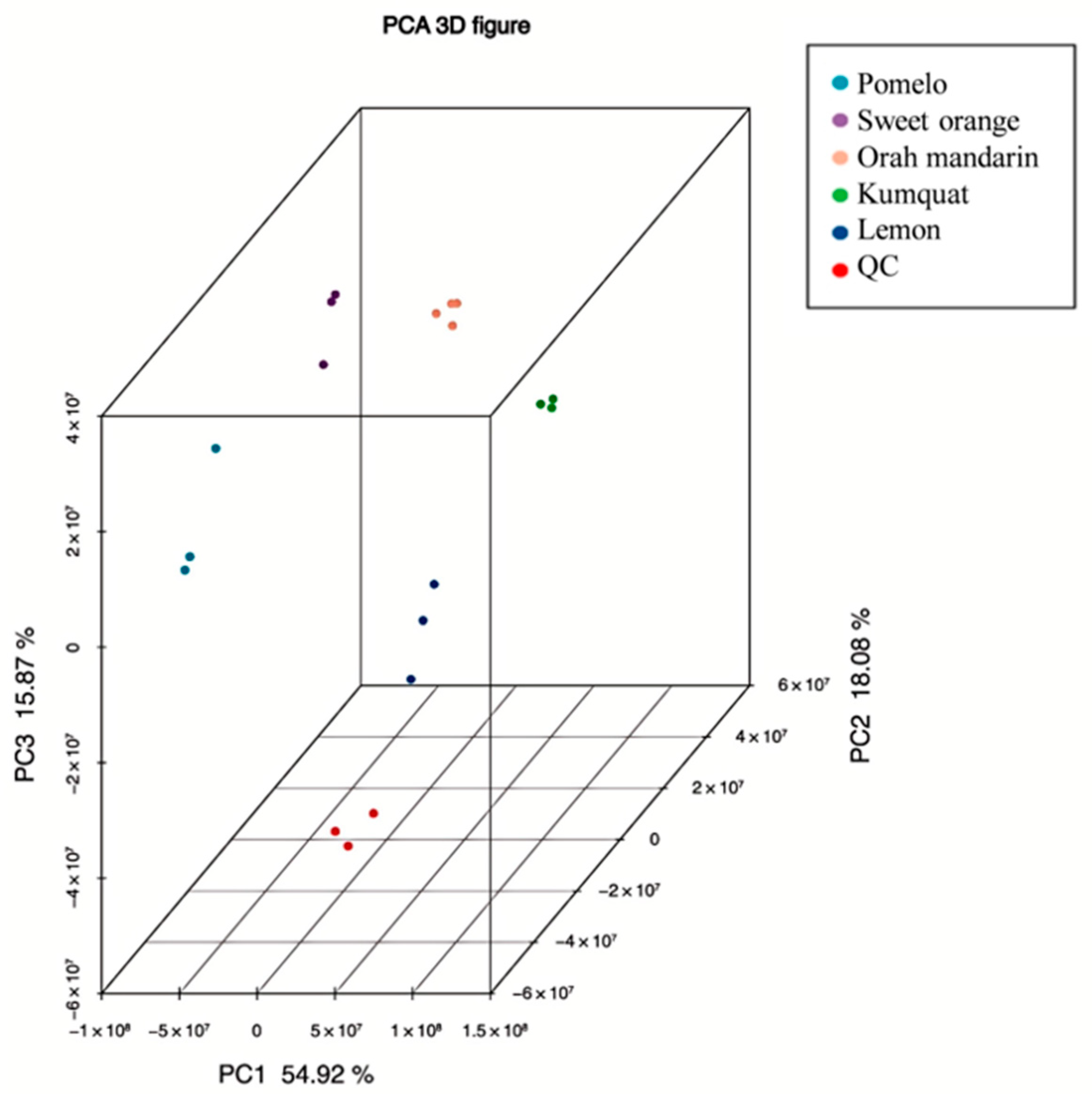
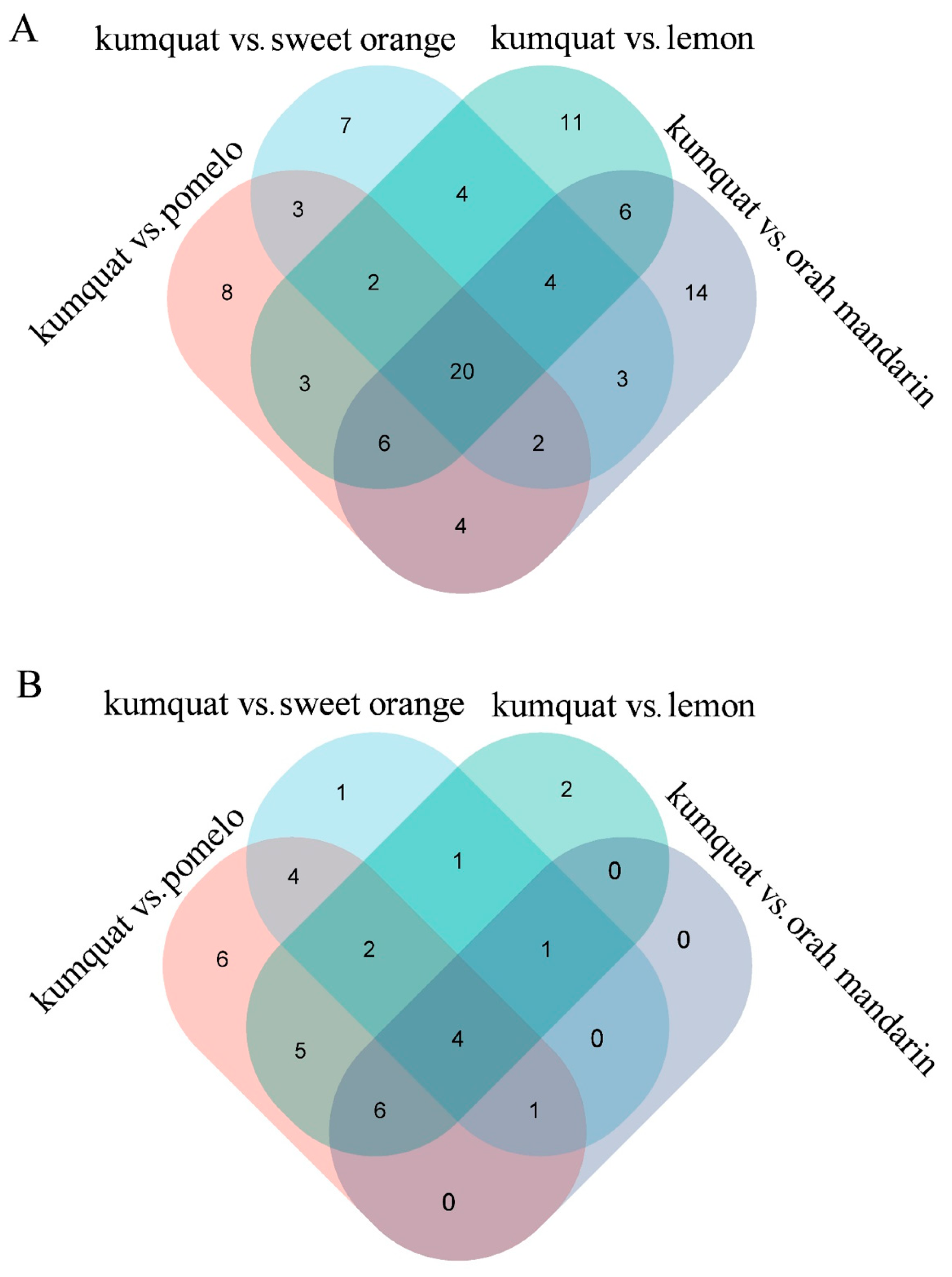
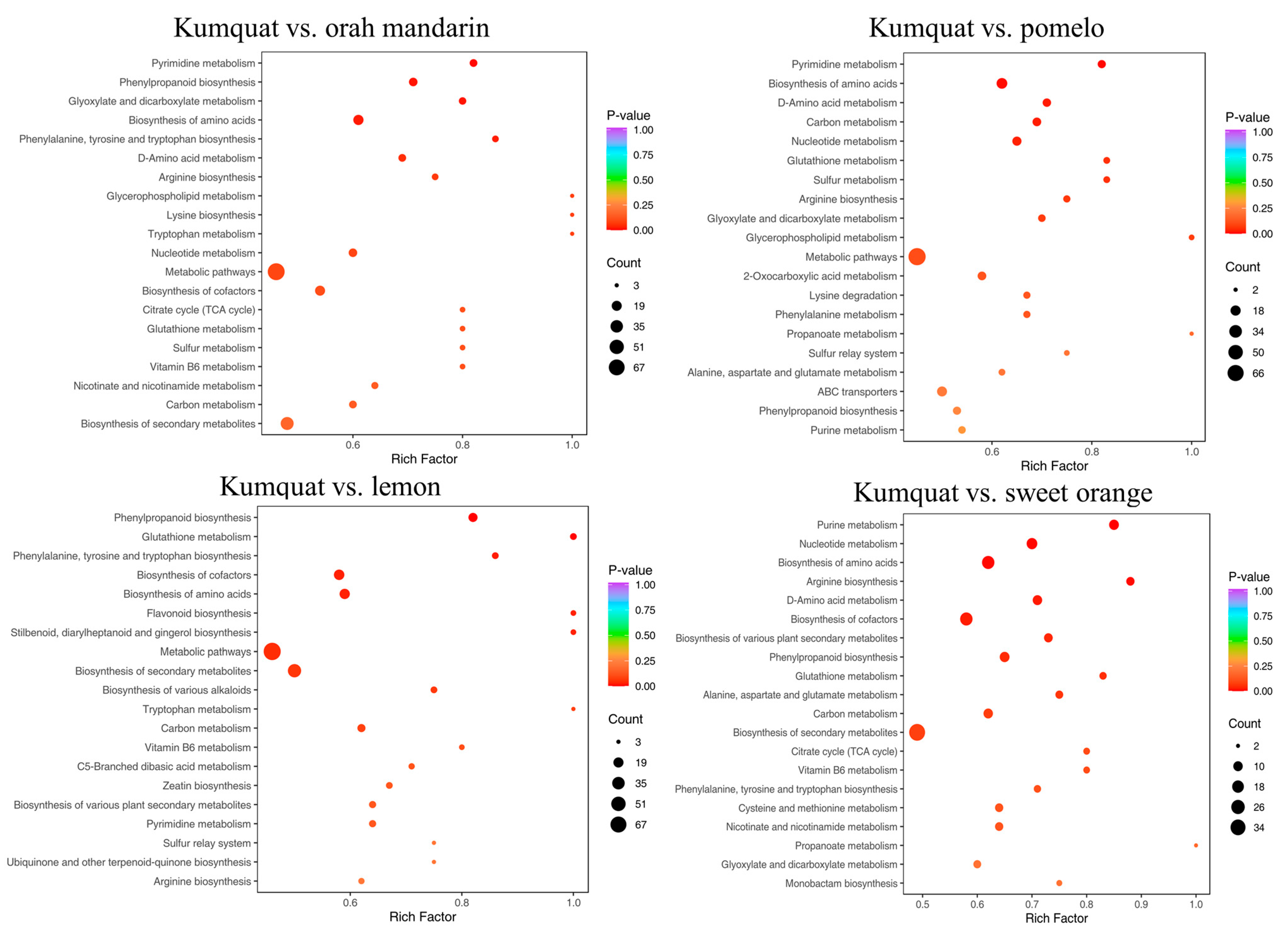
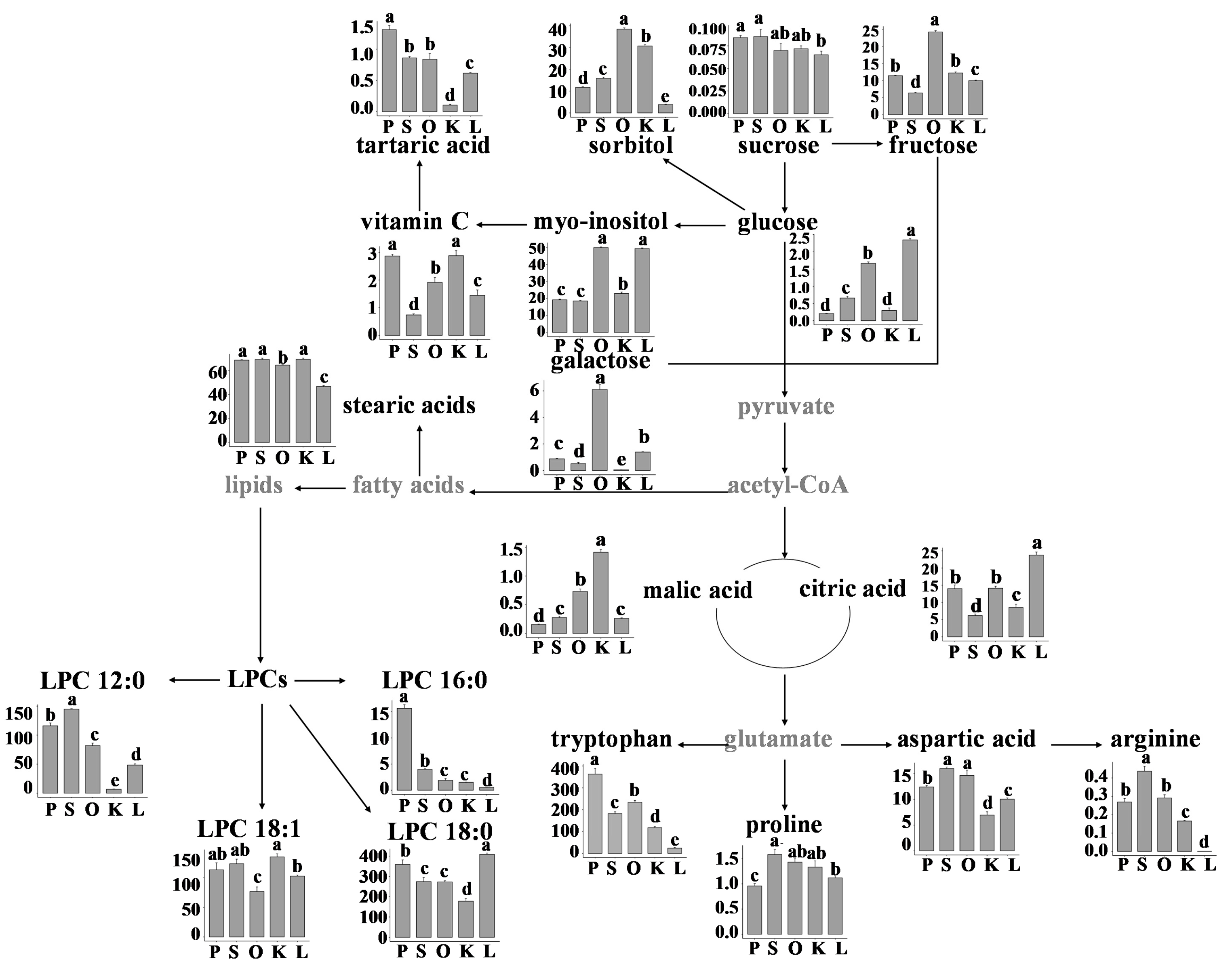
| Class I | Class II | Pomelo | Sweet Orange | Orah Mandarin | Kumquat | Lemon | Total |
|---|---|---|---|---|---|---|---|
| Amino acids and derivatives | Amino acids and derivatives | 67 | 67 | 66 | 63 | 65 | 67 |
| Nucleotides and derivatives | Nucleotides and derivatives | 38 | 38 | 38 | 37 | 38 | 38 |
| Lipids | Free fatty acids | 55 | 55 | 55 | 52 | 53 | 55 |
| Phosphatidylcholine | 1 | 1 | 1 | 1 | 1 | 1 | |
| Glycerol ester | 15 | 16 | 16 | 14 | 15 | 16 | |
| Lysophosphatidyl choline | 29 | 29 | 29 | 29 | 29 | 29 | |
| Lysophosphatidyl ethanolamine | 28 | 28 | 28 | 22 | 24 | 28 | |
| Others | Saccharides and alcohols | 45 | 45 | 44 | 40 | 44 | 45 |
| Vitamin | 13 | 13 | 13 | 13 | 12 | 13 | |
| Phenolic acids | 77 | 76 | 77 | 70 | 75 | 77 | |
| Organic acids | Organic acids | 50 | 50 | 50 | 46 | 43 | 50 |
| Total | 418 | 418 | 417 | 387 | 399 | 419 |
| Comparisons | Higher | Lower | Sum | Comparisons | Higher | Lower | Sum |
|---|---|---|---|---|---|---|---|
| Sweet orange vs. Pomelo | 30 | 28 | 58 | Kumquat vs. Sweet orange | 10 | 29 | 39 |
| Orah mandarin vs. Pomelo | 40 | 21 | 61 | Lemon vs. Sweet orange | 23 | 36 | 59 |
| Kumquat vs. Pomelo | 12 | 49 | 61 | Kumquat vs. Orah mandarin | 7 | 51 | 58 |
| Lemon vs. Pomelo | 28 | 39 | 67 | Lemon vs. Orah mandarin | 5 | 49 | 54 |
| Orah mandarin vs. Sweet orange | 42 | 20 | 62 | Lemon vs. Kumquat | 43 | 14 | 57 |
| Pomelo | Sweet Orange | Orah Mandarin | Kumquat | Lemon | |
|---|---|---|---|---|---|
| Pomelo | / | ||||
| Sweet orange | 73 | / | |||
| Orah mandarin | 78 | 77 | / | ||
| Kumquat | 79 | 79 | 78 | / | |
| Lemon | 70 | 76 | 70 | 75 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Cui, X.; Sun, M.; Huang, X.; Tang, K.; Hu, B.; Liao, H. Liquid Chromatography‒Tandem Mass Spectrometry Analysis of Primary Metabolites and Phenolic Acids Across Five Citrus Species. Curr. Issues Mol. Biol. 2025, 47, 223. https://doi.org/10.3390/cimb47040223
Peng Y, Cui X, Sun M, Huang X, Tang K, Hu B, Liao H. Liquid Chromatography‒Tandem Mass Spectrometry Analysis of Primary Metabolites and Phenolic Acids Across Five Citrus Species. Current Issues in Molecular Biology. 2025; 47(4):223. https://doi.org/10.3390/cimb47040223
Chicago/Turabian StylePeng, Yujiao, Xueyu Cui, Manman Sun, Xiaojuan Huang, Ke Tang, Baoqing Hu, and Hongze Liao. 2025. "Liquid Chromatography‒Tandem Mass Spectrometry Analysis of Primary Metabolites and Phenolic Acids Across Five Citrus Species" Current Issues in Molecular Biology 47, no. 4: 223. https://doi.org/10.3390/cimb47040223
APA StylePeng, Y., Cui, X., Sun, M., Huang, X., Tang, K., Hu, B., & Liao, H. (2025). Liquid Chromatography‒Tandem Mass Spectrometry Analysis of Primary Metabolites and Phenolic Acids Across Five Citrus Species. Current Issues in Molecular Biology, 47(4), 223. https://doi.org/10.3390/cimb47040223






